Abstract
Mucins are gel-forming proteins that are responsible for the characteristic viscoelastic properties of mucus. Mucin overproduction is a hallmark of asthma, but the cellular requirements for airway mucin production are poorly understood. The endoplasmic reticulum (ER) protein anterior gradient homolog 2 (AGR2) is required for production of the intestinal mucin MUC2, but its role in the production of the airway mucins MUC5AC and MUC5B is not established. Microarray data were analyzed to examine the relationship between AGR2 and MUC5AC expression in asthma. Immunofluorescence was used to localize AGR2 in airway cells. Coimmunoprecipitation was used to identify AGR2-immature MUC5AC complexes. Agr2−/− mice were used to determine the role of AGR2 in allergic airway disease. AGR2 localized to the ER of MUC5AC- and MUC5B-producing airway cells and formed a complex with immature MUC5AC. AGR2 expression increased together with MUC5AC expression in airway epithelium from “Th2-high” asthmatics. Allergen-challenged Agr2−/− mice had greater than 50% reductions in MUC5AC and MUC5B proteins compared with allergen-challenged wild-type mice. Impaired mucin production in Agr2−/− mice was accompanied by an increase in the proportion of mucins contained within the ER and by evidence of ER stress in airway epithelium. This study shows that AGR2 increases with mucin overproduction in individuals with asthma and in mouse models of allergic airway disease. AGR2 interacts with immature mucin in the ER and loss of AGR2 impairs allergen-induced MUC5AC and MUC5B overproduction.
Keywords: asthma, airway epithelium, mucus, endoplasmic reticulum stress, protein disulfide isomerase
Clinical Relevance
This article highlights the role of a specialized endoplasmic reticulum molecule in mucus overproduction induced by allergic airway inflammation. A more complete understanding of the cellular mechanisms supporting mucin processing may yield novel targets for therapeutic intervention in allergic airway disease.
Mucus hypersecretion is a central pathophysiologic feature of asthma. Airway occlusion by mucus is a major contributor to fatal asthma, and substantial increases in airway mucus are seen even in subjects with mild or moderate stable asthma (1, 2). Despite the known contribution of mucus hypersecretion to morbidity and mortality, therapeutic interventions directed at reducing mucin production are lacking (3). The characteristic viscoelastic properties of mucus depend upon gel-forming mucins, such as the airway mucins MUC5AC and MUC5B and the intestinal mucin MUC2 (4). These mucins are massive (∼ 5,000 amino acid residue) glycoproteins with highly O-glycosylated central domains. The N- and C-terminal domains contain approximately 200 cysteine residues that facilitate folding and multimerization of mucins through intra- and interchain disulfide bonding. Mucin maturation is achieved through an array of posttranslational modifications initiated in the endoplasmic reticulum (ER) where nascent mucin monomers undergo N-glycosylation and dimerize before moving to the Golgi apparatus for O-glycosylation and higher-order multimerization (5–7). This series of posttranslational modifications can be accomplished in less than 15 minutes (6), but the identities of the ER and Golgi components required to complete this complex and demanding task are largely unknown.
We recently reported that the ER-resident protein anterior gradient 2 (AGR2) is required for production of the intestinal mucin MUC2 (8). AGR2 is a member of the protein disulfide isomerase (PDI) family. There are 19 PDI family members in humans, and all are highly conserved in mouse (9). Whereas many PDI family members are widely expressed, mouse Agr2 was initially identified in a screen for genes that were selectively expressed in intestinal mucous cells (goblet cells) (10). PDI family members reside in the ER and have one or more thioredoxin-like domains. Some members of this family, including PDI, use active site cysteines within thioredoxin-like domains to facilitate rearrangement of disulfide bonds in incorrectly folded substrate proteins that are transiting through the ER (11). AGR2 has an active site cysteine that can form mixed disulfide bonds with MUC2 (8). Although it is not known whether AGR2 functions as an isomerase, we found that loss of AGR2 led to a loss of intestinal MUC2 protein and the development of colitis. Mucin and mucous cell structure and function differ significantly between the intestine and other organs (12–14), and the requirement for AGR2 in other mucus-producing organs has not been previously examined.
In this report we address the role of AGR2 in airway mucus production. We previously reported that Agr2 mRNA is highly induced during mucous metaplasia in a mouse model of allergic asthma and that the critical asthma mediator IL-13 induces Agr2 and mucins (Muc5ac and Muc5b) via a signal transducer and activator of transcription 6 (STAT6)-dependent pathway in airway epithelial cells in vivo (15, 16). Direct effects of IL-13 on airway epithelium are thought to be especially important in a large “Th2-high” subset of individuals with asthma that can be identified by measuring expression of IL-13–responsive genes (17). Compared with “Th2-low” individuals with asthma, these “Th2-high” individuals with asthma have different clinical characteristics, increased airway mucin gene expression, and more favorable responses to inhaled corticosteroids and anti–IL-13 antibody treatment (18, 19). Recent reports demonstrated that AGR2 induction is dependent upon SAM-pointed domain–containing Ets-like factor, a transcription factor shown to play a key role in airway mucous cell differentiation in mice and humans, and showed AGR2 expression within mucus-producing cells in the airways (20, 21). However, these reports do not address whether AGR2 expression changes in asthma or whether AGR2 is required for airway mucin production. In the current report, we analyze AGR2 expression in asthma, characterize AGR2 localization in human and mouse airway cells in detail, analyze the ability of AGR2 to form complexes with immature forms of the major airway mucin MUC5AC, and use Agr2−/− mice to determine whether AGR2 is required for airway mucin synthesis in an allergic mouse model of asthma.
