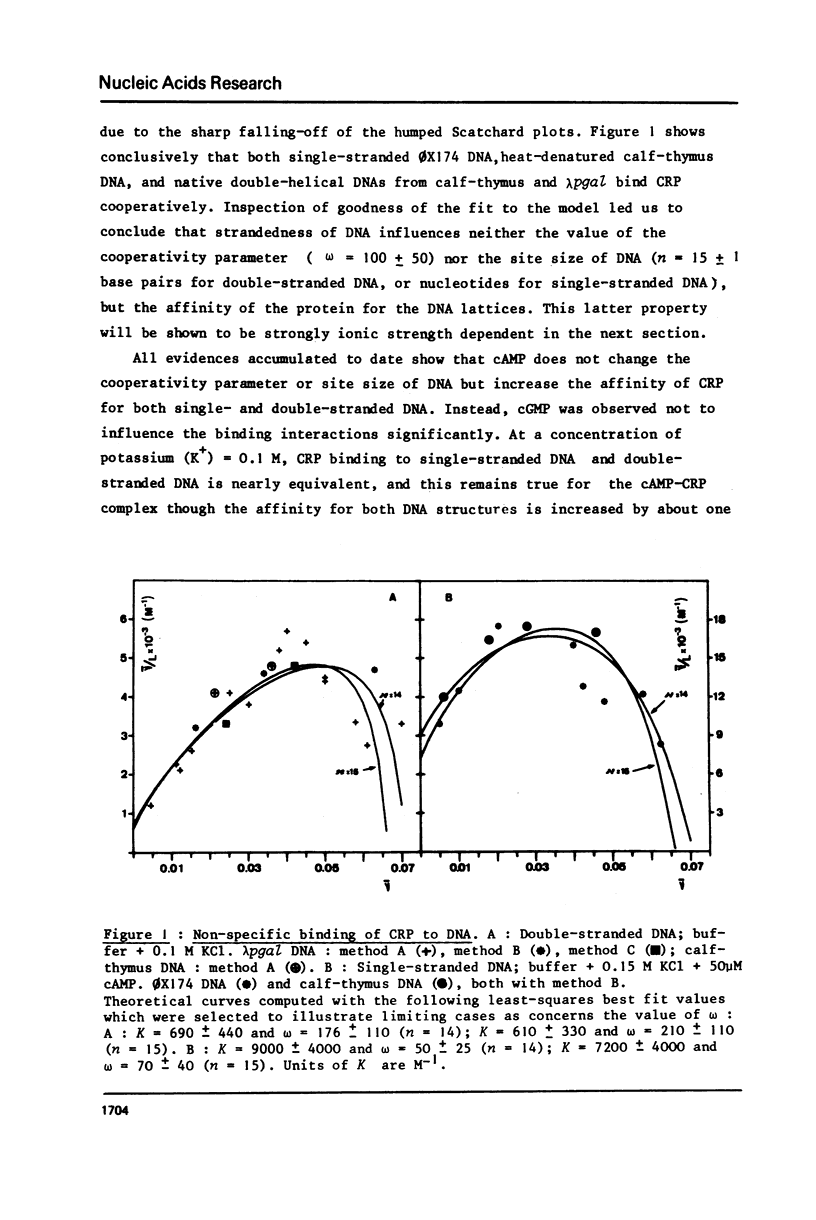
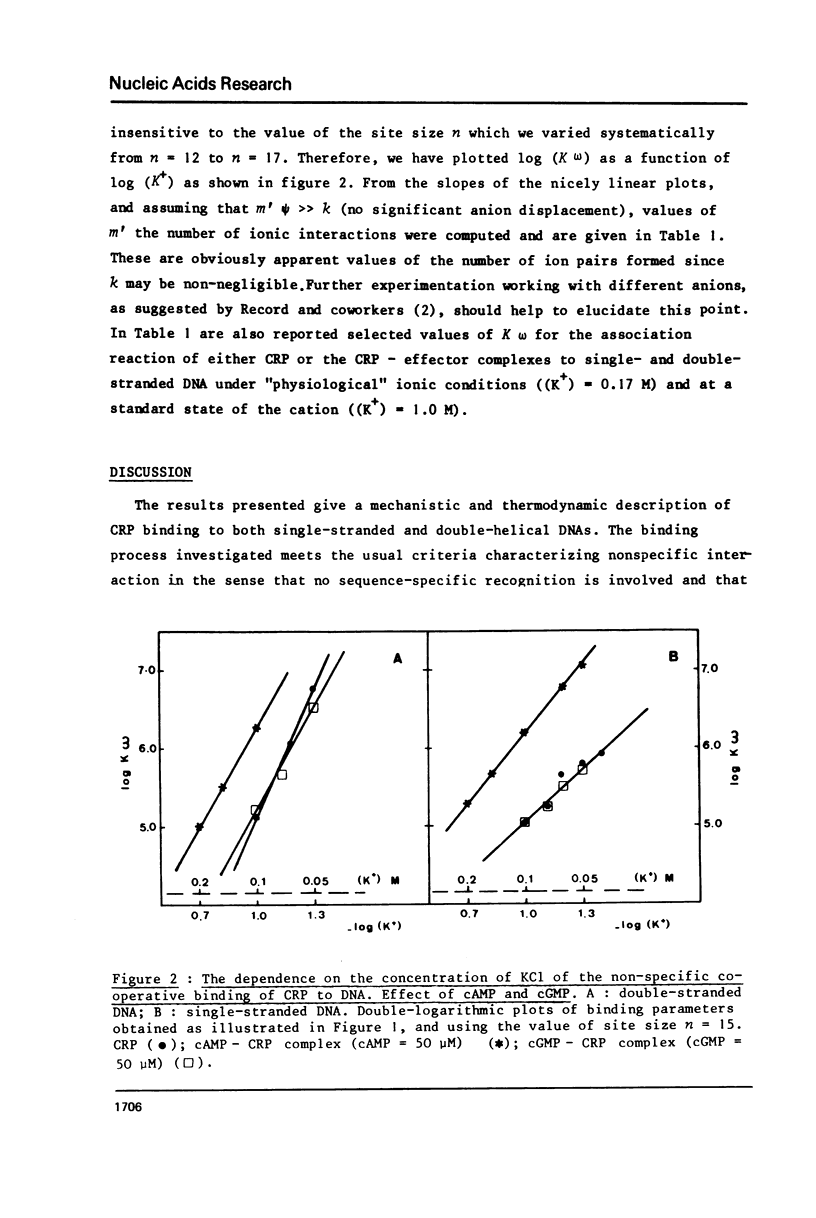
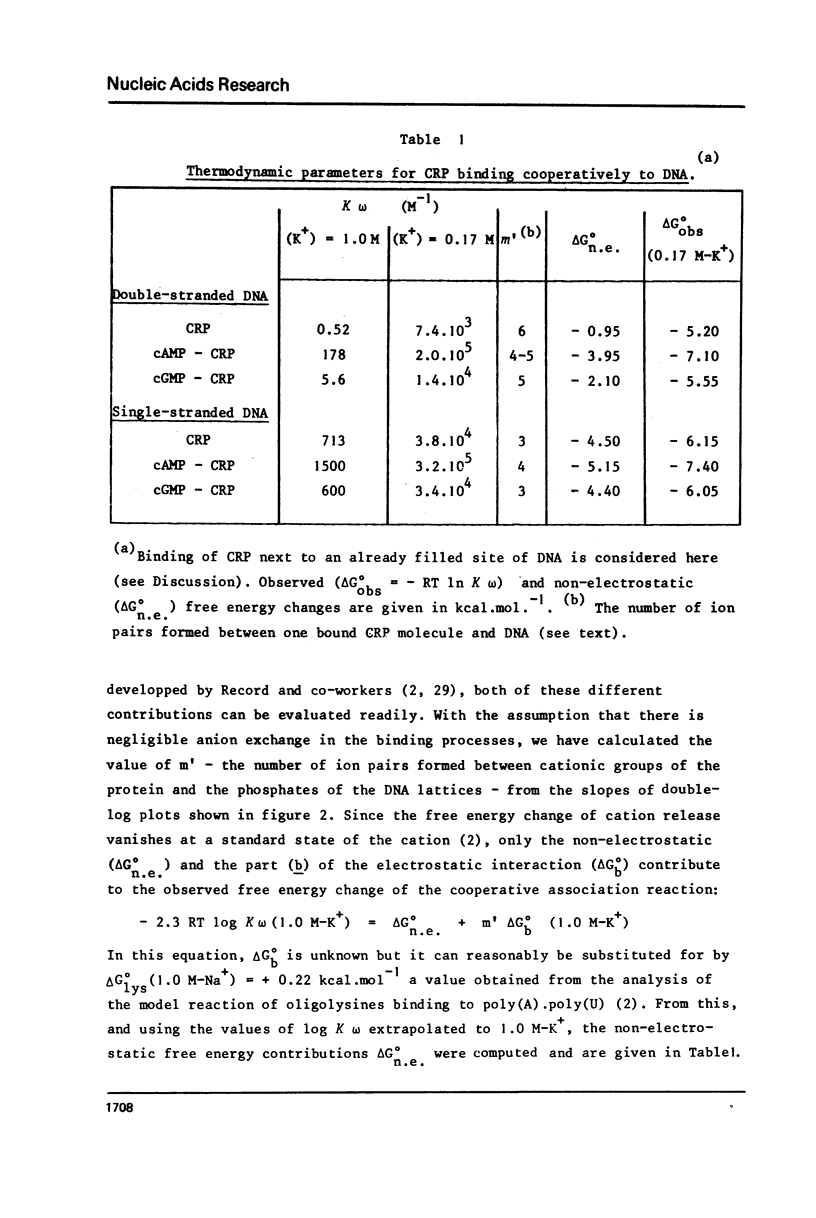
Abstract
The cyclic adenosine 3',5'-monophosphate receptor protein of Escherichia coli (CRP) binds cooperatively to single- and double-stranded DNA. Binding data could be fitted to the model of McGhee and von Hippel (1) and show that neither strandedness of DNA, nor the effectors cAMP and cGMP or the ionic strength (KCl) do change appreciably the cooperativity parameter omega (omega approximately or equal to 100), and site size of DNA. Instead, distinctly different slopes were observed for the linear decrease of log K omega (a measure of the overall affinity) as a function of log (K+). From these double-log plots (2), the number of cations released and the non-electrostatic contributions to the binding free energy could be determined. Binding of CRP to single-stranded DNA is slightly favored under physiological ionic conditions (0.15-0.20 M), but such a preferential binding is almost abolished in the presence of cAMP which increases the strength of the interaction of the protein with both forms of DNA. CGMP does not change the binding properties and interactions of CRP with DNA. These observations do not support the proposal that the cAMP-CRP complex could stimulate transcription via some "melting" property unless its interactions be dramatically changed when it binds specifically to promoter DNA.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Berkowitz S. A., Day L. A. Molecular weight of single-stranded fd bacteriophage DNA. High speed equilibrium sedimentation and light scattering measurements. Biochemistry. 1974 Nov 5;13(23):4825–4831. doi: 10.1021/bi00720a022. [DOI] [PubMed] [Google Scholar]
- Blazy B., Takahashi M., Baudras A. Coopérativité de la fixation non spécifique de la protéine réceptrice de l'adénosine 3'-5'-monophosphate cyclique (CRP) d'Escherichia coli sur les ADN double brin de thymus et lambda pgal. C R Seances Acad Sci D. 1979 May 7;288(17):1327–1330. [PubMed] [Google Scholar]
