Abstract
Altered mitochondrial DNA (mtDNA) levels have been associated with common diseases in humans. We investigated the genetic mechanism that controls mtDNA levels using genome-wide linkage analyses in families from the Genetic Analysis of Idiopathic Thrombophilia Project (GAIT). We measure mtDNA levels by quantitative real-time PCR in 386 subjects from 21 extended Spanish families. A variance component linkage method using 485 microsatellites was conducted to evaluate linkage and to detect quantitative trait loci (QTLs) involved in the control of mtDNA levels. The heritalibility of mtDNA levels was 0.33 (p = 1.82e-05). We identified a QTL on Chromosome 2 (LOD = 2.21) using all of the subjects, independently on their sex. When females and males were analysed separately, three QTLs were identified. Females showed the same QTL on Chromosome 2 (LOD = 3.09), indicating that the QTL identified in the analysis using all of the subjects was a strong female QTL, and another one on Chromosome 3 (LOD = 2.67), whereas in males a QTL was identified on Chromosome 1 (LOD = 2.81). These QTLs were fine-mapped to find associations with mtDNA levels. The most significant SNP association was for the rs10888838 on Chromosome 1 in males. This SNP mapped to the gene MRPL37, involved in mitochondrial protein translation. The rs2140855 on Chromosome 2 showed association in the analysis using all of the subjects. It was near the gene CMPK2, which encodes a mitochondrial enzyme of the salvage pathway of deoxyribonucleotide synthesis. Our results provide evidence of a sex-specific genetic mechanism for the control of mtDNA levels and provide a framework to identify new genes that influence mtDNA levels.
Introduction
Mitochondria are the only cellular organelles (apart from the nucleus) that contain a genome, the mitochondrial DNA (mtDNA). It is inherited exclusively through the maternal line. Human mtDNA consist of one circular double-stranded DNA molecule of 16569 bp, which presents different characteristics compared with the nuclear genome. It is about 0.5–1% of the total DNA human cell content. A variable number of mtDNA molecules are located within the mitochondrial matrix, attached to the inner mitochondrial membrane near the oxidative phosphorilation (OXPHOS) system. The mitochondrial genome encodes few, but essential proteins of the OXPHOS system (13 polypeptides, 22 tRNA and 2 rRNA). Then, any defect of mtDNA can lead finally to mitochondrial dysfunction and energetic cell impairment. Thus, mitochondria play a pivotal role in cell life and death in almost all eukaryotic cells, as they are vital organelles involved in cellular energy production and apoptosis [1]. However, most of the mitochondrial proteins are encoded by the nuclear DNA and synthesized into the cytoplasm, from which they are imported to mitochondria. Consequently, the mitochondrial biogenesis depends on the coordinated expression of both nuclear and mitochondrial genomes.
Mitochondria are considered the major source of reactive oxygen species (ROS) and mtDNA is one of the main targets of oxidative stress. During the last years, a large number of clinical disorders have been linked to mitochondrial dysfunction where oxidative stress was the main pathophysiologic underlying mechanism. In addition, sequence variation in mtDNA contributes to the risk of disease and it has been associated with serious human disorders [2], [3], [4]. Also, sequence variation of the mtDNA may be linked to functional differences and thereby lead to different oxidative phenotypes [5]. Interestingly, the variation of appropriate mtDNA levels has been linked also to a variety of common disorders [6], [7], [8]. In addition, it has been reported that altered mtDNA levels correlates with malignancy [9], [10], [11] and even with prognosis [12], [13]. Moreover, there are several reports that attempt to establish mtDNA quantity as an oxidative stress biomarker to predict the risk for cancer [14], [15].
Some of the mitochondria-associated disorders include obesity, cancer, diabetes, neurodegenerative diseases, sepsis, ischemia/reperfusion injury as well as the physiologic process of ageing [16], [17], [18]. In addition, there is also increasing evidence supporting that oxidative stress plays a key role in the development of cardiovascular diseases and atherosclerosis [19], [20], [21]. As a consequence, there is a growing interest in describing the mechanisms that regulate the mitochondrial biogenesis. Particularly, much effort is being put towards increasing our knowledge about the maintenance of the mitochondrial genome, since both the integrity and quantity of mtDNA are critical for maintaining mitochondrial respiratory capacity to reach the ATP levels necessary for cell viability.
Despite the identification of a number of nuclear DNA-encoded trans-acting factors that regulate mitochondrial biogenesis, the exact biological mechanisms responsible for the control of mtDNA levels are unknown. The aim of our study was to identify genes that influence the variation of mtDNA levels and to determine if sex modulates the genetic mechanism that controls these levels. For this purpose, we conducted genome-wide linkage analyses in families from the Genetic Analysis of Idiopathic Thrombophilia Project (GAIT). In addition, we fine-maped the genetic determinants detected by the linkage analyses.
Methods
Subjects
Our study included 386 subjects belonging to 21 Spanish families from the GAIT Project. An extensive description of the GAIT Project has been published previously [22]. Each family consisted of 3 to 5 generations of subjects that ranged in age from 0.34 to 87.9 years, with a mean of 37.6 years. There were a similar number of males and females in our study. Twelve families were selected through a proband with idiopathic thrombophilia and the remaining 9 families were randomly selected without regard to phenotype. Thrombophilia was defined as recurrent thrombotic episodes (at least one of which was spontaneous) a single spontaneous thrombotic event with a first-degree relative also affected, or early-onset thrombosis (≤45 years). The proband's thrombophilia was considered idiopathic when all known biological causes of thrombophilia (i.e. Protein S and Protein C deficiencies, antithrombin deficiency, activated protein C resistance, plasminogen deficiency) at the time of recruitment were excluded. Subjects that had a personal or familiar history suggestive of mitochondrial disease or neuromuscular disorders were excluded.
Ethics Statement
The Institutional Review Board of the Hospital de la Santa Creu i Sant Pau (Barcelona) approved all protocols of the study. Adult subjects gave informed consent for themselves and for their minor children.
Blood Collection and DNA Extraction
Blood was collected by venipuncture in Vacutainer® Citrate tubes from fasting subjects. Thrombophilic participants were not using oral anticoagulants at the time of sampling.
DNA was extracted by a standard salting-out procedure from the buffy coat [23] and used for quantifying and genotyping the mtDNA.
Genotyping with Microsatellites and SNPs
All subjects were genotyped for a genome-wide scan including 485 microsatellite markers distributed through the autosomal genome at an average interval of 7.1 cM. The average heterozygosity of the microsatellite markers was 0.79. Microsatellites consisted primarily of the ABI Prism Linkage Mapping Set MD-10 (Applied Biosystems, Foster City, CA). Linkage mapping was undertaken with the PE LMS II fluorescent marker set (ABI Prism, Foster City, CA) with multiplex polymerase chain reaction (PCR) [24]. The products from the PCR were analyzed on the PE 310, PE 377, and PE 3700 automated sequencers, and genotyped using the Genotyper software. Information on microsatellite markers can be found in the public-accessible genomic database (http://www.gdb.org). Marker maps for multipoint analyses were obtained from the Marshfield Medical Research Organization (http://research.marshfieldclinic.org/genetics/).
Fine-mapping of linkage regions was carried out using 4,448 SNPs from the Illumina platform (San Diego, CA, USA). The SNPs with a genotype call rate<0.95, a minor allele frequency <0.025 or failed the Hardy-Weinberg equilibrium (HWE) test (p<5e-7) were excluded from the analysis. HWE was ascertained by a standard χ2 with 1 degree of freedom and was tested using parental data only.
