Abstract
Aims
Alcohol and marijuana are the most widely used intoxicants among adolescents, yet their potential unique and interactive influences on the developing brain are not well established. Brain regions subserving learning and memory undergo continued maturation during adolescence, and may be particularly susceptible to substance-related neurotoxic damage. Here, we characterize brain response during verbal learning among adolescent users of alcohol and marijuana.
Design
Participants performed a verbal paired associates encoding task during fMRI scanning.
Setting
Adolescent subjects were recruited from local public schools and imaged at a University-based fMRI Center.
Participants
Participants were 74 16- to 18-year-olds, divided into four groups: (1) 22 controls with limited alcohol and marijuana experience, (2) 16 binge drinkers, (3) 8 marijuana users, and (4) 28 binge drinking marijuana users.
Measurements
Diagnostic interview assured that all teens were free from neurologic or psychiatric disorders; urine toxicology and breathalyzer verified abstinence for 22–28 days before scanning; a verbal paired associates task was administered during fMRI.
Findings
Groups demonstrated no differences in performance on the verbal encoding task, yet exhibited different brain response patterns. A main effect of drinking pointed to decreased inferior frontal but increased dorsal frontal and parietal fMRI response among binge drinkers (corrected p < .05). There was no main effect of marijuana use. Binge drinking × marijuana interactions were found in bilateral frontal regions (corrected p < .05), where users of either alcohol or marijuana showed greater response than non-users, but users of both substances resembled non-users.
Conclusions
Adolescent substance users demonstrated altered fMRI response relative to nonusing controls, yet binge drinking appeared associated with more differences in activation than marijuana use. Alcohol and marijuana may have interactive effects that alter these differences, particularly in prefrontal brain regions.
Keywords: adolescence, functional magnetic resonance imaging, verbal learning, cannabis, alcohol, binge drinking
Introduction
Alcohol is the most used intoxicant among adolescents, with 25% of 12th graders having drunk 5+ drinks during the previous 2 weeks, indicating high rates of heavy episodic, or binge, drinking [1]. Marijuana is the most widely used illicit drug among teenagers [1]. Among 12th graders, 19% report past-month and 5% daily use, reflecting substantial regular intake [1]. Little is known about the differential influence of binge drinking and marijuana use on the developing brain. Regions maturing throughout adolescence include the hippocampus and prefrontal cortex [2–4], implicated in learning and memory [5, 6] and potentially susceptible to damage from chronic alcohol and marijuana exposure. Understanding the influence of adolescent alcohol and marijuana use on learning and memory is important, as education and future opportunities could be impacted.
Alcohol and marijuana use in adolescence are each associated with altered learning and memory. Heavy alcohol use in adolescence has been associated with poorer verbal and object recall [7]. Adults who began chronic marijuana use as young adolescents performed worse on verbal recall than late-onset users, even after 28 days of abstinence [8]. Several studies have characterized verbal learning decrements among adolescent marijuana users [9–12], which appear to be greater among adolescent than adult users, persisting up to six weeks following discontinuation [13].
Recent neuroimaging studies have reported effects of adolescent alcohol and marijuana use. Adolescents with alcohol use disorders demonstrate smaller hippocampi [14–16] and prefrontal volumes [17, 18] than non-users. Adolescent binge drinkers show poorer white matter integrity than non-drinkers, including projections from hippocampal and frontal regions [19], and increased frontal and parietal activation and lack of hippocampal response during verbal learning [20]. In contrast, corpus callosum microstructure appears normal among individuals with adolescent-onset alcohol use disorders [21]. Functional magnetic resonance imaging (fMRI) studies of adolescent marijuana users have characterized increased prefrontal and parietal activation among users during spatial working memory [22, 23] and inhibitory processing [24]. During verbal working memory, adolescent nicotine and marijuana users failed to deactivate the hippocampus [25], but demonstrated increased frontal, parietal, and hippocampal activation with greater task difficulty during nicotine withdrawal [12].
The interactive effects of concomitant alcohol and marijuana use are less clear. Adolescent users of both substances actually demonstrate normal hippocampal volumes and asymmetry as compared to controls, but abnormal relationships between verbal learning and hippocampal volume asymmetry [16]. Although adolescents with alcohol use disorders show smaller prefrontal cortex volumes than controls [17], heavy marijuana using teens show only subtle volume abnormalities [26]. Similarly, binge drinking heavy marijuana users demonstrate fewer microstructural white matter abnormalities than those who binge drank alone [27]. In contrast, adolescents with comorbid alcohol and marijuana use disorders have greater activation aberrations during spatial working memory than adolescents with alcohol use disorders alone, suggesting a unique effect of marijuana use on frontoparietal networks [23]. However, none of these studies included a marijuana-only group.