Materials and Methods
Gene Expression in Human Airway Epithelial Cells from Patients with Asthma and Healthy Control Subjects
Microarray data obtained using RNA extracted from airway epithelial brush biopsies from 42 subjects with mild and moderate asthma and 28 healthy control subjects (18) were downloaded from Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/, GSE4302). Individuals with asthma were divided into 20 “Th2-low” and 22 “Th2-high” subgroups according to levels of IL-13–induced genes and clinical characteristics (17).
Human Airway Tissue
Human trachea sections were generously provided by the Department of Pathology, San Francisco General Hospital (San Francisco, CA). Images from human trachea are representative of images from multiple deidentified tissue donors. The UCSF Committee on Human Research approved the involvement of human subjects.
Antibodies
Antibodies used for immunofluorescence, immunoprecipitation, and immunoblotting are summarized in the online supplement.
Immunofluorescence
Immunofluorescence staining methods are described in the online supplement. Images were acquired at the UCSF Nikon Imaging Center with a Yokogawa CSU22 spinning-disk confocal (Yokogawa Electric Corporation, Tokyo, Japan) on a Nikon Ti-E (Nikon Corporation, Tokyo, Japan). Images were processed using ImageJ 1.43u and the McMasters Biophotonics Facility Microscopy for ImageJ collection.
For quantitative analysis of colocalization of mucins and the ER marker GRP78/GRP94 in Agr2−/− and control mice, a blinded observer selected at least three Z-stacks from each of at least two slides per animal. Fields were selected by following conducting airways to the most proximal segments and imaging epithelium where the plane of section was approximately perpendicular to the surface of the epithelium. The Mander's colocalization ImageJ plugin (22, 23) was used to calculate the fraction of MUC5AC or MUC5B antibody fluorescence that localized to pixels stained by anti-GRP78/GRP94. Background correction and thresholds for each channel were standardized for all image stacks.
Immunoprecipitation and Immunoblotting
A549 lung carcinoma and MG63 osteosarcoma cell lines (American Type Culture Collection, Manassas, VA) were lysed in 25 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, 0.5% Tween20 2× mini-Complete protease inhibitor (Roche, Indianapolis, IN), 1 mM phenylmethylsulfonyl fluoride (Sigma, St. Louis, MO), and 25 mM N-ethyl maleimide. A Dynabead-protein G slurry (Invitrogen, Carlsbad, CA) was used to immunoprecipitate anti-AGR2 bound complexes. Agarose/acrylamide gel electrophoresis and immunoblotting methods are described in the online supplement.
Quantitative Morphometry
Measurements of intraepithelial mucus volume and numbers of mucus cells were performed on periodic acid Schiff (PAS)-stained mouse lung sections as described in the online supplement.
Mouse Allergic Airway Disease Model
Mouse experiments were approved by the UCSF Committee on Animal Research and performed in accordance with NIH guidelines. Agr2−/− mice (8) and wild-type littermate control animals were sensitized on Days 0, 7, and 14 and challenged on Days 21, 22, and 23 with ovalbumin (OVA) or saline as previously described (16). One day after the final challenge, airway reactivity, serum OVA-specific IgE, and bronchoalveolar lavage fluid leukocytes were measured as previously described (24). Epithelial brushing was performed on a separate cohort of mice as described in the online supplement.
PCR Analysis
Quantitative PCR (16, 25) and analysis of Xbp1 splicing (26, 27) were performed as previously described.
Statistical Analyses
Data are reported as mean ± SEM. Significance testing was performed by Student's t test or by ANOVA and Tukey-Kramer posttest for multiple group comparisons unless otherwise specified.
Results
AGR2 Is Localized to the ER of MUC5AC- and MUC5B-Producing Cells in Human Airways
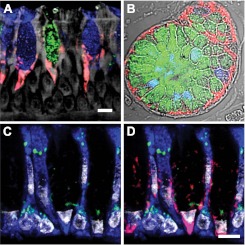
We first explored a potential AGR2 contribution to airway mucin processing by investigating AGR2 protein expression in postmortem normal human trachea. In airway epithelium, AGR2-stained mucous cells in a perinuclear pattern adjacent to apical mucin collections (Figure 1A). AGR2 was found in cells containing only MUC5AC mucin, only MUC5B mucin, and both mucins. We were unable to identify mucous cells where AGR2 staining was absent and did not find AGR2 staining in ciliated or basal cells. In submucosal glands, AGR2 staining was found in the basal aspect of mucous cells, which predominantly express MUC5B (Figure 1B). AGR2 staining was not detected in serous cells or other nonmucous cells. We explored the subcellular localization of AGR2 in mucous cells using the ER-marker GRP78/GRP94 and the Golgi-marker giantin (Figures 1C and 1D). We found that AGR2 expression in airway and submucosal gland was concentrated in the ER with little if any colocalization with the Golgi marker.
Figure 1.
AGR2 localizes to the endoplasmic reticulum (ER) of mucous cells in human airway and submucosal glands. (A) AGR2 (red) localized to cells containing MUC5AC (blue) and MUC5B (green) in airway epithelium. (B) AGR2 and mucins in submucosal glands stained as in A. (C, D) Staining with the ER marker GRP78/GRP94 (blue) and the Golgi marker giantin (green) revealed that AGR2 (red in D) is confined largely to the ER. Gray indicates autofluorescence in A, differential interference contrast in B, and nuclear (DAPI) staining in C and D. Scale bars: 5 μm.