The program INFER (PEDSYS) [25] was used to check for Mendelian inconsistencies in the genotypic data. Mistypings and markers for discrepant subjects were either corrected or excluded from the analyses.
Phenotyping: Quantitative Analysis of mtDNA
Quantitative real-time PCR (ABI Prism 7000 Sequence Detector System, Applied Biosystems, Foster City, CA) was used to determine mtDNA levels in total DNA from the buffy coat of each GAIT individual. For each DNA extract, the highly conserved mitochondrial ND2 gene (mtDNA) and the housekeeping 18S rRNA nuclear gene (nDNA) were quantified separately (Power SYBR® Green PCR Master Mix, Applied Biosystems, Foster City, CA). A double-stranded DNA dye (SYBR Green I) was used to monitor product formation continuously [26] and ROX dye was used as reference.
The PCR amplification of a 235 bp fragment length of the ND2 gene was performed by using the forward 5′-GCCCTAGAAATAAACATGCTA-3′ and the reverse 5′-GGGCTATTCCTAGTTTTATT-3′ primers. For the 18S rRNA gene, the forward 5′-ACGGACCAGAGCGAAAGCAT-3′ and the reverse 5′-GGACATCTAAGGGCATCACAGAC-3′ primers were used for the amplification of a 531 bp fragment length.
PCR conditions for the mitochondrial gene
The PCR reactions for the amplification of the ND2 gene contained 250 nM of each primer, 10 µl of the Power SYBR® Green PCR Master Mix (2×) and 2 ng of the DNA extract in 20 µl volume. The ABI Prism absolute quantification programme conditions for ND2 consisted of a single denaturation-enzyme-activation of 10 min at 95°C, followed by 35 cycles of amplification. Each cycle consisted of a denaturation step of 15 sec at 95°C, an annealing step of 20 sec at 53°C and an extension step of 30 sec at 72°C.
PCR conditions for the nuclear gene
The PCR reactions for the amplification of the 18S rRNA gene contained 300 nM of each primer, 10 µl of the Power SYBR® Green PCR Master Mix (2×) and 2 ng of the DNA extract in 20 µl volume. The ABI Prism absolute quantification programme conditions for 18S rRNA consisted of a single denaturation-enzyme-activation of 10 min at 95°C, followed by 37 cycles of amplification. Each cycle consisted of a denaturation step of 15 sec at 95°C, an annealing step of 20 sec at 66°C and an extension step of 40 sec at 72°C.
After the amplification of each gene, a dissociation curve analysis was carried out to determine the specificity of the PCR product by the melting temperature [27].
A standard curve for each gene was always included in each PCR run by amplifying serial dilutions of a control human genomic DNA (TaqMan® Control Genomic DNA (Human; 10 ng/ul), Applied Biosystems, Foster City, CA). The quantity (pg) of each target sequence (mitochondrial and nuclear) was calculated from the corresponding standard curve. Two nanograms of the control human genomic DNA was also added in each PCR run as a positive control. As a negative control, the template DNA was replaced with PCR-grade water. The results of the mtDNA levels were expressed as mtDNA quantity to nuclear DNA quantity ratio (mtDNA/nDNA) [28], [29].
Linkage and Association Analyses
A standard multipoint variance component linkage method was used to assess linkage between autosomal markers and mtDNA levels using the Sequential Oligogenic Linkage Analysis Routines (SOLAR) v. 4.0 software package [30]. Age, sex, smoking behaviour, oral contraceptives use and the household effect were included in the variance component framework as linear predictors of the phenotype. Previous studies suggested that such a method might be vulnerable to deviations from multivariate normality and particularly to high levels of kurtosis in the trait's distribution, giving inflated logarithm of odds (LOD) scores [31]. Levels of mtDNA in the GAIT sample exhibited a kurtosis of 0.57, which does not affect the distribution of LOD scores. Thus, the standard nominal p-values for LOD scores are appropiate for mtDNA linkage screen [32]. Moreover, as 12 of the GAIT families were ascertained through thrombophilic probands, all analyses included an ascertainment correction achieved by conditioning the likelihood of these pedigrees on the likelihoods of their respective probands [33]. Finally, to control the multiple testing effect, genome-wide p-values were calculated using the method of Feingold et al. [34].
The association between SNPs and mtDNA levels was tested using a measured genotype association analysis assuming an additive model of allelic effect [35]. The p-values for each SNP association test were evaluated under two different approaches of correction for multiple testing. First, we applied the generally more stringent Bonferroni correction, which establishes a significance threshold corresponding to a family-wise error rate of 0.05. Second, the more lenient Benjamini-Hochberg (B-H) adjustment was also applied to the p-values, assuming a 10% false discovery rate [36].
To investigate the potential influence of sex on the genetic mechanism of mtDNA levels, we performed both genome-wide linkage and SNP association analyses based on the whole sample, on females only and on males only.
Results
Phenotypic Data and Heritability of mtDNA
Table 1 summarises the phenotypic data of all of the subjects in our study. A similar number of males and females were included in the analysis. No significant differences were found between the two sexes with regard to age, but not with regard to smoking behaviour (p = 0.0022). Oral contraceptives use was the only covariate that showed a significant effect on mtDNA levels (p = 0.0072). The estimated proportion of mtDNA levels variance due to oral contraceptives use that was included in the analysis was 1.57%, and its effects were estimated simultaneously with the genetic effects. We found no significant differences in mtDNA levels between males and females (0.25±0.11 and 0.24±0.11, respectively; p = NS). Genetic heritability (h2) of mtDNA levels was 0.33±0.09 (p = 1.82e-5), indicating that 33% of the phenotypic variation in this trait is due to the additive effect of genes.
Table 1. Phenotypic Characteristics of the 386 Subjects Included in the GAIT Project.
| Phenotypic characteristics | Phenotypic data | P-value* | ||
| Number of families | 21 | |||
| All subjects | Males | Females | ||
| Number of subjects, n (%) | 386 (100) | 173 (45) | 213 (55) | NS |
| Median age, years ± SD | 37.6±19.94 | 38.3±19.99 | 36.7±19.91 | NS |
| Current smokers†, n (%) | 144 (37) | 79 (46) | 65 (31) | 0.0022 |
| Oral contraceptives‡, n (%) | 15 (4) | — | 15 (7) | — |
| mtDNA levels§, ratio ± SD | 0.24±0.11 | 0.25±0.11 | 0.24±0.11 | NS |
Subjects in the study were defined as currently smokers when they smoke independently of the number of cigarettes.
Oral contraceptives use at inclusion.
mtDNA levels were expressed as the mtDNA to nuclear DNA ratio (mtDNA/nDNA).
SD: standard deviation.
NS: not significant.
P-value<0.05 was considered statistically significant.
