Abstract
Purpose
To review the literature describing patterns of out-patient prescription drug use during pregnancy by therapeutic category, potential for fetal harm, and overall.
Methods
We conducted a systematic review of peer-reviewed literature published from 1989 to 2010. We included studies evaluating individual-level exposures to prescription medicines during pregnancy. We selected only studies conducted in developed (OECD) countries and published in English.
Results
Published drug utilization studies reveal wide variation in estimates of overall prescription drug use in pregnancy (27% to 93% of pregnant women filling at least one prescription excluding vitamins and minerals). Among studies of similar design, estimates were lowest in Northern European countries (44% to 47%) and highest in France (93%) and Germany (85%). Measured rates of use of contraindicated medicines in pregnancy ranged from 0.9% (Denmark; 1991–1996) to 4.6% (USA; 1996–2000). The use of medicines with positive evidence of risk (FDA category D) ranged from 2.0% (Italy; 2004) to 59.3% (France; 1995–2001).
Conclusion
Avoidable inconsistencies in study design and reporting attenuate conclusions that can be drawn from the literature on antenatal drug utilization. Nevertheless, the body of published research shows that antenatal prescription drug use is common, with many studies finding that a majority of women use one or more prescription medicine during pregnancy. Similarly, studies consistently report the use of drugs recognized as having potential risks in pregnancy. Given this widespread use, it is particularly important to develop standards for calculating and reporting antenatal exposures to improve the value of future research in this area.
Keywords: Drug utilization, prescription drugs, pregnancy, review
Introduction
Given the potential for harmful effects and the paucity of safety information for many medicines in pregnancy, prescription drug use is approached with caution by pregnant women and their health care providers. Studies of antenatal drug use provide important indicators of which drugs are most commonly used by pregnant women, for which conditions, and whether this use might be problematic for mothers and infants. By monitoring the use of drugs with known risks, these studies may catalyze programs to optimize antenatal prescribing. By identifying frequently used medicines with unknown risks, they may help to establish priorities for epidemiological research.
A number of antenatal drug use studies have been conducted in developed countries. To our knowledge, Bonati et al published the only review of this literature, examining thirteen studies published from 1960–1988 (1). At that time, the majority of studies originated from the United States and used maternal interviews to ascertain exposures. Several studies have been published since that time, many of which draw on previously unavailable prescription claims databases. Thus, we aimed to update this review. We primarily examine estimates of drug use overall, but also consider use by therapeutic categories, and by potential for fetal risk. In order to investigate potential sources of heterogeneity among study estimates and inform methodology in this field, we compare findings by country and research method.
Methods
Inclusion Criteria
We sought original English language studies that evaluated individual-level exposures to prescription drugs in a community setting for the entire gestational period. To improve inter-country comparability, we limited studies to those examining populations residing in the 34 member countries of the Organization of Economic Co-operation and Development (OECD) (2). We excluded studies that did not report outpatient utilization rates of prescription drugs, for example those that only analyzed pharmaceuticals available over-the-counter, illicit drugs, drugs used in hospital; or those that failed to distinguish between utilization rates reported for these types of drugs and prescription drugs. We also excluded studies that analyzed only a single period of gestation or specific therapeutic categories without providing an estimate of drug use for all prescription drugs. The full review protocol including documentation of the search strategy and data abstraction is available upon request.
Search and Screening Process
Our literature search was conducted in May/June 2010 in the CINAHL (Ebsco), EMBASE (Ovid), International Pharmaceutical Abstracts (Ovid), MEDLINE (Ovid), Web of Science (SCI & SSCI, ISI/Thompson) and POPLINE databases. Our search strategy combined the concepts of pharmaceuticals and pregnancy. We included only peer reviewed journal articles published between January 1989 to April 2010, and we limited results to studies of human subjects. Reference lists of retrieved articles were also hand-searched for other articles that might have met study inclusion criteria.
Articles identified in our search strategy were subject to a three-stage process for study selection: title, abstract, and full text review. At each stage, two independent reviewers (JD, GH) assessed citations against inclusion criteria. Differences in inclusion assessment were resolved by consensus.
Study Assessment
We developed a data abstraction form designed to collect comparable data on study characteristics and outcomes. This template was pilot-tested on three randomly-selected articles by three independent reviewers (JD, GH, SM) and refined accordingly. Data from each study was abstracted by a minimum of two reviewers. Disagreements were resolved by consensus. We successfully contacted the authors of eleven studies via email for further clarification or additional data (3–15).
