Abstract
Objectives
Activated protein C (APC) has a protective efficacy against ischemia-reperfusion (I/R) injury in several organs. The objective of this study was to investigate effect of APC in myocardium with possible mechanism.
Methods
We used regional and global myocardial I/R injury models of rats. They consisted of I/R injuries (1) by ligation of left coronary artery, or (2) using Langendorff apparatus. Langendorff was used to focus the mechanism of APC excluding coagulation cascade in a working heart. Each experiment had an APC group (n = 10) and a control group with normal saline (n = 10). Injections of these solutions into rats were performed 30 minutes before the planned-I/R injury. Cardiac performance after the procedure was evaluated by echocardiography or indices with Langendorff apparatus. Coronary flow (CF) was measured in the global I/R injury model. Western blotting was performed to detect the change of AKT1 signal in myocardium after global I/R injury.
Results
LV function improved significantly in the APC group: %EF at 2 weeks after procedure, 70.8%±4.5% vs. 56.5%±0.7%; APC vs. control; p<0.01. Percent LV development pressure (LVDP) also improved in the APC group significantly, 88.8%±45.3% vs. 28.1%±15.4%; APC vs. control; p<0.01. In APC group, %CF improved significantly, 88.5%±15.8% vs. 65.0%±13.4%; APC vs. control; p<0.01. It was enhanced significantly when acetylcholine was administered; % CF: 103.5%±9.9% vs. 87.0%±12.1%; APC vs. control; p<0.05. Western blotting revealed that APC significantly induced activation of phosphorylated AKT1 in myocardium (p<0.05).
Conclusions
APC has a novel effect to protect myocardium and cardiac performance against I/R injury through improvement of endothelial function and activation of AKT1.
Introduction
Standard treatment for ischemic heart disease is revascularization of coronary artery. It is performed by percutaneous intervention or surgical procedure, using cardiopulmonary bypass (CPB). But revascularization induces ischemia-reperfusion (I/R) injury in the regional myocardium. And CPB and cardiac arrest during cardiac surgery can be a cause of global myocardial I/R injury. I/R injury relate to coagulation cascade in abnormal condition, inflammation, endothelial dysfunction, apoptosis and necrosis. To prevent organs from I/R injury, many effective drugs have been discovered by regulating these mechanisms [1], [2].
Activated protein C (APC) is a serine/threonine protease and a physiological anticoagulant. It regulates coagulation system by inactivating coagulation factors Va and VIIIa. And it also possesses anti-inflammatory and anti-apoptotic effect. These factors have been investigated using a variety of organs of animal models with I/R injury [3]–[14]. In particular, some reports focus anti-apoptotic effect of APC against myocardial I/R injury [15]. However details of the mechanism or signal of anti-apoptotic cascade are obscure. Our group has reported that APC improved I/R injury in spinal cord as neuroprotective effect through insulin like growth factor (IGF) and AKT1 cascade. AKT1 is one of signals that prevent from heart failure [16]. Therefore we focused APC and AKT1 signal against myocardial I/R injury in this study. We assessed the effect of APC for heart following regional or global I/R injury model of rat at first. And at next, we investigated the possible mechanisms including AKT1 in the global I/R injury model.
Materials and Methods
1. Animals and drugs
We used male Sprague-Dawley (SD) rats [body weight (BW) 250–300 g] provided by Nihon Dobutsu, Osaka, Japan. All experiments were approved by the animal care committee of Osaka University School of Medicine with ethics. We used recombinant human activated protein C (Anact C®; Teijin Pharmacy, Osaka, Japan).
2. Experimental design
We used regional and global myocardial I/R injury model of rat. They consisted of (1) ligation of left coronary artery (LCA) model and (2) isolated heart using Langendorff apparatus model. Langendorff apparatus was used to focus the mechanism of APC. This experiment had no component of coagulation cascade, because the solution in this model was not blood. We considered that LCA ligation model supported clinical situation that was not able to be experimented by Langendorff model alone. Each experiment had 10 rats in APC group and same numbers of rats in control group with normal saline.