Autosomal QTLs Influencing mtDNA Levels
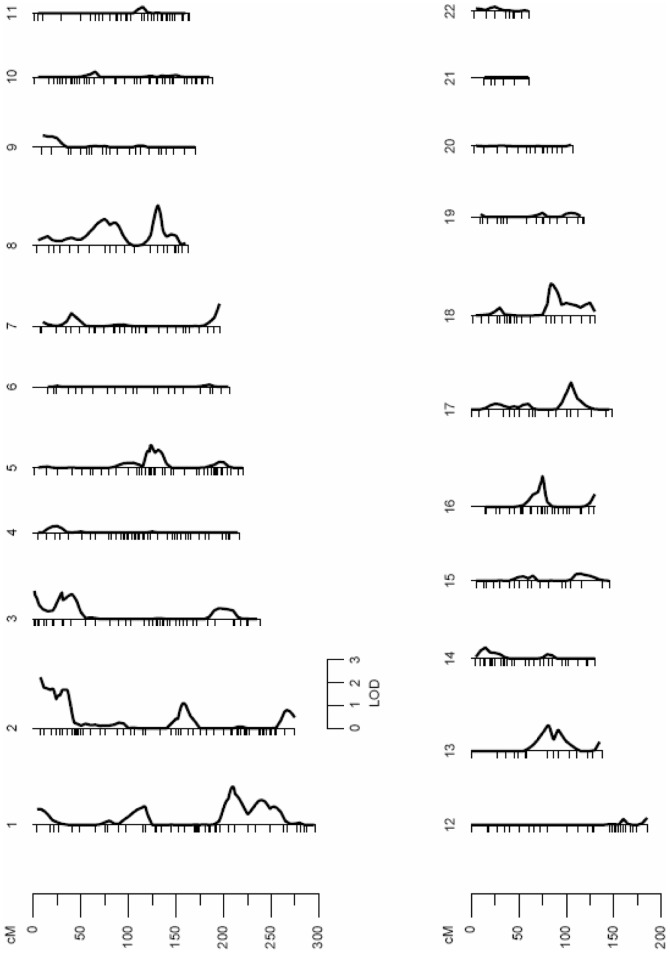
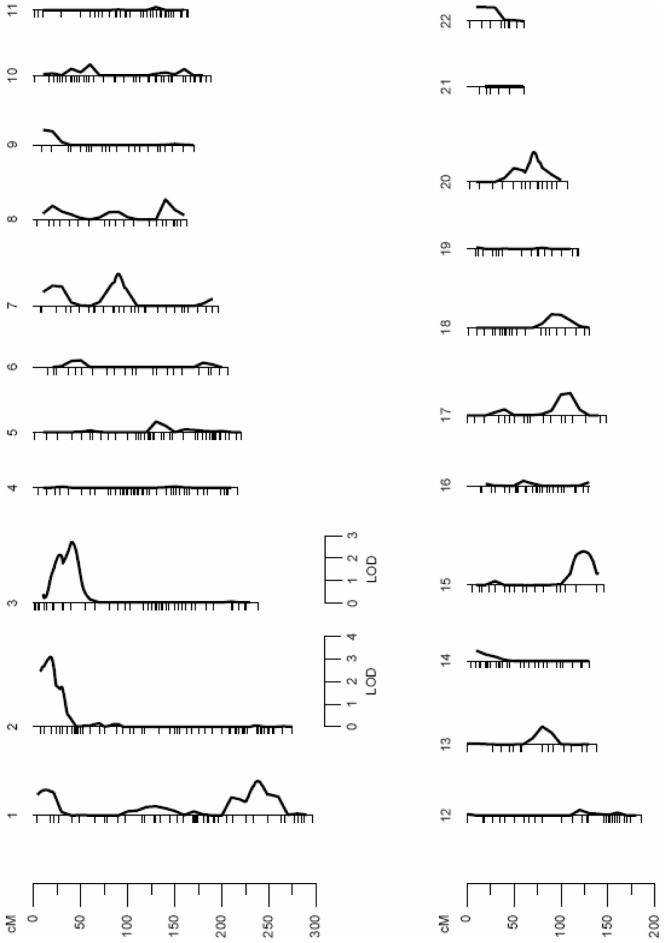
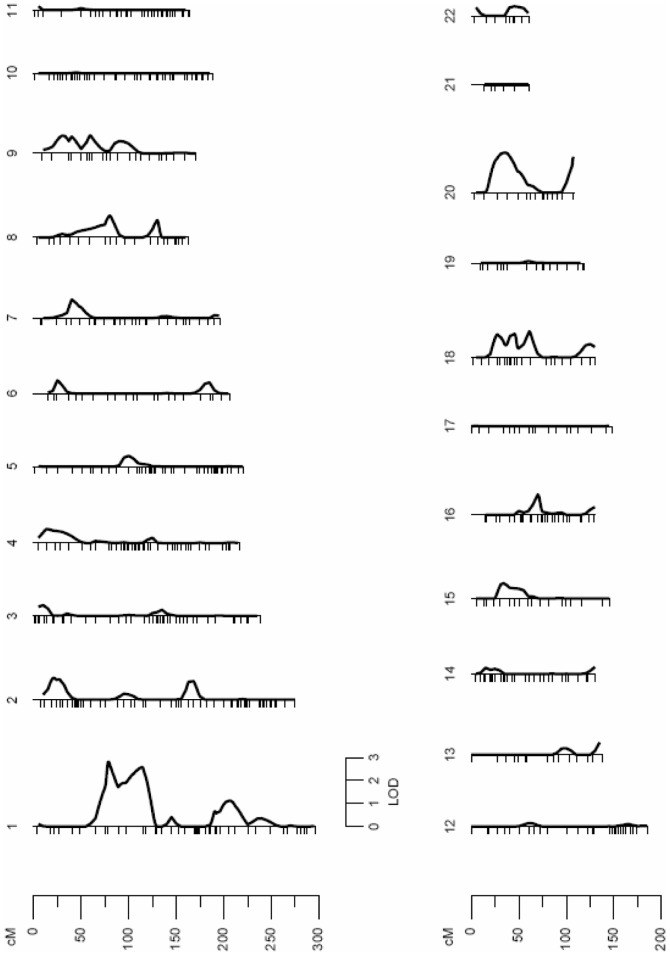
A standard multipoint variance-component method was used to assess linkage between microsatellites and mtDNA levels. A first linkage analysis was carried out using all of the subjects. The results revealed a suggestive quantitative trait locus (QTL) on the short arm of Chromosome 2 (2p), which may influence the variation of mtDNA levels (LOD score = 2.21; p = 7.09e-04) (Figure 1). The QTL on Chromosome 2 was detected through a peak LOD score located between 3137230 bp and 16522359 bp, in a region that maps to 2p25.3-2p24.3. Specific data from the linkage analysis are shown in Table 2. Further genetic linkage analyses were conducted separating the subjects according to their sex to detect sex-specific genomic regions that may regulate the variation of mtDNA levels. The linkage analysis performed with females only revealed two QTLs (Figure 2). One significant linkage signal was detected on Chromosome 2 (LOD score = 3.09; p = 8.11e-05), at the same genomic region (2p25.3-2p25.1) that the signal previously detected in the analysis with the subjects of both sexes. This signal defined a QTL for mtDNA levels that comprises a region between 3137230 bp and 8605209 bp on Chromosome 2 (Table 2). Another linkage region was detected in females on the short arm of Chromosome 3 (3p) with a LOD score of 2.67 (p = 2.27e-04) (Figure 2). This linkage signal was located between 7544027 bp and 23223909 bp, in a region that maps to 3p26.2-3p24.2 (Table 2). Interestingly, the results from the linkage analysis performed in males only differed from those obtained in females. The linkage signal previously detected on Chromosome 2 completely disappeared in males. However, a new genomic region was detected on the short arm of Chromosome 1 (1p) (LOD score = 2.81; p = 1.57e-04), suggesting a sex-specific genetic mechanism for the regulation of mtDNA levels (Figure 3). The male-specific QTL was detected through a peak LOD score located between 45992690 bp and 56878848 bp, in a region that maps to 1p34.1-1p32.2 (Table 2).
