Abstract
The delivery of cystic fibrosis transmembrane conductance regulator (CFTR) to airway epithelia is a goal of many gene therapy strategies to treat cystic fibrosis. Because the native regulatory elements of the CFTR are not well characterized, the development of vectors with heterologous promoters of varying strengths and specificity would aid in our selection of optimal reagents for the appropriate expression of the vector-delivered CFTR gene. Here we contrasted the performance of several novel gene-regulatory elements. Based on airway expression analysis, we selected putative regulatory elements from BPIFA1 and WDR65 to investigate. In addition, we selected a human CFTR promoter region (∼ 2 kb upstream of the human CFTR transcription start site) to study. Using feline immunodeficiency virus vectors containing the candidate elements driving firefly luciferase, we transduced murine nasal epithelia in vivo. Luciferase expression persisted for 30 weeks, which was the duration of the experiment. Furthermore, when the nasal epithelium was ablated using the detergent polidocanol, the mice showed a transient loss of luciferase expression that returned 2 weeks after administration, suggesting that our vectors transduced a progenitor cell population. Importantly, the hWDR65 element drove sufficient CFTR expression to correct the anion transport defect in CFTR-null epithelia. These results will guide the development of optimal vectors for sufficient, sustained CFTR expression in airway epithelia.
Keywords: gene transfer, lung, cystic fibrosis
Clinical Relevance
Our results indicate that the novel endogenous genetic element from the human WDR65 gene may provide a suitable alternative to the stronger viral promoters used in current gene-transfer vectors. Furthermore, our findings suggest that this promoter is active in progenitor cell populations in the airway. This promoter has the potential to mediate long-term cystic fibrosis transmembrane conductance regulator expression in the airway, an important step toward treating cystic fibrosis.
Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene cause cystic fibrosis, a multiorgan disease that affects the sinuses, lung, sweat glands, intestines, liver, pancreas, and reproductive tracts (1–3). However, most of the morbidity and mortality associated with the disease results from chronic bacterial infections and inflammation in the lung. Developing gene transfer vectors for efficient, safe, and effective therapeutic expression in the appropriate cell types of the airways is an important consideration in the treatment of cystic fibrosis.
CFTR mRNA is present in low copy numbers (1–2 copies per cell) in nasal, tracheal, and bronchial epithelia (4, 5). Previous work in Cftr-null murine models showed that achieving as little as 5% of normal CFTR concentrations resulted in a much larger correction of the chloride transport defect (50% of normal), suggesting that modest levels of transgene expression may confer a significant therapeutic benefit (6). Conversely, CFTR overexpression in airway epithelia may cause protein mislocalization to the basal surface and an overall net decrease in chloride current (7). The entire suite of regulatory elements for the CFTR locus is unknown, but several regions have been identified that span several hundred kilobases (8–12), a size too large to package efficiently in standard viral vectors. Thus, a need exists for vectors with appropriate transgene expression levels in the airway.
To that end, we sought to develop vectors carrying transgenes driven by novel promoters from endogenous genes expressed in the airway. Transcript profiling in human airway epithelia indicated that a member of the bactericidal/permeability-inducing fold containing family A (BPIFA1, also known as PLUNC) is highly expressed in human airway epithelia (13). The in situ hybridization of murine nasal sections revealed that the expression of tryptophan, aspartic acid repeat protein 65 (Wdr65) is restricted to the respiratory epithelium (14). Based on the expression profile of BPIFA1 and Wdr65, we selected putative gene-regulatory elements from these genes to investigate. In addition, we selected an approximately 2-kb human CFTR promoter region to study, which presumably encompasses the basic cis-regulatory elements.
In addition to achieving appropriate levels of transgene expression in the airway, expression in progenitor cell types is important for long-term therapeutic potential. Previously, we reported stable luciferase expression in human airway epithelia in vitro and in murine nasal airways in vivo for over a year after the administration of feline immunodeficiency virus (FIV) vector (15–17). This result may indicate that progenitor cells were transduced. Expression in these studies was driven by the ubiquitous and relatively strong Rous sarcoma virus (RSV) promoter. Here we show that firefly luciferase reporter expression from the candidate gene-regulatory elements human (h) BPIFA1 and hWDR65 also persists long term. In subsequent experiments, we used a murine model carrying a floxed termination codon directly upstream of the initiation codon (AUG) of luciferase expressed from the endogenous Rosa26 promoter (18). We delivered FIV-expressing Cre-recombinase under the control of our candidate promoter elements to these mice. After intranasal administration of the detergent polidocanol, expression was ablated in the nasal airways. By 2 weeks after polidocanol administration, luciferase expression in the airways of these animals returned to pretreatment levels, consistent with the activity of our candidate elements in airway progenitor populations. Moreover, an hWDR65-driven CFTR transgene corrected the chloride current defect in CFTR-null airway epithelia.
