Abstract
Rationale: The immune response in sepsis is characterized by overt immune dysfunction. Studies indicate immunostimulation represents a viable therapy for patients. One study suggests a potentially protective role for interleukin 5 (IL-5) in sepsis; however, the loss of eosinophils in this disease presents a paradox.
Objectives: To assess the protective and eosinophil-independent effects of IL-5 in sepsis.
Methods: We assessed the effects of IL-5 administration on survival, bacterial burden, and cytokine production after polymicrobial sepsis. In addition, we examined the effects on macrophage phagocytosis and survival using fluorescence microscopy and flow cytometry.
Measurements and Main Results: Loss of IL-5 increased mortality and tissue damage in the lung, IL-6 and IL-10 production, and bacterial burden during sepsis. Therapeutic administration of IL-5 improved mortality in sepsis. Interestingly, IL-5 administration resulted in neutrophil recruitment in vivo. IL-5 overexpression in the absence of eosinophils resulted in decreased mortality from sepsis and increased circulating neutrophils and monocytes, suggesting their importance in the protective effects of IL-5. Furthermore, novel data demonstrate IL-5 receptor expression on neutrophils and monocytes in sepsis. IL-5 augmented cytokine secretion, activation, phagocytosis, and survival of macrophages. Importantly, macrophage depletion before the onset of sepsis eliminated IL-5–mediated protection. The protective effects of IL-5 were confirmed in humans, where IL-5 levels were elevated in patients with sepsis. Moreover, neutrophils and monocytes from patients expressed the IL-5 receptor.
Conclusions: Taken together, these data support a novel role for IL-5 on noneosinophilic myeloid populations, and suggest treatment with IL-5 may be a viable therapy for sepsis.
Keywords: macrophages, neutrophils, innate immunity, immunotherapy
At a Glance Commentary
Scientific Knowledge on the Subject
Currently, there is no established role for IL-5 in sepsis or any effects on neutrophils and macrophages.
What This Study Adds to the Field
These data support a novel, eosinophil-independent role for IL-5 in promoting a protective innate immune response in sepsis. This supports the theory that augmenting the immune response, specifically through improving host defense, is a viable therapy for patients with sepsis.
Sepsis is the leading cause of death in the intensive care unit, costing more than $17 billion annually. It has a mortality rate of 25%, which increases with the severity of disease (1, 2). With the withdrawal of activated protein C, currently there are no Food and Drug Administration–approved adjunct therapies for sepsis, highlighting the need for novel treatment modalities (3).
Robust activation of the innate immune system is a hallmark of sepsis and numerous strategies to treat this disorder focused on modulation of this response. However, the host inflammatory response in sepsis is associated with a complex interplay between proinflammatory and antiinflammatory mediators, which have divergent effects on pathogen control and inflammation. Numerous clinical trials targeting individual cytokines or pathogen recognition (i.e., tumor necrosis factor-α, IL-1Ra, Toll-like receptor [TLR]-4, and CD14) have been unsuccessful (4–9). Although these treatments limited the deleterious effects of the inflammatory response, they ultimately impaired host defense and pathogen control.
It is well established that monocytes-macrophages and neutrophils are essential for host control of infection and that the presence of leukopenia and granulocytopenia is an independent risk factor for mortality in sepsis (10, 11). Sepsis is associated with immunoparalysis and deactivation of the innate immune system, through reduced HLA-DR expression on circulating monocytes and impaired bacterial killing by neutrophils from patients with sepsis (12–16). This has generated interest in targeting factors responsible for myeloid cell maturation and function in sepsis. Administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) while recruiting additional and potentially proinflammatory leukocytes improved pathogen control and bacterial clearance in sepsis (17–19). In addition, administration of IL-33, which stimulates production of Th2 cytokines (including IL-5 and IL-13), improved survival in septic mice by neutrophil recruitment and improved pathogen control (20). These studies support the therapeutic benefit of targeting and activating myeloid cells in sepsis. However, the broad and constitutive expression of these receptors reduces the specificity of activating agents during the acute phase of sepsis.
Growing data suggest eosinophils play a protective role in the innate immune response to sepsis (21, 22). Interleukin 5 (IL-5) is the primary hematopoietic cytokine responsible for eosinophil growth and maturation. One study found that sepsis survivors had a trend toward elevated levels of IL-5 compared with nonsurvivors (23). However, sepsis and bacterial infections are associated with a marked eosinopenia (24, 25). This apparent paradox suggests differential and independent functions for eosinophils and IL-5 in sepsis. Here, we demonstrate a novel and eosinophil-independent role for IL-5 and its receptor in sepsis. IL-5 administration represents a potential adjuvant therapy for patients with sepsis, as a means to improve host control of infection and improve survival. Moreover, the limited and specific expression of the IL-5 receptor on these cells provides a targeted means to augment myeloid function in sepsis. Some of these results have been reported in the form of an abstract (26, 27).
Methods
Animals and Reagents
Female 8- to 12-week-old C57Bl/6 and macrophage Fas-induced apoptosis (MaFIA) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and allowed to acclimatize at a specific-pathogen-free facility at Oregon Health and Science University (OHSU) for 1 week. NJ.1638 (28), PHIL (29), and IL-5−/− (30) mice were provided by James J. Lee and were backcrossed onto the C57Bl/6 background. NJ.1638/PHIL mice were bred at OHSU. Genotyping was performed as previously described (28, 29). All animal experiments were in accordance with guidelines set by the Institutional Animal Care and Use Committee (OHSU). Experiments were performed using age- and sex-matched littermate controls. RAW264.7 and THP-1 cell lines, and Pseudomonas aeruginosa (Boston 41501) were purchased from ATCC (Manassas, VA). Escherichia coli were obtained from a clinical isolate at OHSU.