We abstracted detailed information on the methodology of all included studies, including: study sample, types of pregnancies included (i.e. birth outcome, location of birth, and parity or plurality), identification of pregnancies, construction of the gestational period, drug exposure data source, and exposure measurement (i.e. the inclusion and classification of prescription drugs).
We abstracted two primary outcome measures: 1) the proportion of women who filled one or more prescriptions during pregnancy, and 2) the mean/median number of different drugs used by pregnant women. If available, we also abstracted three secondary outcome measures: 1) the proportion of women who filled one or more prescription during pregnancy by trimester, 2) the most frequently used therapeutic categories and the proportion of women using drugs within each category; and 3) the proportion of women who filled one or more prescriptions during pregnancy for a specific drug with potential risks (identified by the authors or a risk classification system). It is important to note that we did not develop our review to capture all studies which reported rates of use for specific trimesters of pregnancy, therapeutic categories or risk classification systems. Our review is thus not a systematic review of studies of drug use for a particular therapeutic class (e.g. antidepressants) or any particular risk classification (e.g. FDA category X drugs). Rather, we systematically included all studies that examined specific therapeutic or risk classifications as part of an overall assessment of prescribing during pregnancy in an area (country, region etc.). Thus, our design provides greater context within which rates by trimester, therapeutic class and risk classification can be compared.
Results
Study Selection
Our search strategy identified 3,309 unique citations. Reviewers of citations (JD and GH) agreed on the elimination of 2,560 citations on the basis of title screening and a further 684 after abstract review. Full-text review of the remaining 65 citations resulted in reviewer agreement on the selection of 19 that met our inclusion criteria.
Two citations using a similar study cohort and covering the same years of data were abstracted as one study (9, 10). One study was excluded during abstraction as the report did not provide any exposure rates overall or by therapeutic categories for the full pregnancy period and the author did not reply with additional data (16). Two studies conducted in the Netherlands studied pregnancies in the same region during overlapping time periods; however, because of the different cohort exclusion criteria and results found, we chose to abstract these studies separately (4, 13).
Study Characteristics
Table 1 presents the study characteristics of the seventeen studies included in the systematic review (3–15, 17–21). The studies were based primarily in Europe (12 studies; 70%) with three studies from the US (18%) and two from Canada (12%). The sampled years of delivery ranged from 1981 to 2006.
Table I.
Summary of studies included in the systematic review
| Study | Sample | Prescription Drug Use in Pregnancy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | Publication Year | Country | Sampling Frame | Sampling Method | Year(s) of Delivery | N | Exposure Data Sourcea | % Users (% excl. vitamins/minerals) | Mean Different Drugs |
| Kulaga et al. (7) | 2009 | Canada | Insurance | Population | 1998–2002 | 109 344 | Database | 56 | . |
| Garriguet (17) | 2006 | Canada | National | Random | 2002–2003 | 20 738 | Survey | (27) | . |
| Olesen et al. (9, 10) | 1999 | Denmark | Region | Population | 1991–1996 | 16 001/15 756 | Database | (44.2) | 2.6e |
| Olesen et al. (11) | 2006 | Denmark | Region | Population | 1991–1998 | 19 874 | Database | (46.8) | 2.6e |
| Malm et al. (8) | 2003 | Finland | National | Population | 1999 | 43 470 | Database | (46.2) | 2.1 |
| Lacroix et al. (14) | 2000 | France | Insurance | Random | 1996 | 1 000 | Chartb | 99 | 13.6f |
| Beyens et al. (18) | 2003 | France | Insurance | Random | 1996–1997 | 911 | Databasei | 91.5 | 10.9 |
| Lacroix et al. (15) | 2009 | France | Insurance | Population | 2004–2005 | 10 008 | Database | 95 (93) | 11.0 |
| Reimann et al. (19) | 1996 | Germany | Multi-centre | Random | 1987 | 300 | Survey + Chartc | 61 | 2.5 |
| Egen-Lappe et al.(20) | 2004 | Germany | Insurance | Population | 2000–2001 | 41 293 | Database | 96.4 (85.2) | 4.0, 2.0g |
| Gagne et al. (6) | 2008 | Italy | Region | Population | 2004 | 33 343 | Database | 70.3 (48.0) | 1.8e |
| Bakker et al. (4) | 2006 | Netherlands | Region | Othera | 1994–2003 | 5 412 | Database | 79.1d | . |
| Schirm et al. (13) | 2004 | Netherlands | Region | Othera | 1997–2001 | 7 500 | Database | 85.6 (69.2) | . |
| Engeland et al. (5) | 2008 | Norway | National | Population | 2004–2006 | 106 329 | Database | 57 | 3.3e |
| Rubin et al. (12) | 1993 | USA | Multi-region | Random | 1981–1987 | 2 752 | Survey | (35)h | 1.8e |
| Andrade et al. (3) | 2004 | USA | Insurance | Population | 1996–2000 | 152 531 | Database | 82 (64) | 1.7g |
| Riley et al. (21) | 2005 | USA | Multi-centre | Convenience | 2001–2002 | 1 626 | Chartb | (56) | 2.2, 1.9e |
The study cohort comprised women believed (on the basis of address of primary residence) to be mothers of all children born during the period.