The current study was designed to understand the influence of adolescent binge drinking and heavy marijuana use on blood oxygen level dependent (BOLD) response measured by fMRI during a verbal paired associates encoding task. Previous studies in adults and adolescents suggested that neurocognitive effects of heavy marijuana use might persist following at least three weeks of abstinence [11, 28–30]. Therefore, participants were required to remain abstinent from marijuana for at least three weeks before scanning to ensure that any differences were not due to residual effects of recent use. Based on previous studies, we predicted that both binge drinking and marijuana use would be associated with increased prefrontal and reduced hippocampal fMRI response during verbal encoding. Based on our previous work comparing users of alcohol alone or combined with marijuana [16, 27], we predicted that heavy episodic drinking would be associated with greater deviations than heavy marijuana use.
Method
Participants
Participants were 74 16- to 18-year-olds recruited from local schools. Written informed assent and consent, approved by the University of California San Diego Human Research Protections Program, were obtained from each teen and parent. Exclusions were teen history of medical or neurological disorders, DSM-IV psychiatric diagnoses other than alcohol or marijuana use disorder, head injury with loss of consciousness ≥2 minutes, learning disabilities, left handedness, and MRI contraindications; and maternal substance use during pregnancy. Parents and youth completed the Diagnostic Interview Schedule for Children Predictive Scales [31] to rule out psychiatric disorders, and the Family History Assessment Module [32]. Parents provided socioeconomic status information [33] to facilitate group matching. Teens provided biweekly urine toxicology and breathalyzer samples for 22–28 days before scanning.
Four groups of adolescents were evaluated: (1) controls (CON; n = 22) with very limited alcohol or marijuana experience (≤5 lifetime uses of marijuana, ≤50 lifetime uses of alcohol); (2) binge drinkers (BD; n = 16) who typically drank ≥4 drinks/occasion for females or ≥5 drinks/occasion for males [19, 34, 35] in the previous 3 months, but had used marijuana <10 times; (3) marijuana users (MJ; n = 8) who had used marijuana ≥180 times, but had no binge drinking episode in the previous 3 months; and (4) binge drinking marijuana users (BD+MJ; n = 28) who typically binge drank (≥4 drinks/occasion for females or ≥5 drinks/occasion for males) in the previous 3 months and had used marijuana ≥180 times in their lives. Approximately half the BD and CON participants were in our study examining encoding in binge drinkers [20].
The groups were not statistically different on demographics, estimated verbal IQ (Vocabulary T-score), or mood (see Table 1). BD and BD+MJ were not statistically different on recency of use, drinking days per month, or age of first drink, and showed more alcohol use than non-binge drinking groups. However, BD+MJ teens reported more lifetime drinking episodes and drinks per occasion than BD teens. MJ and BD+MJ showed no statistical differences in degree of marijuana involvement, but had more extensive marijuana use than non-marijuana users. Marijuana users reported more lifetime use of other drugs than non-marijuana users. No participant was a regular smoker, and all had low Fagerstrom Nicotine Dependence scores [36].
Table 1.
Demographics, Substance Use Characteristics, and Task Performance among Adolescent Participants
| CON (n = 22) | BD (n = 16) | MJ (n = 8) | BD+MJ (n = 28) | |
|---|---|---|---|---|
| M (SD) or % | M (SD) or % | M (SD) or % | M (SD) or % | |
| Demographics and Mood | ||||
| Age (range 16 – 18) | 17.6 (0.8) | 18.1 (0.7) | 18.1 (0.9) | 18.0 (1.0) |
| % Female | 27.3 | 18.8 | 50.0 | 17.9 |
| % Caucasian | 59.1 | 56.2 | 50.0 | 60.7 |
| % Family history negative * | 86.4 | 87.5 | 62.5 | 78.6 |
| Parent annual salary (US$ thousands) | 114.5 (88.9) | 107.9 (73.4) | 86.3 (34.2) | 133.0 (134.4) |
| Vocabulary T-score † | 58.3 (8.7) | 60.6 (9.8) | 52.5 (4.7) | 59.5 (8.0) |
| Spielberger State Anxiety T-score | 37.6 (8.7) | 35.1 (4.1) | 39.5 (5.7) | 39.2 (7.1) |
| Beck Depression Inventory Total | 2.5 (3.2) | 2.2 (2.1) | 4.0 (4.2) | 3.3 (2.8) |
|
| ||||
| Substance Use Characteristics | ||||
| Lifetime drinking episodes c,e | 5.8 (8.2) | 50.1 (47.3) | 126.5 (119.8) | 209.5 (165.8) |
| Age of first drink c | 15.7 (1.2) | 15.0 (1.8) | 13.6 (2.3) | 13.5 (2.3) |
| Drinking days/month, past 3 monthsa,c,e,f | 0.2 (0.7) | 4.1 (2.3) | 3.1 (4.9) | 9.7 (5.7) |
