Abstract
Proteoglycans (PGs) and their associated glycosaminoglycan side chains are effectors of inflammation, but little is known about changes to the composition of PGs in response to lung infection or injury. The goals of this study were to identify changes to heparan sulfate PGs in a mouse model of gram-negative pneumonia, to identify the Toll-like receptor adaptor molecules responsible for these changes, and to determine the role of the heparan sulfate PG in the innate immune response in the lungs. We treated mice with intratracheal LPS, a component of the cell wall of gram-negative bacteria, to model gram-negative pneumonia. Mice treated with intratracheal LPS had a rapid and selective increase in syndecan-4 mRNA that was regulated through MyD88-dependent mechanisms, whereas expression of several other PGs was not affected. To determine the role of syndecan-4 in the inflammatory response, we exposed mice deficient in syndecan-4 to LPS and found a significant increase in neutrophil numbers and amounts of CXC-chemokines and total protein in bronchoalveolar lavage fluid. In studies performed in vitro, macrophages and epithelial cells treated with LPS had increased expression of syndecan-4. Studies performed using BEAS-2B cells showed that pretreatment with heparin and syndecan-4 decreased the expression of CXCL8 mRNA in response to LPS and TNF-α. These findings indicate that the early inflammatory response to LPS involves marked up-regulation of syndecan-4, which functions to limit the extent of pulmonary inflammation and lung injury.
Keywords: innate immunity, macrophages, neutrophils, proteoglycan, syndecan
Lung infections place a much higher burden on public health than better recognized diseases such as HIV/AIDS, cancer, heart attacks, and stroke (1). The innate immune system is the body's first line of defense against lung infection (2, 3). LPS, a component of the cell wall of gram-negative bacteria, is used to study lung infection and injury caused by gram-negative bacteria because it results in a potent and reproducible activation of the innate immune system (4, 5). Recognition of LPS by the innate immune system occurs via the Toll-like receptor (TLR)4/MD-2/CD14 receptor complex (2, 6). Upon binding LPS, TLR4 signals through two adaptor molecules, MyD88 and TRIF, which are required for the pulmonary inflammatory response and clearance of gram-negative bacteria from lungs (7, 8). An essential step in the clearance of most gram-negative bacteria from lungs is the pulmonary recruitment of neutrophils, whose influx is mediated by CXC chemokines, such as CXCL8 in humans and macrophage inflammatory peptide (MIP)-2 and KC in rodents (9–11). Although neutrophils are an important component of pulmonary host defenses, in excess numbers they contribute to tissue damage, leading to lung injury (12–14).
Once thought to be the molecular glue that provides structural support and imparts biomechanical properties to lung tissue, it is now recognized that proteoglycans (PGs) are important biological modifiers that regulate a number of processes such as tissue inflammation, lung development, homeostasis, and wound healing (15–17). Growing evidence supports important roles for PGs in the innate immune response to lung infection (17). Heparan sulfate (HS) is the most abundant glycosaminoglycan (GAG) in healthy lungs, and HS proteoglycans (HSPGs) play a key role in modulating tissue inflammation (16, 18). The binding of the CXC chemokines CXCL8, KC, and MIP-2 to GAGs such as HS in lungs provides fine-tune control of chemokine gradient formation and neutrophil migration (19, 20). The binding of chemokines and cytokines to GAGs protects them from proteolysis, thereby increasing their stability, which is thought to promote the inflammatory response in patients with cystic fibrosis (21–23). In addition, the shedding of syndecan-1, a cell-surface HSPG, plays an important role in regulating neutrophil influx into lungs in animal models (24, 25).
Changes in the composition of GAGs and PGs in lungs have been reported in animal models and human lung disease (26–30). After the treatment of lungs with LPS, the composition of GAGs changes from the healthy lung where HS predominates to inflamed lungs where chondroitin sulfate is the predominant GAG (26, 27). The changes that occur to HSPGs during the early stages of gram-negative pneumonia are not known. Therefore, identifying these changes and characterizing how they alter the inflammatory response could lead to a better understanding of the mechanisms controlling the innate immune response and the development of lung injury in patients with gram-negative pneumonia.