Materials and Methods
Primary Epithelial Cultures and Electrophysiology Studies
Tracheal epithelial cells from CFTR-null pigs were isolated by enzymatic digestion, seeded onto permeable filters, and grown at an air–liquid interface, as previously described (19). Adenoviral vectors expressing porcine CFTR from either the RSV or hWDR65 promoter elements were administered basolaterally to CFTR-null porcine tracheal epithelia cultures. Three days after transduction, cultures were studied in modified Ussing chambers, as previously described (20). Briefly, epithelia were bathed on both surfaces with solution containing 135 mM NaCl, 2.4 mM K2HPO4, 0.6 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgCl2, 10 mM dextrose, and 5 mM Hepes (pH 7.4) at 37°C, and gassed with compressed air. Baseline transepithelial currents were measured. After an apical addition of 100 μM amiloride (Amil) and 100 μM 4,4′-diisothiocyanoto-stilbene-2,2′-disulfonic acid, currents were allowed to stabilize, and the apical solution was replaced with a 4.8-mM Cl− solution containing 135 mM d-gluconic Acid, 2.4 mM K2HPO4, 0.6 mM KH2PO4, 1.2 mM CaCl2, 1.2 mM MgCl2, 10 mM dextrose, and 5 mM Hepes (pH, 7.4) at 37°C, and gassed with compressed air. Cyclic adenosine monophosphate (cAMP)–dependent chloride current was stimulated by the apical addition of 10 μM forskolin and 100 μM 3-isobutyl-1-methylxanthine (IBMX), and CFTR-specific chloride transport was inhibited with 100 μM GlyH-101 (a generous gift from Cystic Fibrosis Foundation Therapeutics and R. Bridges) was measured. Transepithelial voltage (Vt) was maintained at 0 mV to measure the short-circuit current (I). Transepithelial electrical conductance was measured by intermittently clamping Vt to +5 and/or −5 mV. Spontaneous values of Vt were measured by transiently removing the voltage clamp.
Murine Studies
All mice for this study were housed at the Animal Care Facilities of the University of Iowa. All animal procedures were previously approved by the Institutional Animal Care and Use Committee of the University of Iowa, in accordance with National Institutes of Health guidelines. The FVB.129S6(B6)-Gt(ROSA)26Sortm1(Luc)Kael/J strain (stock number 005125; Jackson Laboratory, Bar Harbor ME) carries a firefly luciferase cDNA, preceded by a LoxP-stop-LoxP cassette, at the ubiquitously expressed ROSA26 locus (18). This strain received FIV vectors expressing our selected promoter-driven Cre. Balb/c mice (strain code 01B05; Charles River Laboratories, Wilmington, MA) received FIV expressing firefly luciferase. Six to 8-week-old mice were transduced intranasally with FIV or adenovirus (Ad) vector formulated 1:1 with methylcellulose (50 μl total volume), as previously described (21). Animals were imaged at various time points, using the In Vivo Imaging Systems (IVIS) system (Caliper Life Sciences, Hopkinton, MA). Two hundred microliters of luciferin substrate (15 mg/ml; Caliper Life Sciences) were administered intraperitoneally, and the mice were imaged for 5 minutes. Data were analyzed using Living Image software (Caliper Life Sciences). The negative controls in all in vivo bioluminescent assays were naive Balb/c mice. The typical background level of bioluminescence in a naive mouse is approximately 104 photons/second/cm2. We included negative controls in every assay, and report only the background-subtracted levels of experimental groups.
Results
Promoters and Putative Upstream Regulatory Regions
To identify novel gene-regulatory regions for airway gene transfer applications, we selected upstream regions of genes expressed in epithelia as well as from viral vectors with airway tropism. The 3′ long terminal repeat (LTR) of the Jaagsietke sheep retrovirus (JSRV) was chosen because the virus bears a tropism for alveolar Type II pneumocytes and Clara cells (22). The minimal promoter region of the murine Clara cell secretory protein (mCC10) drives expression in Clara cells of the airway epithelia (23). We chose the upstream sequences of two novel endogenous genes (human BPIFA1 and human WDR65), based on expression analysis. BPIFA1 mRNA is highly expressed in human airway epithelia, as shown by transcript profiling using a microarray (13). In situ hybridization showed that Wdr65 expression was restricted to the respiratory epithelia in murine nasal passages (14). However, the upstream regions of these genes are not characterized.
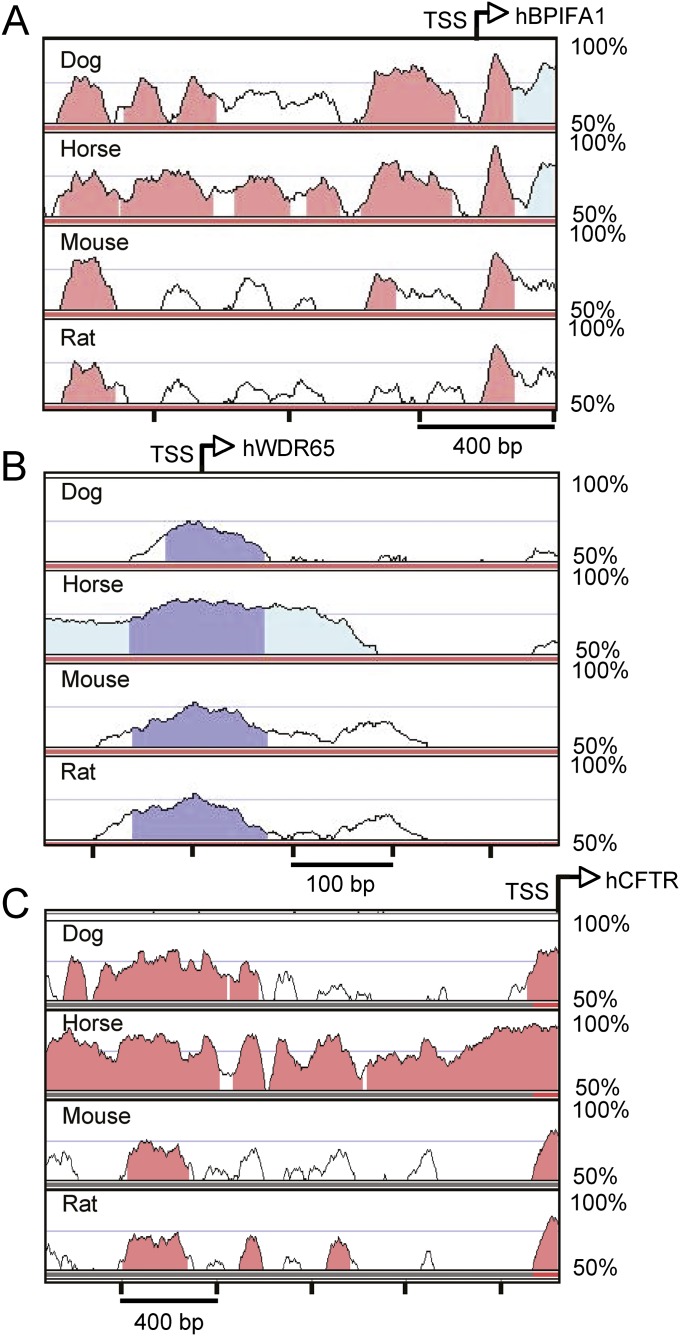