3. Generation of the regional I/R injury model by LAD ligation
Surgical procedure
In APC group, 50 U (approximately 150 U/kg) of APC was injected into the abdominal cavity of each rat through the peritoneum (i.p.) 30 minutes before the procedure for I/R injury. After that, these rats were anesthetized with isoflurane. Endotracheal intubation was performed to them and mechanical ventilation was done during the procedure. After left thoracotomy in the fifth intercostal space, left ventricle was exposed. And transient myocardial ischemia was induced by ligation around LCA. The point of ligation was approximately 2–3 mm away from the origin of LCA, using a piece of 8-0 nylon suture. We confirmed the transient ischemia of LV by finding color of the area turned pale [17]. Ischemic time was 30 minutes. And then blood flow was re-perfused, resulting from removal of suture around LCA. At last, the wound was closed with 4-0 nylon sutures as rapidly as possible. After extubation, we observed rats for 4 hours after surgery. Then we kept these rats for evaluation of cardiac performance for 2 weeks.
Evaluation of regional LV function
LV function was evaluated by echocardiography (SONOS 7200®; Hewlett Packard, Loveland, CO, USA). The evaluator was another investigator who had no information about the experiment group, whether APC was used to rats or not. Ejection fraction (EF) (%), fractional shortening (FS) (%), end-diastolic area (EDA) (cm2) and end-systolic area (ESA) (cm2) were measured in the short axis view at the papillary muscle level, 1 and 2 weeks after the procedure. After that, rats were sacrificed for histological examination. EF and FS were calculated as %EF and %FS, with the values at each time were divided by those before ischemia.
Measurement of myocardial infarct area
We made five slices per heart after sacrifice of rat. And we measured infarct area at the level under the papillary muscles. Size of infarction was assessed by hematoxylin-eosin (HE) and Sirius red (SR) staining 2 weeks after the surgery. Staining allowed us to visualize the part of the myocardial infarction with fibrosis. We assessed reperfused area of LCA by injection of blue ink from the ostium of LCA. The size of infarct area was calculated as percentage of reperfused area using Image J (version 1.42).
4. Generation of the global I/R injury model by Langendorff apparatus with isolated heart
Surgical procedure and determination of LV function
Age-matched male SD rats (BW 250–300 g) were pretreated with heparin (100 U/kg, i.v.), followed injection of pentobarbital sodium. As soon as deep anesthesia had been induced to rats, their hearts were rapidly isolated in ice-slush and mounted in Langendorff system [18]. The method was as follows: the time required for initial stabilization was 20 minutes with perfusion of solution at a speed of 10 ml/min. The solution consists of NaCl, KCl, NaHCO3, Glucose, KH2PO4, MgSO4·7H2O and CaCl2. Several indices those are LV development pressure (LVDP) (mm Hg), the derivative of LV pressure (maximum (+) and minimum (−) dP/dt) (mm Hg/s), heart rate (HR) (bpm) and coronary flow (CF) (ml/min) were measured. +/− dP/dt reflects systolic and diastolic function of LV. After stabilization, the heart was arrested by stop the perfusion. Heart was maintained at 37°C for 30 minutes in an organ bath, filled with a degassed solution. After that, re-perfusion of gassed-solution to the heart was started for 120 minutes. Level of lactic acid dehydrogenase (LDH) in leakage from isolated heart during reperfusion was checked in all collected samples using the SRL© immuno-inhibition method. LDH is an index of myocardial damage.
As in experiment 1, rats in APC group were administered the drug i.p. 30 min before heart excision. The dose of APC was 50 U (approximately 150 U/kg; APC group), whereas controls were administered normal saline i.p.