- Boone T., Wilcox G. A rapid high-yield purification procedure for the cyclic adenosine 3',5'-monophosphate receptor protein from Escherichia coli. Biochim Biophys Acta. 1978 Jul 17;541(4):528–534. doi: 10.1016/0304-4165(78)90162-9. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Hammes G. G. Activation of beef heart mitochondrial adenosine triphosphatase by 2,4-dinitrophenol. Biochemistry. 1973 Nov 20;12(24):4900–4904. doi: 10.1021/bi00748a014. [DOI] [PubMed] [Google Scholar]
- Eilen E., Krakow J. S. Cyclic AMP-mediated intersubunit disulfide crosslinking of the cyclic AMP receptor protein of Escherichia coli. J Mol Biol. 1977 Jul;114(1):47–60. doi: 10.1016/0022-2836(77)90282-0. [DOI] [PubMed] [Google Scholar]
- Eilen E., Pampeno C., Krakow J. S. Production and properties of the alpha core derived from the cyclic adenosine monophosphate receptor protein of Escherichia coli. Biochemistry. 1978 Jun 27;17(13):2469–2473. doi: 10.1021/bi00606a001. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. Helix-random coil transitions in synthetic DNAs of alternating sequence. J Mol Biol. 1962 Aug;5:172–184. doi: 10.1016/s0022-2836(62)80082-5. [DOI] [PubMed] [Google Scholar]
- Jensen D. E., von Hippel P. H. DNA "melting" proteins. I. Effects of bovine pancreatic ribonuclease binding on the conformation and stability of DNA. J Biol Chem. 1976 Nov 25;251(22):7198–7214. [PubMed] [Google Scholar]
- Kao-Huang Y., Revzin A., Butler A. P., O'Conner P., Noble D. W., von Hippel P. H. Nonspecific DNA binding of genome-regulating proteins as a biological control mechanism: measurement of DNA-bound Escherichia coli lac repressor in vivo. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4228–4232. doi: 10.1073/pnas.74.10.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow J. S. Cyclic adenosine monophosphate receptor: effect of cyclic AMP analogues on DNA binding and proteolytic inactivation. Biochim Biophys Acta. 1975 Apr 2;383(4):345–350. doi: 10.1016/0005-2787(75)90303-2. [DOI] [PubMed] [Google Scholar]
- Krakow J. S., Pastan I. Cyclic adenosine monophosphate receptor: loss of cAMP-dependent DNA binding activity after proteolysis in the presence of cyclic adenosine monophosphate. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2529–2533. doi: 10.1073/pnas.70.9.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G. S. On the application of polyelectrolyte "limiting laws" to the helix-coil transition of DNA. I. Excess univalent cations. Biopolymers. 1972;11(5):937–949. doi: 10.1002/bip.1972.360110502. [DOI] [PubMed] [Google Scholar]