Figure 1. Linkage analysis for mtDNA levels in subjects of both sexes.
There is a linkage signal in Chromosome 2 with a peak LOD score of 2.21 (p = 7.09e-04) defining a quantitative trait locus for mtDNA levels in a region that maps to 2p25.3-2p24.3.
Table 2. Data of the Linkage Analysis for mtDNA Levels in GAIT.
| All subjects | Males | Females | |||||
| Linkage signal | Chr. Position | LOD score | Genome-wide P-value | LOD score | Genome-wide P-value | LOD score | Genome-wide P-value |
| Chr. 2 | 2p25.3-2p24.3* | 2.21 | 7.09e-04 | — | — | 3.09 | 8.11e-05 |
| Chr. 3 | 3p26.2-3p24.2 | — | — | — | — | 2.67 | 2.27e-04 |
| Chr. 1 | 1p34.1-1p32.2 | — | — | 2.81 | 1.57e-04 | — | — |
Chr.: Chromosome.
This Chromosome position refers to the linkage signal from the analysis using all of the subjects. Chromosome position for the linkage signal detected in Chromosome 2 only in females is 2p25.3-2p25.1. All Chromosome positions were based on the National Center for Biotechnology Information (NCBI) build 36.
Figure 2. Linkage analysis for mtDNA levels in females only.
The linkage signal detected in Chromosome 2 in the first analysis including the subjects of both sexes remains in the analysis performed with females only. However, this linkage signal in the sex-specfic analysis becomes better defined and more significant (LOD score of 3.09; p = 8.11e-05). In addition, a new quantitative trait locus for mtDNA levels is detected in Chromosome 3 only in females (LOD score of 2.67; p = 2.27e-04). Fine-mapping of the female-specific QTLs detected on Chromosome 2 and Chromosome 3 was carried with a set of 790 and 2687 SNPs, respectively.
Figure 3. Linkage analysis for mtDNA levels in males only.
The linkage signal detected in Chromosome 2 in the first analysis including the subjects of both sexes completely disappears in the analysis performed with males only. However, a new significant linkage signal for mtDNA levels is detected in Chromosome 1 only in males (LOD score of 2.81). Fine-mapping in this linkage region with 971 SNPs reveals the most significant SNP association with mtDNA levels for the rs10888838 (MAF = 0.1133; p = 4.01e-06) in the analysis with males only. This SNP was located in the gene MRPL37, which emerges as a strong candidate gene for the control of mtDNA levels in males.
A bioinformatic search in these linkage regions showed several potential candidate genes for the sex-specific control of mtDNA levels. A brief summary of these data can be found in Table 3. Broad data regarding biological function of each candidate gene and their potential role in disease have been compiled in a supplementary table (Table S1).
Table 3. Candidate Genes Proposed for Sex-specific Variation of mtDNA Levels in GAIT.
| Gene symbol | Description | Chr. Position | Sex-specificity | Subcellular location | Biological process |
| CMPK2 | cytidine monophosphate (UMP-CMP) kinase 2 | 2p25.2 | Females | Mitochondrion | Pyrimidine biosynthesis of the salvage pathway |
| RNASEH1 | ribonuclease H1 | 2p25.3 | Females | Nucleus Mitochondrion | RNA degradation during DNA replication |
| MRPS25 | mitochondrial 28S ribosomal protein S25 | 3p25.1 | Females | Mitochondrion | Mitochondrial translation |
| TIMM40 | coiled-coil-helix-coiled-coil-helix domain containing 4 (CHCHD4) | 3p25.1 | Females | Mitochondrion | Protein transport from the intermembrane space to the mitochondrial matrix |
| C3orf31 | chromosome 3 open reading frame 31 (MMP37-like protein) | 3p25.2 | Females | Mitochondrion | Protein import into mitochondrial matrix |
| PPARG | peroxisome proliferator-activated receptor gamma | 3p25.2 | Females | Nucleus | Nuclear transcription |
| OGG1 | 8-oxoguanine DNA glycosylase | 3p25.3 | Females | Nucleus Mitochondrion | DNA repair |
| MRPL37 | mitochondrial 39S ribosomal protein L37 | 1p32.3 | Males | Mitochondrion | Mitochondrial translation |
| PARS2 | prolyl-tRNA synthetase 2 | 1p32.3 | Males | Mitochondrion | Prolyl-tRNA aminoacylation during protein biosynthesis |
| ATPAF1 | ATP synthase mitochondrial F1 complex assembly factor 1 | 1p33 | Males | Mitochondrion | Protein complex assembly |
| CPT2 | carnitine palmitoyltransferase 2 | 1p32.3 | Males | Mitochondrion | Fatty acid metabolism, β-oxidation |
| UQCRH | ubiquinol-cytochrome c reductase hinge protein | 1p34.1 | Males | Mitochondrion | Mitochondrial electron transport |
| CMPK1 | cytidine monophosphate (UMP-CMP) kinase 1 | 1p33 | Males | Cytoplasm | Pyrimidine ribonucleotide biosynthesis |
Chr.: Chromosome.
Fine-mapping of the Sex-specific QTLs
We conducted a specific search for genotype-phenotype associations in the sex-specific QTLs detected in our genome-wide linkage analyses to detect common variants susceptible to affect mtDNA levels. The most significant SNPs with their p-values included in these genomic regions are shown in Table 4.
Table 4. Top Significant SNP-associations with mtDNA Levels in GAIT.
| Gene | SNP | Chr | Sex-specificity | MAF† | beta‡ | P-value* |
| MRPL37 | rs10888838 | 1 | Males | 0.1133 | 0.806 | 4.01e-06(a) |
| RNF144 | rs2140855 | 2 | No | 0.3879 | 0.321 | 1.85e-05(a) |
| RNF144 | rs2056634 | 2 | No | 0.1928 | 0.344 | 3.50E-04(b) |
| RNF144 | rs11682769 | 2 | No | 0.2819 | −0.275 | 4.90E-04(b) |
| FLJ41046 | rs2609101 | 2 | No | 0.2282 | 0.317 | 2.14E-04(b) |
| FLJ41046 | rs2564023 | 2 | No | 0.0692 | −0.454 | 3.40E-04(b) |
| FLJ41046 | rs1880800 | 2 | No | 0.4739 | −0.248 | 3.81e-04(b) |
| FLJ41046 | rs7571496 | 2 | No | 0.3032 | 0.241 | 9.27E-04(b) |
| SOX11 | rs813779 | 2 | No | 0.2315 | −0.323 | 2.97E-04(b) |
| TSSC1 | rs2305491 | 2 | No | 0.0805 | 0.447 | 4.91e-04(b) |
| VENTXP7 | rs2731943 | 3 | Females | 0.2541 | 0.489 | 6.93e-05(b) |
| ZNF659 | rs161196 | 3 | No | 0.0301 | 0.903 | 6.94E-05(b) |
| SATB1 | rs7635386 | 3 | Females | 0.3835 | 0.385 | 9.47e-05(b) |
Chr.: Chromosome.
Minimal Allele Frequency of the SNP in our sample.
represents the effect of one copy of the rare allele in mtDNA levels when it is expressed in original scale.
P-value of the association with mtDNA levels.
indicates statistical significance after Bonferroni correction for multiple testing.
indicates statistical significance after B-H adjustment for multiple testing assuming a 10% of false discovery rate.