Assessment of endothelial function with %CF
Percent CF was measured as an index of endothelial function. It was calculated as follows: the CF value during reperfusion was divided by that before ischemia [19]. Additional tests with nitroglycerin (NTG) or acetylcholine (Ach) were conducted during reperfusion. We administered each drug to the heart through the aorta 15 minutes after beginning of reperfusion, when their condition was stable. We assessed endothelial function of coronary artery with APC by these CF change.
Western blot analysis
A Western blot analysis was performed to investigate changes of AKT1 and phosphorylated AKT1 (p-AKT1) [7]. They are indicators of cell for survival signal [20]. As primary antibodies, goat polyclonal AKT1 (1∶1000; sc-7126; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse monoclonal p-AKT1 (Ser. 473) (1∶1000; 05-669; Upstate, Bedford, MA, USA) were used. Secondary antibodies were horseradish peroxidase-conjugated anti-goat IgG (1∶1000; sc-2020; Santa Cruz Biotechnology) and anti-mouse IgG (1∶1000). The blots were developed using the ECL Plus detection method (RPN2132; Amersham Bioscience Fairfield, CT, USA). A quantitative protein analysis was performed by Image J (1.42 version), followed Western blotting.
5. Statistical analysis
All data are shown as mean ± standard deviation. The statistical analysis was performed using an unpaired t-test for comparisons between two groups. Differences at p<0.05 were considered statistically significant.
Results
1. Effects of APC in the regional I/R injury model by LCA ligation
All of rats were alive until being sacrificed 2 weeks after the induction of regional I/R injury.
Regional LV function
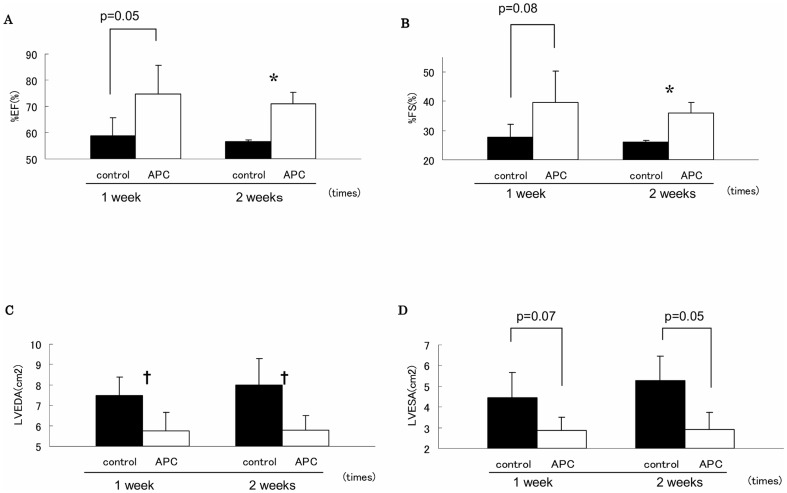
At 1 week after the procedure of I/R injury, %EF and %FS were higher in APC group than controls (%EF: APC group, 74.6%±10.9% vs. controls, 58.8%±6.9%; p = 0.05; %FS: APC group, 39.7%±10.5% vs. controls, 27.6%±4.5%; p = 0.08). LVEDA and LVESA were smaller in APC group than controls (LVEDA: APC group, 5.74±0.92 cm2 vs. controls, 7.47±0.93 cm2; p<0.05. LVESA: APC group, 2.85±0.65 cm2 vs. controls, 4.43±1.25 cm2; p = 0.07). At 2 weeks after the procedure, there were more significant differences in cardiac performance between these two groups (%EF: APC group, 70.8%±4.5% vs. controls, 56.5%±0.7%; p<0.01. %FS: APC group 35.9%±3.6% vs. controls, 26.0%±0.49%; p<0.01; LVEDA: APC group, 5.79±0.7 cm2 vs. controls, 8.00±1.3 cm2; p<0.05; LVESA: APC group, 2.89±0.85 cm2 vs. controls, 5.28±1.18 cm2; p = 0.05, Figure 1).
Figure 1. Assessment of cardiac performance in the regional ischemia/reperfusion injury model.