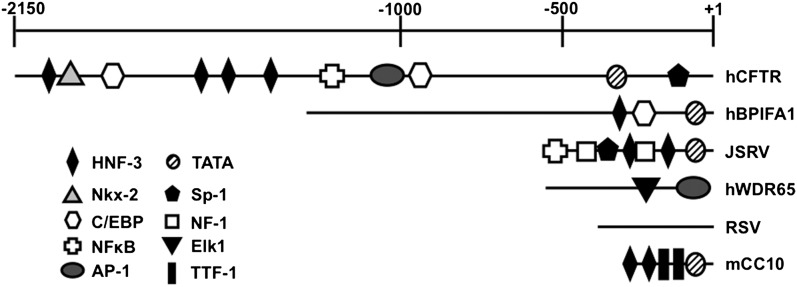
To identify elements to clone and test, we aligned upstream sequences from humans, mice, rats, dogs, and horses, using the University of California at Santa Cruz (UCSC) program for visualizing global DNA sequence alignments of arbitrary length (VISTA) browser (www.ucsc.org). We selected approximately 1 kb and 500 base pairs (bp) of the sequence upstream from BPIFA1 and WDR65, respectively, based on multispecies homology (Figures 1A and 1B, respectively). We also tested approximately 2,150 bp of the sequence upstream from the human CFTR gene (Figure 1C). As we previously demonstrated (24), the 5′ LTR from the RSV drives strong, persistent expression from viral vectors in the airway epithelia, and thus serves as a control for comparisons. The analysis of predicted transcription factor–binding sites in the candidate promoter element sequences, using TFSearch (www.cbrc.jp/research/db/TFSEARCH.html), revealed several predicted motifs important in gene regulation in the airways, such as hepatocyte nuclear factor–3 (HNF-3) (22), CAAT/enhancer binding protein (25), and thyroid transcription factor–1 (22, 25, 26) (Figure 2).
Figure 1.
Visualizing global DNA sequence alignments of arbitrary length (VISTA) plot shows the genomic loci of novel genetic elements. Genomic loci from the human bacterialcidal/permeability-inducing protein family A 1 (BPIFA1) (A), tryptophan and aspartic acid (WD) repeat protein 65 (WDR65) (B), and cystic fibrosis transmembrane conductance regulator (CFTR) (C) genes were aligned to the homologous loci in dogs, horses, mice, and rats, using the VISTA Genome Browser (www.ucsc.org). The human locus is shown at the top of each plot, and the other vertebrate genomes are shown with their relative conservation (70% identity over 100 bp) to the human locus below, in separate tracks. The lower threshold of each track represents 50% conservation. Dark blue represents exons, pink represents the conserved nucleotide sequence, and light blue represents untranslated regions. The displayed region represents the portion of the sequence cloned for analysis in the airway (BPIFA1, chromosome 20:31286263–7563; WDR65, chromosome 1:43410454–962; CFTR, chromosome 7:116905142–7292 on the University of California Santa Cruz human genome 19 assembly). TSS, transcription start site.
Figure 2.
Transcription factor binding sites in novel genetic elements. Selected genetic element sequences were analyzed for predicted transcription factor binding sites (TFBs), using TFSearch (http://www.cbrc.jp/research/db/TFSEARCH.html). TFBs were selected using the default threshold score of 85.0. Only TFBs sharing 85% identity with the TFMATRIX transcription factor binding site database were predicted (31). TFBs corresponding to transcription factors relevant in the airway are indicated. C/EBP, CAAT/enhancer binding protein; mCC10, Clara cell secretory protein; h, human; RSV, Rous sarcoma virus; HNF-3, hepatocyte nuclear factor 3; Nkx-2, NK2 transcription factor–related locus 2; AP-1, activator protein 1; TATA, TATA box; Sp-1, specificity protein 1; NF-1, neurofibromatosis type 1; Elk1, E twenty-six (ETS)-like transcription factor 1; TTf, thyroid transcription factor 1; JSRV, Jaagsietke sheep retrovirus.
In Vitro Candidate Gene-Regulatory Element Activity in Human Airway Cells
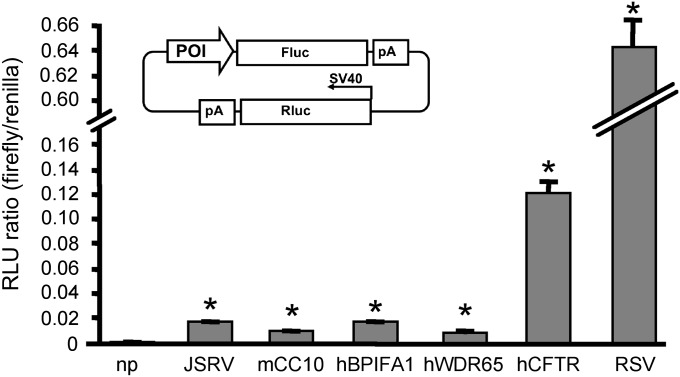
To verify the activity of the novel elements used in this study, we cloned promoter and/or upstream sequences of RSV, Jaagsietke sheep retrovirus (JSRV), mCC10, hPLUNC, hWDR65, and hCFTR upstream from a firefly luciferase reporter gene (please refer to the online supplement's Materials and Methods). Renilla luciferase expression under the control of the SV40 promoter served as an internal control in each plasmid (Figure 3, inset). We transiently transfected the adenocarcinoma cell line A549 with the indicated reporter constructs, and collected cell lysates 48 hours later. Luciferase assays determined the relative activity from each element. Each gene-regulatory element drove significantly higher reporter expression in airway cells than did he control plasmid lacking a promoter (Figure 3). Functional promoter activity was also replicated in the airway epithelial lung adenocarcinoma cell line, Calu-3. However, the transfection efficiency and resulting luciferase expression were substantially lower than in A549 cells (data not shown).
