Abstract
Rationale: Patients with isolated mediastinal lymphadenopathy (IML) are a common presentation to physicians, and mediastinoscopy is traditionally considered the “gold standard” investigation when a pathological diagnosis is required. Endobronchial ultrasound–guided transbronchial needle aspiration (EBUS-TBNA) is established as an alternative to mediastinoscopy in patients with lung cancer.
Objective: To determine the efficacy and health care costs of EBUS-TBNA as an alternative initial investigation to mediastinoscopy in patients with isolated IML.
Methods: Prospective multicenter single-arm clinical trial of 77 consecutive patients with IML from 5 centers between April 2009 and March 2011. All patients underwent EBUS-TBNA. If EBUS-TBNA did not provide a diagnosis, then participants underwent mediastinoscopy.
Measurements and Main Results: EBUS-TBNA prevented 87% of mediastinoscopies (95% confidence interval [CI], 77–94%; P < 0.001) but failed to provide a diagnosis in 10 patients (13%), all of whom underwent mediastinoscopy. The sensitivity and negative predictive value of EBUS-TBNA in patients with IML were 92% (95% CI, 83–95%) and 40% (95% CI, 12–74%), respectively. One patient developed a lower respiratory tract infection after EBUS-TBNA, requiring inpatient admission. The cost of the EBUS-TBNA procedure per patient was £1,382 ($2,190). The mean cost of the EBUS-TBNA strategy was £1,892 ($2,998) per patient, whereas a strategy of mediastinoscopy alone was significantly more costly at £3,228 ($5,115) per patient (P < 0.001). The EBUS-TBNA strategy is less costly than mediastinoscopy if the cost per EBUS-TBNA procedure is less than £2,718 ($4,307) per patient.
Conclusions: EBUS-TBNA is a safe, highly sensitive, and cost-saving initial investigation in patients with IML.
Clinical trial registered with ClinicalTrials.gov (NCT00932854).
Keywords: endobronchial ultrasound, mediastinal lymphadenopathy, sarcoidosis, tuberculosis, lymphoma
At a Glance Commentary
Scientific Knowledge on the Subject
In patients with isolated mediastinal lymphadenopathy, mediastinoscopy is recommended as an initial investigation. Endobronchial ultrasound–guided transbronchial needle aspiration (EBUS-TBNA) has emerged as an important alternative to mediastinoscopy in patients with non–small cell lung cancer. However, it is unclear whether EBUS-TBNA can be used as an initial investigation in patients with isolated mediastinal lymphadenopathy.
What This Study Adds to the Field
This multicenter prospective trial demonstrates that EBUS-TBNA may be recommended as an initial investigation in patients with isolated mediastinal lymphadenopathy. EBUS-TBNA was able to spare mediastinoscopy in 87% of patients and was less costly than mediastinoscopy if the cost per EBUS-TBNA procedure was less than £2,718 ($4,307) per patient. The negative predictive value of EBUS-TBNA was low, at 40%, and therefore negative EBUS-TBNA should still be followed by mediastinoscopy.
Isolated mediastinal lymphadenopathy (IML) is a common presentation to pulmonologists. Final diagnoses often include sarcoidosis, tuberculosis, lymphoma, and metastatic carcinoma. However, symptoms are nonspecific and fevers, night sweats, and weight loss are a common feature of each diagnosis. A pathological and microbiological diagnosis is therefore commonly obtained to differentiate these conditions and guide further management.
Mediastinoscopy has traditionally been considered the “gold standard” for lymph node sampling in these patients. Previous retrospective studies have demonstrated a high diagnostic yield for the procedure in this patient group, with few complications (1–3). However, mediastinoscopy requires general anesthesia, allows access only to the paratracheal and anterior subcarinal lymph nodes, and in many cases requires an inpatient stay. Patients are left with a visible scar above the suprasternal notch, which can be a cosmetic issue in young people. Although complications from mediastinoscopy are rare they may be catastrophic and include vocal cord palsy, innominate vein damage, and even death (2).
Endobronchial ultrasound–guided transbronchial needle aspiration (EBUS-TBNA) was developed for the mediastinal staging of lung cancer, and studies have demonstrated that it has a similar sensitivity to mediastinoscopy for detecting mediastinal metastases from non–small cell lung cancer (4). In the only prospective direct comparison of EBUS-TBNA and mediastinoscopy to date in patients with suspected lung cancer (5), EBUS-TBNA demonstrated significantly superior sensitivity (91 vs. 78%; P = 0.007). Data have now emerged on the sensitivity of EBUS-TBNA in the diagnosis of benign mediastinal lymph nodes (6, 7), lymphoma (8), and extrathoracic malignancy (9).