We evaluated cardiac function by ultrasound cardiography at 1 and 2 weeks after the procedure in controls and the activated protein C (APC) group (n = 10/group). The parameters were percent ejection fraction (EF), percent fractional shortening (FS), left ventricular end diastolic area (LVEDA) and left ventricular end systolic area (LVESA). Significant differences were observed between the two groups at 2 weeks after the procedure. Open circle indicate APC group and closed circle indicate controls. (*P<0.05; APC group versus controls, **P<0.01; APC group versus controls).
Size of infarction
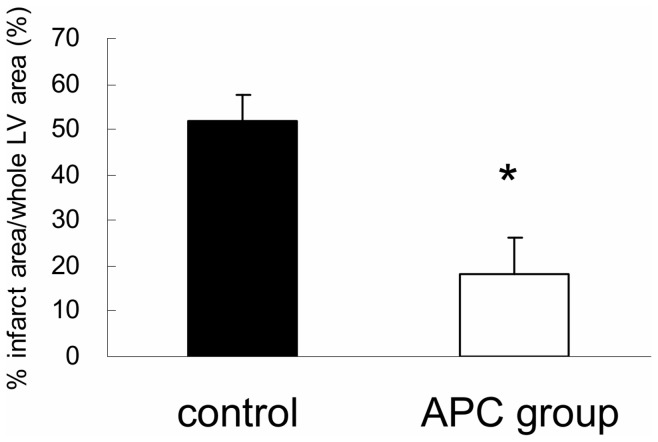
Infarction which was induced by LCA ligation were not lost following APC administration. However, size of infarction was significantly smaller in APC group than controls. It was based on HE and SR staining (% infarction area/whole LV area: APC group, 18.3%±7.6% vs. controls, 51.7%±5.8%; p<0.05, Fig. 2).
Figure 2. Assessment of myocardial infarct size after regional ischemia/reperfusion injury.
We stained slices of left ventricular (LV) tissue with hematoxylin and eosin and Sirius red 2 weeks after inducing transient ischemia. The size of the infarct area was assessed by calculating the percentage of total LV area (% infarction) using Image J. The bar graph shows that the percent infarction in the activated protein C (APC) group was significantly smaller than the controls (* p<0.05; APC versus controls).
2. Effects of APC in the global I/R injury model by Langendorff apparatus
Global LV function
Percent LVDP, % +LV dP/dt, and %−LV dP/dt increased significantly in APC group than controls (% LVDP: APC group, 88.8%±45.3% vs. controls, 28.1%±15.4%; p<0.01. %+dP/dt: APC group, 102.9%±40.4% vs. controls, 23.1%±6.4%; p<0.01. %−dP/dt: APC group, 88.7%±45.8% vs. controls, 20.9%±5.4%; p<0.01) (Table 1). No statistical change was observed between these the two groups for the occurrence of arrhythmia, an index of myocardial damage.
Table 1. Assessment of cardiac performance in the global ischemia/reperfusion (I/R) injury model.
| Group | Controls | APC group | P value |
| %LVDP (%) | 28.1±15.4 | 61.5±44.8 | <0.01 |
| %+dP/dt (%) | 23.1±6.4 | 39.6±13.2 | <0.01 |
| %−dP/dt (%) | 20.9±5.4 | 36.7±12.9 | <0.01 |
| LDH (U/L) | 9.5±4.2 | 4.4±2.5 | 0.08 |
Percent left ventricular development pressure (LVDP), maximum dP/dt (+dP/dt), and minimum dP/dt (−dP/dt) were used as indices of LV function in the global I/R injury model. Significant differences were observed between controls and the activated protein C (APC) group for all indices (p<0.01). No significant difference was found between the two groups for lactate dehydrogenase (LDH), an index of tissue injury (p = 0.08), but a tendency for a decrease in leakage of LDH from the myocardium after APC administration was observed.