| Drinks/occasion, past 3 monthsa,c,e,f | 0.2 (0.7) | 9.4 (4.5) | 1.3 (1.6) | 12.6 (6.5) |
| Days since last alcohol use a,c | 206.9 (203.2) | 32.4 (11.5) | 131.5 (123.4) | 25.0 (12.6) |
| % with alcohol use disorder § | 0 | 0 | 0 | 35.7 |
| Lifetime marijuana use episodesb,c,d,e | 0.6 (1.2) | 2.0 (2.9) | 426.5 (280.1) | 517.6 (451.3) |
| Age of first weekly marijuana use | - | - | 14.5 (2.5) | 14.9 (3.4) |
| Marijuana days/month, past 3 monthsb,c,d,e | 0.0 (0.0) | 0.3 (0.6) | 8.4 (9.3) | 11.8 (9.3) |
| Days since last marijuana useb,c,d,e | 528.0 (257.1) | 397.3 (401.0) | 117.6 (153.9) | 43.4 (37.1) |
| % with marijuana use disorder ∞ | 0 | 0 | 50.0 | 71.4 |
| Fagerstrom Nicotine Dependence ‡ | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.4) | 0.3 (0.8) |
| Lifetime other drug use episodes b,c,d,e | 0.0 | 1.1 | 11.9 | 9.9 |
|
| ||||
| Verbal Paired Associates Task Performance | ||||
| % correct recall during scan | 81.8 (13.1) | 80.7 (11.6) | 81.3 (14.5) | 83.7 (11.4) |
| % requiring 2nd pre-scan training | 13.6 | 31.3 | 37.5 | 10.7 |
No first-degree biological relative with alcohol or drug abuse or dependence
Based on Wechsler Abbreviated Scale of Intelligence [64]
Max possible score = 10; range 0–3 in this sample [36]
Based on DSM-IV current alcohol abuse or dependence; BD+MJ different from others
Based on DSM-IV current marijuana abuse or dependence; MJ and BD+MJ different from others
CON significantly different from BD (Tukey α = .05)
CON significantly different from MJ (Tukey α = .05)
CON significantly different from BD+MJ (Tukey α = .05)
BD significantly different from MJ (Tukey α = .05)
BD significantly different from BD+MJ (Tukey α = .05)
MJ significantly different from BD+MJ (Tukey α = .05)
Measures
Substance Involvement
The Customary Drinking and Drug Use Record (CDDR) [37] assessed lifetime and current use of alcohol, marijuana, tobacco, and other drugs, withdrawal symptomatology, and DSM-IV substance use disorder criteria. The Timeline Followback [38] assessed substance use patterns covering the 22 days prior to scanning and the month preceding being asking to stop.
Verbal Paired Associates Task [20, 39–41]
Subjects were instructed to memorize highly associated pairs of monosyllabic nouns (presented for 5 seconds each) before and during fMRI scanning. Before scanning, participants memorized a list of 16 word pairs. Recall was immediately tested by presenting the first member of a pair and asking subjects to verbalize the second member of that pair. Learning and recall trials were repeated until the participant correctly recalled ≥10 of 16 pairs. During scanning, participants viewed 32 pairs of associated words in a block design, including 16 previously learned pairs and 16 novel pairs. Participants were to learn all the word pairs. The task included blocks of fixation trials with variable durations of 8, 12, and 16 seconds. The order of conditions was pseudo-random and fixed across subjects. After scanning, recall was assessed as in the pre-scan session.
Procedures
Toxicology screening
To ensure abstinence from marijuana and other drugs before scanning, all teens underwent biweekly urine toxicology screening for 22–28 days [22, 24]. Urine samples were tested for evidence of recent use of cannabis, amphetamines, methamphetamines, barbiturates, benzodiazepines, cocaine, codeine, morphine, phencyclidine, and ethanol. THCCOOH, the main secondary metabolite of THC, can be detected in urine several weeks after last use [42, 43], so participants were determined abstinent from marijuana if values decreased with each sample [44]. Two participants in the MJ+BD group reported drug use during the toxicology screening period: one used dextroamphetamine 15 days before scanning, and one used cocaine 21 days before scanning. One MJ+BD participant used prescription codeine per medical guidelines on the day of scanning.
Image Acquisition
Images were collected on a 3-Tesla GE scanner with a sagittally acquired structural image collected with an inversion recovery prepared T1-weighted SPGR sequence (repetition time = 8 ms, echo time = 3 ms, field of view = 240 mm, resolution = 1 mm3, 176 continuous slices, acquisition time = 7:19). During the verbal encoding task, T2*-weighted echo planar imaging was acquired (repetition time = 4000 ms, echo time = 30 ms, flip angle = 90°, field of view = 240 mm, 32 axial slices covering the whole brain, slice thickness = 3.8 mm, in-plane resolution = 3.75 × 3.75 mm, 69 repetitions, acquisition time = 4:36). We have detected hippocampal signal contrast using a similar protocol [20, 39, 41].