AGR2 Associates with Immature MUC5AC in the ER of Mucous Cells
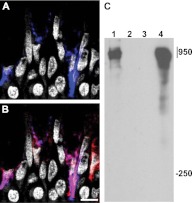
Immature mucins undergo initial processing in the ER before transiting to the Golgi, where the central repeat domains become heavily O-glycosylated (6). MUC5AC antibodies used in the prior experiments were generated against mature, glycosylated MUC5AC (28, 29). To examine whether AGR2 colocalizes with immature (non-O-glycosylated) mucins, we used the monoclonal antibody CLH2. CLH2 recognizes peptide sequences within MUC5AC central repeat domains that become inaccessible after glycosylation (30). We found that immature MUC5AC was located exclusively within AGR2-containing areas of mucus cells (Figures 2A and 2B). Some AGR2-stained regions did not contain immature MUC5AC. Because our other analyses showed that AGR2 staining was confined to the ER of MUC5AC- and MUC5B-producing cells (Figure 1), AGR2-stained regions lacking immature MUC5AC may represent the ER of cells producing MUC5B but not MUC5AC. To determine whether AGR2 physically associates with immature MUC5AC, we immunoprecipitated AGR2-containing complexes from the mucus-producing human lung cancer cell line A549 (31). Immunoblotting with CLH2 demonstrated that nonglycosylated MUC5AC precursor was physically associated with AGR2 (Figure 2C). We conclude that immature MUC5AC associates with AGR2 during its transit through the ER of mucous cells.
Figure 2.
AGR2 physically associates with MUC5AC. (A, B) Immature MUC5AC (blue in A) was restricted to AGR2 stained areas (red in B) in tracheal epithelial cells. Purple-stained areas in B represent areas of AGR2/immature MUC5AC colocalization. Gray indicates nuclear (DAPI) staining. Scale bar: 5 μm. (C) Immunoblot probed with antiimmature MUC5AC antibody. Immature MUC5AC was detected in AGR2-containing complexes immunoprecipitated from A549 cell lysates (lane 1). MUC5AC was not detected after immunoprecipitation from A549 cell lysates using nonimmune rabbit serum (lane 2) or after immunoprecipitation of AGR2 from lysates of MG63 fibroblasts, which do not produce MUC5AC (lane 3). Lane 4 contained A549 cell lysate. Samples were subjected to agarose-acrylamide gel electrophoresis under nonreducing conditions, and numbers at the right indicate positions of molecular weight markers (mass in kD). The predicted molecular mass of MUC5AC monomer based on its amino acid composition is approximately 610 kD (41). Covalent modifications, including mannosylation (42), N-glycosylation (6), dimerization, and associations with AGR2 (and possibly other ER proteins), might add to the molecular mass of the immature MUC5AC complex.
AGR2 and MUC5AC Increase Together in Airway Epithelium from “Th2-High” Individuals with Asthma
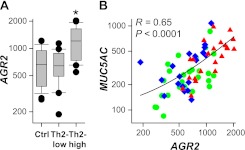
Increased MUC5AC expression and mucus production are characteristic features of asthma (1, 2, 17). Microarray analyses of airway epithelial cells from individuals with asthma and healthy control subjects revealed that MUC5AC expression was elevated in a subset of “Th2-high” individuals with asthma compared with “Th2-low” individuals with asthma or healthy control subjects (18). To investigate the effects of asthma on AGR2 expression in humans, we reanalyzed these published microarray data (Figure 3A). We found that AGR2 mRNA was increased in “Th2-high” individuals with asthma compared with control subjects without asthma and “Th2-low” individuals with asthma. In addition, we found a highly significant correlation between AGR2 and MUC5AC expression in the entire study population (Figure 3B) and within the “Th2-high” subset (R = 0.65; P = 0.0012). Similar results were obtained when we analyzed data for alternate AGR2 and MUC5AC probe sets included on these microarrays (not shown). No MUC5B probe sets were included on these arrays. However, a previous report used PCR to analyze airway epithelial cell MUC5B mRNA levels in the same subjects and found that expression of MUC5B, unlike MUC5AC, is decreased in “Th2-high” asthma (17). Our analysis indicates that AGR2 expression increases in individuals with “Th2-high” asthma and that AGR2 expression increases with MUC5AC expression.
Figure 3.
AGR2 mRNA levels increase in “Th2-high” asthma and correlate with MUC5AC mRNA levels. (A) Airway epithelial cell AGR2 mRNA levels in 28 control subjects without asthma, 20 “Th2-low” individuals with asthma, and 22 “Th2-high” individuals with asthma (18). *P ≤ 0.0001 compared with other groups by Wilcoxon rank sum test. (B) Correlation between AGR2 and MUC5AC levels in samples from control subjects (green circles), “Th2-low” individuals with asthma (blue diamonds), and “Th2-high” individuals with asthma (red triangles) (R = 0.65; P < 0.0001 using Pearson's correlation).