Level of LDH during reperfusion
The level of LDH leakage from heart decreased in APC group than controls (APC group, 4.4±2.5 U vs. controls, 9.5±4.2 U; p = 0.08) (Table 1).
Endothelial function of coronary arteries
Percent CF increased significantly in APC group than controls (APC group, 88.5%±15.8% vs. controls, 65.0%±13.4%; p<0.01). And %CF after administration of Ach. also increased significantly in APC group than controls (%CF: APC group, 103.5%±9.9% vs. controls, 87.0%±12.1%; p<0.05). In the contrast, there was no difference of %CF after administration of NTG between these two groups (Table 2).
Table 2. Assessment of endothelial function in the global injury model.
| group | controls | APC group | P value |
| %CF (%) | 65.0±13.4 | 88.5±15.8 | <0.01 |
| %CF with Ach. (%) | 87.0±12.1 | 103.5±9.9 | <0.01 |
| %CF with NTG. (%) | 109.8±15.0 | 112.4±8.0 | 0.74 |
Percent coronary flow (CF) increased in the activated protein C (APC) group compared with controls (p<0.01). Different vasodilator drugs were administered to investigate the response of enhanced endothelial function. One of them, acetylcholine (Ach), increased %CF only in the APC group (p<0.01, vs. controls), whereas nitroglycerine (NTG) increased %CF in both groups (p = 0.74).
Activation of AKT by Western blotting
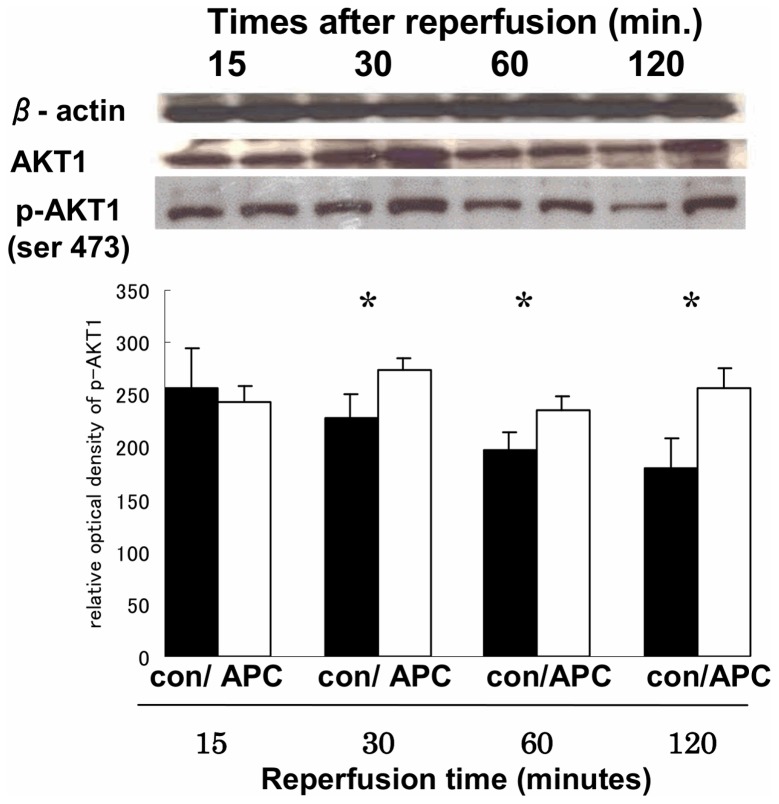
In APC group, p-AKT1 (phosphorylation at serine 473 residues) was significantly expressed at 30, 60, and 120 minutes after reperfusion than controls (p<0.05 vs. controls) (Fig. 3).
Figure 3. Representative Western blots for AKT1 and p-AKT1.
The photograph shows Western blots of myocardium for β-actin, whole AKT1, and p-AKT1in the global ischemia/reperfusion injury model during the time course. AKT1 was strongly detectable 30 min after reperfusion in the activated protein C (APC) group (p = 0.08, vs. control). The bar graph shows that p-AKT1 in the APC group was strongly and significantly expressed 30, 60, and 120 min after reperfusion, (* p<0.05; APC group versus controls).