Figure 3.
In vitro activity in an airway cell line. A549 cells were transfected with the dual luciferase plasmid (inset). Candidate promoter activity (firefly luciferase activity) was normalized to the internal renilla luciferase control activity. POI, promoter of interest; Fluc, firefly luciferase; Rluc, Renilla luciferase; SV40, spleen necrosis virus 40; RLU, relative light units; np, no promoter; pA, polyadenylation signal. Bars represent mean RLU ratios. Error bars represent standard errors (n = 3). *P < 0.05, as determined by Student t test.
In Vivo Activity and Persistence in Murine Airways
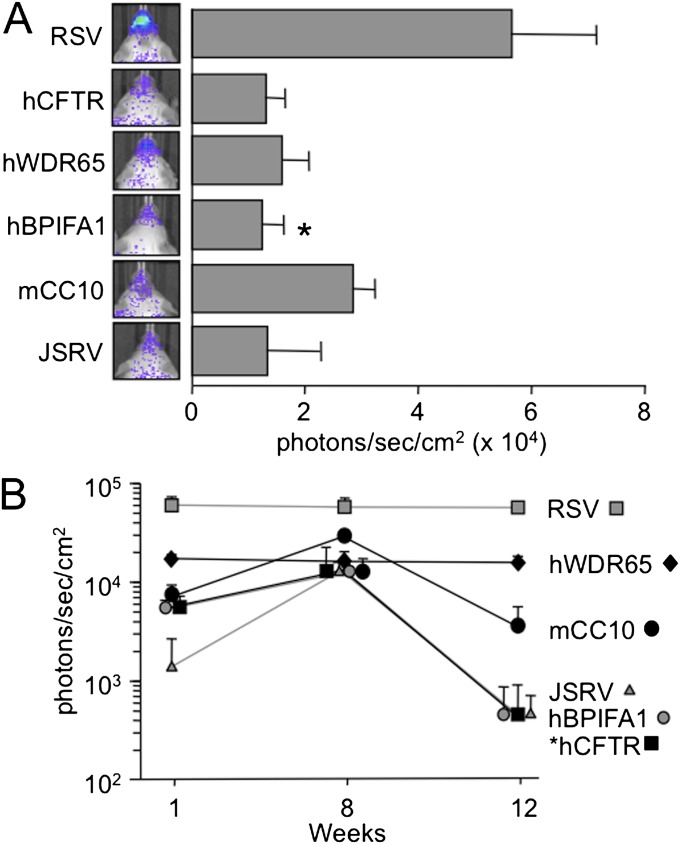
To determine the expression levels and persistence of each of the promoters in vivo, we cloned the promoter/upstream sequences upstream of the reporter firefly luciferase in a GP64-pseudotyped FIV lentiviral vector (Figure 4). We formulated 2.5 × 105 transducing units (TU) of each vector with methylcellulose (1:1) (21), and delivered it intranasally once a day for 5 consecutive days. Expression was monitored noninvasively, using the Xenogen IVIS200 bioluminescent imaging system (Caliper Life Sciences, Hopkinton, MA). Expression directed by the RSV promoter remained at a relatively high level (∼ 6 × 104 photons/second/cm2) for 3 months (i.e., the duration of the study). Expression from the hWDR65 genetic element persisted for 3 months at a lower level (∼ 2 × 104 photons/second/cm2) than the RSV. Expression from the other genetic element vectors was reduced to background levels (≤ 104 photons/second/cm2) by the end of the study (Figure 4B). We furthered these studies by cloning each candidate promoter element upstream from an alternate reporter gene, β-galactosidase, in a lentiviral FIV vector. We were unable to detect β-galactosidase activity in murine nasal sections after intranasal delivery, as previously described in our experiments using FIV vectors expressing luciferase (data not shown). The luciferase data suggest that RSV and hWDR65 can direct persistent expression in the nasal airways. However, because of the variable gene activity driven by each of the candidate promoters, it is unclear whether the lack of persistence resulted from the limits of our ability to detect reporter activity.
Figure 4.
In vivo expression from novel genetic elements persists for 3 months. GP64-pseudotyped feline immunodeficiency virus (FIV) vector expressing firefly luciferase under the control of the candidate promoters and formulated with 1% methylcellulose was delivered to mice intranasally. Mice were imaged at indicated time points, and we measured photons/second/cm2 as described in Materials and Methods. (A) Bars represent expression levels at 8 weeks. The RSV promoter drove significantly higher expression than the hBPIFA1 genetic element (P = 0.04, according to Student t test). (B) Expression was monitored over time. Expression from the hCFTR genetic element was significantly lower at the conclusion of the experiment, compared with expression at 1 week (P = 0.04, according to Student t test). Error bars represent standard errors (n = 3–4 animals per group).
