Abstract
Rationale: In the normal lung, breathing and deep inspirations potently antagonize bronchoconstriction, but in the asthmatic lung this salutary effect is substantially attenuated or even reversed. To explain these findings, the prevailing hypothesis focuses on contracting airway smooth muscle and posits a nonlinear dynamic interaction between actomyosin binding and the tethering forces imposed by tidally expanding lung parenchyma.
Objective: This hypothesis has never been tested directly in bronchial smooth muscle embedded within intraparenchymal airways. Our objective here is to fill that gap.
Methods: We designed a novel system to image contracting intraparenchymal human airways situated within near-normal lung architecture and subjected to dynamic parenchymal expansion that simulates breathing.
Measurements and Main Results: Reversal of bronchoconstriction depended on the degree to which breathing actually stretched the airway, which in turn depended negatively on severity of constriction and positively on the depth of breathing. Such behavior implies positive feedbacks that engender airway instability.
Overall conclusions: These findings help to explain heterogeneity of airflow obstruction as well as why, in people with asthma, deep inspirations are less effective in reversing bronchoconstriction.
Keywords: airway, smooth muscle, bronchoconstriction, stretch, asthma
At a Glance Commentary
Scientific Knowledge on the Subject
Deep breathing substantially reverses induced bronchoconstriction in the normal lung, but in asthma this salutary response is attenuated or even reversed. It has been inferred that these effects are determined by the extent of airway stretch, but this hypothesis has never been tested directly.
What This Study Adds to the Field
We used a novel system to study the contraction of human airways situated within near-normal lung architecture and subjected to dynamic conditions that simulate breathing. Our results suggest that breathing-induced reversal of bronchoconstriction does indeed depend on the degree to which each breath actually stretches the airway tidally, which in turn depends on both the depth of breathing and severity of bronchoconstriction.
Among all factors known to antagonize bronchoconstriction in a healthy lung, a deep breath is among the most effective (1–5). In the asthmatic lung, however, this protective phenomenon is substantially attenuated, and during a spontaneous asthmatic attack it is sometimes even reversed (1, 6, 7). Some have suggested that the inability of a deep breath to dilate the constricted asthmatic airway might be an important cause of excessive airway narrowing (1, 6, 8).
To explain these observations, a new conceptual framework has called attention to the role of airway smooth muscle (ASM) and the dynamic load against which it must contract (9). With each breath (10), lung parenchyma exerts a distending force on intrapulmonary airways and stretches the bands of ASM that they contain. In this conceptual framework, these tidal stretches perturb the binding of myosin to actin, causing the myosin molecule to detach from actin much sooner than it would have otherwise and thus reducing the myosin duty cycle (11–13). As a result, the contracted ASM band within a bronchoconstricted airway relengthens and thus partially relieves the bronchoconstriction. Importantly, such force fluctuation–induced muscle relengthening has molecular determinants that differ from those that determine isometric force (9, 14–17). As such, the length of contracting ASM becomes equilibrated dynamically, not statically as assumed in earlier models (18, 19), and the force generated by the muscle at any instant can be dramatically less than the force predicted by the isometric force length curve (11, 20).
This mechanistic framework provides a plausible basis to explain how the effects of deep breathing are blunted in asthma. For example, increased contractile stimulus, increased muscle mass (21, 22), increased airway wall thickness (23–25), or decreased lung recoil (26, 27) could each act to limit the tidal stretch of contracted airway smooth muscle. Acting alone or in concert, these effects should cause the muscle to stretch less and thus become stiffer and stiffer until, eventually, a tipping point is reached (28, 29) beyond which the ASM has become so stiff that it is virtually frozen (9, 30) and therefore no longer stretches with each breath. When that occurs, ASM would thereafter remain refractory to the beneficial effects of deep inspirations, stuck in a shortened state until the contractile stimulus is removed or bronchodilator drugs take effect.
Although this hypothetical framework is attractive, it is based entirely on measurements of shortening dynamics of tracheal smooth muscle mounted in a muscle bath. To date, its tenets have not been tested in the intact lungs of living people, because available imaging technology cannot safely provide sufficiently detailed temporal or spatial resolution to discern the posited relationships in smaller intraparenchymal airways. Furthermore, recent studies in isolated central airways have called into question the effectiveness of airway stretch in antagonizing bronchospasm (31).
Here, we used a novel system to study the contraction of intraparenchymal human airways situated within near-normal lung architecture and subjected to dynamic conditions that simulate breathing. Our results demonstrate that breathing-induced reversal of bronchoconstriction does indeed depend on the degree to which each breath actually stretches the airway tidally, which in turn depends on both the depth of breathing and the severity of bronchoconstriction. Notably, quiet tidal breathing caused little if any bronchodilatation; rather, substantial reversal of bronchoconstriction occurred only when deeper breathing was applied, with the greatest reversal observed with simulated tidal breaths to full lung inflation. Furthermore, complete reversal of bronchoconstriction occurred only when bronchoconstriction was modest; even simulated deep breathing had little efficacy in reversing severe bronchoconstriction. These findings have mechanistic implications that could potentially explain more complex integrative behaviors of bronchoconstricted normal and asthmatic lungs (32–38). Some of the results of these studies have been previously reported in the form of an abstract (39).
Methods
Human donor lungs that could not be transplanted were obtained from deceased donors (7 men, 10 women; aged 27–70 yr) through Gift of Hope/Regional Organ Bank of Illinois and were stored at 4°C for up to 2 days before use. Limited medical history was available for most donors; none were reported to have asthma or other pulmonary disease, although eight were known to have smoked tobacco products. The right lung was removed and a portion of its middle lobe was infused with approximately 120 to 180 ml of 1.5% low melting temperature agarose (Type IX; Sigma, St. Louis, MO) in Hanks balanced salt solution (pH = 7.4; Invitrogen, Carlsbad, CA) (40, 41). After solidifying at 4°C for 1 hour, the infused lung was cubed (∼1 cm) and 250-μm precision-cut lung slices (PCLS) were cut using a VT1200S vibrating blade microtome (Leica Microsystems, Bannockburn, IL). Slices were incubated at 37°C in Dulbecco’s modified Eagle medium/F12 media (Invitrogen) supplemented with 1× antibiotic-antimycotic (Invitrogen) overnight to remove residual agarose from the airways. PCLS were maintained at 37°C in the above medium and were studied within 3 days.