Discussion
We demonstrated that APC preserved myocardial function against I/R injury in the current study. The experiment with LCA ligation as a regional I/R injury model is nearly to a clinical situation of ischemic heart disease. It revealed that APC improved LV function and decreased infarct area significantly. In the next experiment with Langendorff apparatus as a global I/R injury model without blood component, APC improved endothelial function of coronary artery and induced activation of AKT1 with phosphorylation (Ser 473 residues) significantly. We newly established an efficacy of APC against myocardial I/R injury by improvement of endothelial function and activation of AKT1 in this time.
APC has been useful for treating severe sepsis through its anti-coagulation and anti-inflammatory effects [3]–[14]. It was reported that APC was associated with improvement of cardiovascular function in the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study [21], [22]. Few studies have indicated that APC exerts a protective effect against myocardial I/R injury in vivo model with possible mechanism, although I/R injury is major damage that heart can sustain [15].
Some mechanism of APC against myocardial I/R injury in vitro model have been reported: anti-apoptosis with reduction of TUNEL-positive cells [21], inhibiting apoptosis and inflammation, which are regulated by protease activated receptor (PAR)-1 [22] and so on. PAR is a receptor of endothelial cell. However, no findings were reportedfor anti-apoptotic signal in detail.
In contrast, a variety of substances or pathways which are triggered by administration of APC have been reported. They are P38 mitogen-activated protein kinase [23], nuclear factor κB [24], tumor necrosis factor-α and p53-mediated pathway [4], [25]. In particular, PI3K-AKT pathway is focused as survival signal during heart failure [26]–[28]. Our group have reported that APC has a neuro-protective effect through AKT cascade after I/R injury of spinal cord in rabbits [9]. Thus we selected AKT1 as one of the possible mechanisms which were induced by APC against myocardial I/R injury. And there was sub-possible cascade with AKT1 in detail. AKT1 is a family of serine/threonine kinases consisting of at least 10 isoforms. It has been reported that different an isoform of AKT1 has counter regulatory on coronary endothelial permeability [29].
In the current study, we used p-AKT1 with serine 473 residues as primary antibody in Western blotting. In addition to this, we performed same experiment using p-AKT1 with threonine 308 residues although we didn't showed in this manuscript. The former experiment showed activation of AKT1 signal with phospholyration after myocardial I/R injury by expression in Western blotting. However the latter one didn't it at all. These results might have a new point of view that APC improve cardiac performance after I/R injury by varied pathways.
In the current study, we also found that APC improved endothelial function as another phenomenon which was related to cardiac performance. Endothelial function is one of the important components maintaining blood fluidity and cardiovascular homeostasis [30], [31]. Of course, activation of AKT1 and improvement of endothelial function induced by APC may be independent factors each others. Finigan et al. reported that APC mediates lung endothelial barrier enhancement with cross talk between endothelial protein C receptor (EPCR) and sphingosine 1-phosphate (S1P1) [13]. Feistritzer et al. stated that p-AKT enhances lung endothelial barrier function by affecting cross activation between the S1P receptor-1 and PAR on endothelium [8]. S1P is a potent endothelial cell barrier-enhancing agonist. PAR and EPCR are component of endothelium. They play a critical role in anti-apoptotic cascade which is induced by APC against myocardial I/R injury [5]. According to these previous reports, we might be able to suggest that APC induced a homeostatic effect on coronary flow by improvement of endothelial function with activation of AKT cascade. And we can add the result that p-AKT1 with serine 473 residues is more possible signal in the mechanism of AKT1-endothelial function for cardiac performance, as mentioned above.