Data Analysis
An automated motion correction algorithm was applied to time series datasets [45]. Two trained raters removed repetitions containing residual visible head motions; all datasets retained >85% of repetitions. Next, datasets were deconvolved with a reference function representing the task design while accounting for hemodynamic delays [46] and covarying for motion and linear trends. This yielded fit coefficients representing BOLD response contrast during encoding for each voxel. Our previous work with this task suggested little BOLD response contrast difference between novel and previously learned word pairs and the most robust contrast was for encoding novel word pairs [41]. To best examine brain regions involved in novel verbal learning, we focused on response contrast to novel word pairs. Data were transformed into standard coordinates [47, 48] and functional data were resampled into 3 mm isotropic voxels and spatially smoothed with a Gaussian filter (5 mm full width half maximum).
Group-level analyses were performed on whole brain fMRI data in a two-factor ANOVA, coding for binge drinking status (BD and BD+MJ vs. CON and MJ) and marijuana use status (MJ and BD+MJ vs. CON and BD). We interpreted only clusters comprised of contiguous activated (α <.05) voxels ≥1512 μl in volume, yielding a 5% brain-wise probability of false positive. Tukey pairwise comparisons (family-wise α = .05), compared average BOLD response within each cluster showing a BD × MJ interaction among groups. Data distributions were examined for normality and outliers within each group. A single sample t-test was performed among CON for interpreting group differences in relation to typical activation to this task. Given the effect sizes previously observed [20,22], α=.05, and cell sizes ≥8, power to detect hypothesized effects was ≥.89.
Due to the importance of hippocampal functioning in learning and memory, we examined BOLD response specifically within the left and right hippocampi. As in [20], we defined left and right hippocampal regions of interest (ROIs) using the Talairach atlas within AFNI. Hippocampal ROIs were resampled to the same grid as functional datasets; left and right hippocampal ROIs were 3.3 cm3 each. BOLD response during novel encoding was averaged across the hippocampal ROIs for each subject, then examined in a two-factor ANOVA.
Exploratory regressions examined whether other substance use characteristics were associated with verbal encoding BOLD response. Average fit coefficients for novel encoding were calculated for each participant within each region exhibiting a significant main effect or interaction. ANCOVA determined whether lifetime uses of other drugs contributed to BOLD response above and beyond the effects of binge drinking, marijuana use, and their interaction.
Results
Behavioral Performance
Neither binge drinking nor marijuana use was associated with verbal encoding task performance (see Table 1). During scanning, participants recalled 82 ± 12% of word pairs, on average. Fourteen adolescents (19%) did not perform to criterion (10/16 correct pairs) on the first learning trial, requiring a second learning trial (evenly distributed across groups).
fMRI Response
BOLD Response in Nonusing Controls
CON demonstrated significant activation during verbal encoding in left middle/inferior frontal gyri, bilateral superior/inferior parietal lobule, bilateral middle/inferior occipital gyri, medial frontal gyrus, and bilateral hippocampi.
Main Effect for Binge Drinking
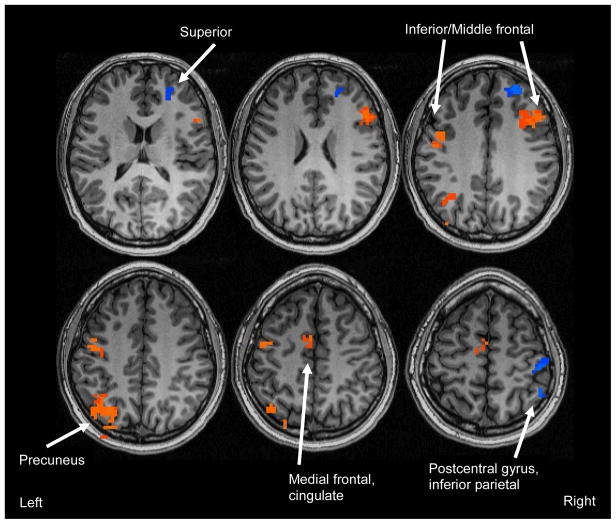
Collapsing across marijuana use group, binge drinkers (n = 44) showed more BOLD response during novel encoding than nondrinkers (n = 30) in two clusters: (1) right superior frontal gyrus (Brodmann’s Area [BA] 10); and (2) right postcentral gyrus and inferior parietal lobule (BA 40; see Table 2 and Figure 1; all clusters >1512 μl, corrected p < .05). Binge drinkers demonstrated less BOLD response than nondrinkers in five clusters: (1) left fusiform gyrus (BA 37); (2) left precentral gyrus (BA 6); (3) right middle and inferior frontal gyri (BA 46, 9); (4) left medial frontal gyrus and cingulate (BA 6, 24); and (5) left inferior parietal lobule and precuneus (BA 19, 39, 40).