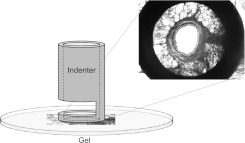
Each PCLS was placed atop, but not physically attached to, a polyacrylamide gel in the bottom of a flow-through chamber. It was loosely held in place under a silicone mesh cut to expose a central 8-mm circle of tissue. A circular indenter (2 mm inner diameter/3 mm outer diameter) was centered over a cross-sectionally cut airway within the PCLS and lowered to contact but not stretch the PCLS (Figure 1). The entire apparatus was placed on an inverted microscope, allowing for monitoring of airway luminal area. The PCLS was equilibrated at approximately 35°C in Krebs-Henseleit solution (14) bubbled with 5% CO2/95% O2. To generate the mechanical forces imposed on the airway by lung parenchyma during breathing, the circular indenter was periodically depressed into the polyacrylamide gel. Depression of the circular indenter caused the confined gel to bulge, which in turn caused a radial stretch of the lung tissue encircled by the indenter (42); in each case, the extent of radial stretch was determined by direct observation under the microscope as described below. Depth of simulated breathing was controlled by adjusting the distance by which the indenter was depressed into the gel; this depth ranged from approximately 40 to 300 μm, chosen for each airway to effect six levels of airway luminal fluctuations (from 3–6% to ∼ 50%) for that airway before contractile stimulation. Note that during contractile stimulation, the same depth of simulated breathing invariably resulted in lesser actual airway luminal fluctuations, presumably reflecting greater stiffness of the contracting airway (see below). Each PCLS was stretched 12 times/min using a quasi-triangle wave pattern that included 2-second cylinder depression, 0.5-second hold, 2-second cylinder retraction, and 0.5-second hold. Indenter motion was controlled by a Micromanipulator 5171 (Eppendorf, Hauppauge, NY) directed by a program created with LabView (National Instruments, Austin, TX). The PCLS was visualized from below on an inverted microscope with a 4× objective. Images were collected at approximately 3.6/s with an Optronics Microfire camera (Goleta, CA). One airway within each PCLS was studied in either of two protocols described below.
Figure 1.
Schema of system used to stretch airways within precision-cut lung slices (PCLS), intended to simulate breathing. Each PCLS was placed on a polyacrylamide gel, and the circular indenter was centered above a cross-sectionally cut airway. The indenter was lowered to come into contact with, but not stretch, the PCLS. The inset is a typical image of a representative nonstimulated airway, as viewed from below through an inverted microscope (not shown). Depression of the circular indenter caused the confined gel to bulge, which in turn caused a radial stretch of the lung tissue encircled by the indenter.
Protocol 1: Acetylcholine Dose–Response Curves at Constant Depth of Breathing
Each PLCS was studied before contractile stimulation and during three additional periods of contractile stimulation with acetylcholine (ACh) at 10−6, 10−5, and 10−4 M (in that order). During each study period, the airway was observed for 30 minutes of constant exposure to ACh (or buffer alone) that included 10 minutes without simulated breathing, 10 minutes simulated breathing at a depth (set for the relaxed airway) that caused 16 to 20% luminal area expansions with each “tidal breath,” and finally 10 minutes without simulated breathing. The PCLS was then superfused with ACh-free buffer and allowed to relax for at least 1 hour before the next ACh dose. In preliminary studies (data not shown), we tested the reproducibility of airway narrowing, area strain, and breathing-induced reversal of bronchoconstriction in five lung slices during two consecutive contractions. Using identical ACh concentrations and cylinder indentation depths during the two contractions, we found that the severity of auxotonic constriction, reversal of such bronchoconstriction, and airway strain were all quite reproducible in the same lung slice (paired t tests; all P values ≥ 0.2).
Protocol 2: Depth of Breathing Dose–Response Curves at Constant ACh Stimulation
In this protocol, each PCLS was exposed to only one concentration of ACh: 10−6, 10−5, or 10−4 M. The airway under study was observed for 80 minutes total during constant ACh exposure, including 10 minutes without simulated breathing; six 10-minute periods of simulated breathing at depths (set for the relaxed airway) that caused luminal area expansions of 3 to 6%, 7 to 10%, 11 to 14%, 16 to 20%, 23 to 26%, and approximately 50%; and finally 10 minutes without simulated breathing. Assuming isotropic expansion of the lung, tidal breathing corresponds to 7 to 10% and full inspiration to total lung capacity approximately 50% luminal area expansion.
Airway luminal area was determined from digitally recorded images using the Magic Wand tool of NIH ImageJ. “Maximum luminal area” refers to the greatest luminal area observed in the absence of simulated breathing during the entire study of an airway (invariably before any contractile stimulation, but on occasion after application of simulated breathing in the absence of contractile stimulation). “Minimum constricted area” is defined as the smallest luminal area during ACh stimulation. The area datum for each 10-minute simulated breathing period was taken as the “end-exhalation” area at the end of the period. “Area strain” refers to the luminal area fluctuation during simulated tidal breathing, taken as the difference between “end-inhalation” and “end-exhalation” areas. “Tidal stress” reflects the depth of simulated breathing applied. Finally, “percent reversal of bronchoconstriction” is used to quantify how effectively simulated breathing antagonizes ACh-induced bronchoconstriction, and is calculated as:
Correlations among these parameters were determined using Spearman rank correlation.