EBUS-TBNA has several advantages for patients compared with mediastinoscopy. The procedure may be performed in the ambulatory care setting under sedation, has a lower complication rate, and provides access to lymph node stations that are inaccessible to standard cervical mediastinoscopy. However, given concerns about smaller biopsy sizes with EBUS-TBNA and inherent selection bias of previous retrospective studies, it is unknown whether EBUS-TBNA can replace mediastinoscopy as a first investigation in patients with IML. In this prospective multicenter clinical trial, we aimed to determine whether EBUS-TBNA should be used as the initial procedure of choice in patients presenting with IML requiring pathological evaluation and describe the economic consequences of this strategy.
Methods
Study Design
This was a multicenter single-arm prospective clinical trial of EBUS-TBNA in patients with IML. If EBUS-TBNA did not give a definitive diagnosis, patients underwent mediastinoscopy. To ascertain whether patients included in this study were representative of patients previously referred for mediastinoscopy, data were collected on 68 patients from our institution who underwent mediastinoscopy between 2007 and 2008, before the introduction of the EBUS-TBNA service. The study protocol is available in the online supplement and was approved by the Moorfields and Whittington Research Ethics Committee (reference 09/H0721/23). The trial registration at ClinicalTrials.gov is NCT00932854.
Participants
Consecutive patients with undiagnosed IML on computed tomography or positron emission tomography–computed tomography, and for whom a pathological diagnosis was required, were approached for trial entry between July 2009 and April 2011. The participating centers were University College London Hospital, Whittington Hospital, North Middlesex University Hospital, Barnet General Hospital, and Princess Alexandra Hospital. Patients with anterior mediastinal lymphadenopathy only, with known or suspected lung cancer, without informed consent, or contraindications to EBUS-TBNA or mediastinoscopy were excluded from the trial.
Intervention
All patients underwent EBUS-TBNA as an initial procedure. Our technique for EBUS-TBNA has been previously described (9). Briefly, the procedure was conducted in the outpatient setting and patients received intravenous sedation with midazolam and fentanyl in addition to topical lidocaine. A linear echoendoscope (BF-UC160F-OL8; Olympus, Keymed, UK) was used in all cases and a systematic assessment of hilar and mediastinal lymph nodes was made. Under ultrasound guidance mediastinal and/or hilar lymph nodes were punctured with a 21- or 22-gauge needle and suction was applied. Samples were transferred onto glass slides and air-dried or directly expelled into liquid fixative for cell block processing. Any cores of tissue were placed in formalin. The site and number of lymph nodes punctured as well as the number of passes per node were at the operator’s discretion. One pass was routinely submitted for acid-fast bacilli smear and culture. On-site evaluation of samples was not employed. Immunohistochemistry was performed as required; however, flow cytometry and serological and urinary antigen tests were not used. Additional transbronchial lung biopsies, after the EBUS-TBNA procedure was complete, were performed at the operator’s discretion. The pathologists were blinded to the fact that the patient was in a clinical trial and were provided with clinical information, reflecting routine clinical practice.
Pathological and microbiological results were reviewed in a multidisciplinary team setting including radiologists, respiratory physicians, thoracic surgeons, and pathologists. If a diagnosis agreed by the multidisciplinary team was not obtained from EBUS-TBNA, then the patient underwent mediastinoscopy. Cervical mediastinoscopy was performed under general anesthesia via an incision above the suprasternal notch, and lymph node stations 2, 4, and 7 were sampled.
When EBUS-TBNA and mediastinoscopy failed to show a definitive diagnosis, the participant underwent serial imaging and clinical follow-up for at least 6 months.
Outcomes
The coprimary outcomes were the proportion of mediastinoscopies saved and health care costs compared with a strategy in which all patients undergo mediastinoscopy alone. Secondary end points were the sensitivity, negative predictive value (NPV), and diagnostic accuracy of EBUS-TBNA in patients with IML. Complications of EBUS-TBNA were recorded if bleeding exceeded 10 ml or if peripheral oxygen saturations were recorded below 90%. Length of inpatient stay was prospectively documented.