Patients
Patients with sepsis in the intensive care unit at (OHSU) meeting the 2001 International Sepsis Definition Criteria (31) were enrolled in this study within 24 hours of admission and compared with healthy volunteers and patients without sepsis in the unit. All subjects gave informed consent. Subjects were excluded for presence of a Do Not Resuscitate order or decision to institute comfort care measures, hemoglobin less than 7 g/dl, or the presence of active bleeding requiring more than 2 units of packed red blood cells. This study was approved by the Human Subjects Institutional Committee at OHSU.
Flow Cytometry and Cytokine Detection
Cells were stained for human IL-5Rα-PE (Beckman Coulter, Brea, CA); CD125-PE (mouse IL-5Rα); F4/80; Ly6 g; CD11b; CD14; and CD16 (BD Biosciences, San Jose, CA). Survival was assessed using LIVE/DEAD fixable violet stain (Invitrogen, Grand Island, NY). Samples were acquired using the LSRII and FACSDiva software (BD Biosciences) and analyzed using FlowJo (Treestar, Ashland, OR). ELISAs (R&D Systems, Minneapolis, MN) were performed according to the manufacturer’s instructions. Myeloperoxidase was detected in peritoneal lavage samples by ELISA (Hycult Biotech, Plymouth Meeting, PA).
Cecal Ligation and Puncture
Cecal ligation and puncture (CLP) was performed as previously described (32, 33). Briefly, an incision was made in the abdominal cavity of female mice. The cecum was ligated with suture and punctured through once with a 19-gauge needle, once with a 22-gauge needle for the sublethal model, or twice with a 19-gauge needle for the MaFIA mice. The cecum was returned to the peritoneal cavity and incisions were closed. Mice received saline for resuscitation.
Statistics
Data were analyzed by Student unpaired t test (GraphPad Prism, La Jolla, CA). One-way analysis of variance with Bonferroni multiple comparisons test was used to compare means across groups. Significance is defined as P < 0.05. All error bars represent means ± SEM.
Additional details on the methods are available in the online supplement.
Results
IL-5 Is Protective in Polymicrobial Sepsis
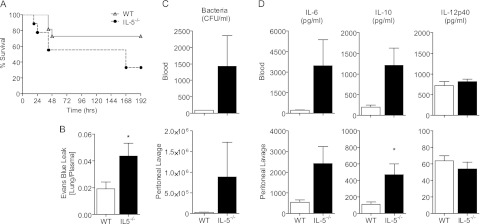
To investigate whether IL-5 was protective in sepsis, we first assessed the effect of IL-5 deletion on survival using the CLP model of polymicrobial sepsis. To detect a potential increase in mortality, we used a sublethal CLP model. IL-5–deficient mice exhibited reduced survival after CLP (Figure 1A). This was accompanied by increased tissue damage, as evidenced by increased pulmonary capillary leak (Figure 1B), and decreased bacterial clearance compared with wild-type control animals (Figure 1C). This reduction in survival and increased organ injury was associated with a trend toward increased IL-6 (blood, P = 0.10; peritoneal lavage, P = 0.08) and IL-10 (blood, P = 0.06) compared with wild-type control animals (Figure 1D). Together, these data indicate endogenous IL-5 is protective in sepsis and support a novel role for IL-5 in this disease.
Figure 1.
Endogenous IL-5 is protective in cecal ligation and puncture (CLP) sepsis. (A) Survival curve of IL-5−/− or wild-type (WT) C57Bl/6 controls after sublethal CLP. Data were analyzed using the log-rank test. n = 5–10 per group. (B and C) Wild-type C57Bl/6 and IL-5−/− were killed 18 hours after CLP for analysis. Bars represent the mean ± SEM. n = 5 per group. (B) Evans Blue dye leak measured by optical density, shown as the ratio of dye in the lung to plasma. (C) Bacterial burden measured by serial dilution and (D) IL-6, IL-10, and IL-12 measured by ELISA in the blood (upper panel) and peritoneal lavage fluid (lower panel). Data were analyzed by Student t test; significance is defined as *P < 0.05.
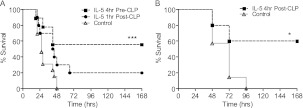
To determine whether IL-5 was sufficient to improve mortality in sepsis and whether it could serve as an immunoadjuvant, we examined the effects of IL-5 administration. Prophylactic administration of IL-5 before the onset of sepsis significantly improved survival compared with vehicle-treated control animals (Figure 2A). To establish the use of IL-5 as a therapeutic modality in CLP, IL-5 was administered 1 hour after CLP (Figure 2A). Although administration of IL-5 at this time point was effective at improving survival, because of the rapid onset of mortality in this model, a sublethal model of CLP was needed to further delay the time to treatment and assess the therapeutic benefits of IL-5 administration at a time when patients are more likely to be treated. Using an attenuated model of sepsis, IL-5 administration 4 hours after CLP significantly improved survival compared with vehicle-treated control animals (Figure 2B). These data indicate that IL-5 administration is a viable therapeutic modality in sepsis as a means of improving survival.
Figure 2.