Results
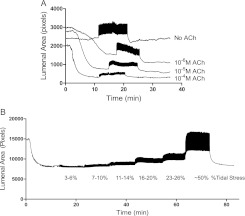
Figure 2 presents typical examples of primary data gathered during study of PCLS under Protocol 1 (Figure 2A) or under Protocol 2 (Figure 2B). A video showing actual images from the 10−5 M contraction in Figure 2A is provided in the online supplement.
Figure 2.
Representative tracings of airway luminal area from protocols 1 (A) and 2 (B). (A) Protocol 1: Acetylcholine (ACh) dose–response curves at constant depth of breathing. Airway luminal area was monitored for 10 minutes before, 10 minutes during, and 10 minutes after simulated breathing at 16 to 20% tidal stress. Each precision-cut lung slice was studied before contractile stimulation and during three additional periods of contractile stimulation with ACh at 10−6, 10−5, and 10−4 M (in that order). (B) Protocol 2: Depth of breathing dose–response curves at constant ACh stimulation. Each PCLS was exposed to only one concentration of ACh: 10−6 (shown), 10−5, or 10−4 M. The airway was observed for 80 minutes total during constant ACh exposure, including 10 minutes without simulated breathing; six 10-minute periods of simulating breathing using tidal stress of 3–6%, 7–10%, 11–14%, 16–20%, 23–26%, and ∼ 50%; and 10 minutes without simulated breathing.
In Protocol 1, each airway exhibited progressively smaller minimum constricted areas as the concentration of ACh was increased during repeated study periods. During simulated breathing, each depression cycle of the indenter tidally increased then decreased luminal area; the magnitude of tidal area fluctuations (area strain) fell with increasing ACh concentration and with more severe airway narrowing, despite the constant depth of breathing (tidal stress) used across ACh concentrations. Thus, with increasing ACh concentration and severity of constriction, the airway manifested greater stiffness (i.e., less area strain for the same tidal stress). End-expiratory luminal area increased during simulated breathing (i.e., breathing antagonized bronchoconstriction), but the extent to which this occurred fell as the ACh concentration and severity of constriction increased. For example, for the airway shown in Figure 2A, the simulated breathing-induced increase in end-expiratory luminal area over minimum luminal area observed during stimulation with 10−6 M ACh was much greater than that observed during stimulation with 10−4 M ACh.
In Protocol 2, each PCLS was exposed to only one concentration of ACh. After initial airway constriction, simulated breaths were then applied at progressively increasing depths. As shown in Figure 2B, the step increases in depth of breathing (tidal stress) caused progressive step increases in area strain, and these were accompanied by progressive step increases in end-expiratory luminal area. Thus, deeper breathing antagonized bronchoconstriction more effectively than did shallow breathing. After cessation of simulated breathing, the airway renarrowed to a degree similar to that observed before initiation of simulated breathing. Thus, the simulated breathing appears not to have damaged the ability of the airway to constrict and instead must have induced airway dilatation through a noninjurious and reversible mechanism.
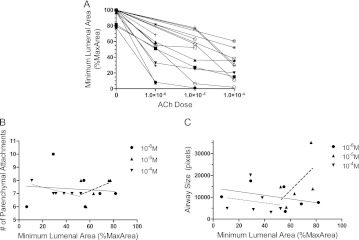
We used data gathered from both protocols to explore how the level of contractile stimulation, the severity of resultant constriction, and the depth of simulated breathing interact to determine the degree to which breathing antagonizes bronchoconstriction. First, there was considerable variation in the cholinergic sensitivity of airways studied (Figure 3A), as reflected in the differing slopes of their ACh dose–response curves and maximum severities of constriction. Because we studied only one airway from each donor, it is uncertain whether this variability reflects heterogeneity among different airways (43), among individuals, or both. However, neither the number of airway alveolar attachments (Figure 3B) nor the size of airways studied (Figure 3C) can account for the variability of ACh sensitivity, as neither correlated with minimum luminal area at constant ACh dose.
Figure 3.
Cholinergic sensitivity is variable among different airways. (A) Bronchoconstriction versus acetylcholine (ACh) concentration dose–response curves for all airways studied under protocols 1 and 2. There was considerable variation in the cholinergic sensitivity, as reflected in the differing slopes of their ACh dose–response curves and maximum severities of constriction. (B) The number of parenchymal attachments to the airway cannot account for the variability in ACh responsiveness, as there was no significant correlation between number of parenchymal attachments and severity of constriction at any ACh concentration (slopes of linear regressions not significantly different from zero, P > 0.05 each concentration). (C) The sizes of airways studied cannot account for the variability in ACh responsiveness, as there was no significant correlation between maximum luminal area and severity of constriction at any ACh concentration (slopes of linear regressions not significantly different from zero, P > 0.05 each concentration). Solid line, regression for 10−6 M ACh; dashed line, regression for 10−5 M ACh; dotted line, regression for 10−4 M ACh.
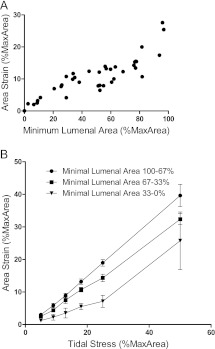
Second, the area strain induced by a fixed amount of stress (16–20% tidal stress) decreased with the severity of bronchoconstriction among all airways studied, seemingly independent of the concentration of ACh required to achieve that level of constriction (Figure 4A). Thus, it appears that the severity of constriction, rather than intensity of cholinergic stimulation, determined the stiffness of the airway. However, for a given level of constriction, area strain increased essentially proportionally to tidal stress (Figure 4B). As shown in Figure 4B, when airway contractions were binned into three levels of severity (minimum luminal areas of 100–67%, 67–34%, and 34–0%), almost linear area strain/tidal stress relationships emerged for each severity of bronchoconstriction, with the slope (= 1/stiffness) smallest for the most severely constricted airways. Together, these data indicate that the area strain actually experienced by a constricted human airway embedded within lung parenchyma depends not only on the depth of breathing but also on the severity of bronchoconstriction.