Fine-mapping of the male-specific QTL on Chromosome 1
A total of 971 SNPs were typed in the linkage region detected on Chromosome 1 in males. Among them, the rs10888838 (p = 4.01e-06) remained significant for the association with mtDNA levels after applying the Bonferroni correction for multiple testing (Table 4). This SNP association was significant in the analysis with males, but not with females (data not shown). The rs10888838 is located in the gene mitochondrial 39S ribosomal protein L37 (MRPL37) on Chromosome 1p32.3. We want to emphasise that MRPL37 was identified by linkage and association analyses. Such genetic convergence, obtained by two different analytical strategies, gives more confidence in our results.
Fine-mapping of the female-specific QTLs on Chromosome 2 and Chromosome 3
A total of 790 SNPs were genotyped to fine-map the linkage region detected on Chromosome 2. Among these SNPs, the rs2140855 (p = 1.85e-05) remained significant after applying the Bonferroni correction. This intergenic polymorphism is located on Chromosome 2p25.2 at ∼170 Kbp downstream of the gene RNF144 (ring finger protein 144A) and ∼380 Kbp downstream of the gene cytidine monophosphate (UMP-CMP) kinase 2 (CMPK2). In addition, other 8 SNPs in this QTL showed significant association with mtDNA levels after applying the B-H adjustment assuming 10% false discovery rate (Table 4). Notably, 2 of these SNPs (rs2056634, p = 3.50e-04 and rs11682769, p = 4.90e-04) are at the same locus as rs2140855, and also around RNF144 and CMPK2. Moreover, 4 of these polymorphisms (rs2609101, p = 2.14e-04; rs2564023, p = 3.40e-04; rs1880800, p = 3.81e-04 and rs7571496, p = 9.27e-04) are in or around FLJ41046, at a distance of ∼700 Kbp upstream of the gene CMPK2. The rs813779 (p = 2.97e-04) and rs2305491 (p = 4.91e-04) were the remaining two SNPs that passed the B-H adjustment with 10% false discovery rate. They are around the gene Transcription factor SOX-11 (SOX11) and in the gene tumor suppressing subtransferable candidate 1 (TSSC1) respectively. Although all these SNPs are located in the female-specific QTL, the association with the phenotype only reached statistical significance in the analysis using all of the subjects.
A total of 2687 SNPs were genotyped to fine-map the linkage region detected on Chromosome 3 in females. None of the analysed SNPs remained significant after applying the Bonferroni correction. However, three SNPs reached statistical significance when applied the B-H adjustment assuming 10% false discovery rate (Table 4). These SNPs were rs2731943 (p = 6.93e-05) located around VENTXP7 (VENT homeobox (Xenopus laevis) pseudogene 7) rs7635386 (p = 9.47e-05) located in SABT1 (special AT-rich sequence binding protein 1) and rs161196 (p = 6.94e-05) located in ZNF659 (zinc finger protein 385D). Despite that ZNF659 is located in a female-specific QTL, the rs161196 in this gene showed significant association with mtDNA levels only in the analysis with all of the subjects.
Discussion
Genetic variation and altered levels of mtDNA have been associated with the risk of disease and many common complex disorders in humans. A perfectly orchestrated interaction between both nuclear and mitochondrial genomes is essential for mitochondrial biogenesis. In particular, the coordinated expression of many proteins in an extremely complex scenario is required for mtDNA maintenance. Nevertheless, we are still far from identifying all of the factors involved in this process as well as the mechanisms involved in the control of mtDNA levels [37]. The identification of an increasing number of human diseases associated with altered levels of mtDNA has stimulated investigations of the mechanisms and factors involved in the regulation of this trait. In addition, mtDNA levels vary among subjects of different ages and among different tissues within the same individual. These levels are largely influenced by genetic factors, with a heritability (h2) ranging between 33% and 65% in different studies [38], [39]. Curran et al. [39] reported a QTL for mtDNA levels on Chromosome 10 (LOD = 3.83) in 1,259 Mexican American subjects from 42 extended families of the Sant Antonio Family Heart Study (SAFHS). They suggested the mitochondrial transcription factor A (TFAM) and the translocase of the inner mitochondrial membrane 23 (TIMM23) as candidate genes involved in mitochondrial processing for the control of mtDNA levels. In agreement with theses studies, we estimated the h2 of mtDNA levels in the Spanish population in 33%. This indicates a substantial genetic component in regulating mtDNA levels and guarantees success in the search for genes that influence this quantitative trait.
The present study aimed to conduct sex-specific genome-wide linkage analyses in a mtDNA-related trait. Accordingly, in our initial linkage scan carried using all of the subjects we found a suggestive linkage signal on Chromosome 2 (LOD = 2.21; p = 7.09e-04). Unlike Curran et al., we did not detect a linkage signal on Chromosome 10. However, when we performed further linkage analyses with subjects according to their sex, we found a strong significant QTL on Chromosome 2 (LOD = 3.09; p = 8.11e-05) and a suggestive QTL on Chromosome 3 (LOD = 2.67; p = 2.27e-04) only in females. Notably, the significant linkage signal from females on Chromosome 2 was at the same region as was the suggestive linkage signal previously detected in the analysis performed using all of the subjects. Interestingly, this genomic region on Chromosome 2 showed no linkage in the analysis performed with males. This demonstrates that the linkage signal detected in the first analysis was the result of the great influence that genes exert over mtDNA levels only in females. In addition, the linkage analysis performed with males only showed a suggestive QTL on Chromosome 1 (LOD = 2.81; p = 1.57e-04). Fine-mapping of these linkage regions revealed significant SNP associations with mtDNA levels. The most significant association was found for the rs10888838 in the linkage region on Chromosome 1 observed in males. This SNP mapped in the MRPL37 gene, which encodes a component of the mitoribosome involved in the synthesis of key proteins of the OXPHOS system. We noted that this gene was detected exclusively in males through two distinct statistical approaches, such as linkage and association. This combined genetic convergence supports our sex-specific family-based studies to find genes that may differently control quantitative traits of complex diseases according to sex. In addition, these results reinforce the hypothesis that MRLP37 may contribute to the variation of mtDNA levels only in males. In this sense, functional variants in MRLP37 having an effect on mtDNA levels needs to be confirmed. The identification of these polymorphisms would be clinically relevant, since it has been reported that a defect in any component of the mitochondrial protein-synthesis machinery might compromise normal mitochondrial function and contribute to disease [40]. On the other hand, fine-mapping of the female-specific linkage region on Chromosome 2 showed a significant association of the rs2140855 with mtDNA levels. However, this SNP association reached significance only in the analysis using all of the subjects. The rs2140855 is intergenic and located ∼170 Kbp downstream of the gene RNF144 and ∼380 Kbp downstream of the gene CMPK2. RNF144 encodes an E3 ubiquitin-protein ligase, involved in proteolysis and little information exists with regard this gene and its involvement in disease. However, CMPK2 deserves special attention, since it is directly related with the mtDNA biology. CMPK2 encodes a novel mitochondrial kinase that participates in the salvage pathway of deoxyribonucleotide synthesis into the organelle [41]. CMPK2 also may be involved in the activation of several pyrimidine nucleoside analogs that are widely used as antiviral and anticancer agents, and can cause mtDNA depletion after long term therapy [42]. In addition, mutations in other enzymes of the salvage pathway that are involved in the maintenance of balanced mitochondrial dNTP pools cause severe mitochondrial DNA depletion syndromes [43], [44]. Unfortunately, our genotyping method did not cover the genomic region in CMPK2, so no SNP data were available. Nevertheless, the rs2056634 and rs11682769 also reached significance for the association with the phenotype. Interestingly, they were also located around RNF144 and CMPK2. This suggests the existence of a genetic variant in or around this