AGR2 and Mucous Metaplasia in a Murine Model of Allergic Asthma
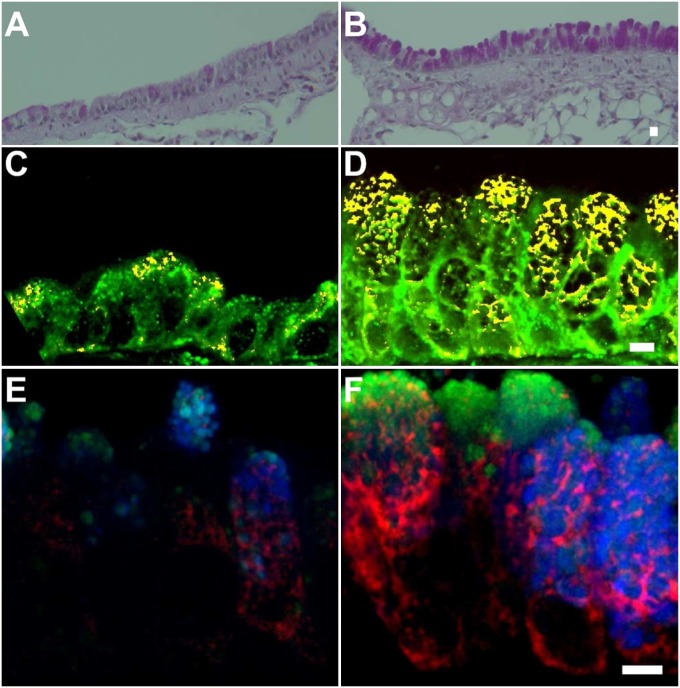
We used a standard mouse model of asthma to assess the role of AGR2 in allergen-induced mucous metaplasia. Muc5ac expression is increased in this model, as it is in “Th2-high” asthma; however, unlike “Th2-high” asthma, allergen challenge also results in an increase in Muc5b expression (24). As expected, we found that allergen sensitization and challenge resulted in an increase in mucous cells identified by PAS staining (Figures 4A and 4B). Consistent with a previous report (16), quantitative RT-PCR analysis revealed a large increase in lung Agr2 mRNA after allergen challenge (15.8-fold higher levels in allergen-challenged mice compared with saline-challenged mice; P < 0.0001). Modest AGR2 staining was seen in the ER of some epithelial cells from saline-challenged mice (Figure 4C). More intense and widespread ER AGR2 staining was seen in epithelium from allergen-challenged mice (Figure 4D). Quantitative analysis revealed a 7-fold increase in the number of AGR2-containing cells after allergen challenge (1.19 ± 0.34 versus 8.37 ± 1.06 [mean ± SEM] cells/Z stack; P < 0.001). AGR2-stained cells in saline- and allergen-challenged mice were mucous cells expressing MUC5AC and/or MUC5B (Figures 4E and 4F). AGR2 staining was prominent within areas of the ER that were closely apposed to mucin granules.
Figure 4.
Mucous metaplasia and increased AGR2 expression in mouse allergic airway disease. Periodic acid Schiff staining of saline-challenged (A) and allergen-challenged (B) mouse airways. (C, D) Immunofluorescence staining of AGR2 (red), the ER-marker GRP78/GRP94 (green), and colocalized pixels (yellow) in saline-challenged (C) and allergen-challenged (D) mice. (E, F) AGR2 (red) staining was present in cells containing MUC5AC (blue) and/or MUC5B (green) in saline-challenged (E) and allergen-challenged (F) mouse airways. Scale bars: 5 μm.
AGR2 Is Not Required for Atopic Sensitization, Airway Inflammation, or Airway Hyperreactivity
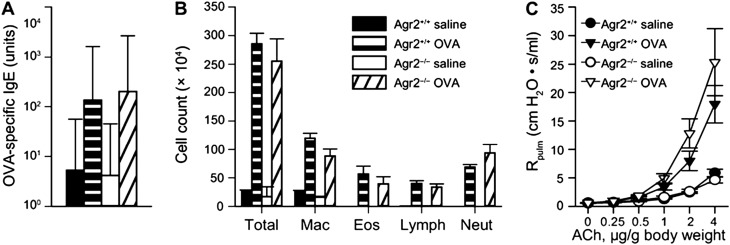
We used Agr2−/− mice and Agr2+/+ littermates (as controls) to determine whether the absence of AGR2 affected features of allergic airway disease, including atopic sensitization, airway inflammation, and airway hyperreactivity. After allergen sensitization and challenge, AGR2-deficient mice and control mice had similar increases in allergen-specific serum IgE (Figure 5A) and inflammatory cells (Figure 5B). This indicates that AGR2-deficient mice have intact atopic and inflammatory responses to allergen. Allergen sensitization and challenge also leads to exaggerated airway narrowing in response to bronchoconstrictor agents (airway hyperreactivity). We found that allergen-induced airway hyperreactivity was not impaired in AGR2-deficient mice (Figure 5C). Although mucin overproduction can cause airway obstruction, we previously showed that airway hyperreactivity is independent of airway epithelial cell mucus production in this model (24). Airway hyperreactivity in the absence of mucus overproduction may be attributable to direct effects of IL-4 and IL-13 on airway smooth muscle (32). We conclude that AGR2 is not required for atopic sensitization, inflammatory cell recruitment, or airway hyperreactivity in an acute allergic airway disease model.
Figure 5.
AGR2-deficient mice have preserved atopic, inflammatory, and airway hyperreactivity responses in an allergic model of asthma. Ovalbumin (OVA)-specific serum IgE (in arbitrary units) (A), cell counts in bronchoalveolar lavage fluid (B), and airway reactivity to acetylcholine (C) were measured in control (Agr2+/+) and AGR2-deficient (Agr2−/−) mice. OVA-sensitized and challenged mice had significant elevations in IgE, total cells, macrophages (Mac), lymphocytes (Lymph), neutrophils (Neut), and pulmonary resistance (Rpulm) at acetylcholine (ACh) doses of 1 μg/g body weight or higher compared with saline-treated control mice (P < 0.05). There were no significant differences in IgE, inflammatory cells, or airway resistance between Agr2−/− mice and Agr2+/+ control mice.