- McConnell B., von Hippel P. H. Hydrogen exchange as a probe of the dynamic structure of DNA. II. Effects of base composition and destabilizing salts. J Mol Biol. 1970 Jun 14;50(2):317–332. doi: 10.1016/0022-2836(70)90195-6. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Nissley P., Anderson W. B., Gallo M., Pastan I., Perlman R. L. The binding of cyclic adenosine monophosphate receptor to deoxyribonucleic acid. J Biol Chem. 1972 Jul 10;247(13):4264–4269. [PubMed] [Google Scholar]
- Pampeno C., Krakow J. S. Cross-linking of the cAMP receptor protein of Escherichia coli by o-phenylenedimaleimide as a probe of conformation. Biochemistry. 1979 Apr 17;18(8):1519–1525. doi: 10.1021/bi00575a020. [DOI] [PubMed] [Google Scholar]
- Paulus H. A rapid and sensitive method for measuring the binding of radioactive ligands to proteins. Anal Biochem. 1969 Oct 15;32(1):91–100. doi: 10.1016/0003-2697(69)90107-9. [DOI] [PubMed] [Google Scholar]
- Perlman R. L., De Crombrugghe B., Pastan I. Cyclic AMP regulates catabolite and transient repression in E. coli. Nature. 1969 Aug 23;223(5208):810–812. doi: 10.1038/223810a0. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Lohman M. L., De Haseth P. Ion effects on ligand-nucleic acid interactions. J Mol Biol. 1976 Oct 25;107(2):145–158. doi: 10.1016/s0022-2836(76)80023-x. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, deHaseth P. L., Lohman T. M. Interpretation of monovalent and divalent cation effects on the lac repressor-operator interaction. Biochemistry. 1977 Nov 1;16(22):4791–4796. doi: 10.1021/bi00641a005. [DOI] [PubMed] [Google Scholar]
- Revzin A., von Hippel P. H. Direct measurement of association constants for the binding of Escherichia coli lac repressor to non-operator DNA. Biochemistry. 1977 Nov 1;16(22):4769–4776. doi: 10.1021/bi00641a002. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Reiness G., Zubay G. Purification and DNA-binding properties of the catabolite gene activator protein. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1222–1225. doi: 10.1073/pnas.68.6.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Wetmur J. G. Studies on the cooperative binding of the Escherichia coli DNA unwinding protein to single-stranded DNA. Biochemistry. 1975 Dec 16;14(25):5529–5534. doi: 10.1021/bi00696a023. [DOI] [PubMed] [Google Scholar]
- Saxe S. A., Revzin A. Cooperative binding to DNA of catabolite activator protein of Escherichia coli. Biochemistry. 1979 Jan 23;18(2):255–263. doi: 10.1021/bi00569a003. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Wu C. W., Wu F. Y. Conformational transitions of cyclic adenosine monophosphate receptor protein of Escherichia coli. A temperature-jump study. Biochemistry. 1974 Jun 4;13(12):2573–2578. doi: 10.1021/bi00709a016. [DOI] [PubMed] [Google Scholar]
- Wu F. Y., Nath K., Wu C. W. Conformational transitions of cyclic adenosine monophosphate receptor protein of Escherichia coli. A fluorescent probe study. Biochemistry. 1974 Jun 4;13(12):2567–2572. doi: 10.1021/bi00709a015. [DOI] [PubMed] [Google Scholar]
- Zubay G., Schwartz D., Beckwith J. Mechanism of activation of catabolite-sensitive genes: a positive control system. Proc Natl Acad Sci U S A. 1970 May;66(1):104–110. doi: 10.1073/pnas.66.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deHaseth P. L., Lohman T. M., Burgess R. R., Record M. T., Jr Nonspecific interactions of Escherichia coli RNA polymerase with native and denatured DNA: differences in the binding behavior of core and holoenzyme. Biochemistry. 1978 May 2;17(9):1612–1622. doi: 10.1021/bi00602a006. [DOI] [PubMed] [Google Scholar]
- deHaseth P. L., Lohman T. M., Record M. T., Jr Nonspecific interaction of lac repressor with DNA: an association reaction driven by counterion release. Biochemistry. 1977 Nov 1;16(22):4783–4790. doi: 10.1021/bi00641a004. [DOI] [PubMed] [Google Scholar]
- von Hippel P. H., Revzin A., Gross C. A., Wang A. C. Non-specific DNA binding of genome regulating proteins as a biological control mechanism: I. The lac operon: equilibrium aspects. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4808–4812. doi: 10.1073/pnas.71.12.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]