Figure 4.
Area strain experienced by a constricted human airway depends on both the severity of bronchoconstriction and the depth of breathing. (A) Area strain induced by a fixed amount of stress (16–20% tidal stress) decreased with the severity of bronchoconstriction (increased severity is reflected in lower minimum luminal area) among all airways studied, seemingly independent of the concentration of acetylcholine (ACh) required to achieve that level of constriction (Spearman rank correlation, r = 0.8667; P < 0.0001). (B) However, for a given level of constriction, area strain increased essentially proportionally to tidal stress. Values are means ± SEM; data are plotted at the nominal midpoint of each tidal stress bin.
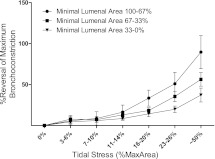
Third, we explored how the depth of breathing influences its ability to antagonize bronchoconstriction, as reflected in breathing-induced increases in end-expiratory luminal area. In Figure 5, results are plotted as the percent reversal of maximal bronchoconstriction versus the depth of simulated breathing and (as in Figure 4B) are grouped according to severity of bronchoconstriction. At each level of bronchoconstriction severity, breathing-induced airway dilatation increased with depth of breathing (tidal stress) in a dose–response fashion. Two features of these results are worthy of special note: (1) Breathing was most effective at antagonizing bronchoconstriction when the bronchoconstriction was least severe, and even deep breathing had only a modest ability to reverse severe bronchoconstriction. (2) At any level of bronchoconstriction, simulated quiet tidal breathing (7–10% tidal stress) had minimal, if any, influence on end-expiratory airway caliber. Instead, substantial reversal of bronchoconstriction was found only during deeper simulated breathing (i.e., greater tidal stress). We did not study the influence of breathing frequency on its ability to antagonize bronchoconstriction.
Figure 5.
Breathing-induced reversal of bronchoconstriction depends on both the severity of bronchoconstriction and the depth of breathing. At each level of bronchoconstriction severity (minimum luminal areas 100–67%, 67–33%, 33–0%), reversal of bronchoconstriction increased with depth of breathing (tidal stress) in a dose–response fashion. Greater reversal occurs when the bronchoconstriction is least severe (minimum luminal area, 100–67%). Furthermore, substantial reversal of bronchoconstriction is found only during deeper simulated breathing (i.e., greater tidal stress). Values are means ± SEM.
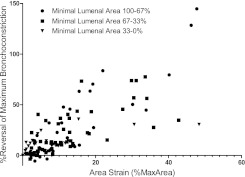
Finally, because area strain reflects both depth of breathing and severity of bronchoconstriction, we wondered whether this consequence of breathing might be an important determinant of breathing-induced antagonism of bronchoconstriction. To explore this possibility, we plotted the percent reversal of bronchoconstriction against area strain, including every observation from this study. As shown in Figure 6, breathing-induced antagonism of bronchoconstriction correlated strongly (Spearman rank correlation) with area strain across all levels of bronchoconstriction. This supports the possibility that circumferential stretching of the airway wall is a primary mechanism by which breathing antagonizes bronchoconstriction.
Figure 6.
Breathing-induced reversal of bronchoconstriction correlates significantly (Spearman rank correlation. 100–67%: r = 0.8153, P < 0.001; 67–33%: r = 0.8453, P < 0.001; 33–0%: r = 0.5971, P = 0.002) with area strain when considered across all levels of bronchoconstriction.
Discussion
In normal people, deep breathing is a potent antagonist of artificially-induced bronchoconstriction. For example, voluntary isocapnic hyperpnea quickly reverses methacholine-induced bronchoconstriction (44). Furthermore, deep breaths performed during inhaled-aerosol bronchial provocation limit the maximal constrictor response in normal individuals, as evidenced by the finding that severe bronchoconstriction occurs only when deep inhalation is prohibited (2, 7). Although deep breathing can also suppress bronchoconstriction in individuals with asthma (45), its beneficial effect is less marked than in those without asthma (37, 38). Indeed, the loss of airway dilatation induced by a deep breath might contribute to persistent airflow obstruction in asthma (6, 7). In the conceptual framework reviewed above, each breath must actually stretch the airway wall in order for breathing to perturb myosin binding and thereby reverse bronchoconstriction (4, 9, 46). Our results provide new experimental validation of this conceptual framework. We demonstrate that breathing-induced reversal of bronchoconstriction in individual intraparenchymal airways depends on the degree to which each breath actually stretches the airway tidally, which in turn depends on both the depth of breathing (tidal volume) (38, 45–50) and the severity of bronchoconstriction (51, 52).
Limitations in spatial and time resolution of current imaging techniques, and/or risks of ionizing radiation, preclude the accurate measurement of the tidal expansion of individual intraparenchymal airways in living people. We therefore developed a new experimental system in which human airways within thin lung slices can be studied under conditions of dynamic load that simulate breathing, while precisely monitoring airway size and its tidal fluctuation (Figure 1). Our approach builds on prior work by Wohlsen and others (40, 41, 53–57), who assessed airway contraction in the absence of simulated breathing, and on that of Dassow and colleagues (58) and Sanderson (59), who have also developed systems to stretch lung slices.