Economic Analysis
The incremental cost of the EBUS-TBNA strategy (where negative EBUS-TBNA is followed by mediastinoscopy if required) versus mediastinoscopy alone in patients with IML was calculated from the perspective of the U.K. National Health Service (NHS). The analysis was based on a decision tree model (see Figure E1 in the online supplement). Patients with IML who underwent EBUS-TBNA either received a diagnosis or did not. In the case of the latter, they proceeded to mediastinoscopy, and if that failed to produce a diagnosis they received clinical and radiological follow-up for at least 6 months. They received treatment depending on their diagnosis. The alternative strategy was that patients with IML underwent mediastinoscopy as their only investigation. We assume in this case that there were no complications from mediastinoscopy and that no patients required clinical and radiological follow-up, which is probably conservative. We also assume that treatment and treatment outcomes after diagnosis were the same irrespective of the method of diagnosis, and therefore treatment costs are omitted from the incremental analysis. Because treatment outcomes were the same, we assume the two strategies were equally effective, and therefore our economic evaluation is a cost-minimization analysis; hence the EBUS-TBNA strategy represents good value for money to the health care system if it is less costly than the mediastinoscopy strategy.
In the decision tree model, the proportion of patients undergoing EBUS-TBNA, mediastinoscopy and clinical follow-up for the EBUS-TBNA strategy was obtained from the prospective trial. Unit costs were taken from manufacturers’ prices, local hospital costs, and 2010–2011 NHS tariffs where available (see Table E1 for further details of the cost of the EBUS-TBNA procedure). Because all costs occurred within 1 year, discounting was unnecessary. We investigated the sensitivity of the economic analysis to the cost of the EBUS-TBNA procedure, varying it between £503 ($797, the 2010–2011 NHS tariff for a flexible bronchoscopy) and £5,259 ($8,337, the 2010–2011 NHS tariff for mediastinoscopy with complications) (10).
Sample Size
The cost associated with mediastinoscopy without complications was £2,157 ($3,419) per patient, based on the NHS tariffs at the time this study was initiated. A difference of £500 ($792) in average cost for EBUS-TBNA compared with that for mediastinoscopy was considered to be important. A sample size calculation based on a one-sample t test suggested that a total of 75 patients would be required to detect a mean difference of £500 ($792) from the average cost of £2,157 ($3,419), assuming 80% power and a 2.5% significance level (using the Bonferroni correction for testing two primary outcomes). A standard deviation of £1,400 ($2,219) was assumed for the cost of EBUS-TBNA, based on clinical experience (a proportion of people may need to undergo mediastinoscopy followed by EBUS-TNA and/or stay in-hospital for additional days because of complications and require clinical follow-up). This sample size is also sufficient to assess whether the proportion of patients undergoing mediastinoscopy is reduced by 40%, assuming the same power and significance level.
Statistical Methods
Demographic and clinical characteristics of the study population were summarized using mean, standard deviation, median, or counts and percentages, depending on their type and distribution. A one-sample z test for proportions was used to determine whether there was a significant reduction in mediastinoscopies because of EBUS-TBNA. A one-sample t test was used to investigate whether the strategy of EBUS-TBNA initially (followed by mediastinoscopy if negative) differs significantly in cost on average from that associated with mediastinoscopy alone. Because of the skewed distribution of cost data, bootstrapping based on 2,000 samples was used to estimate a bias corrected and accelerated 95% confidence interval for the mean cost of EBUS-TBNA (11). Test accuracy of EBUS-TBNA and mediastinoscopy was calculated, using the sensitivity and NPV with 95% binomial confidence intervals. Specificity of EBUS-TBNA and mediastinoscopy samples was assumed to be 100%. All statistical analyses were performed with STATA version 11 (Statacorp, College Station, TX). The design, conduct, analysis, and report of this study conform to the Standard of Reporting Diagnostic Accuracy Guidelines (12).
Results
The 77 patients recruited had a median age of 42 years, and their characteristics are summarized in Table 1. To confirm a representative cohort within the study the characteristics of patients undergoing mediastinoscopy between 2007 and 2008 were documented (Table E2). Patients in the prospective trial and the historical control subjects had similar age, symptom distributions, and final diagnoses.
TABLE 1.