Review of original prescription records
Chart refers to the “Mutter-pass”, a document given to expectant mothers in Germany, where prescription medications used may be recorded by the attending gynecologist, midwife, or the pregnant woman herself.
Excluding oral contraceptives.
Among women using one or more prescription medicines.
Proprietary medicines only.
Excluding vitamins and minerals
Proportion calculated from Table 4 in study report
Database of original prescriptions (not dispensed medicines)
Pregnancies were most often identified through pregnancy registries (5, 7, 8, 14, 15, 18) or hospital records (3, 6, 19). Most studies appeared to analyze pregnancies ending in live births only (4, 9–13, 15, 17). A small number reported including live and still births (3, 6, 8, 14), two studies explicitly reported the inclusion of therapeutic abortions (5, 7), and only one indicated inclusion of spontaneous abortions (7).
Prescription drug information was most often obtained from administrative prescription databases, which often only included medications reimbursed by a specific insurer (3–11, 13, 15, 18, 20). Only two included studies used maternal survey to measure drug exposure (12, 17). Indeed, many studies based on maternal self-report did not meet our inclusion criteria as they rarely distinguish between the use of over-the-counter and prescription medicines.
Measuring antenatal drug use with administrative databases requires the accurate construction of the pregnancy period. To do so, many included studies assumed all pregnancies were full-term (3, 4, 6, 8, 13, 20), employing gestational age assumptions ranging from 270 to 280 days. Others used the actual gestational age of the infant available from the birth record or pregnancy registry (5, 7, 9–11, 14, 15).
All studies based on administrative data classified an exposed pregnancy as one in which the dispensed date of at least one prescription fell within the constructed pregnancy period. The mean or median number of different drugs prescribed during each pregnancy was often reported; however, the level of granularity at which drugs were considered “different” was not explicitly stated in any study, making these measures difficult to compare. None of the included studies provided more complex constructions of drug use, such as the duration of exposure, adherence, or persistence. Based on descriptions of methods and reported rates of use by therapeutic category, the majority of studies appeared to include prescribed vitamins and minerals in counts of overall prescription drug exposures (11 studies; 65%) (3–7, 13–15, 18–20). Five of these provided separate estimates including and excluding vitamins and minerals (46%) (3, 6, 13, 15, 20).
Nearly half of the included studies on overall prescription drug use also applied a risk classification system. The US Food and Drug Administration (FDA) system was the most common and was used in both American and European contexts (3, 6, 14, 21). Three other studies drew on other major risk classification systems: the Australian system in the Netherlands (13), the Swedish system in Denmark (9), and a combination of the Australian and FDA system in Italy (6). Researchers in Canada developed a unique list of drugs with potential risks based on established literature and consultation with an expert panel (7).