Allergen-Induced Mucus Production Is Attenuated in AGR2-Deficient Mice
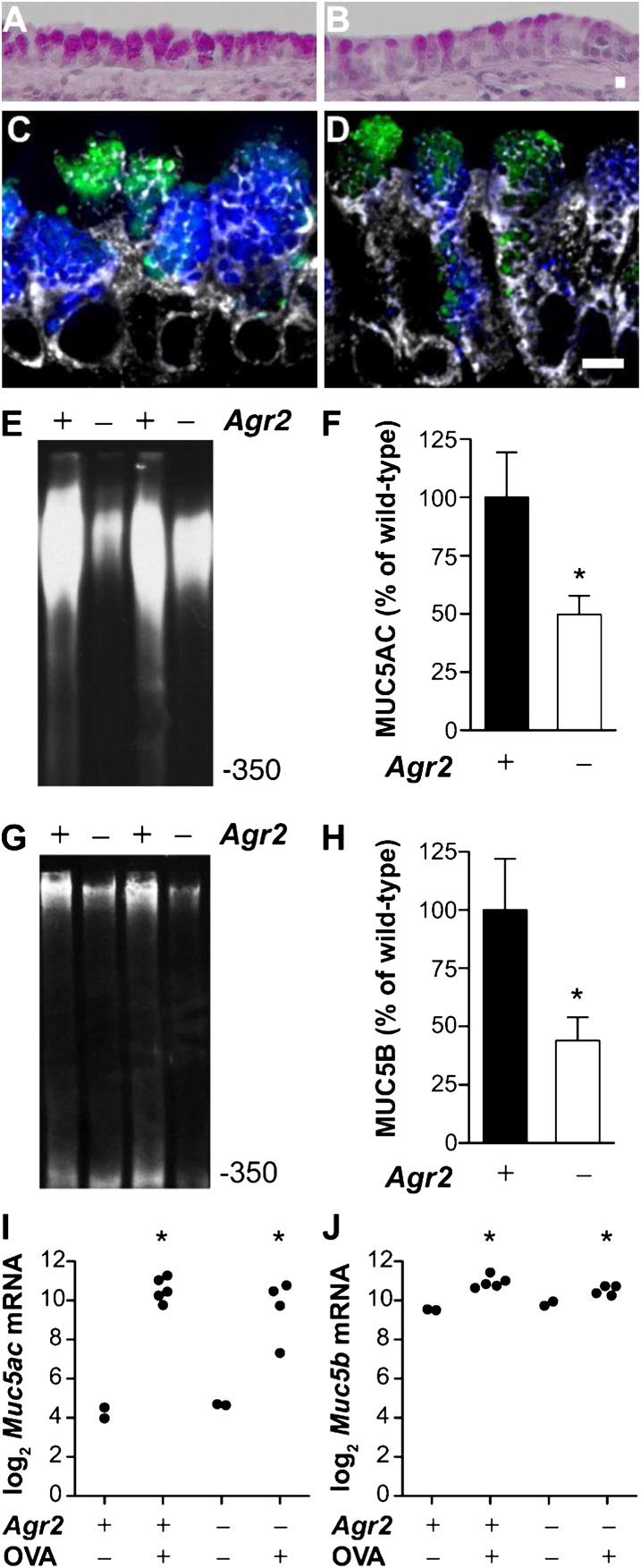
We determined the effect of AGR2 deficiency on allergen-induced mucous metaplasia using PAS-stained sections (Figures 6A and 6B). Quantitative analysis of these sections revealed very similar numbers of mucous cells in airway epithelium from wild-type and AGR2-deficient mice (3.92 ± 0.40 versus 3.93 ± 0.67 [mean ± SEM] mucus cells per high-power field). However, the volume of stored mucus per mucous cell was reduced by 42% in AGR2-deficient mice (305.4 ± 39.1 μm3 versus 179.5 ± 29.3 μm3; P = 0.001). These data indicate that the absence of AGR2 decreases the volume of mucus per mucous cell but does not result in loss of mucous cells.
Figure 6.
Mucin is reduced in airway epithelium from allergen-challenged Agr2−/− mice. Periodic acid Schiff staining of airways from allergen-sensitized and -challenged wild-type (A) and Agr2−/− (B) mice. Immunofluorescence staining of airways from allergen-sensitized and -challenged wild-type (C) and Agr2−/− (D) mice with antibodies against MUC5AC (blue) and MUC5B (green) and the ER-marker GRP78/GRP94 (gray). Scale bar: 5 μm. Representative immunoblots and densitometry for MUC5AC (E, F) and MUC5B (G, H) protein from allergen-challenged wild-type control (+) and Agr2−/− (−) mice. Mucin signal (mean ± SEM for ≥ 5 mice per group) is represented as a percentage of the mean signal from allergen-challenged wild-type mice. *P < 0.05 versus allergen-challenged wild-type mice. (I, J) qRT-PCR was used to measure Muc5ac (A) and Muc5b (B) mRNA in epithelial brushings from wild-type and AGR2-deficient mice after saline (OVA−) or allergen (OVA+) challenge. Each point represents data from one mouse. *P < 0.05 versus wild-type saline-challenged mice. Mucin transcript levels in allergen-challenged Agr2−/− and control groups were not significantly different.
Immunofluorescent staining of MUC5AC and MUC5B suggested that allergen-challenged AGR2-deficient mice produced less of both of these mucins than wild-type mice (Figures 6C and 6D). To quantify the effect of Agr2 deletion on MUC5AC and MUC5B levels in the lungs, we analyzed immunoblots of lung lysates by densitometry. Compared with allergen-challenged wild-type control animals, allergen-challenged AGR2-deficient mice had a 50.3% reduction in MUC5AC (Figures 6E and 6F) and a 56.1% reduction in MUC5B (Figures 6G and 6H). AGR2 deficiency had no detectable effect on the relatively low levels of MUC5B found in nonallergic lungs. Relative to MUC5B levels in allergen-challenged wild-type mice (defined as 100%), MUC5B levels were 6 ± 1% in saline-challenged wild-type mice and 5 ± 2% in saline-challenged AGR2-deficient mice (P = 0.6). MUC5AC levels in saline-challenged mice were too low for accurate quantification. We conclude that MUC5AC and MUC5B production is attenuated but not abolished in allergen-sensitized and challenged AGR2-deficient mice.