CLINICAL CHARACTERISTICS OF PATIENTS WITH ISOLATED MEDIASTINAL LYMPHADENOPATHY: THE REMEDY TRIAL
| Characteristic | EBUS-TBNA (n = 77) |
| Age, yr | |
| <30 | 15 (19%) |
| 30–49 | 34 (44%) |
| 50–69 | 16 (21%) |
| >69 | 12 (16%) |
| Median (range) | 42 (17–79) yr |
| Sex | |
| Male | 45 (58%) |
| Female | 32 (42%) |
| Ethnicity | |
| White | 25 (32%) |
| Asian | 29 (38%) |
| Black | 15 (19%) |
| Caribbean | 6 (8%) |
| Other | 2 (3%) |
| Symptom | |
| Cough | 27 (35%) |
| Dyspnea | 11 (14%) |
| Weight loss | 13 (17%) |
| Fevers/night sweats | 13 (17%) |
| Chest pain | 3 (4%) |
| Other | 2 (3%) |
| None | 8 (10%) |
| Final diagnosis | |
| Sarcoidosis stage 1 | 31 (40%) |
| Sarcoidosis stage 2 | 3 (4%) |
| Tuberculosis | 28 (36%) |
| Lymphoma | 3 (4%) |
| Extrathoracic malignancy | 4 (5%) |
| Lung cancer | 4 (5%) |
| Reactive lymphadenopathy | 4 (5%) |
Definition of abbreviations: EBUS-TBNA = endobronchial ultrasound–guided transbronchial needle aspiration; REMEDY = Clinical Trial of Endobronchial Ultrasound for the Diagnosis of Mediastinal Lymphadenopathy.
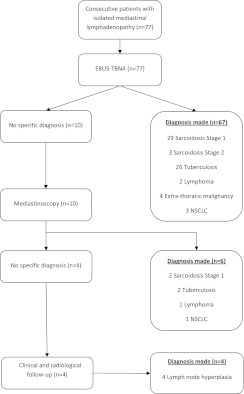
EBUS-TBNA prevented 87% of mediastinoscopies (95% confidence interval [CI], 77–94%), failing to provide a diagnosis in 10 of the 77 patients (13%; Figure 1). All 10 patients proceeded to mediastinoscopy as per protocol, which provided a specific diagnosis in 6 cases (8%). The remaining four patients (5%) had further clinical and radiological follow-up lasting at least 6 months. There was no loss to follow-up and all patients were included in the analysis. The final diagnosis was correctly determined by EBUS-TBNA in 67 cases, giving an overall diagnostic sensitivity of 92% (95% CI, 83–97%). The NPV was 40% (95% CI, 12–74%) and the diagnostic accuracy was 92% (95% CI, 84–97%). EBUS-TBNA successfully diagnosed sarcoidosis in 32 (94%) of 34 patients with the condition. Twenty-eight patients in the trial had a final diagnosis of tuberculosis, and EBUS-TBNA provided pathological evidence of tuberculosis in 26 (93%) and cultured Mycobacterium tuberculosis in 11 (40%) cases. Two patients were diagnosed with Hodgkin’s lymphoma and avoided the need for mediastinoscopy. Of these, EBUS-TBNA provided a conclusive diagnosis in one patient and enough information to prevent mediastinoscopy in another (who underwent bone marrow biopsy to confirm the diagnosis). A further patient with lymphoma was not definitively diagnosed by EBUS-TBNA and required mediastinoscopy to establish the diagnosis.
Figure 1.
Flowchart of patients in the REMEDY (Clinical Trial of Endobronchial Ultrasound for the Diagnosis of Mediastinal Lymphadenopathy) trial. NSCLC = non–small cell lung cancer.
Of the 77 patients undergoing EBUS-TBNA, 4 patients experienced transient hypoxia and 1 patient had self-limiting bleeding. These complications did not result in early termination of the procedure in any case. One patient with a final diagnosis of sarcoidosis developed a fever after the procedure and was admitted to hospital for a total inpatient stay of 5 nights. Ninety-nine lymph nodes were sampled in the 77 patients, with a mean lymph node size of 23 mm. The median number of passes per lymph node was four. The 10 patients undergoing mediastinoscopy (after negative EBUS-TBNA) accumulated a total of 16 inpatient nights and no serious complications were observed.
In the retrospective study of mediastinoscopy in patients with IML, mediastinoscopy provided a specific diagnosis in 53 of 68 patients. In the 15 patients with no specific diagnosis, the final diagnosis was sarcoidosis in 4, adenocarcinoma in 1, and reactive lymphadenopathy in the remainder. The sensitivity of mediastinoscopy was 91% (95 CI, 81–97%) and the NPV of mediastinoscopy was 67% (95% CI, 42–85%).