Table 2.
Regions Showing Significant Main Effects for Binge Drinking on BOLD Response during Novel Verbal Encoding (clusters > 1512 μl, p < .05)
| Anatomic Region | Brodmann Areas | Volume (μl) | Talairach Coordinates
|
Effect Size Partial η2 | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| BD and BD+MJ > CON and MJ | ||||||
| Right postcentral gyrus/inferior parietal lobule | 40 | 2430 | −47 | 47 | 54 | 0.20 |
| Right superior frontal gyrus | 10 | 1539 | −29 | −50 | 27 | 0.16 |
|
| ||||||
| CON and MJ > BD and BD+MJ | ||||||
| Left fusiform/parahippocampal gyrus/cuneus | 17, 30 | 4050 | 26 | 74 | −16 | 0.17 |
| Left precuneus/inferior parietal lobule | 19, 39, 40 | 3888 | 32 | 80 | 39 | 0.20 |
| Left inferior frontal/precentral gyri | 6, 9 | 2106 | 47 | −5 | 30 | 0.14 |
| Right middle/inferior frontal gyri | 9, 46 | 1944 | −50 | −20 | 27 | 0.16 |
| Left medial frontal/cingulate gyrus | 6, 24 | 1890 | 2 | 8 | 54 | 0.13 |
Note: Coordinates refer to maximum signal intensity voxel within the cluster. Effect sizes refer to main effect of drinking averaged over the cluster.
Figure 1.
Clusters showing significant main effects for binge drinking (clusters > 1512 μl, corrected p < .05). Orange indicates regions where CON and MJ (non-drinkers; n = 30) showed greater BOLD response during novel encoding than BD and BD+MJ (binge drinkers; n = 44); blue clusters represent regions where BD and BD+MJ showed greater BOLD response during novel encoding than CON and MJ.
Main Effect for Marijuana Use
Collapsing across drinking group, marijuana users (n = 36) did not differ significantly from nonusers (n = 38) in any brain region on BOLD response during novel encoding.
Drinking × Marijuana Interaction
Four clusters showed a significant drinking × marijuana interaction for BOLD response during novel encoding: (1) left superior frontal and middle frontal gyri (BA 6, 8); (2) right superior and middle frontal gyri (BA 6,8); (3) right inferior and middle frontal gyri (BA 10, 45); and (4) medial bilateral cuneus and lingual gyrus (BA 18; see Table 3 and Figure 2). Pairwise comparisons characterized the nature of these interactions. In the left superior/middle frontal cluster, MJ demonstrated significantly greater response than CON and BD+MJ. In the right superior/middle frontal cluster, both MJ and BD groups showed significantly more response than CON. In the right middle/inferior frontal cluster, MJ showed greater response than CON and BD+MJ, and BD showed more activation than CON. In the bilateral cuneus and lingual gyrus cluster, both MJ and BD groups showed significantly less activation than CON, and BD also showed less response than BD+MJ. In no region did CON significantly differ from BD+MJ, or BD differ from MJ.
Table 3.
BOLD Response During Novel Verbal Encoding in Each Group for Regions of Significant Binge × Marijuana Interactions and the Hippocampal a Priori Region of Interest
| Anatomic Region | Brodmann Areas | Volume (μl) | Talairach Coordinates | Activation Mean (SD) | Effect Size Partial η2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| x | y | z | CON (n = 22) | BD (n = 16) | MJ (n = 8) | BD+MJ (n = 28) | ||||
| Significant Interaction Clusters | ||||||||||
|
| ||||||||||
| Left superior/middle frontal gyri b,c | 6, 8 | 3996 | 29 | −20 | 51 | 0.37 (1.37) | 5.39 (1.60) | 7.96 (2.27) | 0.48 (1.21) | 0.17 |
| Right inferior/middle frontal gyri a,b,c | 10, 45 | 3645 | −44 | −41 | −1 | −1.88 (1.41) | 4.41 (1.65) | 6.39 (2.33) | −0.76 (1.25) | 0.18 |
| Right superior/middle frontal gyri a,b | 6, 8 | 3159 | −26 | −26 | 54 | −4.79 (1.11) | 0.90 (1.31) | 2.08 (1.85) | −2.58 (0.99) | 0.17 |
| Medial cuneus/lingual gyrus a,b,d | 18 | 2700 | 2 | 89 | 9 | 7.48(2.25) | −7.20(2.64) | −5.25 (3.74) | 1.93(2.00) | 0.19 |
|
| ||||||||||
| Hippocampal Regions of Interest | ||||||||||
| Left hippocampus BOLD Response |
2.52(2.60)* | 1.67(2.08)* | 0.82 (3.09) | 2.60(3.23)* | ||||||
| Left hippocampus Effect Size Cohen’s d |
1.37 | 1.14 | 0.38 | 1.14 | ||||||
| Right hippocampus BOLD Response |
1.57(2.42)* | 1.50(2.34)* | 1.00 (2.64) | 1.56(2.80)* | ||||||
| Right hippocampus Effect Size Cohen’s d |
0.92 | 0.91 | 0.54 | 0.79 | ||||||
Note: Coordinates refer to maximum signal intensity group difference within cluster. Mean activations represent average fit coefficients within each cluster, positive values indicate greater response during encoding than fixation. Partial η2 refers to effect sizes for interaction within the cluster; Cohen’s d refers to effect sizes for single sample t-tests within hippocampal regions of interest.