Progenitor Populations Transduced
To address concerns regarding our limits of detection, we used a murine model in which luciferase is expressed from the endogenous Rosa26 promoter. This mouse carries a floxed termination codon directly upstream from the AUG initiation codon (18). The JSRV, mCC10, hBPIFA1, hCFTR, or hWDR65 candidate promoter elements driving Cre recombinase were packaged in a GP64-pseudotyped FIV vector. About 2.5 × 105 TU of each vector (maximum dose) were formulated with 1% methylcellulose (21) and delivered intranasally once a day for 3 days. Cre recombinase delivered to murine nasal epithelia excises the termination codon, which results in luciferase expression. This model allowed us to normalize candidate promoter strength. In addition, this model facilitated our ability to determine whether our candidate promoter elements were active in progenitor cell populations. To this end, we transiently ablated the surface epithelium with detergent. Polidocanol (26) was administered intranasally at 24 weeks after transduction. Bioluminescent expression was monitored over a 3-week period after polidocanol administration.
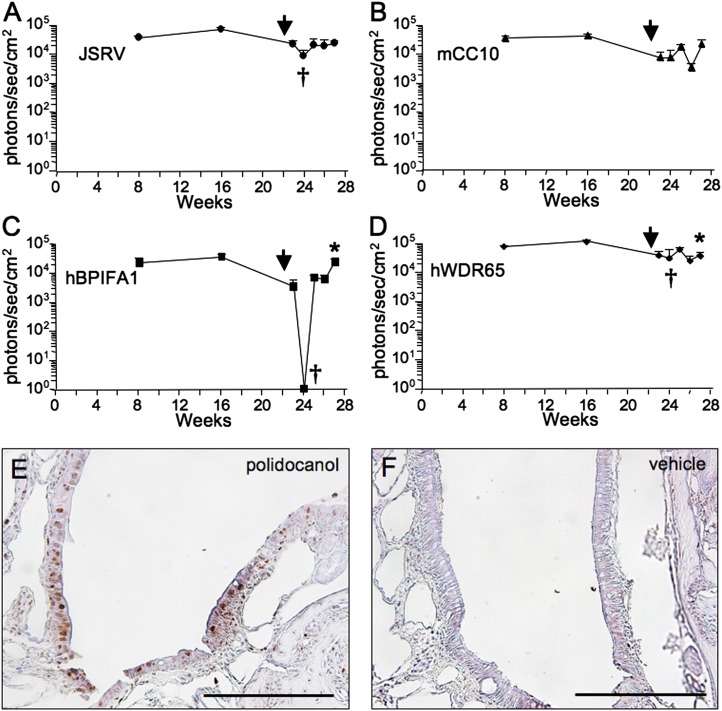
Luciferase expression in mice receiving the JSRV–Cre vector was stable for 23 weeks. After polidocanol administration, expression decreased to 47% of pretreatment levels (Figure 5A). Expression returned to pretreatment levels by 2 weeks in these mice. Expression at 1 day after polidocanol administration was significantly decreased in these mice, and did not significantly increase by Day 21 after treatment. Expression in the mice treated with the mCC10–Cre vector was reduced to 14% of pretreatment levels by 2 weeks after polidocanol administration, and returned to pretreatment levels by 21 days after treatment (Figure 5B). Expression in the mice receiving CFTR–Cre was not significantly different from that in naive control mice (data not shown). Expression in mice receiving the hBPIFA1–Cre vector was significantly reduced to 0.04% after 1 day. However, expression recovered to pretreatment levels after 14 days (Figure 5C). The increase in expression to pretreatment levels was significant (P < 0.05). Luciferase expression in mice receiving the hWDR65–Cre vector decreased to 30% of pretreatment levels (P < 0.05), and returned to approximately 59% of pretreatment levels (P < 0.05) by 3 weeks (Figure 5D). Although the expression in these animals did not completely disappear after polidocanol treatment, the decrease in luciferase expression was significant for each promoter except mCC10. These data suggest that the candidate promoter elements driving Cre are integrated in progenitor cell populations.
Figure 5.
Progenitor cell populations were transduced in murine nasal epithelia. GP64-pseudotyped FIV vector expressing Cre recombinase under the control of the JSRV (A), mCC10 (B), hBPIFA1 (C), and hWDR65 (D) candidate promoter elements and formulated with 1% methylcellulose was delivered to FVB.129S6(B6)-Gt(ROSA)26Sortm1(Luc)Kael/J mice intranasally. Animals were imaged, and luciferase activity was measured at various time points over 30 weeks. Luciferase activity reflects naive-subtracted values at each time point. At 24 weeks after administration, 2% polidocanol was delivered intranasally (arrow), and animals were imaged at various time points. Error bars represent standard errors (n = 3 animals per group). †P < 0.05, relative to values before polidocanol treatment. *P < 0.05, relative to values 1 day after polidocanol treatment, as determined by one-tailed t test. Bromodeoxyuridine (BrdU) staining of nasal respiratory epithelia of mice that received polidocanol (E) or vehicle (F) was undertaken, as described in the online supplement's Materials and Methods. Sections were counterstained with hematoxylin and eosin. Scale bar = 100 μm.
To examine proliferation after epithelial ablation, sections of nasal airways from animals receiving an intranasal delivery of polidocanol were stained for bromodeoxyuridine (BrdU) to indicate dividing cells (please see the online supplement's Materials and Methods). In contrast to animals receiving vehicle only (Figure 5F), abundant BrdU signal was detected in the nasal epithelia of animals receiving the detergent (Figure 5E). In addition, epithelia were less well differentiated in polidocanol-treated mice, as evidenced by an absence of ciliated cells. The rapid drop and recovery of expression after detergent delivery was consistent with the rapid stripping and regeneration of surface epithelia.