Study limitations
We were unable to clearly elucidate the overall mechanism that APC protected myocardium against I/R injury [32]. We should further investigate the dose depending on the effect induced by APC. We also did not analyze the activity of a pivotal vasodilator, nitric oxide (NO). Further studies of NO may be needed to develop the mechanism for endothelial function. Especially the correlation between endothelial function and AKT1 should be focused in detail [33].
Conclusion
APC has a novel effect to protect myocardium and cardiac performance against I/R injury, possibly through improvement of endothelial function and activation of AKT1.
Funding Statement
No current external funding sources for this study.
References
- 1. Liakopoulos OJ, Teucher N, Muhlfeld C (2006) Prevention of TNFalpha-associated myocardial dysfunction resulting from cardiopulmonary bypass and cardioplegic arrest by glucocorticoid treatment. Eur J Cardiothorac Surg 30: 263–270. [DOI] [PubMed] [Google Scholar]
- 2. Aikawa R, Nawano M, Gu Y (2000) Insulin prevents cardiomyocytes from oxidative stress-induced apoptosis through activation of PI3 kinase/Akt. Circulation 102: 2873–2879. [DOI] [PubMed] [Google Scholar]
- 3. Mosnier LO, Zlokovic BV, Griffin JH (2007) The cytoprotective protein C pathway. Blood 109: 3161–3172. [DOI] [PubMed] [Google Scholar]
- 4. Griffin JH, Fernandez JA, Liu D, Cheng T, Guo H, et al. (2004) Activated protein C and ischemic stroke. Crit Care Med 32: S247–253. [DOI] [PubMed] [Google Scholar]
- 5. Mosnier LO, Griffin JH (2003) Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem J 373: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nick JA, Coldren CD, Geraci MW (2004) Recombinant human activated protein C reduces human endotoxin-induced pulmonary inflammation via inhibition of neutrophil chemotaxis. Blood 104: 3878–3885. [DOI] [PubMed] [Google Scholar]
- 7. Abraham E (2005) Effects of recombinant human activated protein C in human models of endotoxin administration. Proc Am Thorac Soc 2: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feistritzer C, Riewald M (2005) Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 105: 3178–3184. [DOI] [PubMed] [Google Scholar]
- 9. Yamauchi T, Sakurai M, Abe K, Takano H, Sawa Y (2006) Neuroprotective effects of activated protein C through induction of insulin-like growth factor-1 (IGF-1), IGF-1 receptor, and its downstream signal phosphorylated serine-threonine kinase after spinal cord ischemia in rabbits. Stroke 37: 1081–1086. [DOI] [PubMed] [Google Scholar]
- 10. Guo H, Liu D, Gelbard H (2004) Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron 41: 563–572. [DOI] [PubMed] [Google Scholar]
- 11. Yamanel L, Mas MR, Comert B (2005) The effect of activated protein C on experimental acute necrotizing pancreatitis. Crit Care 9: R184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mizutani A, Okajima K, Uchiba M, Noguchi T (2000) Activated protein C reduces ischemia/reperfusion-induced renal injury in rats by inhibiting leukocyte activation. Blood 95: 3781–3787. [PubMed] [Google Scholar]
- 13. Finigan JH, Dudek SM, Singleton PA (2005) Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem 280: 17286–17293. [DOI] [PubMed] [Google Scholar]
- 14. Jakson CJ, Xue M, Thompson P (2005) Activated protein C prevents inflammation yet stimulates angiogenesis to promote cutaneous wound healing. Wound Repair Regen 13: 284–294. [DOI] [PubMed] [Google Scholar]
- 15. Fernandez JA, Vento AE, Jormalainen M (2006) Activated protein C in the cardioplegic solution on a porcine model of coronary ischemia-reperfusion has deleterious hemodynamic effects. Cardiovasc Drugs Ther 20: 113–121. [DOI] [PubMed] [Google Scholar]