Overall Drug Use
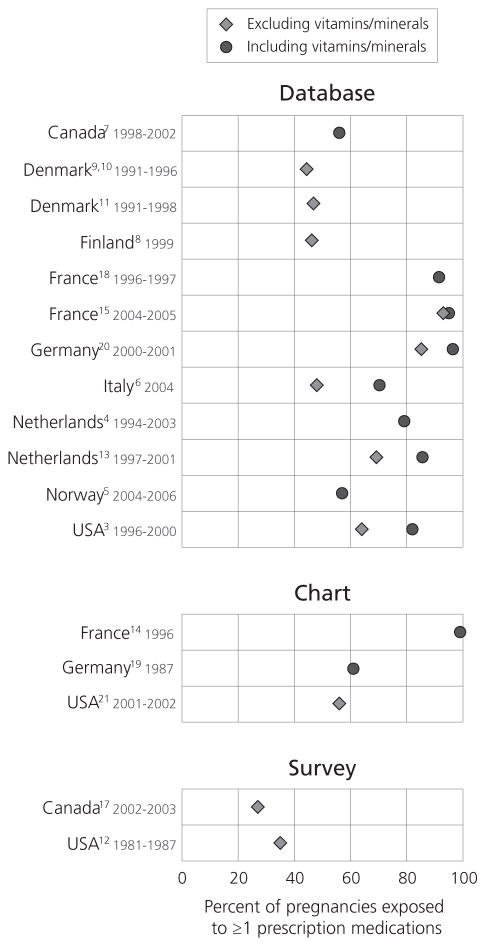
Overall estimates of prescription drug use during pregnancy ranged from 27% to 99% (Figure 1). Estimates including vitamins and minerals ranged from 57% to 99%, while those excluding vitamins and minerals ranged from 27% to 93%. On average, among studies that provided separate estimates including and excluding vitamins and minerals, the inclusion of vitamins and minerals increased estimated exposure rates by 14% (range: 2 to 22%). The two studies relying solely on maternal self-report found the lowest estimates of drug use (27% in the US, and 35% in Canada) (12, 17). The mean number of different drugs reported to be used among pregnant women ranged widely from 1.7 to 13.6.
Figure 1.
Among the eleven most comparable studies using administrative prescription databases, estimated rates of prescription drug use in pregnancy excluding vitamins and minerals ranged from 44.2% to 93% across countries. Among these, studies from Northern Europe reported the lowest rates of prescription drug use during pregnancy ranging from 44.2% to 57%.. Studies of pregnancies in the Netherlands (69.2%), Germany (85.2%), and France (93%) found the highest rates of exposure to prescription drugs, excluding vitamins and minerals. Moreover, the mean number of different prescription drugs, vitamins, and minerals used per pregnancy in French studies ranged from 10.9 to 13.6, far above estimates in all other countries (all below 4.0). Population-based studies from North America found use rates of approximately 64% excluding vitamins for privately insured Americans (82% including vitamins); and 56% for publicly insured Canadians, including vitamins (though vitamins are rarely prescribed in Canada).
Prescription drug use by trimester
Seven studies using prescription databases reported overall drug use by trimester (Table 2). Four studies found that the proportion of women receiving at least one prescription medicine increased from the first to third trimester of pregnancy (4, 6, 13, 20). All four of these studies included vitamins and minerals; however, one study found an attenuated trend when vitamins and minerals were excluded (20). Two other studies, excluding vitamins and minerals, found that rates of prescription drug use were highest in the first trimester of pregnancy (3, 10).
Table II.