CON significantly different from BD (Tukey α = .05)
CON significantly different from MJ (Tukey α = .05)
MJ significantly different from BD+MJ (Tukey α = .05)
BD significantly different from BD+MJ (Tukey α = .05)
Novel verbal encoding activation significantly different from zero (p < .01)
Figure 2.
Clusters showing significant interactions between binge drinking and marijuana use (N=74; clusters >1512 μl, corrected p < .05).
Within both left and right hippocampal ROIs, significant BOLD response contrast during verbal encoding was observed in BD, BD+MJ, and CON, but not in the MJ group (see Table 3). No main effects or interactions of alcohol or marijuana use on right or left hippocampal BOLD response were seen. Hippocampal response was not related to task performance.
We examined the possible influence of other drug use on BOLD response in regions that differed between groups. BOLD response was not associated with other drug use in any cluster except for the left precentral gyrus, where BD had shown less response than non-drinkers. Here, ANCOVA revealed that total lifetime other drug use was positively associated with BOLD response above and beyond the effects of binge drinking, marijuana use, and their interaction (F(1,69) = 9.25, p < .005). After including lifetime other drug use in the model, binge drinking remained a significant predictor of BOLD response in this region.
Given previous findings of gender differences in neurocognitive function related to alcohol [49] and marijuana [50], we explored the potential influence of gender on group differences. For all clusters, gender was not a contributor to the model, and all results remained when gender was included. Boys and girls did not perform significantly differently on the task.
Discussion
This study examined the neural underpinnings of verbal encoding among adolescent binge drinkers and marijuana users after >3 weeks of monitored marijuana abstinence. Results indicated a main effect of binge drinking and an interaction between binge drinking and marijuana use, but no main effect of marijuana use, on activation.
Binge drinking was associated with less encoding-related activation than non-bingers, independent of marijuana use, in medial cuneus and left posterior parietal cortices, yet greater activation in lateral right parietal and anterior right superior frontal areas, overlapping with our previous findings among non-marijuana using adolescent drinkers [20]. Binge drinkers in the current study showed diminished BOLD response in bilateral dorsolateral prefrontal regions typically involved in verbal rehearsal [for review, see 51]. Others have observed diminished bilateral dorsolateral prefrontal response among adult alcoholics during spatial working memory [52]. This altered frontoparietal functioning could indicate different use of strategies, or reorganization of networks for verbal rehearsal and encoding. For instance, left prefrontal activation has been associated with elaborative learning during novel encoding [53], which could suggest that binger drinkers utilize less sophisticated rehearsal strategies than controls. Recent developmental work has observed increasing dorsolateral prefrontal activation with age during working memory, as youth become more dependent on working memory networks [54]. Thus, reduced dorsolateral prefrontal response may indicate less mature responding among binge drinkers.
Results revealed interactions between binge drinking and marijuana use, particularly in frontal regions. Interestingly, within these areas, both marijuana- and binge-drinking-only groups evinced similar degrees of increased BOLD response than controls, while teens who binge drank plus used marijuana had control-like levels. This could indicate compensatory recruitment among users of alcohol or marijuana alone in regions involving working memory, selecting and monitoring information, and processing subgoals [e.g., 55].
Counter-intuitively, in clusters with interactions, users of both substances showed intermediate response patterns between controls and single substance use groups. This is consistent with fewer neurocognitive differences among users of both marijuana and alcohol than those who use alcohol alone [16, 17, 26, 27, 56]. The mechanism is unclear, but it is possible that cannabinoids may yield some neuroprotective anti-inflammatory properties that counteract effects of heavy alcohol exposure [57–59]. Alternatively, the differential effect of concomitant use may relate to the recency of intoxication for each substance. Adolescents in the current study had been abstinent from marijuana for >22 days, but may have used alcohol more recently. We previously identified unique brain response patterns during spatial working memory among recent heavy users of both substances in comparison to heavy drinkers [23]. Thus, it is important to consider the time course of normalization following cessation [20]. Another alternative is that simultaneous but opposing processes may exist in proximal regions. For example, if the frontal areas showing interactions contained small neural beds of impaired neurons as well as overactive neurons compensating for insults, the net result would be similar overall activation as in controls.