Two results of our study are salient. First, for any level of initial bronchoconstriction, the reversal of that bronchoconstriction by breathing increased in a dose-dependent fashion with depth of breathing (Figure 5). Notably, simulated tidal breathing (tidal stress 7–10%) caused little if any bronchodilatation; rather, substantial reversal of bronchoconstriction occurred only when deeper breathing was applied, with the greatest reversal observed with simulated tidal breaths to full lung inflation (tidal stress ∼ 50%). This observation mirrors the clinical observations of Freedman and colleagues (44) and Skloot and colleagues (7), who found that deep breathing but not quiet tidal breathing suppressed experimentally induced bronchoconstriction in normal subjects, and confirms parallel findings by Noble and colleagues (60), who subjected excised human airways to transmural pressure fluctuations to simulate breathing. Second, for any depth of simulated breathing, the reversal of bronchoconstriction fell progressively with the initial level of that bronchoconstriction (Figure 5). For example, simulated inhalations to total lung capacity (tidal stress ∼ 50%) almost fully reversed bronchoconstriction when the level of bronchoconstriction was relatively mild (minimal luminal area 100–67%), but these deep breaths were only minimally effective in reversing bronchoconstriction that was severe (minimal luminal area 33–0%). Thus, depth of breathing and severity of bronchoconstriction interact to determine the ability of breathing to reverse bronchoconstriction.
As shown in Figure 4, more severely constricted airways are stiffer (i.e., exhibit less area strain at each level of tidal stress). We therefore wondered whether, as postulated in the conceptual framework, area strain actually achieved by simulated breathing integrates the influences of depth of breathing and severity of constriction on breathing-induced reversal of bronchoconstriction. Although by design our system did not allow for direct manipulation of constricted airway circumference, it was nonetheless possible to test this idea by examining the relationship between breathing-induced reversal of bronchoconstriction and area strain. Indeed, these parameters are strongly correlated (Figure 6).
This relationship also provides experimental support for mechanisms proposed to explain two complex integrative behaviors of bronchoconstricted normal and asthmatic lungs (32–38). First, spontaneous or induced airflow obstruction is typically heterogeneous throughout the respiratory tree, resulting in heterogeneous ventilation of regions subtended by the variously constricted airways (32–36, 61). As proposed by Anafi and Wilson (62), Winkler and Venegas (28), Venegas and colleagues (29), and Tgavalekos and colleagues (34), an airway coursing through the lung region it ventilates is subjected to tidal stresses determined by local regional ventilation. Thus, lung regions served by more constricted airways have relatively smaller regional tidal volumes and so exert smaller tidal stresses on their subtending airways; consequently, these more constricted airways experience relatively little breathing-induced reversal of bronchoconstriction. Conversely, less severely constricted airways allow greater tidal ventilation of their subtended lung regions, which in turn exert greater tidal stresses on their feeding airways (28, 29, 34, 62). Because they are less severely constricted and subject to greater tidal stress, these airways experience more effective reversal of bronchoconstriction. This heterogeneity of breathing-induced reversal defines a self-reinforcing positive feedback that acts to amplify heterogeneity of airway narrowing. Our experimental results support this possibility. Second, deep breathing is less efficacious in reversing bronchoconstriction in individuals with asthma than in normal individuals (37, 38, 63). If bronchoconstriction of intraparenchymal airways were generally more severe in individuals with asthma than in normal individuals (6), then perhaps the greater initial narrowing might be one potential mechanism that could account for a lesser response of asthmatic lungs to deep breathing. That is, there might be no innate derangement of the mechanism by which deep breathing reverses bronchoconstriction in individuals with asthma; rather, the more severely constricted airways of the patient with asthma would respond essentially normally in the sense that these airways are constricted more severely, therefore are stiffer, and thus would distend less (Figure 4B). Studies of airways from individuals with asthma would be needed to test this possibility. In this regard, Pyrgos and colleagues (64) reported that the ability of deep inhalations to reverse methacholine-induced bronchoconstriction in individuals with asthma increased with the distensibility of their airways; however, the latter was assessed in the absence of bronchoconstriction induced a priori. As such, their study did not directly address the conceptual framework outlined above.
Our results confirm and extend the works of LaPrad and colleagues (31, 65) and Noble and colleagues (60), who studied porcine, bovine, and human fluid-filled airways dissected free from other lung architecture and used transmural pressure fluctuations to mimic breathing. In each of these studies, a simulated deep breath increased the diameter of the constricted fluid-filled airway. Pressure fluctuations meant to simulate quiet tidal breathing did not reverse bronchoconstriction, but in a more recent study (31) deep breathing also failed to reverse bronchoconstriction. This difference from our results might be explained by differences in species, airway size, or the experimental system.
There are other potential applications for our experimental system. Future studies could address the potential influences of prolonged bronchoconstriction or prolonged “breathhold” on breathing-induced reversal of bronchoconstriction. Our system could also be used to study the bronchoprotective effect of deep inspiration observed in people (66). Because the airways remain within their native architecture, and because fluctuation-induced relengthening has molecular determinants that differ from those of isometric force (9, 14–17), it may be a useful platform for discovering novel pharmacologic agents that potentiate breathing-induced airway dilatation (67). For example, we have previously shown that latrunculin B (14), dexamethasone (17), and mitogen-activated protein kinase kinase inhibition (15) potentiate force fluctuation-induced relengthening of contracted canine tracheal smooth muscle strips, and unpublished observations in our laboratory suggest that latrunculin B potentiates breathing-induced reversal of bronchoconstriction. In addition, the broad range of lung cell types within thin lung slices might facilitate discovery of potential lung toxicities of agents under study. Finally, genetic manipulations of human lung slices ex vivo, or lung slices from genetically engineered mice, might provide further mechanistic insights into the effects of breathing on constricted airways.
It is important to consider the limitations of our study. In our system, lung slices were submerged and the alveoli fluid filled; this would have reduced surface tension below that of air-filled lungs, and the transmission of force from the parenchyma to the airway adventitia may be somewhat reduced. However, for each airway we adjusted cylinder depression depths to accomplish the desired airway strain in its relaxed state under the same airless lung condition; as such, any alteration in force transmission should have been compensated. On occasion, a pulmonary blood vessel was present in close proximity to the airway being studied; this anatomical arrangement also occurs in the intact lung, so we analyzed data from these airways together with all other results. There was variability among airways in the sensitivity and magnitude of their constrictor responses to acetylcholine (Figure 3). Because we studied only one airway from each donor lung, we could not discern the extent to which this reflects heterogeneity among airways within an individual versus among individuals. Also, we stretched lung slices isotropically in two directions but not in the third dimension perpendicular to the plane of the slice. We suspect that adding axial stretch would have had little effect on our results, because airway smooth muscle shortening undoubtedly dominated the contraction dynamics we observed, and the helical angle of muscle fibers within the airways is small. Last, we did not directly study airways in lung slices from individuals with asthma.