Percentage of pregnancies in which one or more prescription(s) was filled, by trimester, country, and year(s) of study
| Trimester | ||||
|---|---|---|---|---|
| Country | Year | 1 | 2 | 3 |
| Including vitamins and minerals | ||||
| Germany (20) | 2000–01 | 69.7 | 80.7 | 85.2 |
| Italy (6) | 2004 | 41.0 | 49.0 | 59.0 |
| Netherlands (4) | 1994–04 | 43.6 | 49.3 | 60.8 |
| Netherlands (13) | 1995–01 | 45.3 | 57.1 | 70.3 |
| Norway (5) | 2004–06 | 33.0 | 29.0 | 29.0 |
| Excluding vitamins and minerals | ||||
| Denmark (9, 10) | 1991–96 | 21.6 | 19.1 | 20.8 |
| Germany (20) | 2000–01 | 53.6 | 55.7 | 59.9 |
| USA (3) | 1996–2000 | 39.0 | 34.4 | 37.9 |
Prescription drug use by therapeutic category
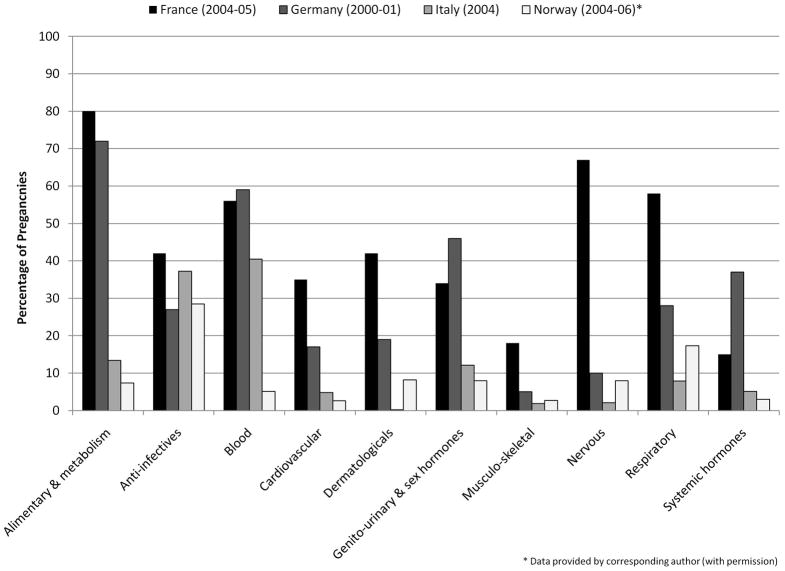
No consistent method was used for reporting drug exposures by therapeutic categories. Figure 2 presents results from four studies of similar design and years of study (post-2000) that provided the percentage of pregnancies exposed to broad therapeutic categories defined at level one (anatomical main group) of the ATC system. Anti-infectives were widely prescribed in each study ranging from 27% in Germany to 42% in France. In other categories, use of prescription drugs during pregnancy varied far more dramatically, often differing by five- to ten-fold across countries.
Figure 2.
Drugs used with the potential for fetal harm
Seven studies reported rates of use for prescription medicines considered to have potential for harm in pregnancy, based on an established or author-defined risk classification system (Table 3). Studies using different systems are difficult to compare: as of 2000, only 26% of the drugs common to all three major systems (US FDA, Swedish, and Australian) were placed in the same risk factor category (22). Fortunately for the purposes of comparison, several studies were based (at least in part) on the FDA system. A US study found that 4.8% of pregnant women filled one or more prescriptions for a drug rated by the FDA as having positive evidence of risk in pregnancy (category D) and that 4.6% filled a prescription for one or more drug rated by the FDA as contraindicated in pregnancy (category X) (3). A study of pregnancies in Italy found that 2.0% of pregnant women filled one or more prescription for a category D drug and 1.0% filled one or more prescription for a category X drug (6). A study from France found that more than half of pregnant women filled a prescription for a category D drug (14). Studies using risk classification systems other than the FDA also found a considerable proportion of women used a drug with positive evidence of fetal risk in Denmark (18.7%), the Netherlands (21.0%), and Canada (6.3%) (4, 7, 11).
Table III.
Percentage of pregnancies exposed to prescription medicines with potential for harm, by risk classification system
| USA (FDA) | D Positive evidence of risk |
X Contraindicated |
|---|---|---|
| USA (1996–00)(3) | 4.8 | 4.6 |
| USA (2001–02) (21) | 3.0 | 1.0 |
| Italy (2004)(6)a | 2.0 | 1.0 |
| France (1996)(14) | 59.3 | 1.6 |
| Swedish (FASS) |
C Positive evidence of risk |
D Primary teratogenic effects |
| Denmark (1991–96)(9) | 18.7 | 0.9 |
| Australian (ADEC) |
B3/C/D/X
Positive evidence of risk/contraindicated |
|
| Netherlands (1997–01)(13) | 21 | |
| Author Definedb | Potential for harmc | |
| Canada (1998–02)(7) | 6.3 | |
The ADEC system was used if a product label with a corresponding FDA risk classification could not be identified.
Based on consultation of established sources, review articles, and an expert panel.
Recognized embyrotoxic, fetotoxic, or teratogenic potential
Discussion
The results of published literature on antenatal prescription drug use confirm that prescription medicine use during pregnancy is the norm in many developed countries. Studies also consistently find that women use medicines with established risks. There appears to be considerable international variation in both overall rates of medicine use and rates of use for drugs with potential harms in pregnancy. While these findings highlight the importance of research on prenatal drug exposures and related health outcomes, differences in study methods and reporting limit general conclusions that may be drawn and highlight a need for improved standards for studies of drug exposures during pregnancy.