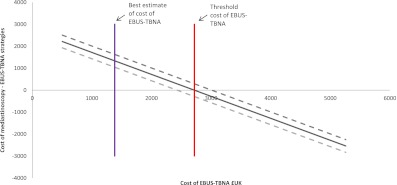
The mean cost of the EBUS-TBNA procedure per patient was £1,382 ($2,190; Table E1). The mean cost per patient of the EBUS-TBNA strategy was £1,892 ($2,998; Table 2), which was significantly different from the mean cost of mediastinoscopy (£3,228, $5,115) using a one-sample t test (P < 0.001). The result was supported by the 95% CI around the cost of the EBUS-TBNA strategy based on 2,000 bootstrapped samples (£1,653 [$2,620] to £2,235 [$3,542]). The mean cost-saving to the NHS per patient was £1,336 ($2,117; Table 2). The EBUS-TBNA strategy was less costly than the mediastinoscopy strategy if the cost per EBUS-TBNA procedure was less than £2,718 ($4,307; Figure 2).
TABLE 2.
MEAN COSTS PER PATIENT OF VARIOUS STRATEGIES FOR INVESTIGATION OF PATIENTS WITH ISOLATED MEDIASTINAL LYMPHADENOPATHY
| Parameter Value (Proportion of Patients) |
Costs (Mean Costs per Patient; UK£) |
|||||
| EBUS Strategy* | Mediastinoscopy Strategy | Unit Cost (UK£) | EBUS Strategy | Mediastinoscopy Strategy | Difference | |
| EBUS-TBNA | 1.00 | 0 | 1,382 | 1,382 | 0 | |
| Mediastinoscopy | 0.13 | 1.00 | 3,228† | 420 | 3,228 | |
| Clinical follow-up | 0.04 | 0 | 500 | 20 | 0 | |
| Additional inpatient stay | 0.14 | 0 | 500 | 70 | 0 | |
| Total cost (SD or 95% CI, P value) | 1,892 (95% CI, 1,653–2,235); P < 0.001‡ | 3,228 | −1,336 | |||
Definition of abbreviations: CI = confidence interval; EBUS-TBNA = endobronchial ultrasound–guided transbronchial needle aspiration; REMEDY = Clinical Trial of Endobronchial Ultrasound for the Diagnosis of Mediastinal Lymphadenopathy.
All costs are measured in 2010–2011 U.K. pounds: 1 £UK = $1.58 USD (February 1, 2012).
Data are from the REMEDY trial.
The cost of mediastinoscopy was obtained from the 2010–2011 U.K. National Health Service tariff for mediastinoscopy and included one night inpatient stay if required.
One-sample t test.
Figure 2.
Threshold sensitivity analysis showing the effect of variation in cost of endobronchial ultrasound–guided transbronchial needle aspiration (EBUS-TBNA). EBUS-TBNA prevented 87% of mediastinoscopies, with a 97.5% confidence interval of 78–96% (dashed lines). Assuming that 87% of mediastinoscopies can be prevented by EBUS-TBNA, the analysis demonstrates that the cost of the mediastinoscopy-alone strategy is more expensive than a strategy of EBUS-TBNA (followed by mediastinoscopy if EBUS-TBNA is negative) as long as EBUS-TBNA costs less than £2,718 (red line). Above this threshold the cost for EBUS-TBNA, mediastinoscopy alone is the less costly strategy. At the best estimate cost of EBUS-TBNA of £1,382 (purple line) the EBUS-TBNA strategy is cost-saving. 1 £UK = $1.58 USD (February 1, 2012).
Discussion
This is the first prospective clinical trial to demonstrate the usefulness and cost-savings of using EBUS-TBNA as an initial investigation for patients with IML requiring pathological diagnosis. The study demonstrates that EBUS-TBNA is a highly effective diagnostic modality and can prevent 87% of mediastinoscopies in this scenario. The negative predictive value, however, is low at 40% in our sample, which had a disease prevalence of 95%. Therefore mediastinoscopy is recommended after negative EBUS-TBNA, which is an important consideration when obtaining consent from patients for the procedure.