IL-5 administration rescues mice from the lethality of sepsis. (A and B) C57Bl/6 mice were treated with 1 μg mouse IL-5 4 hours before (A, dashed square line), 1 hour before lethal cecal ligation and puncture (CLP) (A, dashed circle line), or 4 hours after (B, dashed square line) sublethal CLP and animals were monitored for survival. Solid gray triangle lines are phosphate-buffered saline–treated controls. Data were analyzed by log-rank test. n = 5–10 per group and significance is defined as *P < 0.05; ***P < 0.001.
IL-5 Protection in Sepsis Is Eosinophil Independent
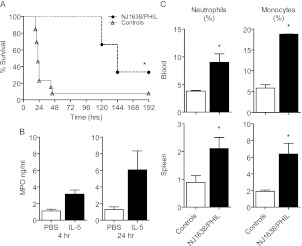
Previous data indicate that mice with constitutive IL-5 overexpression (NJ.1638) have profound eosinophilia and improved survival in CLP (21). This was believed to be caused by the antibacterial properties of eosinophils, because we have previously demonstrated that eosinophil-deficient (PHIL) mice have increased susceptibility to bacterial sepsis (22, 34). To establish whether eosinophils were necessary for the protective effects of IL-5 in sepsis, NJ.1638 mice were crossed with PHIL+/− mice, which express a diphtheria toxin transgene under control of the eosinophil peroxidase promoter (28, 29). NJ.1638/PHIL mice constitutively overexpress IL-5, but have a congenital deficiency in eosinophils thereby enabling accurate assessment of the independent effects of IL-5 in sepsis (28, 29, 35). Unexpectedly, NJ.1638/PHIL mice had improved survival over littermate controls in CLP (Figure 3A), suggesting an important role in vivo for IL-5 in the absence of eosinophils.
Figure 3.
IL-5 affects noneosinophilic myeloid cells in sepsis. (A) Survival curve of NJ.1638/PHIL or littermate (NJ.1638-/PHIL−) controls after cecal ligation and puncture. Data were analyzed using the log-rank test. n = 5–10 per group. (B) Myeloperoxidase (MPO) measured by ELISA in the peritoneal lavage fluid of animals treated with IL-5 or phosphate-buffered saline (PBS) control 4 and 24 hours after treatment. n = 5 per group. (C) Blood (upper panel) and spleen (lower panel) from NJ.1638/PHIL mice (black bars) and littermate controls (white bars) were analyzed by flow cytometry to assess Ly6 g+ neutrophils and Ly6 g-CD11b+ monocytes. Bars represent means ± SEM. n = 3 per group. Data were analyzed by Student t test; significance is defined as *P < 0.05.
Interestingly, NJ1638/PHIL mice had a significant expansion of neutrophils and monocytes (Figure 3C), suggesting that IL-5 has unappreciated effects on noneosinophilic myeloid subsets. When we reexamined the effects of acute IL-5 administration in wild-type mice, we observed no change in eosinophil recruitment into the peritoneal cavity as determined by direct visualization and flow cytometry (FSChiSSChiCCR3+) (data not shown). Instead, IL-5 administration resulted in increased neutrophil and monocyte recruitment as determined by flow cytometry (data not shown) and myeloperoxidase release (Figure 3B), confirming that IL-5 acts in an eosinophil-independent manner on leukocyte chemotaxis. Combined, these data demonstrate for the first time that IL-5 acts in an eosinophil-independent manner during sepsis.
The IL-5 Receptor Is Expressed on Neutrophils and Macrophages in Sepsis
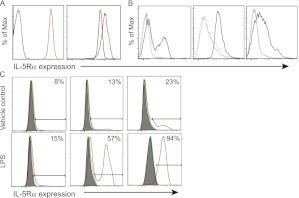
It has been documented that under noninflammatory conditions, neither mouse neutrophils nor monocytes-macrophages express the IL-5 receptor α (IL-5Rα) subunit, which is the unique ligand-binding portion of the receptor. However, there is evidence that these cells respond to IL-5 by chemotaxis (36, 37). Given the expansion of neutrophils and monocytes observed in NJ.1638/PHIL mice, we sought to determine if IL-5Rα was present on these cell populations in sepsis. Neutrophils isolated from the blood and the lungs of septic mice expressed IL-5Rα (Figure 4A), as did bone marrow neutrophils isolated from healthy mice (see Figure E1 in the online supplement). In addition, blood monocytes and macrophages in the lung and spleen of septic mice expressed IL-5Rα (Figure 4B). These data demonstrate novel expression of the IL-5Rα on neutrophils and monocytes-macrophages in sepsis.
Figure 4.
Mouse neutrophils and monocytes-macrophages express the IL-5Rα in sepsis. (A) Lung and serum samples taken 18 hours after cecal ligation and puncture (CLP) were analyzed by flow cytometry for IL-5Rα, looking at receptor expression on neutrophils (Ly6 g+). The plots show IL-5Rα expression on neutrophils from a representative mouse that underwent CLP in red, and an unoperated control animal in black. Left plot represents serum neutrophils; right plot represents lung neutrophils. (B) IL-5Rα expression was examined using flow cytometry on circulating Ly6 g−CD11b+ monocytes (left panel), F4/80+ macrophages in the lung (middle panel), and F4/80+ macrophages in the spleen (right panel) 18 hours after CLP. Gray lines are unstained and isotype controls, IL-5Rα staining from a representative mouse is in black. (C) RAW264.7 mouse macrophages were stimulated with LPS, and IL-5Rα expression was examined by flow cytometry. Gray histograms are unstained controls, green is a staining control in a different channel, and red is IL-5Rα stained cells. Representative plots shown for n = 3 experiments.