Some variations in rates of prescription drug use – overall and by specific classes of medicines - do have explanations. A notable consideration is the inclusion of vitamins and minerals in some studies and by differences in the prescription versus over-the-counter status of certain medicines. For example, the relatively high utilization rate for nervous system drugs found in France (67%) is inflated by prescriptions filled for paracetamol (63%) (15), which women may more commonly purchase over-the-counter in other countries. Wide variations found in the use of alimentary and metabolism drugs and blood-related agents may be explained by differences in inclusion or exclusion of vitamins (ATC codes A11A- A11J), minerals (A12A -A12C), iron (B03A), and folic acid (B03B), all of which may be attainable without a prescription in some countries. Some inter-country variation may also reflect differences in the health needs of pregnant populations. For example, Germany’s high rate of systemic hormone use (37% of women) appears to be driven by the use of iodide, of which the German population has a low dietary intake (20). Other differences may indicate varied approaches and norms in the treatment of certain conditions in pregnancy, such as the widespread use of domperidone in France for nausea.
Further comparative studies should be pursued to investigate inter-country (and perhaps also intra-country) differences in the use of specific medicines including those with potential risks, with attention paid to factors such as the prevalence of indicated maternal conditions, prescription status of medicines, and insurance coverage. Such research could illustrate the extent to which women in different countries are indeed receiving different care (and perhaps varying quality of care) during pregnancy.
In 1990, Bonati and colleagues concluded that there is a “challenging need” for a research protocol on drug use in pregnancy with standardized outcome variables (1). Twenty years later, the results of our review suggest that a need for such a research protocol still exists. Despite widespread availability and use of comparable pharmacy claims databases, we found significant inter-study differences in cohort definitions, gestational period construction, categorization of vitamins and minerals, inclusion of off-formulary drugs, and risk classification as well as in the nature and extent of reporting of methods and findings in published studies. We therefore propose that researchers in this field collaborate to reach consensus on best practice methods for sampling, inclusion and exclusion criteria for pregnancies, exposure measurement, classification of prescription medicines, and construction of therapeutic categories. This may include working with regulators to address some of the widely recognized limitations of established risk classification systems for medicines in pregnancy or the development of an independent system through consultations with experts in teratogenesis (22–24).
We further propose that authors pay special attention to reporting the methods and data sources used to identify pregnancies, included pregnancy outcomes, gestational age and delivery date assumptions, treatment of vitamins and minerals, classification of prescription medicines, and the construction of measures of drug use. There is a particular need for authors to collaborate to establish consistent exposure reporting standards (i.e. the specificity of reporting by therapeutic categories) that are aligned with the practical needs of knowledge users in the research, policy, and clinical communities.
To our knowledge, this is the only review of antenatal drug utilization studies since the one published in 1990 by Bonati and colleagues, and the only review of exclusively prescription drug use in pregnancy (based on a search of the same databases as our review) (1). Our approach was not without limitations. Our inclusion of only English-language peer-reviewed articles may have resulted in us missing information published in other languages and forms. Due to the considerable heterogeneity in study methodology and exposure reporting, we were unable to calculated pooled estimates of exposures and thus were limited to reporting the range of estimates among similar studies.
Finally, it should be noted that only two included studies reported the inclusion of pregnancies ending in spontaneous and therapeutic abortions. While this is likely due to data limitations, missing abortion information weakens the conclusions that can be drawn from this literature. Given that one study able to capture therapeutic abortions found that 47% of these pregnancies had been exposed to a potentially teratogenic medicine (7), we have reason to suspect that estimates presented in this review for use of medicines with potential risks are in fact underestimations. It may also be the case that overall estimates of prescription drug use would be higher among those whose pregnancies resulted in spontaneous or therapeutic abortion. The further development of pregnancy registries that capture all pregnancies beyond a specific gestational age, regardless of outcome, would strengthen research capacity in this field and allow for the construction of a more accurate picture of antenatal drug use.
Conclusion
The use of prescription medicines in pregnancy is widespread. Avoidable differences in study methods and reporting make results of drug utilization studies difficult to compare. However, the magnitude of difference in estimates of overall use and those seen by therapeutic category suggest that there is notable international variation in both the extent and content of prescription drug use during pregnancy that deserves further attention.