Considerable evidence is now available on the efficacy of EBUS-TBNA in patients with lung cancer (13). Data are also expanding on the role of EBUS-TBNA in patients with mediastinal sarcoidosis (14, 15), tuberculosis (16), lymphoma (8), and extrathoracic malignancy (9). However, many of the data are from retrospective cohort studies and are therefore subject to selection bias. In many of the studies, patients referred for mediastinoscopy were excluded and therefore their characteristics are unknown. In addition, in prior studies, many patients undergoing EBUS-TBNA may not have been otherwise referred for mediastinoscopy. Therefore, previous inferences on mediastinoscopies prevented are prone to bias. In the current prospective trial, only patients who were referred for pathological diagnosis were included and the trial patients were similar to historical control subjects undergoing mediastinoscopy.
The diagnosis of lymphoma by EBUS-TBNA is an area of controversy because the management of lymphoma often depends on pathological subtype and grade and EBUS-TBNA obtains smaller specimens than mediastinoscopy. EBUS-TBNA may be particularly useful for the mediastinal staging of lymphoma and in the diagnosis of lymphoma recurrence; however, its role for primary diagnosis is under debate. In one study of EBUS-TBNA in patients with lymphoma, EBUS had a sensitivity of 76%; however, 19% of the diagnosed patients still required a further invasive procedure (17). In the current study of 77 consecutive patients, only 3 patients had a lymphoma. EBUS-TBNA provided a conclusive diagnosis in one patient and prevented mediastinoscopy in another (who went on to have the diagnosis confirmed by bone marrow biopsy). The low prevalence of lymphoma in this cohort of patients, however, means that EBUS-TBNA can be a good initial test for patients with IML.
EBUS-TBNA was diagnostic of tuberculosis in 26 of 28 cases in this study. Of these, 11 (40%) were culture positive and 1 isolate of Mycobacterium tuberculosis was resistant to isoniazid. This is consistent with a larger multicenter cohort of patients with mediastinal lymph node tuberculosis, which demonstrated an overall diagnostic sensitivity of 94% in 156 patients and a culture rate of 47% (16). The emergence of drug-resistant tuberculosis emphasizes the importance of sampling mediastinal lymph nodes in this scenario and the usefulness of EBUS-TBNA in this group of patients. In some cases, however, diagnostic difficulty remains in distinguishing sarcoid from tuberculosis, as noncaseating granulomas obtained from EBUS-TBNA may also be consistent with tuberculosis (16). Four patients in this trial who were culture positive for Mycobacterium tuberculosis also yielded noncaseating granulomas on EBUS-TBNA pathology. The merit of PCR-based investigations on samples obtained from EBUS-TBNA is currently under evaluation. The advent of miniforceps and transbronchial needle forceps may improve diagnostic yield further in patients with suspected mediastinal lymphoma or tuberculosis.
This study is the first health economic analysis of EBUS-TBNA in patients with IML. The cost of EBUS-TBNA to the NHS in this trial was estimated at £1,382 per procedure. This is slightly higher than the costs of EBUS-TBNA estimated in Singapore (18) of SGD 2,623 (£1337) and the United States (19) of $1,711 (£1,051). A decision tree analysis in patients with lung cancer from an Australian perspective (20) employed a mean cost of EBUS-TBNA of $1,361 (£905). The current NICE (National Institute for Health and Clinical Excellence, London, UK) guidance on lung cancer uses a cost of £1,252 per EBUS-TBNA (21). If we used these lower costs per procedure then the cost savings achieved by the EBUS-TBNA strategy would clearly increase.
In the current analysis, a cost-minimization approach was considered the most appropriate as the same final diagnosis, treatment, and treatment outcomes would have been reached regardless of whether EBUS-TBNA or mediastinoscopy was employed as the initial procedure. Complications from mediastinoscopy were not observed or included in the model; this is also a conservative assumption. A systematic review of studies of EBUS-TBNA in patients with lung cancer up to 2008 (22) has demonstrated that the procedure is safe, with only 2 complications recorded in 1,299 procedures (1 patient with a pneumothorax and 1 patient with hypoxia). In a large retrospective single-center study of 2,145 patients undergoing mediastinoscopy the complication rate was 1%, which comprised 1 death, 7 hemorrhages, 2 tracheal injuries, 2 pneumothoraces, and 12 patients with vocal cord dysfunction (1). Complications from mediastinoscopy were not included in the decision tree model, but in view of reported complication rates in the literature being higher for mediastinoscopy, their inclusion would have favored the EBUS-TBNA strategy. A sensitivity of 100% for mediastinoscopy has also been assumed in the decision tree model and is unlikely to be the case in clinical practice. Furthermore, as the use of EBUS-TBNA spreads, it is likely that thoracic surgeons will, in the years ahead, become less skilled. The assumption of 100% sensitivity for the procedure is therefore likely to favor the mediastinoscopy strategy in the economic evaluation in this trial.