To address whether loss of Agr2 impairs mucin mRNA transcription, we measured Muc5ac and Muc5b transcript levels in airway brushings by qRT-PCR (Figures 6I and 6J). Consistent with previous reports (16), we found a significant increase in Muc5ac and Muc5b expression in control animals after allergen sensitization. There was no significant difference in Muc5ac/Muc5b expression between Agr2−/− and control mice after saline or allergen challenge. This suggests that AGR2 deficiency causes a posttranscriptional defect in mucin production.
The Lack of AGR2 Alters the Intracellular Localization of Mucins and Increases ER Stress
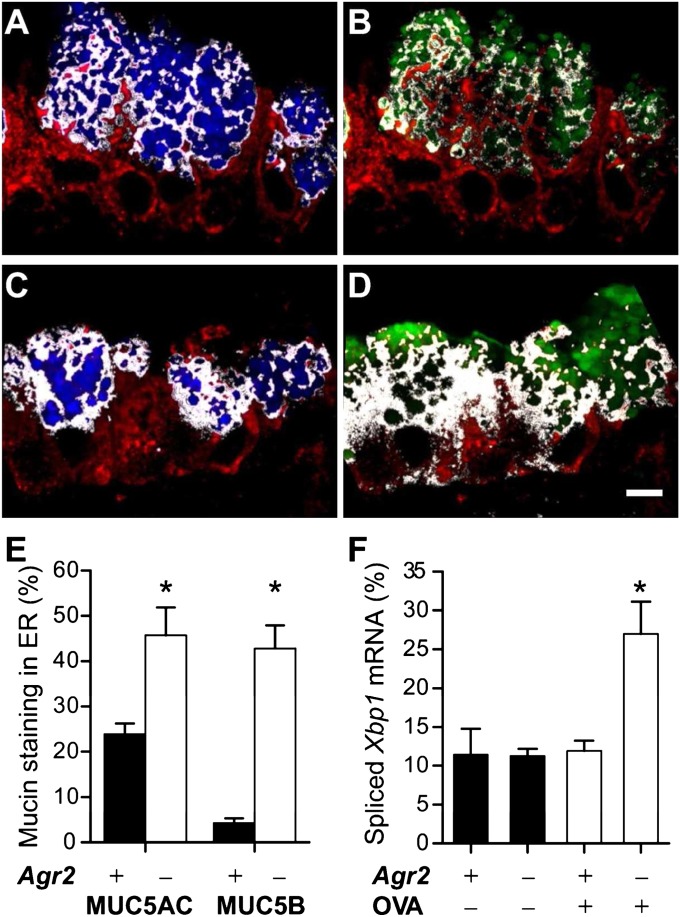
We hypothesized that compromised mucin processing in AGR2-deficient mucous cells relates to impaired mucin transit through the ER and predicted that a lack of AGR2 would lead to activation of the unfolded protein response, a conserved response to disturbances within the ER (33). To test this hypothesis, we first measured the fractions of intracellular mucins present within the ER of mucous cells from allergen-sensitized and challenged wild-type and Agr2−/− mice (Figures 7A–7E). In wild-type mice, the large majority of MUC5AC and MUC5B staining was outside of the ER, consistent with the idea that most mucins had exited the ER and were located in the Golgi or in secretory granules (Figures 7A, 7B, and 7E). As predicted by our hypothesis, the fractions of MUC5AC and MUC5B found within the ER were markedly increased in allergen-challenged Agr2−/− mice (Figures 7C–7E).
Figure 7.
Redistribution of mucins and induction of the unfolded protein response in airways of allergen-challenged AGR2-deficient mice. (A–D) ER localization of mucins in mucous cells from allergen-challenged mice. Airway epithelium from allergen-challenged wild-type control (A, B) and Agr2−/− (C, D) mice was stained for GRP78/GRP94 (A–D, red), MUC5AC (A, C, blue), and MUC5B (B, D, green). White indicates regions staining for both GRP/78/GRP94 (ER) and mucin. Scale bar: 5 μm. (E) The percentage of cellular mucin staining within the ER was determined by quantitative analysis of GRP78/GRP94 and mucin-stained images from OVA-challenged wild-type mice (closed bars, mean ± SEM for 18 total microscopic fields from six mice) and AGR2-deficient mice (open bars, 17 fields from six mice). *P < 0.05 versus wild-type mice (for the same mucin). (F) Xbp1 mRNA splicing (spliced Xbp1 mRNA amount as a percentage of total Xbp1 mRNA) was determined by PCR analysis of samples harvested by epithelial brushing from wild-type (Agr2+) and AGR2-deficient (Agr2–) mice challenged with saline (OVA–) or ovalbumin (OVA+). *P < 0.05 versus other groups.
Disturbances in the ER lead to a stress response, the unfolded protein response, which involves induction of genes that enhance ER protein folding capacity and promote degradation of unfolded proteins (34). An important ER stress signal is mediated by inositol-requiring enzyme 1 (Ire1)-dependent splicing of Xbp1 mRNA, leading to production of the XBP-1 transcription factor. We found low levels of spliced Xbp1 in airway epithelial samples from saline- and allergen-challenged wild-type mice and in saline-challenged Agr2−/− mice but significantly higher levels of spliced Xbp1 in allergen-challenged Agr2−/− mice (Figure 7F). We conclude that allergen challenge induces an unfolded protein response in airway epithelium from AGR2-deficient mice.