- 16. Morissette MR, Rosenzweig A (2005) Targeting survival signaling in heart failure. Curr Opin Pharmacol 5: 165–170. [DOI] [PubMed] [Google Scholar]
- 17. Fukui S, Kitagawa-Sakakida S, Sawa Y (2008) Therapeutic effect of midkine on cardiac remodeling in infarcted rat hearts. Ann Thirac Surg 85: 562–70. [DOI] [PubMed] [Google Scholar]
- 18. Ono M, Sawa Y, Ryugo M (2005) BH4 peptide derivative from Bcl-xL attenuates ischemia/reperfusion injury thorough anti-apoptotic mechanism in rat hearts. Eur J Cardiothorac Surg 27: 117–121. [DOI] [PubMed] [Google Scholar]
- 19. Chang WL, Chung CH, Wu YC, Su MJ (2004) The vascular and cardioprotective effects of liriodenine in ischemia-reperfusion injury via NO-dependent pathway. Nitric Oxide 11: 307–315. [DOI] [PubMed] [Google Scholar]
- 20. Nagoshi T, Matsui T, Aoyama T (2005) PI3K rescues the detrimental effects of chronic Akt activation in the heart during ischemia/reperfusion injury. J Clin Invest 115: 2128–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Isobe H, Okajima K, Uchiba M (2001) Activated protein C prevents endotoxin-induced hypotension in rats by inhibiting excessive production of nitric oxide. Circulation 104: 1171–1175. [DOI] [PubMed] [Google Scholar]
- 22. Favory R, Lancel S, Marechal X, Tissier S, Neviere R (2006) Cardiovascular protective role for activated protein C during endotoxemia in rats. Intensive Care Med 32: 899–905. [DOI] [PubMed] [Google Scholar]
- 23. Pirat B, Muderrisoglu H, Unal MT (2007) Recombinant human-activated protein C inhibits cardiomyocyte apoptosis in a rat model of myocardial ischemia-reperfusion. Coron Artery Dis 18: 61–66. [DOI] [PubMed] [Google Scholar]
- 24. Loubele ST, Spek A, Leenders P, van Oerle R, Aberson HL, et al. (2009) Activated Protein C Protects Against Myocardial Ischemia/Reperfusion Injury via Inhibition of Apoptosis and Inflammation. Arterioscler Tromb Vasc Biol 29: 29: 1087–1092. [DOI] [PubMed] [Google Scholar]
- 25. Uchiba M, Okajima K, Oike Y (2004) Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ Res 95: 34–41. [DOI] [PubMed] [Google Scholar]
- 26. Joyce DE, Grinnell BW (2002) Recombinant human activated protein C attenuates the inflammatory response in endothelium and monocytes by modulating nuclear factor-kappaB. Crit Care Med 30: S288–293. [DOI] [PubMed] [Google Scholar]
- 27. Cheng T, Liu D, Griffin JH (2003) Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med 9: 338–342. [DOI] [PubMed] [Google Scholar]
- 28. Matsui T, Tao J, del Monte F (2001) Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104: 330–335. [DOI] [PubMed] [Google Scholar]
- 29. Gao F, Gao E, Yue TL (2002) Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation 105: 1497–1502. [DOI] [PubMed] [Google Scholar]
- 30. Gaudreault N, Perrin RM, Guo M, Clanton CP, Wu MH, et al. (2008) Counter regulatory effets of PKCβII and PKCδ on coronary endothelial permeability. Arterioscler Thromb Vas Biol 28: 1527–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rubanyi GM (1993) The role of endothelium in cardiovasucular homeostasis and diseases. J Cardiovasc Pharmacol 22: S1–14. [DOI] [PubMed] [Google Scholar]
- 32. Swaidi JA, Hamasaki S, Higano ST (2000) Long-Term Follow-Up of Patients With Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation 101: 948–954. [DOI] [PubMed] [Google Scholar]
- 33. Dossumbekova A, Berdyshev EV, Gorshkova I (2008) Akt activates NOS3 and separately restores barrier integrity in H2O2-stressed human cardiac microvascular endothelium. Am J Physiol Heart Circ Physiol 295: 2417–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]