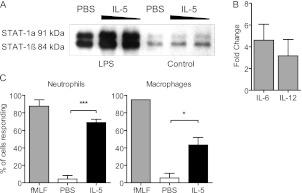
Moreover, we were able to model IL-5 receptor expression on macrophages in vitro through stimulation with various Toll ligands relevant to sepsis, including LPS (Figure 4C) and CpG (see Figure E2). To determine if the receptor was functional, we assessed nuclear translocation of STAT1, because it is known to be downstream of IL-5 signaling in eosinophils (38). IL-5 stimulation of primary mouse macrophages resulted in increased STAT1 nuclear translocation compared with controls (Figure 5A). Moreover, these cells secreted IL-6 and IL-12 after IL-5 stimulation (Figure 5B), further demonstrating receptor functionality. We wished to further examine the effects of IL-5 signaling on specific neutrophil and macrophage functions. It is known that changes in intracellular calcium occur during phagocytosis, chemotaxis, and bacterial killing by monocytes-macrophages and neutrophils (39, 40). To examine cytosolic changes in calcium after IL-5 stimulation, cells were loaded with a calcium-sensitive fluorescent dye and stimulated with IL-5. Mouse macrophages and neutrophils responded to IL-5 by releasing stored intracellular calcium (Figure 5C). Taken together, these data demonstrate that the IL-5 receptor is expressed on neutrophils and macrophages in sepsis and may represent a unique way to modulate the function of these leukocytes in sepsis.
Figure 5.
IL-5 signaling in macrophages induces activation, STAT1 phosphorylation, and cytokine production. (A and B) Primary mouse peritoneal macrophages were stimulated with LPS to induce IL-5Rα expression, followed by either 100 ng or 1 μg IL-5, or phosphate-buffered saline (PBS) control. (A) Western blot for STAT-1 performed on nuclear extracts. n = 3 experiments. (B) IL-6 or IL-12 were detected by ELISA in supernatants from IL-5–stimulated macrophages. Data are represented as fold change over vehicle-treated controls. (C) Primary mouse neutrophils or macrophages were stimulated with IL-5 and intracellular calcium changes were measured by fluorescence microscopy. fMLF is a positive control. Time-lapse images (for a total of 200 cells per experiment) were quantitated by counting the total number of cells per frame that responded to IL-5 stimulation. n = 3 experiments. Data were analyzed by Student t test; significance is defined as *P < 0.05; ***P < 0.001.
Macrophages Are Required for the Protective Effects of IL-5
Because calcium flux is responsible for bacterial killing and phagocytosis by macrophages and neutrophils, and these functions are impaired in sepsis, we wanted to examine the effects of IL-5 on these important functions. Surprisingly, neutrophil-mediated bacterial killing was unaffected by IL-5 (Figure 6A). This was also true for human neutrophils (data not shown); however, the efficiency of bacterial killing by these cells (>98%) made it impossible to detect enhancement in killing. In addition, IL-5 had no effect on survival of primary mouse neutrophils in culture (Figure 6B).
Figure 6.
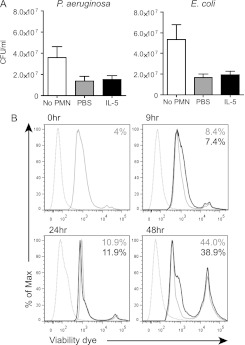
Neutrophil function is not affected by IL-5. (A) Thioglycollate-elicited neutrophils (PMN) were cultured with Pseudomonas aeruginosa (left panel) or Escherichia coli (right panel) and bacterial killing was assessed by serial dilution. Data were analyzed by Student t test. There was no significant difference in bacterial killing between PMN treated with phosphate-buffered saline (PBS) control and IL-5. n = 3 experiments. (B) Bone marrow neutrophils were stimulated with IL-5 and survival was assessed by flow cytometry using a viability dye. Dashed line is unstained control; solid gray line is PBS control; black line is IL-5 treatment. The percentage listed is dead cells at that time point. n = 3 experiments.
Although IL-5 did not impact the function of neutrophils, we observed a significant increase in macrophage phagocytosis of Escherichia coli after IL-5 stimulation (Figure 7A, see Figure E3). Additionally, there was a moderate increase in bacterial killing from IL-5–stimulated macrophages (Figure 7B). Although this was not statistically significant the increase in phagocytosis may be masking an effect on bacterial killing. Finally, mouse macrophages treated with IL-5 had prolonged survival over control (Figure 7C). These data indicate that macrophage function and survival is augmented by IL-5 stimulation, whereas neutrophils are unaffected.
Figure 7.
Macrophages are required for the protective effects of IL-5 in polymicrobial sepsis. (A) RAW264.7 macrophages were stimulated and allowed to phagocytose fluorescently labeled Escherichia coli; total internal fluorescence (for 100 cells per experiment) was measured to determine the level of phagocytosis. This was quantitated after subtracting background fluorescence. n = 3 experiments. FITC = fluorescein isothiocyanate; PBS = phosphate-buffered saline. (B) Bacterial killing by macrophages after IL-5 stimulation was quantitated by serial dilution. n = 3 experiments. (C) RAW264.7 macrophages were stimulated with LPS to induce receptor expression, then stimulated with IL-5. Survival was assessed by flow cytometry using a viability dye. Dashed line is unstained control; solid gray line is PBS-treated control; black histogram is IL-5–treated cells. The percentage listed is dead cells at that time point. n = 3 experiments. (D) Macrophage fas-induced apoptosis (MaFIA) mice were depleted of macrophages (dashed lines) or remained intact (solid lines) and treated with either mouse IL-5 (triangles) or PBS (open circles) 4 hours before cecal ligation and puncture and animals were monitored for survival. n = 5 per group. Data were analyzed by Student t test; significance is defined as *P < 0.05; ***P < 0.001. In D, significance is noted for undepleted/IL-5 treated versus Mac depleted/IL-5 treated. No significant difference between PBS or IL-5 treated/Mac depleted groups.