Limitations of the study are acknowledged. EBUS-TBNA was performed in a tertiary center with a high volume of procedures performed by physicians with expertise in the procedure. The reporting pathologists also have considerable experience in the interpretation of EBUS-TBNA specimens. The sensitivity obtained and proportion of mediastinoscopies prevented in this study therefore may not be immediately reproducible in other centers. This trial was conducted in southeast England, which has a low incidence of endemic fungal diseases and so the case mix of diseases in this trial may not be representative of areas with a higher incidence of endemic fungal diseases. Last, the trial excluded patients with anterior mediastinal lymphadenopathy (inaccessible by EBUS-TBNA) and therefore the results cannot be applied to these patients.
In conclusion, EBUS-TBNA is a safe, highly sensitive, and cost-saving initial investigation in patients with IML. The low negative predictive value of EBUS-TBNA in this setting indicates that mediastinoscopy should be performed in cases of negative EBUS-TBNA.
Supplementary Material
Acknowledgments
The REMEDY trial management group thanks the patients who participated in this study and the participating centers: (1) University College London Hospital (H. Booth, J. Porter, K. Ardeshna, R. Miller, P. Gothard); (2) Whittington Hospital (S. Lock, N. Johnson); (3) North Middlesex University Hospital (H. Makker, I. Moonsie, S. Lozewicz, B. Sheinman); (4) Barnet Hospital (S. Khan, D. Creer, R. Vancheesewaran); and (5) Princess Alexandra Hospital (P. Russell, J. Waller, S. Sundaram).
Footnotes
Supported by a grant to N.N. and S.M.J. from the UK Medical Research Council (G0800465/1). S.M.J. is a Wellcome Trust Senior Research Fellow in Clinical Science. This study was undertaken at UCLH/UCL, which received a proportion of funding from the Department of Health NIHR Biomedical Research Centres funding scheme (N.N., S.M., R.Z.O., S.M.J.).
Author Contributions: N.N., conception of project, performance of procedures, compilation and analysis of data, production of manuscript; D.R.L., performance of procedures, review of manuscript; S.K., performance of procedures, review of manuscript; M.H., performance of procedures, review of manuscript; D.M., compilation of data, review of manuscript; G.K., review of pathological specimens, review of manuscript; M.F., review of pathological specimens, review of manuscript; A.C., review of pathological specimens, review of manuscript; P.S., review of patient imaging, review of manuscript; S.M., health economic analysis, review of manuscript; R.Z.O., statistical analysis of data, production of manuscript; S.M.J., conception and supervision of project, performance of procedures, review of manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201203-0393OC on May 31, 2012
This article has an online supplement, which is available from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lemaire A, Nikolic I, Petersen T, Haney JC, Toloza EM, Harpole DH, Jr, D’Amico TA, Burfeind WR. Nine-year single center experience with cervical mediastinoscopy: complications and false negative rate. Ann Thorac Surg 2006;82:1185–1189 [DOI] [PubMed] [Google Scholar]
- 2.Porte H, Roumilhac D, Eraldi L, Cordonnier C, Puech P, Wurtz A. The role of mediastinoscopy in the diagnosis of mediastinal lymphadenopathy. Eur J Cardiothorac Surg 1998;13:196–199 [DOI] [PubMed] [Google Scholar]
- 3.McManus TE, Haydock DA, Alison PM, Kolbe J. Isolated mediastinal adenopathy: the case for mediastinoscopy. Ulster Med J 2008;77:97–101 [PMC free article] [PubMed] [Google Scholar]
- 4.Navani N, Spiro SG, Janes SM. Mediastinal staging of NSCLC with endoscopic and endobronchial ultrasound. Nat Rev Clin Oncol 2009;6:278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ernst A, Anantham D, Eberhardt R, Krasnik M, Herth FJ. Diagnosis of mediastinal adenopathy–real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol 2008;3:577–582 [DOI] [PubMed] [Google Scholar]