In summary, we have developed a novel system for stretching small human airways embedded within their normal lung architecture. Using this system, we found that simulated breathing reversed bronchoconstriction most effectively when the severity of bronchoconstriction was small and the depth of breathing large, conditions that together result in the greatest tidal fluctuation in airway smooth muscle length in response to breathing. Reduction in breathing-induced airway wall stretching under circumstances of excessive airway constriction and/or reduced regional lung ventilation in asthma may explain why a deep inspiration is less effective at reversing bronchoconstriction in the presence of airways disease.
Acknowledgments
The authors thank Kavitha Rajendran and Greeshma Manomohan for their help in preparing the gels and Dr. Richard Mitchell, Paul Kogut, and Jonathan Learoyd for their technical advice. Tissue used for this research project was provided by Gift of Hope Organ and Tissue Donor Network through the generous gift of donor families. Advice on biostatistical analysis was provided by the Biostatistics Laboratory at the University of Chicago, under support from the University of Chicago Institute for Translational Medicine and NCRR CTSA UL1 RR024999.
Footnotes
Supported by National Institutes of Health grants P50 HL107171, R01 HL097805, and UL1 RR024999, and by a research grant from Astra-Zeneca, Inc.
Author Contributions: Conceived and designed the experiments: T.L.L., R.K., J.J.F., J.S., M.L.D. Performed the experiments: T.L.L., R.K., H.R.S., E.D.M., M.L.D. Analyzed the data: T.L.L., R.K., J.S., M.L.D. Wrote the paper: T.L.L., R.K., J.J.F., J.S., M.L.D.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201202-0368OC on June 7, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Salter HH. On asthma: its pathology and treatment. New York: William Wood and Company; 1859 [Google Scholar]
- 2.Moore BJ, Verburgt LM, King GG, Pare PD. The effect of deep inspiration on methacholine dose–response curves in normal subjects. Am J Respir Crit Care Med 1997;156:1278–1281 [DOI] [PubMed] [Google Scholar]
- 3.Nadel JA, Tierney DF. Effect of a previous deep inspiration on airway resistance in man. J Appl Physiol 1961;16:717–719 [DOI] [PubMed] [Google Scholar]
- 4.Gump A, Haughney L, Fredberg J. Relaxation of activated airway smooth muscle: relative potency of isoproterenol vs. Tidal stretch. J Appl Physiol 2001;90:2306–2310 [DOI] [PubMed] [Google Scholar]
- 5.Green M, Mead J. Time dependence of flow-volume curves. J Appl Physiol 1974;37:793–797 [DOI] [PubMed] [Google Scholar]
- 6.Lim TK, Pride NB, Ingram RH., Jr Effects of volume history during spontaneous and acutely induced air-flow obstruction in asthma. Am Rev Respir Dis 1987;135:591–596 [DOI] [PubMed] [Google Scholar]
- 7.Skloot G, Permutt S, Togias A. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J Clin Invest 1995;96:2393–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fish JE, Ankin MG, Kelly JF, Peterman VI. Regulation of bronchomotor tone by lung inflation in asthmatic and nonasthmatic subjects. J Appl Physiol 1981;50:1079–1086 [DOI] [PubMed] [Google Scholar]
- 9.Oliver MN, Fabry B, Marinkovic A, Mijailovich SM, Butler JP, Fredberg JJ. Airway hyperresponsiveness, remodeling, and smooth muscle mass: right answer, wrong reason? Am J Respir Cell Mol Biol 2007;37:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendixen HH, Smith GM, Mead J. Pattern of ventilation in young adults. J Appl Physiol 1964;19:195–198 [DOI] [PubMed] [Google Scholar]
- 11.Latourelle J, Fabry B, Fredberg JJ. Dynamic equilibration of airway smooth muscle contraction during physiological loading. J Appl Physiol 2002;92:771–779 [DOI] [PubMed] [Google Scholar]
- 12.Mijailovich SM, Butler JP, Fredberg JJ. Perturbed equilibria of myosin binding in airway smooth muscle: bond-length distributions, mechanics, and ATP metabolism. Biophys J 2000;79:2667–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredberg JJ, Inouye DS, Mijailovich SM, Butler JP. Perturbed equilibrium of myosin binding in airway smooth muscle and its implications in bronchospasm. Am J Respir Crit Care Med 1999;159:959–967 [DOI] [PubMed] [Google Scholar]
- 14.Dowell ML, Lakser OJ, Gerthoffer WT, Fredberg JJ, Stelmack GL, Halayko AJ, Solway J, Mitchell RW. Latrunculin B increases force fluctuation-induced relengthening of ACh-contracted, isotonically shortened canine tracheal smooth muscle. J Appl Physiol 2005;98:489–497 [DOI] [PubMed] [Google Scholar]
- 15.Dowell ML, Lavoie TL, Lakser OJ, Dulin NO, Fredberg JJ, Gerthoffer WT, Seow CY, Mitchell RW, Solway J. MEK modulates force-fluctuation-induced relengthening of canine tracheal smooth muscle. Eur Respir J 2010;36:630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakser OJ, Lindeman RP, Fredberg JJ. Inhibition of the p38 map kinase pathway destabilizes smooth muscle length during physiological loading. Am J Physiol Lung Cell Mol Physiol 2002;282:L1117–L1121 [DOI] [PubMed] [Google Scholar]