To establish that macrophages were required for the protective effects of IL-5 in vivo, we used MaFIA mice, which allow for systemic macrophage depletion through Fas ligand-mediated apoptosis (41, 42). Similar to observations with C57BL/6 mice, IL-5 protected macrophage-intact (nondepleted) MaFIA mice. However, macrophage depletion eliminated the protective effects of IL-5 administration in sepsis compared with macrophage-depleted vehicle-treated control animals (P = 0.48) (Figure 7D). These data demonstrate that macrophages are required for IL-5–mediated protection in polymicrobial sepsis.
Endogenous IL-5 Is Protective in Human Sepsis
To assess the clinical implications of these data, we first examined levels of IL-5 and IL-5Rα in patients with sepsis. Of note, similar to multiple prior studies, no patients with sepsis had detectable levels of circulating eosinophils (data not shown). Data establish IL-5 levels were significantly elevated in patients with sepsis compared with healthy control subjects (Figure 8A). In particular, increased IL-5 was associated with improved outcomes in patients with sepsis. This was evident through elevated levels of IL-5 in survivors compared with nonsurvivors, and also in patients who did not require mechanical ventilation compared with those who did (Figure 8B). There was no correlation between levels of IL-5 and Acute Physiology and Chronic Health Evaluation II, Sequential Organ Failure Assessment, or Simplified Acute Physiology Score II score (data not shown). Furthermore, CD14+ neutrophils and CD16+ monocytes from patients with sepsis expressed IL-5Rα (Figure 8C). IL-5Rα expression was unique to sepsis, because no receptor expression was observed on monocytes from ICU patients without sepsis (Figure 8D). Expression of this receptor waned over time in surviving patients (Figure 8D). In addition, we were able to model expression of this receptor in vitro. Stimulation of THP-1 monocytes in vitro with either LPS (Figure 8E) or CpG (data not shown) induced up-regulation of IL-5Rα, in addition to stimulation of peripheral blood mononuclear cells from healthy volunteers with heat-killed E. coli (see Figure E4). Finally, the soluble isoform of IL-5Rα may be acting as a cytokine trap, because it was found at greater levels in plasma from patients with septic shock compared with those without (Figure 8F). However, there was no difference in sIL-5Rα levels between survivors and nonsurvivors (data not shown). Collectively, these data indicate that IL-5 is protective in human sepsis and that expression of IL-5Rα on monocytes suggests the mechanism for this protection is through modulation of monocyte-macrophage function.
Figure 8.
IL-5 is protective in human sepsis. (A) Serum IL-5 was measured by ELISA and compared among patients with sepsis (n = 75) and healthy volunteers (n = 15). (B) Serum IL-5 levels in patients with sepsis were subdivided based on their survival (left panel) or the need for mechanical ventilation (right panel). (C) Peripheral CD14+ neutrophils (left panel) or CD16+ (monocytes) from patients with sepsis were analyzed for IL-5Rα expression by flow cytometry. Unstained control is in black, IL-5Rα stained cells are in red. Histograms are representative plots of a subset of patients with sepsis. (D) The percentage of IL-5Rα expressing monocytes was assessed by flow cytometry and compared among patients with sepsis (n = 20) and nonseptic intensive care unit (ICU) control subjects (n = 5) (left panel) and was monitored over time in those patients with sepsis (right panel). Day 1 is at the time of admission. (A and D) Each symbol represents one individual subject. Error bars represent the means ± SEM. (E) THP-1 human monocytes were stimulated with LPS and IL-5Rα expression was assessed by flow cytometry. Unstained control is in black, IL-5Rα stained cells are in red. n = 3 experiments. (F) Serum levels of soluble IL-5Rα in patients with sepsis (n = 50) was measured by ELISA. Patients were subdivided based on the need for vasopressors (shock). Error bars represent the means ± SEM. Data were analyzed by Student t test; significance is defined as *P < 0.05; ***P < 0.001.
Discussion
Data in this study indicate that IL-5 enhances survival in a clinically relevant model of polymicrobial sepsis. More importantly, the ability of IL-5 administration after the onset of sepsis to significantly improve survival in mice suggests that IL-5 is an effective rescue therapy. Interestingly, IL-5 has been administered safely to patients with asthma as part of studies investigating leukocyte recruitment, further emphasizing its viability as a therapeutic adjuvant (43). Although numerous studies demonstrate a role for IL-5 in modulation of Th2 polarization in allergic diseases and a protective role in response to various parasitic infections, to our knowledge this is the first time it has been investigated in any acute bacterial or infectious disorder. These results are consistent with studies that demonstrate that GM-CSF, whose receptor shares the same common β-subunit with the IL-5 receptor, is capable of improving monocyte function in patients with sepsis and in animal models of sepsis (19, 44).
IL-5 is traditionally involved in eosinophil recruitment and maturation, and expression of the receptor is believed to be restricted to eosinophils and some B-cell subsets (45, 46). Interestingly, IL-5 treatment mediated these protective effects in an eosinophil-independent manner. The continued protection of IL-5 overexpression in eosinophil-deficient mice implies a noneosinophil target for IL-5 bioactivity in sepsis. The basal expansion of monocytes and neutrophils in these mice, and the recruitment of both of these cellular subsets with exogenous IL-5 administration, implicates these leukocytes as potential targets of IL-5 in vivo. This observation is consistent with one prior report suggesting IL-5 is necessary for eosinophil and neutrophil recruitment in a mouse model of filariasis and may explain the phenomenon of anti-IL-5Rα–mediated neutropenia in a recent clinical study (47, 48).