CFTR Expression from the hWDR65 Genetic Element Corrects the Chloride Current Defect in CFTR-Null Airway Epithelia
Based on our in vivo luciferase expression experiments, we selected the hBPIFA1 and hWDR65 candidate promoters as candidates for CFTR gene addition studies. To demonstrate the expression of an alternative transgene in human cells, we transduced A549 cells with Ad5-based vectors expressing β-galactosidase from each of the hBPIFA1 and hWDR65 candidate gene-regulatory elements. These vectors achieved β-galactosidase expression in a dose-dependent manner (Figure 6A). These results indicate that the candidate elements tested can direct expression in an airway cell line from multiple vector platforms.
Figure 6.
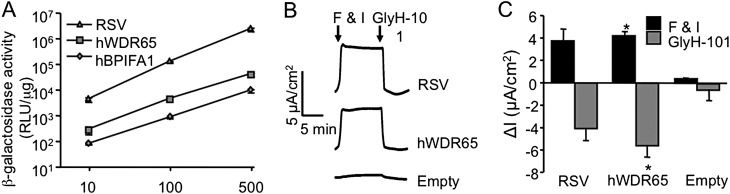
hWDR65-driven CFTR corrects chloride current defect in CFTR-null tracheal epithelia. (A) A549 cells were transduced with adenovirus vectors expressing β-galactosidase from each of RSV, hBPIFA1, and hWDR65 candidate promoter elements at multiplicities of infection [MOIs] of 10, 100, and 500. Two days after transduction, β-galactosidase activity was measured via GalactoLight assay (Applied Biosystems, Bedford, MA; see Materials and Methods). (B) Representative chloride current tracings from each of RSV, hWDR65, and empty control measurements. (C) Well-differentiated tracheal epithelia cultures from CFTR-null piglets were transduced basolaterally with adenovirus carrying porcine CFTR (pCFTR) driven by the hWDR65 genetic element (MOI = 500) or the RSV promoter (MOI = 50). Adenovirus vector without pCFTR was used as a control (empty; MOI = 50). Three days after transduction, transepithelial Cl− currents were measured in Ussing chambers (see Materials and Methods). Bars represent means of change in chloride current upon the addition of forskolin (10 μM) and IBMX (100 μM) (black; F & I) or GlyH-101 (gray). Error bars represent SEMs. n = 3. *P < 0.05, according to t test. The asterisk represents significant change (P < 0.05 according to t test) relative to empty control cultures.
Murine models of cystic fibrosis (CF) do not spontaneously develop lung disease, as seen in human patients, possibly because of the presence of alternative chloride channels in the airways. Thus, we chose a porcine model system to investigate corrections of the anion transport defect. CFTR-null pigs recapitulate the disease progression characteristic of CF in humans (27). To determine if the anion secretion defect in CFTR-null airway epithelial cells could be rescued with wild-type (WT) CFTR driven by the novel genetic elements, well-differentiated primary tracheal epithelia from CFTR-null pigs, cultured at an air–liquid interface (19), were transduced with an adenoviral vector expressing porcine CFTR (pCFTR) under the control of the hWDR65 or hBPIFA-1 genetic elements (multiplicity of infection [MOI] = 500). An Ad vector expressing pCFTR driven by the RSV promoter served as a positive control. As a negative control, an Ad vector without a transgene (empty) (MOI = 50 each) was delivered to cultured airway epithelia. Three days after transduction, chloride secretion was measured in modified Ussing chambers, as previously reported (20). Upon the addition of forskolin and IBMX, cAMP-stimulated chloride secretion was observed in cultures treated with hWDR65-pCFTR (Figure 6B). No chloride secretion was evident in cultures treated with hBPIFA1-pCFTR (data not shown). The chloride current (I) increased approximately 4 μA/cm2 in these cultures. This increase in cAMP-stimulated chloride secretion was significant, compared with the empty control–treated cultures (Figure 6C; P < 0.05, according to Student t test). This observation indicates that the hWDR65 genetic element can drive sufficient expression to achieve functional correction in airway epithelia.
Discussion
Here we demonstrate that several novel, putative promoters have activity in the airway. We show that the 5′ genetic regions from the human BPIFA1 and WDR65 genes direct persistent expression in the airway. The expression from these promoters was approximately 30% that of the control RSV promoter (2 × 104 photons/second/cm2 versus 6 × 104 photons/second/cm2, respectively). Expression from the upstream region of CFTR was also similar to that of the hBPIFA1 and hWDR65 promoters (2 × 104 photons/second/cm2). The relative in vivo expression levels from the novel promoters correlate with our in vitro experiments. We note that the values of Renilla expression varied among dual promoter constructs from 2–40-fold. This range likely resulted from variability in the transfection efficiency, plasmid quality, and potential promoter read-through that could occur when both expression cassettes are delivered on a single plasmid. In the absence of Renilla normalization, the relative firefly luciferase activities were similar. Our results using 2,150 bases upstream of CFTR are supported by previous work using a luciferase reporter construct including 2,244 bases upstream from the transcription start site. Yoshimura and colleagues reported that this sequence supported expression in airway cells at less than 5% of that from the RSV promoter (5). Lower expression of the transgene may be desirable when attempting to correct the anion transport defect in CFTR-deficient cells, because the overexpression of CFTR in airway epithelia leads to mislocalization to the basolateral surface, thereby decreasing the net chloride conductance (7).
One of the goals of gene therapy for cystic fibrosis involves the long-term, efficient correction of the anion transport defect. Thus, developing vectors with persistent transgene expression is an important step toward achieving this goal. When we analyzed luciferase expression in animals receiving vectors carrying luciferase driven by the novel promoters hBPIFA1 and hWDR65, expression remained stable over the course of the experiment (3 months), indicating that the promoter activity persists. However, expression from the hCFTR gene element significantly decreased at the conclusion of the study (5.5 × 103 photons/second/cm2 versus 4.8 × 102 photons/second/cm2, respectively). The transcriptional regulation of CFTR remains under investigation, and studies indicate that the regulatory elements are spread over several kilobases (10–12). The 2,150 base fragment we used in these studies may not have contained the necessary regulatory elements to confer sustained expression, or else human-specific regulatory mechanisms were missing in our murine models. The novel hWDR65 promoter may offer a suitable alternative because it drives expression at a level similar to that of CFTR, and it also persists in airway epithelia.