- 17.Lakser OJ, Dowell ML, Hoyte FL, Chen B, Lavoie TL, Ferreira C, Pinto LH, Dulin NO, Kogut P, Churchill J, et al. Steroids augment relengthening of contracted airway smooth muscle: potential additional mechanism of benefit in asthma. Eur Respir J 2008;32:1224–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Pare PD. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol 1993;74:2771–2781 [DOI] [PubMed] [Google Scholar]
- 19.Macklem PT. Mechanical factors determining maximum bronchoconstriction. Eur Respir J Suppl 1989;6:516s–519s [PubMed] [Google Scholar]
- 20.Wang L, Pare PD, Seow CY. Selected contribution: effect of chronic passive length change on airway smooth muscle length-tension relationship. J Appl Physiol 2001;90:734–740 [DOI] [PubMed] [Google Scholar]
- 21.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis 1993;148:720–726 [DOI] [PubMed] [Google Scholar]
- 22.Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, Carter R, Wong HH, Cadbury PS, Fahy JV. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med 2004;169:1001–1006 [DOI] [PubMed] [Google Scholar]
- 23.Macklem PT. A theoretical analysis of the effect of airway smooth muscle load on airway narrowing. Am J Respir Crit Care Med 1996;153:83–89 [DOI] [PubMed] [Google Scholar]
- 24.Moreno RH, Hogg JC, Pare PD. Mechanics of airway narrowing. Am Rev Respir Dis 1986;133:1171–1180 [DOI] [PubMed] [Google Scholar]
- 25.James AL, Pare PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis 1989;139:242–246 [DOI] [PubMed] [Google Scholar]
- 26.Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol 1987;62:1324–1330 [DOI] [PubMed] [Google Scholar]
- 27.Gelb AF, Licuanan J, Shinar CM, Zamel N. Unsuspected loss of lung elastic recoil in chronic persistent asthma. Chest 2002;121:715–721 [DOI] [PubMed] [Google Scholar]
- 28.Winkler T, Venegas JG. Self-organized patterns of airway narrowing. J Appl Physiol 2011;110:1482–1486 [DOI] [PubMed] [Google Scholar]
- 29.Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature 2005;434:777–782 [DOI] [PubMed] [Google Scholar]
- 30.Fredberg JJ, Jones KA, Nathan M, Raboudi S, Prakash YS, Shore SA, Butler JP, Sieck GC. Friction in airway smooth muscle: mechanism, latch, and implications in asthma. J Appl Physiol 1996;81:2703–2712 [DOI] [PubMed] [Google Scholar]
- 31.LaPrad AS, Szabo TL, Suki B, Lutchen KR. Tidal stretches do not modulate responsiveness of intact airways in vitro. J Appl Physiol 2010;109:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tzeng YS, Lutchen K, Albert M. The difference in ventilation heterogeneity between asthmatic and healthy subjects quantified using hyperpolarized 3He MRI. J Appl Physiol 2009;106:813–822 [DOI] [PubMed] [Google Scholar]
- 33.Winkler T, Venegas JG. Complex airway behavior and paradoxical responses to bronchoprovocation. J Appl Physiol 2007;103:655–663 [DOI] [PubMed] [Google Scholar]
- 34.Tgavalekos NT, Musch G, Harris RS, Vidal Melo MF, Winkler T, Schroeder T, Callahan R, Lutchen KR, Venegas JG. Relationship between airway narrowing, patchy ventilation and lung mechanics in asthmatics. Eur Respir J 2007;29:1174–1181 [DOI] [PubMed] [Google Scholar]
- 35.Harris RS, Winkler T, Tgavalekos N, Musch G, Melo MF, Schroeder T, Chang Y, Venegas JG. Regional pulmonary perfusion, inflation, and ventilation defects in bronchoconstricted patients with asthma. Am J Respir Crit Care Med 2006;174:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venegas JG, Schroeder T, Harris S, Winkler RT, Melo MF. The distribution of ventilation during bronchoconstriction is patchy and bimodal: a PET imaging study. Respir Physiol Neurobiol 2005;148:57–64 [DOI] [PubMed] [Google Scholar]
- 37.Lutchen KR, Jensen A, Atileh H, Kaczka DW, Israel E, Suki B, Ingenito EP. Airway constriction pattern is a central component of asthma severity: the role of deep inspirations. Am J Respir Crit Care Med 2001;164:207–215 [DOI] [PubMed] [Google Scholar]
- 38.Jackson AC, Murphy MM, Rassulo J, Celli BR, Ingram RH., Jr Deep breath reversal and exponential return of methacholine-induced obstruction in asthmatic and nonasthmatic subjects. J Appl Physiol 2004;96:137–142 [DOI] [PubMed] [Google Scholar]
- 39.Lavoie TL, Krishnan R, Maston ED, Nixon JA, Siegel HR, Kogut P, Solway J, Dowell ML. “Breathing” enhances bronchodilation in ACh-contracted human airways from precision cut lung slices. Am J Respir Crit Care Med 2011;183:A3654 [Google Scholar]
- 40.Wohlsen A, Martin C, Vollmer E, Branscheid D, Magnussen H, Becker WM, Lepp U, Uhlig S. The early allergic response in small airways of human precision-cut lung slices. Eur Respir J 2003;21:1024–1032 [DOI] [PubMed] [Google Scholar]
- 41.Ressmeyer AR, Bai Y, Delmotte P, Uy KF, Thistlethwaite P, Fraire A, Sato O, Ikebe M, Sanderson MJ. Human airway contraction and formoterol-induced relaxation is determined by Ca2+ oscillations and Ca2+ sensitivity. Am J Respir Cell Mol Biol 2010;43:179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan R, Park CY, Lin YC, Mead J, Jaspers RT, Trepat X, Lenormand G, Tambe D, Smolensky AV, Knoll AH, et al. Reinforcement versus fluidization in cytoskeletal mechanoresponsiveness. PLoS ONE 2009;4:e5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minshall E, Wang CG, Dandurand R, Eidelman D. Heterogeneity of responsiveness of individual airways in cultured lung explants. Can J Physiol Pharmacol 1997;75:911–916 [PubMed] [Google Scholar]