locus that influences the quantity of mtDNA. In keeping with this, 4 SNPs (rs2609101, rs2564023, rs1880800 and rs7571496) are in or around FLJ41046, at a distance of ∼700 Kbp upstream of the gene CMPK2. With all the above-mentioned, CMPK2 emerged as a strong candidate gene in the QTL on Chromosome 2 for the control of mtDNA levels. However, we can not rule out other potential candidates genes in this region until functional studies are performed. In addition, the rs813779 and rs2305491 located around SOX11 and in TSSC1, respectively, also were significant in the fine-mapping of the female-specific QTL on Chromosome 2. Notably, TSSC1 is ∼200 Kbp upstream of the RNASEH1 gene. RNASEH1 is also a potential candidate gene in this region. It encodes an endonuclease that specifically degrades the RNA of RNA-DNA hybrids, and it may exert a key function during mtDNA replication [45]. Although all these SNPs are located in the female-specific QTL, it seems that the association with the phenotype occurs when all of the subjects are considered together, but not when males and females are analyzed separately. This observation could be explained by the fact that linkage and association analyses detect different types of genetic variants. In particular, this significant linkage signal could be due to a rare variant present in a group of subjects in these extended pedigrees, whereas the same sample does not provide enough power to detect the effect of the same variant by association. Finally, in the QTL region on Chromosome 3 observed in females, three SNPs showed significant association with mtDNA levels. These polymorphisms were rs2731943 in VENTXP7, rs7635386 in SATB1 and rs161196 in ZNF659. SATB1 encodes a DNA-binding protein involved in transcription regulation, as well as in chromatin organization and nuclear architecture during apoptosis. VENTXP7 is a non-coding protein gene that is a member of the Vent homeobox gene family. ZNF659 encodes a nuclear protein involved in nuclear acid binding and zinc ion binding at the transcription level. Although the biological function of these three genes is not directly associated with mtDNA biology, this area deserves further attention given that the linkage and association signals at the same genetic region were significant. Moreover, despite ZNF659 is located in a female-specific QTL, the rs161196 in this gene showed significant association with mtDNA levels only in the analysis using all of the subjects.
In support of our findings, we detected two haplotypes in the QTL regions on Chromosome 2 and Chromosome 3 in females. These haplotypes have a significant positive effect on mtDNA levels in females only (see File S1, Figure S1, Figure S2 and Figure S3).
Our results emphasize the usefulness of linkage maps in extended pedigrees to detect sex-specific genomic regions for complex traits. This agrees with publications that suggest a strong influence of sex on the susceptibility to common complex diseases in humans [46]. Accordingly, the genetic architecture differs significantly among subjects according to their sex for a large number of quantitative phenotypes [47]. This is consistent with data from other studies of sex-specific heritabilities of common disease-associated quantitative phenotypes such as the case of six sex-specific obesity-related traits in the Chinese population [48] and a set of polymorphisms that influence plasma fibrinogen concentration in a sex-specific manner [49].
It is important to note that despite the sex-specific genetic architecture found in our study, our results show no significant differences in mtDNA levels between males and females. In fact, sex-specific regulation of mtDNA levels does not necessarily imply different quantity of mtDNA between the two sexes. However, if there were significant variations in mtDNA levels between sexes there would be the need for sex-specific mechanisms controlling mtDNA quantity. Accordingly, and in support of our findings, the study of Curran et al. revealed a significant decrease in mtDNA content in males with respect to females in the Caucasian population [39]. In addition, a case-control study including patients affected by renal cell carcinoma reported lower mtDNA content in lymphocytes in males than in females in both cases and controls [38]. Furthermore, other mitochondrial parameters also showed differences between male and female rats for various tissues, thus supporting the existence of sex-specific mechanisms in the control of mitochondrial biogenesis [50], [51]. This agrees with epidemiological data, that reveal less prevalence of some chronic and neurodegenerative diseases associated with mitochondrial dysfunction and oxidative damage in human females (i.e. Parkinson disease). Accordingly, the mitochondrial effects of estrogens may play a role in breast cancer, cardiovascular function, and neuroprotection [52]. Moreover, there is evidence that sex hormones modulate the expression and the activity of proteins that control mitochondrial biogenesis [53], [54]. Thus, estrogens regulate the expression of a number of nuclear- and mitochondrial-encoded genes, which are involved in the control of the OXPHOS system. Also, mitochondria are an important target for the action of estrogens [55]. Accordingly, it is currently well-established that estrogens have direct and indirect effects on mitochondrial activity and biogenesis, but the mechanisms mediating these effects remain to be clarified [56]. Recently, it has been shown that estrogens ameliorate mitochondrial dysfunction in cybrid cells carrying the mtDNA mutation that cause Leber's hereditary optic neuropathy (LHON) and may be responsible for the high prevalence of LHON disease in males [57]. Interestingly, these cybrids were able to increase mtDNA levels after treatment with 17β-estradiol. Consistently with these data, the significant effect of the use of oral contraceptives on mtDNA levels in our sample could be indicative of the control of steroid hormones on mtDNA levels.
Given the growing number of common diseases that are associated with altered mtDNA levels, it has become necessary to investigate the mechanisms that control the quantity of mtDNA in humans. To our knowledge, the present work is the first genome-wide study in extended pedigrees providing data for the existence of sex-specific linkage and association in the control of mtDNA levels. Our results showed a clear sex-specific genetic architecture that may regulate the variation of mtDNA levels in subjects from the GAIT Project. In addition, we provide extensive information regarding potential candidate genes that could modulate mtDNA levels. Our findings may contribute to the understanding of the mechanisms that control mtDNA maintenance. However, these results should be confirmed and extended. Particularly, functional analyses are essential.
Supporting Information
Haplotyping analysis in the linkage region detected on Chomosome 2 in females. Merlin pedigree analysis package was used to perform haplotyping analysis in the family with the maximum contribution to the LOD score in the QTL detected on Chromosome 2 in females (family number 12). Haplotyping analysis in this family revealed the haplotype 5/8/2/8 for the microsatellite genetic polymorphism markers D2S319/D2S2166/D2S2211/D2S162. This haplotype corresponds to the alleles 136/248/249/137 for these markers, respectively; and it was significantly associated with higher levels of mtDNA exclusively in female subjects.
(DOC)
Haplotyping analysis in the linkage region detected on Chomosome 3 in females. Merlin pedigree analysis package was used to perform haplotyping analysis in the family with the maximum contribution to the LOD score in the QTL detected on Chromosome 3 in females (family number 12). Haplotyping analysis in this family revealed the haplotype 8/10/12 for the microsatellite markers D3S3589/D3S1263/D3S2338. This haplotype corresponds to the alleles 241/204/114 for these markers, respectively; and it was significantly associated with higher levels of mtDNA exclusively in female subjects.
(DOC)
Haplotyping analysis in the linkage region detected on Chomosome 1 in males. Merlin pedigree analysis package was used to perform haplotyping analysis in the family with the maximum contribution to the LOD score in the QTL detected on Chromosome 1 in males. Family number 12 also contributed to the linkage signal identified on Chromosome 1 in males. However, no clear haplotype associated with mtDNA levels was detected in these individuals.