When the detergent polidocanol was administered intranasally, bioluminescent imaging indicated that expression transiently decreased in the nasal passages of mice transduced with vectors carrying JSRV-driven, hBPIFA1-driven, and hWDR65-driven transgenes. Two weeks after detergent administration, significant expression was again detected only in the animals receiving vectors carrying hBPIFA1-driven and hWDR65-driven transgenes. The polidocanol treatment of nasal airways causes epithelial cell disruption and ablation, whereas the basal cell layer and basement membrane remain intact (28). This is followed by epithelial regeneration within 7 days, mediated by progenitor cells (29). Luciferase expression decreased after polidocanol administration. We can only speculate about the reasons why polidocanol treatment exerted a much more dramatic effect in hBPIFA1–Cre–treated mice, compared with hWDR65–Cre–treated mice. Based on previous evidence using FIV-expressing RSV–β-galactosidase (15), hBPIFA1–Cre has likely integrated into a broad range of airway epithelial cells, including ciliated and nonciliated surface cells and basal cells. Human BPIFA-1 may only be active in terminally differentiated surface epithelial cells, which are more susceptible to removal by polidocanol. Perhaps hWDR65–Cre is active in a broader range of epithelial cells, including surface and basal epithelia. In this case, polidocanol would not ablate all luciferase-expressing cells. The return of luciferase expression in mice treated with the detergent supports the conclusion that a progenitor cell population was transduced. The transduced cell population may include basal cells, because they are a source of multipotent stem cells (30) in the airways of both humans and mice. Stable expression from a progenitor cell population would lessen the need for repeated administration. However, the overexpression of CFTR in progenitor cells may be detrimental to progenitor cell function. This is an important question to consider for future studies.
When we delivered an adenoviral vector carrying porcine CFTR under the control of the novel genetic element from the hWDR65 gene to CFTR-null airway epithelia, we observed a rescue of the anion transport defect. The cAMP-stimulated increase was specific to CFTR expression, because the addition of the CFTR inhibitor GlyH-100 decreased the current to levels measured before forskolin/IBMX treatment. This level of chloride current is approximately 10% of that measured in WT epithelia (20). However, our results are similar to those observed by Zabner and colleagues (30), using the lower-expressing Elongation Factor 1 alpha (EF1a) promoter to drive CFTR expression in airway epithelia. EF1α-driven CFTR sufficiently and persistently corrected the chloride secretion defect in human CF airway epithelia (31). Because CFTR overexpression in the airway results in a net decrease in anion transport (7), lower therapeutic transgene expression from endogenous gene internal promoters may be desirable when investigating strategies to treat CF.
Taken together, our results indicate that the novel endogenous genetic element from the hWDR65 gene may provide a suitable alternative to the stronger viral promoters used in current gene transfer vectors. Furthermore, our findings suggest this novel promoter is active in progenitor cell populations in the airway. This promoter has the potential to mediate long-term CFTR expression in the airway, an important step toward treating CF.
Supplementary Material
Acknowledgments
The authors thank Dr. Barry Stripp for providing the mCC10 promoter sequence. The authors also thank John Fisher, Sarah Ernst, and Phil Karp for technical assistance. The authors acknowledge the assistance of the DNA Sequencing, Central Microscopy, and Gene Transfer Vector Core Facilities at the University of Iowa.
Footnotes
This work was supported by National Institutes of Health grants P01 HL-51670 (P.B.M. and P.L.S.), P01 HL-091842 (P.B.M.), and HL-007638 (E.R.B.), by the Children's Miracle Network (P.L.S.), and by the Roy J. Carver Charitable Trust. The authors also acknowledge the support of the In Vitro Models and Cell Culture Core, the Gene Transfer Vector Core, and the Cell Morphology Core, partly supported by the Center for Gene Therapy for Cystic Fibrosis (through National Institutes of Health grant P30 DK-54759) and the Cystic Fibrosis Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0444OC on March 23, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, Buchwald M, Tsui LC. Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073–1080 [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066–1073 [DOI] [PubMed] [Google Scholar]
- 3.Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, Rozmahel R, Cole JL, Kennedy D, Hidaka N, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 1989;245:1059–1065 [DOI] [PubMed] [Google Scholar]
- 4.Trapnell BC, Chu CS, Paakko PK, Banks TC, Yoshimura K, Ferrans VJ, Chernick MS, Crystal RG. Expression of the cystic fibrosis transmembrane conductance regulator gene in the respiratory tract of normal individuals and individuals with cystic fibrosis. Proc Natl Acad Sci USA 1991;88:6565–6569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshimura K, Nakamura H, Trapnell BC, Dalemans W, Pavirani A, Lecocq JP, Crystal RG. The cystic fibrosis gene has a “housekeeping”-type promoter and is expressed at low levels in cells of epithelial origin. J Biochem 1991;266:9140–9144 [PubMed] [Google Scholar]
- 6.Dorin JR, Farley R, Webb S, Smith SN, Farini E, Delaney SJ, Wainwright BJ, Alton EW, Porteous DJ. A demonstration using mouse models that successful gene therapy for cystic fibrosis requires only partial gene correction. Gene Ther 1996;3:797–801 [PubMed] [Google Scholar]