Discussion
Airway mucins are important contributors to normal host defense and to airway diseases, but the processes involved in the assembly of these large and unusual proteins are not well understood. Our studies indicate that the specialized protein disulfide isomerase family member AGR2 is important for mucin processing in airways. We found that AGR2 is expressed in the ER of MUC5AC- and MUC5B-producing mucus cells in the airway epithelium and submucosal glands. Coimmunoprecipitation studies demonstrated a physical association between AGR2 and MUC5AC proteins, suggesting that AGR2 has a direct role in MUC5AC processing. Analysis of airway epithelial gene expression data showed that AGR2 expression correlates with mucin (MUC5AC) expression and that “Th2-high” individuals with asthma have higher levels of AGR2 and MUC5AC expression than “Th2-low” individuals with asthma or healthy control subjects. Finally, we found that AGR2-deficient mice had impaired export of mucins from the ER and activation of the unfolded protein response and produced less airway mucin than control mice in a model of “Th2-high” asthma. These results indicate that AGR2 plays an important role in mucin production in allergic airway disease.
Despite important differences between the major airway mucins MUC5AC and MUC5B and the major intestinal mucin MUC2, AGR2 has a role in the production of all of these mucins. Although these mucins have a common ancestor, they have evolved different expression patterns and functional capabilities (35). For example, MUC5AC is not normally present in the intestine but is induced during intestinal nematode infections, is toxic to worms, and is required for nematode expulsion; although there is abundant MUC2 in the intestine, MUC2 is not toxic to worms and does not lead to nematode expulsion (13). AGR2 is present in MUC5AC-only, MUC5B-only, and dual MUC5AC/MUC5B-producing mucous cells in the airway epithelium and submucosal glands (Figure 1) and in MUC2-producing cells in the intestine (8, 36). Mice deficient in AGR2 have impaired production of MUC5AC and MUC5B (Figure 6) and MUC2 (8). Because AGR2 plays roles in cells producing each of these mucins but is generally not present in non–mucus-producing cells, it seems likely that AGR2 evolved to serve unique processing requirements shared by the polymeric gel-forming mucins.
AGR2 associated with mucins in the ER and loss of AGR2 impaired mucin production in allergen-challenged mouse airways. The availability of an antibody that recognizes immature (non-O-glycosylated) MUC5AC allowed us to show that AGR2 and immature MUC5AC form a complex in the ER of mucous cells. The precise molecular function of AGR2 remains to be defined. A few widely expressed members of the PDI family have been shown to have enzymatic activity in in vitro isomerization assays (37), but the biochemical functions of most PDI family members have not been determined. However, our studies of AGR2-deficient mice show that AGR2 is required for normal ER mucin processing after allergen challenge. The proportion of mucins localized to the ER was substantially higher in mucous cells from allergen-challenged, AGR2-deficient mice compared with wild-type control animals. This could be accounted for by delayed export of mucins from the ER to the Golgi or by increased degradation of mucins via the ER-associated protein degradation pathway. Allergen-challenged AGR2-deficient mice also had increased Xbp-1 splicing, indicating that attempts to produce large amounts of airway mucin in the absence of AGR2 trigger an unfolded protein response. Similar results have been reported in intestine of mice lacking AGR2 (8, 36) or carrying Muc2 mutations that result in mucin misfolding (38). Although the unfolded protein response can in some cases trigger apoptosis, we saw very few apoptotic epithelial cells in wild-type or AGR2-deficient mice by terminal deoxynucleotidyl transferase dUTP nick-end labeling (not shown), and AGR2 deficiency did not reduce the number of mucous cells in the epithelium. MUC5AC and MUC5B production was reduced but not eliminated in AGR2-deficient mice, suggesting that other ER molecules can partially compensate for the loss of AGR2 in airway mucous cells. In fact, in nonallergic mice, AGR2 deficiency had no detectable effect MUC5B levels or on ER stress, suggesting that other molecules are sufficient for basal mucin processing in the mouse airway. However, our results demonstrate that AGR2 induction during mucous metaplasia enables ER processing of large amounts of airway mucins without the development of ER stress.
Mucus overproduction is a prominent feature of asthma and other airway diseases. Mucus contributes to airway occlusion in the large majority of patients with fatal asthma (1). Mucus abnormalities are not limited to individuals with severe asthma because epithelial mucus stores were increased 3-fold even in subjects with stable mild or moderate asthma (2). Our analysis of AGR2 and MUC5AC gene expression in patients with asthma (Figure 3) implicates AGR2 in mucus overproduction in this disease. There is intense interest in developing therapeutic strategies to reduce airway mucins (39, 40), but our understanding of the mechanisms involved in the production of these enormous glycoproteins remains incomplete. This study adds to our knowledge of specialized molecules that participate in this important process by identifying AGR2 as a PDI family member that contributes to mucin overproduction in allergic airway inflammation.
Supplementary Material
Acknowledgments
The authors thank Sara Goetz, Xin Bernstein, Xin Ren, and Katherine Huang for technical assistance; Dan Han, Feroz Papa, Kamran Atabai, and Dean Sheppard for providing advice; Alice Thwin, Kurt Thorn, and the UCSF Nikon Imaging Center and Prescott Woodruff for assistance with microscopy and image analysis; and Walter Finkbeiner, John Fahy, Prescott Woodruff, and the UCSF Airway Tissue Bank for assistance with human specimens.