Key Points.
The use of prescription medicines in pregnancy is widespread in OECD countries
Excluding vitamins and minerals, available estimates of overall drug use are lowest in Northern European countries (44–47%) and highest in France (93%)
The use of medicines with known risks is commonly reported
The magnitude of difference among estimates of overall prescription drug use and differences by therapeutic category suggest international differences in prenatal prescribing practices exist and should be further evaluated
Administrative health databases in multiple OECD countries provide immense capacity to conduct rigorous pharmacoepidemiological research in pregnant populations
This potential is currently limited by a lack of standards for calculating and reporting antenatal exposures
Acknowledgments
Sponsors
This research was supported, in part, by a Canadian Institutes for Health Research (CIHR)/Health Canada Emerging Team Grant and by a Catalyst Grant from the CIHR Drug Safety and Effectiveness Network. Jamie Daw was supported in part by the CIHR and the Western Regional Training Centre for Health Services Research (WRTC). Gillian Hanley was supported in part by the CIHR, the Michael Smith Foundation for Health Research, and the WRTC. The funding organizations had no role in the conduct of the study, analysis or interpretation of the data, or in the preparation of this manuscript. The authors thank Drs. Barbara Mintzes and Michael Law for their thoughtful review of earlier versions of this manuscript.
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to declare.
References
- 1.Bonati M, Bortolus R, Marchetti F, Romero M, Tognoni G. Drug use in pregnancy: an overview of epidemiological (drug utilization) studies. European Journal of Clinical Pharmacology. 1990;38(4):325–8. doi: 10.1007/BF00315569. [DOI] [PubMed] [Google Scholar]
- 2.Organization for Economic Co-operation and Development. Members and partners. 2010 Available from: http://www.oecd.org/pages/0,3417,en_36734052_36761800_1_1_1_1_1,00.html.
- 3.Andrade S, Gurwitz J, Davis R, Chan K, Finkelstein J, Fortman K, et al. Prescription drug use in pregnancy. American Journal of Obstetrics & Gynecology. 2004;191(2):398–407. doi: 10.1016/j.ajog.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Bakker M, Jentink J, Vroom F, Van Den Berg P, De Walle H, De Jong-Van Den Berg L. Drug prescription patterns before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in the Netherlands. BJOG. 2006;113(5):559–68. doi: 10.1111/j.1471-0528.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 5.Engeland A, Bramness JG, Daltveit AK, Ronning M, Skurtveit S, Furu K. Prescription drug use among fathers and mothers before and during pregnancy: a population-based cohort study of 106,000 pregnancies in Norway 2004–2006. British Journal of Clinical Pharmacology. 2008;65(5):653–60. doi: 10.1111/j.1365-2125.2008.03102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gagne J, Maio V, Berghella V, Louis D, Gonnella J. Prescription drug use during pregnancy: a population-based study in Regione Emilia-Romagna, Italy. European Journal of Clinical Pharmacology. 2008;64(11):1125–32. doi: 10.1007/s00228-008-0546-y. [DOI] [PubMed] [Google Scholar]
- 7.Kulaga S, Zargarzadeh A, Berard A. Prescriptions filled during pregnancy for drugs with the potential of fetal harm. BJOG. 2009;116(13):1788–95. doi: 10.1111/j.1471-0528.2009.02377.x. [DOI] [PubMed] [Google Scholar]
- 8.Malm H, Martikainen J, Klaukka T, Neuvonen PJ. Prescription drugs during pregnancy and lactation - a Finnish register-based study. European Journal of Clinical Pharmacology. 2003;59(2):127–33. doi: 10.1007/s00228-003-0584-4. [DOI] [PubMed] [Google Scholar]
- 9.Olesen C, Sorensen HT, de Jong-van den Berg L, Olsen J, Steffensen FH, Grp E. Prescribing during pregnancy and lactation with reference to the Swedish classification system -A population-based study among Danish women. Acta Obstetricia Et Gynecologica Scandinavica. 1999;78(8):686–92. doi: 10.1034/j.1600-0412.1999.780805.x. [DOI] [PubMed] [Google Scholar]