Although no main effect for marijuana use was seen, interactions pointed to altered BOLD response patterns among marijuana users who did not drink, particularly in lack of hippocampal response contrast during novel encoding. This is consistent with observed hippocampal hypoactivity during associative learning among adult marijuana users following a week of abstinence [60]. We also identified increased frontal response among users of marijuana alone. In contrast, previous reports have characterized reduced prefrontal activation during learning among adult recent marijuana users [60–62]. The differences in the current study were observed following >22 days of confirmed cannabinoid abstinence. Thus, the pattern of compensation or using alternate strategies may change in early abstinence [28], such that recent users reduce brain response, while more abstinent users exhibit hyperactivity.
Our previous fMRI studies of abstinent adolescent marijuana users identified alterations after statistically controlling for alcohol use, but were unable to examine brain response among marijuana using nondrinkers [e.g., 22, 24]. Similarly, an fMRI study of verbal working memory characterized disrupted frontoparietal connectivity among abstinent adolescent marijuana users during nicotine withdrawal [12], but it is unclear what patterns would be observed among nonsmoking marijuana users. The current investigation provides preliminary evidence of subtle differences during verbal learning among abstinent users of marijuana alone, which could suggest persisting differences associated with heavy marijuana use independent of drinking.
These differences in activation were observed despite similar task performance. Because groups performed the task at the same level, comparison of the underlying activation pattern is less likely confounded by differential achievement or motivation. Our previous studies found adolescent heavy drinkers and marijuana users to perform poorly on more sensitive behavioral tasks of verbal memory [7, 11]. Because these populations show deficits on related tests with a broader range of difficulty, the activation patterns in the current study can be interpreted as possibly inefficient.
Several limitations warrant consideration. To our knowledge, this is the first fMRI study to disentangle the possible effects of alcohol and marijuana use using a full factorial design. However, the sample of marijuana-only subjects was small, since few adolescents only use this substance [63]. The finding of intermediate brain response among users of both substances remains counterintuitive and the underlying mechanism deserves further exploration. Other drug use, although relatively limited, may have contributed to results, although only one region with effects showed a relationship to other drug use, and the effect remained after controlling for other drug use. Longitudinal studies, currently underway, will clarify whether participants differed in neural functioning prior to the onset of substance use. Finally, this study had limited power to detect gender differences, yet this represents an important question for future studies.
In general, each substance-using group displayed deviations in BOLD response relative to non-using controls. In addition, binge drinking and marijuana use appear to have independent as well as interactive effects on brain functioning. Overall, results indicate more brain regions associated with binge drinking than with marijuana use. Results may have implications for public health and prevention strategies. Altered memory functioning associated with adolescent substance use may impact school performance and development. Although alcohol use is legal in most countries, its possible harmful effects should be recognized, particularly at doses exceeding 4 or 5 drinks in an episode.
Acknowledgments
This research was made possible by grant support from the National Institute on Alcohol Abuse and Alcoholism (R01 AA13419, PI: Tapert), and the National Institute on Drug Abuse (R01 DA021182, PI: Tapert; P20 DA024194, PI: Mason).
The authors would like to thank Dr. Sunita Bava, Christina Burke, Diane Goldenberg, Joanna Jacobus, Dr. Omar Mahmood, Dr. Krista Medina, Dr. MJ Meloy, Tim McQueeny, Ann Park, Anthony Scarlett, Rachel Thayer, Jennifer Winward, and the participating adolescents and parents.
Footnotes
Declarations of Interest: None.