Our finding of IL-5Rα expression on monocytes and neutrophils in vivo during sepsis further establishes these cellular subsets as targets for IL-5 activity in vivo. Our ability to induce IL-5Rα expression on macrophages using various TLR agonists involved in bacterial sepsis confirmed receptor expression and also provided a possible explanation for IL-5Rα expression on monocytes and neutrophils from patients with sepsis. Finally, the effects of IL-5 stimulation on macrophages cytokine production, intracellular calcium mobilization, increased phagocytosis, and delayed cell death are consistent with the known effects of IL-5 on eosinophils and common β-subunit receptor stimulation on macrophages by GM-CSF. Although our findings are the first to demonstrate IL-5Rα expression on neutrophils and macrophages in vitro and in vivo, these findings are consistent with other reports that document IL-5Rα mRNA up-regulation in neutrophils and a known chemotactic effect of IL-5 on monocytes and neutrophils (although receptor expression was never established in these studies) (36, 47, 49). Furthermore, these data also provide an explanation for the reduction in circulating neutrophils and monocytes in patients with asthma treated with anti-IL5Rα (48).
The finding of IL-5Rα on noneosinophil leukocytes may explain the paradox of how elevated IL-5 levels are associated with improved survival in sepsis in the absence of circulating eosinophils. Indeed, the failure of IL-5 to rescue macrophage-depleted mice further supports that this leukocyte subset is necessary for mediating the protective effects of IL5 in vivo. Although our data establish a protective role for IL-5 modulation of macrophages in vivo and in vitro, they fail to establish whether activation of neutrophils is necessary and sufficient for the protective effects of IL-5. Further studies are necessary to examine the role of IL-5Rα on neutrophils. Although IL-5 induced intracellular calcium flux in neutrophils, it failed to increase either phagocytosis or bacterial killing. This was in part caused by the high level of killing by neutrophils at baseline, something that may not be true for septic neutrophils, which have impaired bacterial killing and phagocytosis compared with those from healthy patients. Furthermore, the ability of IL-5 to induce neutrophil recruitment and induce intracellular calcium flux implies that although IL-5 did not augment their function, it may still improve patient outcomes purely through increased neutrophil recruitment (20).
In the development of any new strategy for the treatment of human sepsis, it is imperative that these findings be confirmed in humans. Importantly, we document for the first time IL-5Rα expression on circulating monocytes and neutrophils in patients with sepsis. The loss of receptor expression with the resolution of sepsis suggests this expression is specific to the acute inflammatory response in sepsis and explains the multitude of other studies that failed to demonstrate significant levels of IL-5Rα expression on these leukocyte subsets in healthy humans. This is also consistent with the need for TLR agonism for maximal IL-5Rα expression on human macrophages in vitro, which we observed in this study. Combined, these data establish that IL-5Rα expression on noneosinophilic myeloid subsets is not species specific and that the cellular targets for IL-5 administration in human sepsis are indeed present in our patient population. Our observation that higher levels of IL-5 were associated with improved survival and a reduction in the incidence of respiratory failure confirms one prior study and supports the hypothesis of a protective effect for IL-5 in sepsis (23). Interestingly, we also describe expression of the soluble isoform of IL-5Rα in our patients with sepsis, with higher levels found in patients with septic shock, suggesting endogenous neutralization of IL-5 may impair host response. However, given the differences in affinity between sIL5Rα and membrane IL-5Rα (50), the ability of sIL-5Rα to independently predict outcome is dependent on a combination of circulating sIL-5Rα, circulating IL-5, and overall membrane-bound IL-5Rα density on circulating and tissue-based granulocytes, which is beyond the scope of the current investigation.
There are a few important limitations to this study. First, we are unable to exclude an important role for other cellular subsets in mediating the effects of IL-5 in vivo. Specifically, some studies document potential IL-5Rα expression on a variety of endothelial and epithelial cell subsets, both of which have also been shown to modulate the host response in sepsis and other diseases (51, 52). In addition, macrophage depletion in MaFIA mice also results in depletion of dendritic cells, which have a potentially important immunomodulatory role in the host response to sepsis (53). We will continue to pursue the role of these additional cellular subsets in subsequent studies. Second, our human studies imply there is a subset of human subjects with higher levels of IL-5, which is associated with improved outcomes in sepsis. However, we are unable to determine whether this is causative or more of a general marker for illness severity. Although the lack of correlation between IL-5 levels and illness severity score suggests a causal effect, we acknowledge this can only be definitively established in humans with a clinical study of IL-5 administration. Furthermore, the source of IL-5 and the factors regulating IL-5 secretion in sepsis remain unknown. The recently described innate helper cells, which secrete high levels of IL-5, may provide a source of and potentiate IL-5 production in sepsis (54, 55). This possibility needs to be further investigated. Finally, it is important to confirm the protective effects of IL-5 in vitro on neutrophils and monocytes from patients with sepsis.
Taken together, these data demonstrate that IL-5 is protective in sepsis through improving survival and host control of infection. Surprisingly, monocytes, not eosinophils, are necessary for these effects in vivo. These studies not only establish the feasibility for the use of IL-5 as an immunoadjuvant in sepsis, but also expand the current paradigm for IL-5 biologic activity in vivo.