- 10.Olesen C, Steffensen F, Nielsen G, de Jong-van den Berg L, Olsen J, Sorensen H. Drug use in first pregnancy and lactation: a population-based survey among Danish women. The EUROMAP group. European Journal of Clinical Pharmacology. 1999;55(2):139–44. doi: 10.1007/s002280050608. [DOI] [PubMed] [Google Scholar]
- 11.Olesen C, Thrane N, Henriksen T, Ehrenstein V, Olsen J. Associations between socioeconomic factors and the use of prescription medication during pregnancy: a population-based study among 19,874 Danish women. European Journal of Clinical Pharmacology. 2006;62(7):547–53. doi: 10.1007/s00228-006-0119-x. [DOI] [PubMed] [Google Scholar]
- 12.Rubin J, Ferencz C, Loffredo C. Use of prescription and non-prescription drugs in pregnancy. The Baltimore-Washington Infant Study Group. Journal of Clinical Epidemiology. 1993;46(6):581–9. doi: 10.1016/0895-4356(93)90132-k. [DOI] [PubMed] [Google Scholar]
- 13.Schirm E, Meijer W, Tobi H, de Jong-van den Berg L. Drug use by pregnant women and comparable non-pregnant women in The Netherlands with reference to the Australian classification system. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2004;114(2):182–8. doi: 10.1016/j.ejogrb.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix I, Damase-Michel C, Lapeyre-Mestre M, Montastruc JL. Prescription of drugs during pregnancy in France. Lancet. 2000;356(9243):1735–6. doi: 10.1016/s0140-6736(00)03209-8. [DOI] [PubMed] [Google Scholar]
- 15.Lacroix I, Hurault C, Sarramon M, Guitard C, Berrebi A, Grau M, et al. Prescription of drugs during pregnancy: a study using EFEMERIS, the new French database. European Journal of Clinical Pharmacology. 2009;65(8):839–46. doi: 10.1007/s00228-009-0647-2. [DOI] [PubMed] [Google Scholar]
- 16.Splinter M, Sagraves R, Nightengale B, Rayburn WF. Prenatal use of medications by women giving birth at a university hospital. Southern Medical Journal. 1997;90(5):498–502. doi: 10.1097/00007611-199705000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Garriguet D. Medication use among pregnant women. Health Reports. 2006;17(2):9–18. [PubMed] [Google Scholar]
- 18.Beyens M, Guy C, Ratrema M, Ollagnier M. Prescription of drugs to pregnant women in France: the HIMAGE study. Therapie. 2003;58(6):505–11. doi: 10.2515/therapie:2003082. [DOI] [PubMed] [Google Scholar]
- 19.Reimann I, Karpinsky C, Hoffmann A. Epidemiological data on drug use during pregnancy in Thuringia, East Germany, 1993. International Journal of Clinical Pharmacology and Therapeutics. 1996;34(2):80–3. [PubMed] [Google Scholar]
- 20.Egen-Lappe V, Hasford J. Drug prescription in pregnancy: analysis of a large statutory sickness fund population. European Journal of Clinical Pharmacology. 2004;60(9):659–66. doi: 10.1007/s00228-004-0817-1. [DOI] [PubMed] [Google Scholar]
- 21.Riley E, Fuentes-Afflick E, Jackson R, Escobar G, Brawarsky P, Schreiber M, et al. Correlates of prescription drug use during pregnancy. Journal of Women’s Health. 2005;14(5):401–9. doi: 10.1089/jwh.2005.14.401. [DOI] [PubMed] [Google Scholar]
- 22.Addis A, Sharabi S, Bonati M. Risk classification systems for drug use during pregnancy: are they a reliable source of information? Drug Safety. 2000;23(3):245–53. doi: 10.2165/00002018-200023030-00006. [DOI] [PubMed] [Google Scholar]
- 23.Feibus K. FDA’s proposed rule for pregnancy and lactation labeling: improving maternal child health through well-informed medicine use. Journal of Medical Toxicology. 2008;4(4):284–8. doi: 10.1007/BF03161214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sannerstedt R, Lundborg P, Danielsson B, Kihlstrom I, Alvan G, Prame B, et al. Drugs during pregnancy: an issue of risk classification and information to prescribers. Drug Safety. 1996;14(2):69–77. doi: 10.2165/00002018-199614020-00001. [DOI] [PubMed] [Google Scholar]