References
- 1.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2008. Bethesda, MD: National Institute on Drug Abuse; 2009. [Google Scholar]
- 2.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 4.Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–22. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- 5.Squire LR, Schacter DL. The neuropsychology of memory. New York: Guilford; 2002. [Google Scholar]
- 6.Budson AE. Understanding memory dysfunction. Neurologist. 2009;15:71–9. doi: 10.1097/NRL.0b013e318188040d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–71. [PubMed] [Google Scholar]
- 8.Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Earlyonset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–10. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. Am J Dis Child. 1989;143:1214–9. doi: 10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- 10.Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana--a comparison with pre-drug performance. Neurotoxicol Teratol. 2005;27:231–9. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007;13:807–20. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 2007;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–90. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–44. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- 16.Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29:141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–94. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- 19.McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered White Matter Integrity in Adolescent Binge Drinkers. Alcohol Clin Exp Res. 2009;33:1278– 1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of fMRI response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111– 117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, Payne ME, MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clin Exp Res. 2008;32:395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201– 210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–83. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Ann N Y Acad Sci. 2004;1021:384–90. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- 26.Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol. 2009;14:457–68. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009 doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, Tapert SF. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. J Psychoactive Drugs. doi: 10.1080/02791072.2010.10400703. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–15. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- 30.Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003;9:679–89. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- 31.Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–9. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–23. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 33.Hollingshead AB. Two-factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- 34.Wechsler H, Isaac N. ‘Binge’ drinkers at Massachusetts colleges. Prevalence, drinking style, time trends, and associated problems. JAMA. 1992;267:2929–31. doi: 10.1001/jama.267.21.2929. [DOI] [PubMed] [Google Scholar]
- 35.Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull. 2009;135:142–56. doi: 10.1037/a0014414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 37.Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): A measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- 38.Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Humana Press, Inc; Totowa, NJ, US: 1992. pp. 41–72. [Google Scholar]
- 39.Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, Brown GG, Corey-Bloom J, Salmon DP, Thal LJ, Bondi MW. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging. 2007;28:238–47. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleisher AS, Houston WS, Eyler LT, Frye S, Jenkins C, Thal LJ, Bondi MW. Identification of Alzheimer disease risk by functional magnetic resonance imaging. Arch Neurol. 2005;62:1881–8. doi: 10.1001/archneur.62.12.1881. [DOI] [PubMed] [Google Scholar]
- 41.Eyler LT, Jeste DV, Brown GG. Brain response abnormalities during verbal learning among patients with schizophrenia. Psychiatry Res. 2008;162:11–25. doi: 10.1016/j.pscychresns.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fraser AD, Coffin L, Worth D. Drug and chemical metabolites in clinical toxicology investigations: the importance of ethylene glycol, methanol and cannabinoid metabolite analyses. Clin Biochem. 2002;35:501–11. doi: 10.1016/s0009-9120(02)00325-9. [DOI] [PubMed] [Google Scholar]
- 43.Huestis MA, Cone E. Urinary Excretion Half-life of 11-Nor-9-carboxy-D9- tetrahydrocannabinol in Humans. 1998;20:570–576. doi: 10.1097/00007691-199810000-00021. [DOI] [PubMed] [Google Scholar]
- 44.Smith ML, Barnes AJ, Huestis MA. Identifying new cannabis use with urine creatinine-normalized THCCOOH concentrations and time intervals between specimen collections. J Anal Toxicol. 2009;33:185–9. doi: 10.1093/jat/33.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 46.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- 47.Talairach J, Tournoux P. Threedimensional proportional system: An approach to cerebral imaging. New York: Thieme; 1988. Coplanar stereotaxic atlas of the human brain. [Google Scholar]
- 48.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skosnik PD, Krishnan GP, Vohs JL, O’Donnell BF. The effect of cannabis use and gender on the visual steady state evoked potential. Clin Neurophysiol. 2006;117:144–56. doi: 10.1016/j.clinph.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 51.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 52.Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- 53.Kopelman MD, Stevens TG, Foli S, Grasby P. PET activation of the medial temporal lobe in learning. Brain. 1998;121:875–87. doi: 10.1093/brain/121.5.875. [DOI] [PubMed] [Google Scholar]
- 54.Thomason ME, Race E, Burrows B, Whitfield-Gabrieli S, Glover GH, Gabrieli JD. Development of spatial and verbal working memory capacity in the human brain. J Cogn Neurosci. 2009;21:316–32. doi: 10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fletcher PC, Henson RN. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–81. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- 56.Nixon SJ, Paul R, Phillips M. Cognitive efficiency in alcoholics and polysubstance abusers. Alcohol Clin Exp Res. 1998;22:1414–20. doi: 10.1111/j.1530-0277.1998.tb03929.x. [DOI] [PubMed] [Google Scholar]
- 57.Hampson AJ, Grimaldi M, Axelrod J, Wink D. Cannabidiol and (−)Delta9- tetrahydrocannabinol are neuroprotective antioxidants. Proc Natl Acad Sci U S A. 1998;95:8268–73. doi: 10.1073/pnas.95.14.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hampson AJ, Grimaldi M, Lolic M, Wink D, Rosenthal R, Axelrod J. Neuroprotective antioxidants from marijuana. Ann N Y Acad Sci. 2000;899:274–82. [PubMed] [Google Scholar]
- 59.Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther. 2005;314:780–8. doi: 10.1124/jpet.105.085779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jager G, Van Hell HH, DeWin MM, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol. 2007;17:289–97. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 61.Nestor L, Roberts G, Garavan H, Hester R. Deficits in learning and memory: parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Deficits in learning and memory: parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. 2008;40:1328–39. doi: 10.1016/j.neuroimage.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 62.Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Boles Ponto LL, Ghoneim MM, Arndt S, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav. 2002;72:237–50. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- 63.Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U. S residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse. 2002;28:643–52. doi: 10.1081/ada-120015873. [DOI] [PubMed] [Google Scholar]
- 64.Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]