Footnotes
This work was supported by National Institutes of Health grants HL085089 and HL099101 (D.J.E.), T32 HL007185 (B.W.S.), and F32 HL107019 (B.W.S.); by the UCSF Sandler Asthma Basic Research (SABRE) Program; and National Natural Science Foundation of China Grant 81170022 (G.Z.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2011-0421OC on March 8, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kuyper LM, Pare PD, Hogg JC, Lambert RK, Ionescu D, Woods R, Bai TR. Characterization of airway plugging in fatal asthma. Am J Med 2003;115:6–11 [DOI] [PubMed] [Google Scholar]
- 2.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001;163:517–523 [DOI] [PubMed] [Google Scholar]
- 3.Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med 2006;38:116–125 [DOI] [PubMed] [Google Scholar]
- 4.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278 [DOI] [PubMed] [Google Scholar]
- 5.Asker N, Axelsson MA, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem 1998;273:18857–18863 [DOI] [PubMed] [Google Scholar]
- 6.Asker N, Axelsson MA, Olofsson SO, Hansson GC. Human MUC5AC mucin dimerizes in the rough endoplasmic reticulum, similarly to the MUC2 mucin. Biochem J 1998;335:381–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheehan JK, Kirkham S, Howard M, Woodman P, Kutay S, Brazeau C, Buckley J, Thornton DJ. Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J Biol Chem 2004;279:15698–15705 [DOI] [PubMed] [Google Scholar]
- 8.Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, Killeen N, Erle DJ. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci USA 2009;106:6950–6955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruddock LW, Freedman RB, Klappa P. Specificity in substrate binding by protein folding catalysts: tyrosine and tryptophan residues are the recognition motifs for the binding of peptides to the pancreas-specific protein disulfide isomerase PDIp. Protein Sci 2000;9:758–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komiya T, Tanigawa Y, Hirohashi S. Cloning of the gene gob-4, which is expressed in intestinal goblet cells in mice. Biochim Biophys Acta 1999;1444:434–438 [DOI] [PubMed] [Google Scholar]
- 11.Laboissiere MC, Sturley SL, Raines RT. The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J Biol Chem 1995;270:28006–28009 [DOI] [PubMed] [Google Scholar]
- 12.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 2008;70:459–486 [DOI] [PubMed] [Google Scholar]
- 13.Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK, et al. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med 2011;208:893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol 2011;9:265–278 [DOI] [PubMed] [Google Scholar]
- 15.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002;8:885–889 [DOI] [PubMed] [Google Scholar]
- 16.Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM, Fahy JV, Erle DJ. Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol 2005;116:305–311 [DOI] [PubMed] [Google Scholar]
- 17.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009;180:388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA 2007;104:15858–15863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med 2011;365:1088–1098 [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 2009;119:2914–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Li Q, Kolosov VP, Perelman JM, Zhou X. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun Adhes 2010;17:83–92 [DOI] [PubMed] [Google Scholar]
- 22.Manders EMM, Verbeek FJ, Aten JA. Measurement of co-localization of objects in dual-colour confocal images. J Microsc 1993;169:375–382 [DOI] [PubMed] [Google Scholar]
- 23.Zinchuk V, Zinchuk O, Okada T. Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochem Cytochem 2007;40:101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuperman DA, Huang X, Nguyenvu L, Holscher C, Brombacher F, Erle DJ. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. J Immunol 2005;175:3746–3752 [DOI] [PubMed] [Google Scholar]
- 25.Park SW, Verhaeghe C, Nguyenvu LT, Barbeau R, Eisley CJ, Nakagami Y, Huang X, Woodruff PG, Fahy JV, Erle DJ. Distinct roles of FOXA2 and FOXA3 in allergic airway disease and asthma. Am J Respir Crit Care Med 2009;180:603–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mrna. Nature 2002;415:92–96 [DOI] [PubMed] [Google Scholar]
- 27.Upton JP, Austgen K, Nishino M, Coakley KM, Hagen A, Han D, Papa FR, Oakes SA. Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol Cell Biol 2008;28:3943–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bara J, Gautier R, Daher N, Zaghouani H, Decaens C. Monoclonal antibodies against oncofetal mucin M1 antigens associated with precancerous colonic mucosae. Cancer Res 1986;46:3983–3989 [PubMed] [Google Scholar]
- 29.Bara J, Gautier R, Mouradian P, Decaens C, Daher N. Oncofetal mucin m1 epitope family: characterization and expression during colonic carcinogenesis. Int J Cancer 1991;47:304–310 [DOI] [PubMed] [Google Scholar]
- 30.Ho JJ, Crawley S, Pan PL, Farrelly ER, Kim YS. Secretion of MUC5AC mucin from pancreatic cancer cells in response to forskolin and VIP. Biochem Biophys Res Commun 2002;294:680–686 [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Nickola TJ, DiFronzo NL, Colberg-Poley AM, Rose MC. Dexamethasone-mediated repression of MUC5AC gene expression in human lung epithelial cells. Am J Respir Cell Mol Biol 2006;34:338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins C, Yanase N, Smulian G, Gildea L, Orekov T, Potter C, Brombacher F, Aronow B, Wills-Karp M, Finkelman FD. Selective stimulation of IL-4 receptor on smooth muscle induces airway hyperresponsiveness in mice. J Exp Med 2011;208:853–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008;134:743–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 2005;115:2656–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desseyn JL, Buisine MP, Porchet N, Aubert JP, Degand P, Laine A. Evolutionary history of the 11p15 human mucin gene family. J Mol Evol 1998;46:102–106 [DOI] [PubMed] [Google Scholar]
- 36.Zhao F, Edwards R, Dizon D, Afrasiabi K, Mastroianni JR, Geyfman M, Ouellette AJ, Andersen B, Lipkin SM. Disruption of paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev Biol 2010;338:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep 2005;6:28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 2008;5:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol 2004;4:241–250 [DOI] [PubMed] [Google Scholar]
- 40.Curran DR, Cohn L. Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am J Respir Cell Mol Biol 2010;42:268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Bovenkamp JH, Hau CM, Strous GJ, Buller HA, Dekker J, Einerhand AW. Molecular cloning of human gastric mucin MUC5AC reveals conserved cysteine-rich D-domains and a putative leucine zipper motif. Biochem Biophys Res Commun 1998;245:853–859 [DOI] [PubMed] [Google Scholar]
- 42.Perez-Vilar J, Randell SH, Boucher RC. C-mannosylation of MUC5AC and MUC5B Cys subdomains. Glycobiology 2004;14:325–337 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.