(DOC)
Haplotyping Analyses of the Linkage Regions.
(DOC)
Biological data of the candidate genes proposed for sex-specific variation of mtDNA levels in the GAIT sample.
(DOC)
Acknowledgments
We would like to acknowledge the advice and helpful discussion of Professor W.H. Stone. We would like to thank R. Pérez for her technical support as well as A. Cárdenas and O. Solà for their administrative help with particular regard to the inclusion of all subjects. Finally, we are indebted to all of the families who participated in the GAIT Project.
Funding Statement
This work was supported by the ‘Instituto de Salud Carlos III-Fondo de Investigación Sanitaria’ [PI-11/00184, PI-08/0420, PI-08/0756, RECAVA-RD06/0014]; the ‘Ministerio de Ciencia e Innovación’ [SAF2008/01859]; and the ‘Agència de Gestió d'ajuts Universitaris i de Recerca’ [SGR 2009-1240]. SL was supported by “Contratos Posdoctorales de Perfeccionamiento Sara Borrell” from ‘Instituto de Salud Carlos III-Fondo de Investigación Sanitaria’ (ISCIII-FIS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kushnareva Y, Newmeyer DD (2010) Bioenergetics and cell death. Ann N Y Acad Sci 1201: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yu X, Koczan D, Sulonen AM, Akkad DA, Kroner A, et al. (2008) mtDNA nt13708A variant increases the risk of multiple sclerosis. PLoS One 3: e1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Czarnecka AM, Klemba A, Krawczyk T, Zdrozny M, Arnold RS, et al. (2010) Mitochondrial NADH-dehydrogenase polymorphisms as sporadic breast cancer risk factor. Oncol Rep 23: 531–535. [PubMed] [Google Scholar]
- 4. Zhang R, Zhang F, Wang C, Wang S, Shiao YH, et al. (2010) Identification of sequence polymorphism in the D-Loop region of mitochondrial DNA as a risk factor for hepatocellular carcinoma with distinct etiology. J Exp Clin Cancer Res 29: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moreno-Loshuertos R, Acin-Perez R, Fernandez-Silva P, Movilla N, Perez-Martos A, et al. (2006) Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat Genet 38: 1261–1268. [DOI] [PubMed] [Google Scholar]
- 6. Song GJ, Lewis V (2008) Mitochondrial DNA integrity and copy number in sperm from infertile men. Fertil Steril 90: 2238–2244. [DOI] [PubMed] [Google Scholar]
- 7. Weng SW, Lin TK, Liou CW, Chen SD, Wei YH, et al. (2009) Peripheral blood mitochondrial DNA content and dysregulation of glucose metabolism. Diabetes Res Clin Pract 83: 94–99. [DOI] [PubMed] [Google Scholar]
- 8. Liu CS, Kuo CL, Cheng WL, Huang CS, Lee CF, et al. (2005) Alteration of the copy number of mitochondrial DNA in leukocytes of patients with hyperlipidemia. Ann N Y Acad Sci 1042: 70–75. [DOI] [PubMed] [Google Scholar]
- 9. Lee HC, Li SH, Lin JC, Wu CC, Yeh DC, et al. (2004) Somatic mutations in the D-loop and decrease in the copy number of mitochondrial DNA in human hepatocellular carcinoma. Mutat Res 547: 71–78. [DOI] [PubMed] [Google Scholar]
- 10. Lee HC, Yin PH, Lin JC, Wu CC, Chen CY, et al. (2005) Mitochondrial genome instability and mtDNA depletion in human cancers. Ann N Y Acad Sci 1042: 109–122. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Liu VW, Xue WC, Tsang PC, Cheung AN, et al. (2005) The increase of mitochondrial DNA content in endometrial adenocarcinoma cells: a quantitative study using laser-captured microdissected tissues. Gynecol Oncol 98: 104–110. [DOI] [PubMed] [Google Scholar]
- 12. Yamada S, Nomoto S, Fujii T, Kaneko T, Takeda S, et al. (2006) Correlation between copy number of mitochondrial DNA and clinico-pathologic parameters of hepatocellular carcinoma. Eur J Surg Oncol 32: 303–307. [DOI] [PubMed] [Google Scholar]
- 13. Yu M, Zhou Y, Shi Y, Ning L, Yang Y, et al. (2007) Reduced mitochondrial DNA copy number is correlated with tumor progression and prognosis in Chinese breast cancer patients. IUBMB Life 59: 450–457. [DOI] [PubMed] [Google Scholar]
- 14. Hosgood HD III, Liu CS, Rothman N, Weinstein SJ, Bonner MR, et al. (2010) Mitochondrial DNA copy number and lung cancer risk in a prospective cohort study. Carcinogenesis 31: 847–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lan Q, Lim U, Liu CS, Weinstein SJ, Chanock S, et al. (2008) A prospective study of mitochondrial DNA copy number and risk of non-Hodgkin lymphoma. Blood 112: 4247–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burhans WC, Weinberger M (2007) DNA replication stress, genome instability and aging. Nucleic Acids Res 35: 7545–7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443: 787–795. [DOI] [PubMed] [Google Scholar]
- 18. Wallace DC (2005) A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 39: 359–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Madamanchi NR, Vendrov A, Runge MS (2005) Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: 29–38. [DOI] [PubMed] [Google Scholar]
- 20. Bonomini F, Tengattini S, Fabiano A, Bianchi R, Rezzani R (2008) Atherosclerosis and oxidative stress. Histol Histopathol 23: 381–390. [DOI] [PubMed] [Google Scholar]
- 21. Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A (2006) Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res 99: 924–932. [DOI] [PubMed] [Google Scholar]
- 22. Souto JC, Almasy L, Borrell M, Gari M, Martinez E, et al. (2000) Genetic determinants of hemostasis phenotypes in Spanish families. Circulation 101: 1546–1551. [DOI] [PubMed] [Google Scholar]
- 23. Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soria JM, Almasy L, Souto JC, Bacq D, Buil A, et al. (2002) A quantitative-trait locus in the human factor XII gene influences both plasma factor XII levels and susceptibility to thrombotic disease. Am J Hum Genet 70: 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyke B (1995) PEDSYS, a pedigree data management system. User's manual. Technical Report No 2 Population Genetics Laboratory, Department of Genetics, Southwest Foundation for Biomedical research, San antonio, TX.