- 6.Tremblay A, Stather DR, Maceachern P, Khalil M, Field SK. A randomized controlled trial of standard vs endobronchial ultrasonography–guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009;136:340–346 [DOI] [PubMed] [Google Scholar]
- 7.Navani N, Booth HL, Kocjan G, Falzon M, Capitanio A, Brown JM, Porter JC, Janes SM. Combination of endobronchial ultrasound guided transbronchial needle aspiration with standard bronchoscopic techniques for the diagnosis of stage I and stage II pulmonary sarcoidosis. Respirology 2011;16:467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall CB, Jacob B, Patel S, Sneige N, Jimenez CA, Morice RC, Caraway N. The utility of endobronchial ultrasound–guided transbronchial needle aspiration biopsy in the diagnosis of mediastinal lymphoproliferative disorders. Cancer Cytopathol 2011;119:118–126 [DOI] [PubMed] [Google Scholar]
- 9.Navani N, Nankivell M, Woolhouse I, Harrison RN, Munavvar M, Oltmanns U, Falzon M, Kocjan G, Rintoul RC, Janes SM. Endobronchial ultrasound–guided transbronchial needle aspiration for the diagnosis of intrathoracic lymphadenopathy in patients with extrathoracic malignancy: a multicenter study. J Thorac Oncol 2011;6:1505–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Department of Health. Payment by results [tariff information] [accessed 2012 June 9]. Available from: http://www.dh.gov.uk/en/Managingyourorganisation/NHSFinancialReforms/index.htm
- 11.Hall P. Theoretical comparison of bootstrap confidence intervals. Ann Stat 1988;16:927–953 [Google Scholar]
- 12.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 2003;326:41–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McComb BL, Wallace MB, Pascual JM, Othman MO. Mediastinal staging of nonsmall cell lung carcinoma by endoscopic and endobronchial ultrasound–guided fine needle aspiration. J Thorac Imaging 2011;26:147–161 [DOI] [PubMed] [Google Scholar]
- 14.Garwood S, Judson MA, Silvestri G, Hoda R, Fraig M, Doelken P. Endobronchial ultrasound for the diagnosis of pulmonary sarcoidosis. Chest 2007;132:1298–1304 [DOI] [PubMed] [Google Scholar]
- 15.Oki M, Saka H, Kitagawa C, Tanaka S, Shimokata T, Kawata Y, Mori K, Kajikawa S, Ichihara S, Moritani S. Real-time endobronchial ultrasound–guided transbronchial needle aspiration is useful for diagnosing sarcoidosis. Respirology 2007;12:863–868 [DOI] [PubMed] [Google Scholar]
- 16.Navani N, Molyneaux PL, Breen RA, Connell DW, Jepson A, Nankivell M, Brown JM, Morris-Jones S, Ng B, Wickremasinghe M, et al. Utility of endobronchial ultrasound–guided transbronchial needle aspiration in patients with tuberculous intrathoracic lymphadenopathy: a multicentre study. Thorax 2011;66:889–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinfort DP, Conron M, Tsui A, Pasricha SR, Renwick WE, Antippa P, Irving LB. Endobronchial ultrasound–guided transbronchial needle aspiration for the evaluation of suspected lymphoma. J Thorac Oncol 2010;5:804–809 [DOI] [PubMed] [Google Scholar]
- 18.Ang SY, Tan RW, Koh MS, Lim J. Economic analysis of endobronchial ultrasound (EBUS) as a tool in the diagnosis and staging of lung cancer in Singapore. Int J Technol Assess Health Care 2010;26:170–174 [DOI] [PubMed] [Google Scholar]
- 19.Harewood GC, Pascual J, Raimondo M, Woodward T, Johnson M, McComb B, Odell J, Jamil LH, Gill KR, Wallace MB. Economic analysis of combined endoscopic and endobronchial ultrasound in the evaluation of patients with suspected non–small cell lung cancer. Lung Cancer 2010;67:366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinfort DP, Liew D, Conron M, Hutchinson AF, Irving LB. Cost-benefit of minimally invasive staging of non–small cell lung cancer: a decision tree sensitivity analysis. J Thorac Oncol 2010;5:1564–1570 [DOI] [PubMed] [Google Scholar]
- 21.Medford AR, Agrawal S, Free CM, Bennett JA. A performance and theoretical cost analysis of endobronchial ultrasound–guided transbronchial needle aspiration in a UK tertiary respiratory centre. QJM 2009;102:859–864 [DOI] [PubMed] [Google Scholar]
- 22.Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH. Endobronchial ultrasound–guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer 2009;45:1389–1396 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.