- 44.Freedman S, Lane R, Gillett MK, Guz A. Abolition of methacholine induced bronchoconstriction by the hyperventilation of exercise or volition. Thorax 1988;43:631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stirling DR, Cotton DJ, Graham BL, Hodgson WC, Cockcroft DW, Dosman JA. Characteristics of airway tone during exercise in patients with asthma. J Appl Physiol 1983;54:934–942 [DOI] [PubMed] [Google Scholar]
- 46.Raqeeb A, Solomon D, Pare PD, Seow CY. Length oscillation mimicking periodic individual deep inspirations during tidal breathing attenuates force recovery and adaptation in airway smooth muscle. J Appl Physiol 2010;109:1476–1482 [DOI] [PubMed] [Google Scholar]
- 47.Shen X, Gunst SJ, Tepper RS. Effect of tidal volume and frequency on airway responsiveness in mechanically ventilated rabbits. J Appl Physiol 1997;83:1202–1208 [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Pare PD, Seow CY. Effects of length oscillation on the subsequent force development in swine tracheal smooth muscle. J Appl Physiol 2000;88:2246–2250 [DOI] [PubMed] [Google Scholar]
- 49.Salerno FG, Shinozuka N, Fredberg JJ, Ludwig MS. Tidal volume amplitude affects the degree of induced bronchoconstriction in dogs. J Appl Physiol 1999;87:1674–1677 [DOI] [PubMed] [Google Scholar]
- 50.Fredberg JJ, Inouye D, Miller B, Nathan M, Jafari S, Raboudi SH, Butler JP, Shore SA. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med 1997;156:1752–1759 [DOI] [PubMed] [Google Scholar]
- 51.Moore BJ, King GG, D’Yachkova Y, Ahmad HR, Pare PD. Mechanism of methacholine dose–response plateaus in normal subjects. Am J Respir Crit Care Med 1998;158:666–669 [DOI] [PubMed] [Google Scholar]
- 52.King GG, Moore BJ, Seow CY, Pare PD. Airway narrowing associated with inhibition of deep inspiration during methacholine inhalation in asthmatics. Am J Respir Crit Care Med 2001;164:216–218 [DOI] [PubMed] [Google Scholar]
- 53.Fisher RL, Smith MS, Hasal SJ, Hasal KS, Gandolfi AJ, Brendel K. The use of human lung slices in toxicology. Hum Exp Toxicol 1994;13:466–471 [DOI] [PubMed] [Google Scholar]
- 54.Wohlsen A, Uhlig S, Martin C. Immediate allergic response in small airways. Am J Respir Crit Care Med 2001;163:1462–1469 [DOI] [PubMed] [Google Scholar]
- 55.Munoz NM, Leff AR. New method for real-time measurements of changes in luminal area of microsection explants of airways by videomicrometry. Chest 1995;107:146S–147S [DOI] [PubMed] [Google Scholar]
- 56.Martin C, Uhlig S, Ullrich V. Cytokine-induced bronchoconstriction in precision-cut lung slices is dependent on cyclooxygenase-2 and thromboxane receptor activation. Am J Respir Cell Mol Biol 2001;24:139–145 [DOI] [PubMed] [Google Scholar]
- 57.Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol 2002;119:187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dassow C, Wiechert L, Martin C, Schumann S, Muller-Newen G, Pack O, Guttmann J, Wall WA, Uhlig S. Biaxial distension of precision-cut lung slices. J Appl Physiol 2010;108:713–721 [DOI] [PubMed] [Google Scholar]
- 59.Sanderson MJ. Exploring lung physiology in health and disease with lung slices. Pulm Pharmacol Ther 2011;24:452–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noble PB, Jones RL, Needi ET, Cairncross A, Mitchell HW, James AL, McFawn PK. Responsiveness of the human airway in vitro during deep inspiration and tidal oscillation. J Appl Physiol 2011;110:1510–1518 [DOI] [PubMed] [Google Scholar]
- 61.Brown RH, Croisille P, Mudge B, Diemer FB, Permutt S, Togias A. Airway narrowing in healthy humans inhaling methacholine without deep inspirations demonstrated by HRCT. Am J Respir Crit Care Med 2000;161:1256–1263 [DOI] [PubMed] [Google Scholar]
- 62.Anafi RC, Wilson TA. Airway stability and heterogeneity in the constricted lung. J Appl Physiol 2001;91:1185–1192 [DOI] [PubMed] [Google Scholar]
- 63.Brown RH, Scichilone N, Mudge B, Diemer FB, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am J Respir Crit Care Med 2001;163:994–1001 [DOI] [PubMed] [Google Scholar]
- 64.Pyrgos G, Scichilone N, Togias A, Brown RH. Bronchodilation response to deep inspirations in asthma is dependent on airway distensibility and air trapping. J Appl Physiol 2011;110:472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LaPrad AS, West AR, Noble PB, Lutchen KR, Mitchell HW. Maintenance of airway caliber in isolated airways by deep inspiration and tidal strains. J Appl Physiol 2008;105:479–485 [DOI] [PubMed] [Google Scholar]
- 66.Scichilone N, Kapsali T, Permutt S, Togias A. Deep inspiration-induced bronchoprotection is stronger than bronchodilation. Am J Respir Crit Care Med 2000;162:910–916 [DOI] [PubMed] [Google Scholar]
- 67.Lavoie TL, Dowell ML, Lakser OJ, Gerthoffer WT, Fredberg JJ, Seow CY, Mitchell RW, Solway J. Disrupting actin-myosin-actin connectivity in airway smooth muscle as a treatment for asthma? Proc Am Thorac Soc 2009;6:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]