- 26. Morrison TB, Weis JJ, Wittwer CT (1998) Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques 24: 954–958, 960, 962. [PubMed] [Google Scholar]
- 27. Ririe KM, Rasmussen RP, Wittwer CT (1997) Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem 245: 154–160. [DOI] [PubMed] [Google Scholar]
- 28. Cote HC, Brumme ZL, Craib KJ, Alexander CS, Wynhoven B, et al. (2002) Changes in mitochondrial DNA as a marker of nucleoside toxicity in HIV-infected patients. N Engl J Med 346: 811–820. [DOI] [PubMed] [Google Scholar]
- 29. Cote HC, Gerschenson M, Walker UA, Miro O, Garrabou G, et al. (2011) Quality assessment of human mitochondrial DNA quantification: MITONAUTS, an international multicentre survey. Mitochondrion 11: 520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Almasy L, Blangero J (1998) Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet 62: 1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allison DB, Neale MC, Zannolli R, Schork NJ, Amos CI, et al. (1999) Testing the robustness of the likelihood-ratio test in a variance-component quantitative-trait loci-mapping procedure. Am J Hum Genet 65: 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blangero J, Williams JT, Almasy L (2000) Robust LOD scores for variance component-based linkage analysis. Genet Epidemiol 19 Suppl 1: S8–14. [DOI] [PubMed] [Google Scholar]
- 33. Boehnke M, Lange K (1984) Ascertainment and goodness of fit of variance component models for pedigree data. Prog Clin Biol Res 147: 173–192. [PubMed] [Google Scholar]
- 34. Feingold E, Brown PO, Siegmund D (1993) Gaussian models for genetic linkage analysis using complete high-resolution maps of identity by descent. Am J Hum Genet 53: 234–251. [PMC free article] [PubMed] [Google Scholar]
- 35. Boerwinkle E, Chakraborty R, Sing CF (1986) The use of measured genotype information in the analysis of quantitative phenotypes in man. I. Models and analytical methods. Ann Hum Genet 50: 181–194. [DOI] [PubMed] [Google Scholar]
- 36. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc B 57: 289–300. [Google Scholar]
- 37. Moraes CT (2001) What regulates mitochondrial DNA copy number in animal cells? Trends Genet 17: 199–205. [DOI] [PubMed] [Google Scholar]
- 38. Xing J, Chen M, Wood CG, Lin J, Spitz MR, et al. (2008) Mitochondrial DNA content: its genetic heritability and association with renal cell carcinoma. J Natl Cancer Inst 100: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Curran JE, Johnson MP, Dyer TD, Goring HH, Kent JW, et al. (2007) Genetic determinants of mitochondrial content. Hum Mol Genet 16: 1504–1514. [DOI] [PubMed] [Google Scholar]
- 40. Jacobs HT, Turnbull DM (2005) Nuclear genes and mitochondrial translation: a new class of genetic disease. Trends Genet 21: 312–314. [DOI] [PubMed] [Google Scholar]
- 41. Xu Y, Johansson M, Karlsson A (2008) Human UMP-CMP kinase 2, a novel nucleoside monophosphate kinase localized in mitochondria. J Biol Chem 283: 1563–1571. [DOI] [PubMed] [Google Scholar]
- 42. Dalakas MC, Semino-Mora C, Leon-Monzon M (2001) Mitochondrial alterations with mitochondrial DNA depletion in the nerves of AIDS patients with peripheral neuropathy induced by 2′3′-dideoxycytidine (ddC). Lab Invest 81: 1537–1544. [DOI] [PubMed] [Google Scholar]
- 43. Marti R, Nascimento A, Colomer J, Lara MC, Lopez-Gallardo E, et al. (2010) Hearing loss in a patient with the myopathic form of mitochondrial DNA depletion syndrome and a novel mutation in the TK2 gene. Pediatr Res 68: 151–154. [DOI] [PubMed] [Google Scholar]
- 44. Freisinger P, Futterer N, Lankes E, Gempel K, Berger TM, et al. (2006) Hepatocerebral mitochondrial DNA depletion syndrome caused by deoxyguanosine kinase (DGUOK) mutations. Arch Neurol 63: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 45. Cerritelli SM, Crouch RJ (2009) Ribonuclease H: the enzymes in eukaryotes. FEBS J 276: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weiss LA, Pan L, Abney M, Ober C (2006) The sex-specific genetic architecture of quantitative traits in humans. Nat Genet 38: 218–222. [DOI] [PubMed] [Google Scholar]
- 47. Seda O, Tremblay J, Gaudet D, Brunelle PL, Gurau A, et al. (2008) Systematic, genome-wide, sex-specific linkage of cardiovascular traits in French Canadians. Hypertension 51: 1156–1162. [DOI] [PubMed] [Google Scholar]
- 48. Chiu YF, Chuang LM, Kao HY, Shih KC, Lin MW, et al. (2010) Sex-specific genetic architecture of human fatness in Chinese: the SAPPHIRe Study. Hum Genet 128: 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carter AM, Catto AJ, Bamford JM, Grant PJ (1997) Gender-specific associations of the fibrinogen B beta 448 polymorphism, fibrinogen levels, and acute cerebrovascular disease. Arterioscler Thromb Vasc Biol 17: 589–594. [DOI] [PubMed] [Google Scholar]
- 50. Guevara R, Santandreu FM, Valle A, Gianotti M, Oliver J, et al. (2009) Sex-dependent differences in aged rat brain mitochondrial function and oxidative stress. Free Radic Biol Med 46: 169–175. [DOI] [PubMed] [Google Scholar]
- 51. Colom B, Alcolea MP, Valle A, Oliver J, Roca P, et al. (2007) Skeletal muscle of female rats exhibit higher mitochondrial mass and oxidative-phosphorylative capacities compared to males. Cell Physiol Biochem 19: 205–212. [DOI] [PubMed] [Google Scholar]
- 52. Gavrilova-Jordan LP, Price TM (2007) Actions of steroids in mitochondria. Semin Reprod Med 25: 154–164. [DOI] [PubMed] [Google Scholar]
- 53. Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, Barch MJ, et al. (2008) Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol 22: 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Klinge CM (2008) Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem 105: 1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Psarra AM, Sekeris CE (2008) Steroid and thyroid hormone receptors in mitochondria. IUBMB Life 60: 210–223. [DOI] [PubMed] [Google Scholar]
- 56. Simpkins JW, Yang SH, Sarkar SN, Pearce V (2008) Estrogen actions on mitochondria–physiological and pathological implications. Mol Cell Endocrinol 290: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Giordano C, Montopoli M, Perli E, Orlandi M, Fantin M, et al. (2011) Estrogens ameliorate mitochondrial dysfunction in Leber's Hereditary Optic Neuropathy. Zaragoza (Spain). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Haplotyping analysis in the linkage region detected on Chomosome 2 in females. Merlin pedigree analysis package was used to perform haplotyping analysis in the family with the maximum contribution to the LOD score in the QTL detected on Chromosome 2 in females (family number 12). Haplotyping analysis in this family revealed the haplotype 5/8/2/8 for the microsatellite genetic polymorphism markers D2S319/D2S2166/D2S2211/D2S162. This haplotype corresponds to the alleles 136/248/249/137 for these markers, respectively; and it was significantly associated with higher levels of mtDNA exclusively in female subjects.
(DOC)
Haplotyping analysis in the linkage region detected on Chomosome 3 in females. Merlin pedigree analysis package was used to perform haplotyping analysis in the family with the maximum contribution to the LOD score in the QTL detected on Chromosome 3 in females (family number 12). Haplotyping analysis in this family revealed the haplotype 8/10/12 for the microsatellite markers D3S3589/D3S1263/D3S2338. This haplotype corresponds to the alleles 241/204/114 for these markers, respectively; and it was significantly associated with higher levels of mtDNA exclusively in female subjects.
(DOC)
Haplotyping analysis in the linkage region detected on Chomosome 1 in males. Merlin pedigree analysis package was used to perform haplotyping analysis in the family with the maximum contribution to the LOD score in the QTL detected on Chromosome 1 in males. Family number 12 also contributed to the linkage signal identified on Chromosome 1 in males. However, no clear haplotype associated with mtDNA levels was detected in these individuals.
(DOC)
Haplotyping Analyses of the Linkage Regions.
(DOC)
Biological data of the candidate genes proposed for sex-specific variation of mtDNA levels in the GAIT sample.
(DOC)