Supplementary Material
Footnotes
Supported by NIH grant R01 AI067522 and a BD Biosciences Research Grant.
Author Contributions: Conception and design, S.N.L. and J.A.G.; analysis and interpretation, S.N.L., E.T.D., A.M.K., R.A.T., and J.A.G.; research reagents, technical expertise, and critical review of the manuscript, J.J.L.
Originally Published in Press as DOI: 10.1164/rccm.201201-0134OC on May 31, 2012
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–150 [DOI] [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007;35:1244–1250 [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Birmingham MC, Balk RA, Scannon PJ, Collins D, Kruse JA, Graham DR, Dedhia HV, Homann S, MacIntyre N. E5 murine monoclonal antiendotoxin antibody in gram-negative sepsis: a randomized controlled trial. E5 Study Investigators. JAMA 2000;283:1723–1730 [DOI] [PubMed] [Google Scholar]
- 4.Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S, Cooney R, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Norasept II study group. Lancet 1998;351:929–933 [PubMed] [Google Scholar]
- 5.Opal SM, Fisher CJ, Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator group. Crit Care Med 1997;25:1115–1124 [DOI] [PubMed] [Google Scholar]
- 6.Echtenacher B, Freudenberg MA, Jack RS, Mannel DN. Differences in innate defense mechanisms in endotoxemia and polymicrobial septic peritonitis. Infect Immun 2001;69:7271–7276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebong SJ, Goyert SM, Nemzek JA, Kim J, Bolgos GL, Remick DG. Critical role of CD14 for production of proinflammatory cytokines and cytokine inhibitors during sepsis with failure to alter morbidity or mortality. Infect Immun 2001;69:2099–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reinhart K, Gluck T, Ligtenberg J, Tschaikowsky K, Bruining A, Bakker J, Opal S, Moldawer LL, Axtelle T, Turner T, et al. CD14 receptor occupancy in severe sepsis: results of a phase i clinical trial with a recombinant chimeric CD14 monoclonal antibody (IC14). Crit Care Med 2004;32:1100–1108 [DOI] [PubMed] [Google Scholar]
- 9.Ely EW, Bernard GR, Vincent JL. Activated protein C for severe sepsis. N Engl J Med 2002;347:1035–1036 [DOI] [PubMed] [Google Scholar]
- 10.Weinstein MP, Murphy JR, Reller LB, Lichtenstein KA. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. II. Clinical observations, with special reference to factors influencing prognosis. Rev Infect Dis 1983;5:54–70 [DOI] [PubMed] [Google Scholar]
- 11.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–1310 [DOI] [PubMed] [Google Scholar]
- 12.Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med 1997;3:678–681 [DOI] [PubMed] [Google Scholar]
- 13.Lekkou A, Karakantza M, Mouzaki A, Kalfarentzos F, Gogos CA. Cytokine production and monocyte HLA-DR expression as predictors of outcome for patients with community-acquired severe infections. Clin Diagn Lab Immunol 2004;11:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adrie C, Pinsky MR. The inflammatory balance in human sepsis. Intensive Care Med 2000;26:364–375 [DOI] [PubMed] [Google Scholar]
- 15.Solomkin JS, Cotta LA, Brodt JK, Hurst JM. Regulation of neutrophil superoxide production in sepsis. Arch Surg 1985;120:93–98 [DOI] [PubMed] [Google Scholar]
- 16.Zimmerman JJ, Millard JR, Farrin-Rusk C. Septic plasma suppresses superoxide anion synthesis by normal homologous polymorphonuclear leukocytes. Crit Care Med 1989;17:1241–1246 [DOI] [PubMed] [Google Scholar]
- 17.Orozco H, Arch J, Medina-Franco H, Pantoja JP, Gonzalez QH, Vilatoba M, Hinojosa C, Vargas-Vorackova F, Sifuentes-Osornio J. Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis: a randomized, double-blind, placebo-controlled clinical trial. Arch Surg 2006;141:150–153, discussion 154 [DOI] [PubMed] [Google Scholar]
- 18.Presneill JJ, Harris T, Stewart AG, Cade JF, Wilson JW. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med 2002;166:138–143 [DOI] [PubMed] [Google Scholar]
- 19.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 2009;180:640–648 [DOI] [PubMed] [Google Scholar]
- 20.Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA, Jr, Auxiliadora-Martins M, Basile-Filho A, McKenzie AN, Xu D, Cunha FQ, et al. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med 2010;16:708–712 [DOI] [PubMed] [Google Scholar]
- 21.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med 2008;14:949–953 [DOI] [PubMed] [Google Scholar]
- 22.Linch SN, Kelly AM, Danielson ET, Pero R, Lee JJ, Gold JA. Mouse eosinophils possess potent antibacterial properties in vivo. Infect Immun 2009;77:4976–4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care 2007;11:R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abidi K, Khoudri I, Belayachi J, Madani N, Zekraoui A, Zeggwagh AA, Abouqal R. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care 2008;12:R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bass DA, Gonwa TA, Szejda P, Cousart MS, DeChatelet LR, McCall CE. Eosinopenia of acute infection: production of eosinopenia by chemotactic factors of acute inflammation. J Clin Invest 1980;65:1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linch S, Kelly A, Danielson E, Gold JA. IL-5 augments macrophage function in polymicrobial sepsis. Am J Respir Crit Care Med 2010;181:A1802 [Google Scholar]