- 7.Farmen SL, Karp PH, Ng P, Palmer DJ, Koehler DR, Hu J, Beaudet AL, Zabner J, Welsh MJ. Gene transfer of CFTR to airway epithelia: low levels of expression are sufficient to correct Cl− transport and overexpression can generate basolateral CFTR. Am J Physiol Lung Cell Mol Physiol 2005;289:L1123–L1130 [DOI] [PubMed] [Google Scholar]
- 8.Blackledge NP, Carter EJ, Evans JR, Lawson V, Rowntree RK, Harris A. CTCF mediates insulator function at the CFTR locus. Biochem J 2007;408:267–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackledge NP, Ott CJ, Gillen AE, Harris A. An insulator element 3′ to the CFTR gene binds CTCF and reveals an active chromatin hub in primary cells. Nucleic Acids Res 2009;37:1086–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ott CJ, Blackledge NP, Kerschner JL, Leir SH, Crawford GE, Cotton CU, Harris A. Intronic enhancers coordinate epithelial-specific looping of the active CFTR locus. Proc Natl Acad Sci USA 2009;106:19934–19939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ott CJ, Blackledge NP, Leir SH, Harris A. Novel regulatory mechanisms for the CFTR gene. Biochem Soc Trans 2009;37:843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ott CJ, Suszko M, Blackledge NP, Wright JE, Crawford GE, Harris A. A complex intronic enhancer regulates expression of the CFTR gene by direct interaction with the promoter. J Cell Mol Med 2009;13:680–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bingle CD, Bingle L. Characterisation of the human PLUNC gene, a gene product with an upper airways and nasopharyngeal restricted expression pattern. Biochim Biophys Acta 2000;1493:363–367 [DOI] [PubMed] [Google Scholar]
- 14.Rorick NK, Kinoshita A, Weirather JL, Peyrard-Janvid M, de Lima RL, Dunnwald M, Shanske AL, Moretti-Ferreira D, Koillinen H, Kere J, et al. Genomic strategy identifies a missense mutation in WD-repeat domain 65 (WDR65) in an individual with Van der Woude syndrome. Am J Med Genet 2011;155A:1314–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinn PL, Burnight ER, Hickey MA, Blissard GW, McCray PB., Jr Persistent gene expression in mouse nasal epithelia following feline immunodeficiency virus–based vector gene transfer. J Virol 2005;79:12818–12827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang G, Slepushkin V, Zabner J, Keshavjee S, Johnston JC, Sauter SL, Jolly DJ, Dubensky TW, Jr, Davidson BL, McCray PB., Jr Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J Clin Invest 1999;104:R55–R62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinn PL, Anthony RM, McCray PB., Jr Genetic therapies for cystic fibrosis lung disease. Hum Mol Genet 2011;20:R79–R86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safran M, Kim WY, Kung AL, Horner JW, DePinho RA, Kaelin WG., Jr Mouse reporter strain for noninvasive bioluminescent imaging of cells that have undergone Cre-mediated recombination. Mol Imaging 2003;2:297–302 [DOI] [PubMed] [Google Scholar]
- 19.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia: methods for establishing primary cultures. Methods Mol Biol 2002;188:115–137 [DOI] [PubMed] [Google Scholar]
- 20.Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 2010;143:911–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinn PL, Shah AJ, Donovan MD, McCray PB., Jr Viscoelastic gel formulations enhance airway epithelial gene transfer with viral vectors. Am J Respir Cell Mol Biol 2005;32:404–410 [DOI] [PubMed] [Google Scholar]
- 22.Palmarini M, Datta S, Omid R, Murgia C, Fan H. The long terminal repeat of Jaagsiekte sheep retrovirus is preferentially active in differentiated epithelial cells of the lungs. J Virol 2000;74:5776–5787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Margraf LR, Finegold MJ, Stanley LA, Major A, Hawkins HK, DeMayo FJ. Cloning and tissue-specific expression of the cDNA for the mouse Clara cell 10 kD protein: comparison of endogenous expression to rabbit uteroglobin promoter-driven transgene expression. Am J Respir Cell Mol Biol 1993;9:231–238 [DOI] [PubMed] [Google Scholar]
- 24.Sinn PL, Penisten AK, Burnight ER, Hickey MA, Williams G, McCoy DM, Mallampalli RK, McCray PB. Gene transfer to respiratory epithelia with lentivirus pseudotyped with Jaagsiekte sheep retrovirus envelope glycoprotein. Hum Gene Ther 2005;16:479–488 [DOI] [PubMed] [Google Scholar]
- 25.Cassel TN, Nord M. C/EBP transcription factors in the lung epithelium. Am J Physiol Lung Cell Mol Physiol 2003;285:L773–L781 [DOI] [PubMed] [Google Scholar]
- 26.Ray MK, Chen CY, Schwartz RJ, DeMayo FJ. Transcriptional regulation of a mouse Clara cell–specific protein (MCC10) gene by the NKX transcription factor family members thyroid transciption factor 1 and cardiac muscle–specific homeobox protein (CSX). Mol Cell Biol 1996;16:2056–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons DW, Grubb BR, Johnson LG, Boucher RC. Enhanced in vivo airway gene transfer via transient modification of host barrier properties with a surface-active agent. Hum Gene Ther 1998;9:2661–2672 [DOI] [PubMed] [Google Scholar]
- 28.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science 2008;321:1837–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 2009;106:12771–12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zabner J, Wadsworth SC, Smith AE, Welsh MJ. Adenovirus-mediated generation of cAMP-stimulated Cl− transport in cystic fibrosis airway epithelia in vitro: effect of promoter and administration method. Gene Ther 1996;3:458–465 [PubMed] [Google Scholar]
- 31.Heinemeyer T, Wingender E, Reuter I, Hermjakob H, Kel AE, Kel OV, Ignatieva EV, Ananko EA, Podkolodnaya OA, Kolpakov FA, et al. Databases on transcriptional regulation: Transfac, TRRD and Compel. Nucleic Acids Res 1998;26:362–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.