- 27.Linch S, Danielson ET, Kelly AM, Lee JJ, Gold JA. The effect of IL-5 on macrophages and pmns in sepsis. Am J Respir Crit Care Med 2009;179:A1024 [Google Scholar]
- 28.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol 1997;158:1332–1344 [PubMed] [Google Scholar]
- 29.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 2004;305:1773–1776 [DOI] [PubMed] [Google Scholar]
- 30.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG, et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 1996;4:15–24 [DOI] [PubMed] [Google Scholar]
- 31.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 2003;31:1250–1256 [DOI] [PubMed] [Google Scholar]
- 32.Gold JA, Parsey M, Hoshino Y, Hoshino S, Nolan A, Yee H, Tse DB, Weiden MD. CD40 contributes to lethality in acute sepsis: in vivo role for CD40 in innate immunity. Infect Immun 2003;71:3521–3528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock: a review of laboratory models and a proposal. J Surg Res 1980;29:189–201 [DOI] [PubMed] [Google Scholar]
- 34.Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, Gleich GJ. Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J Immunol 1989;142:4428–4434 [PubMed] [Google Scholar]
- 35.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, Wang H, O'Neill KR, Colbert DC, Colby TV, et al. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol 2007;178:7879–7889 [DOI] [PubMed] [Google Scholar]
- 36.Hakansson L, Venge P. Priming of eosinophil and neutrophil migratory responses by interleukin 3 and interleukin 5. APMIS 1994;102:308–316 [DOI] [PubMed] [Google Scholar]
- 37.Ringheim GE. Mitogenic effects of interleukin-5 on microglia. Neurosci Lett 1995;201:131–134 [DOI] [PubMed] [Google Scholar]
- 38.Pazdrak K, Stafford S, Alam R. The activation of the JAK-STAT 1 signaling pathway by IL-5 in eosinophils. J Immunol 1995;155:397–402 [PubMed] [Google Scholar]
- 39.Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, Kusser K, Goodrich S, Howard M, Harmsen A, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat Med 2001;7:1209–1216 [DOI] [PubMed] [Google Scholar]
- 40.Stephens CG, Snyderman R. Cyclic nucleotides regulate the morphologic alterations required for chemotaxis in monocytes. J Immunol 1982;128:1192–1197 [PubMed] [Google Scholar]
- 41.Karulf M, Kelly A, Weinberg AD, Gold JA. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. J Immunol 2010;185:4856–4862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, Cohen DA. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol 2004;75:612–623 [DOI] [PubMed] [Google Scholar]
- 43.Shi HZ, Xiao CQ, Zhong D, Qin SM, Liu Y, Liang GR, Xu H, Chen YQ, Long XM, Xie ZF. Effect of inhaled interleukin-5 on airway hyperreactivity and eosinophilia in asthmatics. Am J Respir Crit Care Med 1998;157:204–209 [DOI] [PubMed] [Google Scholar]
- 44.Gennari R, Alexander JW, Gianotti L, Eaves-Pyles T, Hartmann S. Granulocyte macrophage colony-stimulating factor improves survival in two models of gut-derived sepsis by improving gut barrier function and modulating bacterial clearance. Ann Surg 1994;220:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hiroi T, Yanagita M, Iijima H, Iwatani K, Yoshida T, Takatsu K, Kiyono H. Deficiency of IL-5 receptor alpha-chain selectively influences the development of the common mucosal immune system independent IGA-producing B-1 cell in mucosa-associated tissues. J Immunol 1999;162:821–828 [PubMed] [Google Scholar]
- 46.Takatsu K, Nakajima H. IL-5 and eosinophilia. Curr Opin Immunol 2008;20:288–294 [DOI] [PubMed] [Google Scholar]
- 47.Al-Qaoud KM, Pearlman E, Hartung T, Klukowski J, Fleischer B, Hoerauf A. A new mechanism for IL-5-dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int Immunol 2000;12:899–908 [DOI] [PubMed] [Google Scholar]
- 48.Busse WW, Katial R, Gossage D, Sari S, Wang B, Kolbeck R, Coyle AJ, Koike M, Spitalny GL, Kiener PA, et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor alpha antibody, in a phase I study of subjects with mild asthma. J Allergy Clin Immunol 2010;125:1237–1244, e1232 [DOI] [PubMed] [Google Scholar]
- 49.Dewachi O, Joubert P, Hamid Q, Lavoie JP. Expression of interleukin (IL)-5 and IL-9 receptors on neutrophils of horses with heaves. Vet Immunol Immunopathol 2006;109:31–36 [DOI] [PubMed] [Google Scholar]
- 50.Tavernier J, Tuypens T, Plaetinck G, Verhee A, Fiers W, Devos R. Molecular basis of the membrane-anchored and two soluble isoforms of the human interleukin 5 receptor alpha subunit. Proc Natl Acad Sci USA 1992;89:7041–7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colotta F, Bussolino F, Polentarutti N, Guglielmetti A, Sironi M, Bocchietto E, De Rossi M, Mantovani A. Differential expression of the common beta and specific alpha chains of the receptors for GM-CSF, IL-3, and IL-5 in endothelial cells. Exp Cell Res 1993;206:311–317 [DOI] [PubMed] [Google Scholar]
- 52.Andrew AS, Warren AJ, Barchowsky A, Temple KA, Klei L, Soucy NV, O'Hara KA, Hamilton JW. Genomic and proteomic profiling of responses to toxic metals in human lung cells. Environ Health Perspect 2003;111:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol 2002;168:2493–2500 [DOI] [PubMed] [Google Scholar]
- 54.Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol 2010;31:407–413 [DOI] [PubMed] [Google Scholar]
- 55.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity 2012;36:451–463 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.