The results provide evidence that expression of syndecan-4, a cell surface HSPG, was rapidly and selectively increased in response to LPS via MyD88-dependent signaling pathways. Experiments performed with syndecan-4–null (Sdc4–/–) mice showed that the lack of this HSPG resulted in a significant increase in pulmonary inflammation and injury after treatment with LPS. Finally, studies performed in vitro showed that macrophages and epithelial cells increase the expression of syndecan-4 in response to treatment with LPS and that pretreatment with heparin and syndecan-4 decreased the expression of CXCL8 mRNA in a human epithelial cell line treated with LPS and TNF-α. These findings suggest that syndecan-4 has a critical role in the early inflammatory response in lungs by limiting tissue inflammation and injury.
Materials and Methods
Reagents
The reagents used in this study were Escherichia coli serotype 0111:B4 LPS (List Biological Laboratories, Campell, CA or Sigma-Aldrich, St. Louis, MO), TaqMan primer-probes for quantitative PCR (Applied Biosystems, Foster City, CA), human TNF-α (Sigma-Aldrich), BEAS-2B cells (ATCC, Manassas, VA), human syndecan-4 (R&D, Minneapolis, MN), and low-molecular-weight heparin (Fragmin Pfizer, Tokyo, Japan).
Animal Protocols
The Animal Research Committees of the Veterans Affairs Puget Sound Health Care System and the Fukushima Medical University approved all animal experiments. C57BL/6 and C57BL/6J-Ticam1Lps2/J (TRIF mutant) mice (Jackson Laboratories, Bar Harbor, ME), MyD88 knockout (MyD88−/−) (Dr. S. Akira at University of Osaka), and Sdc4−/− mice (Dr. T. Kojima) were housed in specific pathogen-free facilities. The intratracheal injection of LPS (1 μg/g), animal death at specified times, bronchoalveolar lavage (BAL), total and differential cell counts, ELISA for murine KC and MIP-2 (R&D) and measurement of total protein (Thermo Scientific, Rockford, IL) were performed as described (20, 31, 32).
Isolation of RNA
RNA was isolated with Absolute RNA Miniprep Kit (Stratagene, La Jolla, CA). Genomic DNA was digested with DNase I (Ambion, Austin, TX), and RNA was reverse transcribed with a High Capacity cDNA Archive Kit (Applied Biosystems).
Measurement of mRNA
Quantitative PCR was performed using TaqMan primer probes or Power SYBR Green PCR master mix and an ABI PRISM 7000 (Applied Biosystems) (32). The threshold cycle (Ct) was calculated using threshold cycles for the target genes and 18-S. Relative mRNA expression was expressed as fold increase over values obtained from RNA from normal lungs, untreated cells, or human reference total RNA (Stratagene).
Cell Culture
Bone marrow–derived macrophages (BMDMs) were cultured in macrophage medium (RPMI 1640, 10% FBS, 30% L929 cell supernatant, 2 mM l-glutamine, 100 IU/mL penicillin, and 100 μg/ml streptomycin) as described (20). After being cultured in macrophage medium for 6 days, BMDMs were isolated, counted, and cultured in macrophage media for 24 hours and then stimulated with LPS (10 or 100 ng/ml), IL-4/IL-13 (10 ng/ml), IL-10 (10 ng/ml), or RPMI 1640 for up to 48 hours. Alveolar macrophages isolated with repeated BAL using PBS were cultured for 24 hours in macrophage media and then stimulated with LPS for 4 hours. BEAS-2B cells were cultured in RPMI 1640 supplemented with 10% BSA, penicillin, and streptomycin for 5 to 6 days until they reached 90% confluence. The culture medium was then replaced, and low-molecular-weight heparin, syndecan-4, or RPMI 1640 was added for 1 hour. Cells were washed with PBS and incubated with LPS (1,000 ng/ml), TNF-α (1 ng/ml), or RPMI 1640 without heparin or syndecan-4 for 3 hours. Mouse tracheal air–liquid interface cultures were performed as described and stimulated with LPS or RPMI 1640 for 4 and 24 hours (33). Supernatants were removed and RNA was harvested.
Flow Cytometric Analysis
BMDMs were detached, resuspended, incubated with Fc block (BD Pharmingen, San Diego, CA), and centrifuged at 1,500 rpm for 5 minutes at 4°C. The supernatant was removed, and BMDMs were resuspended in 100 μl binding buffer (PBS and 1% BSA) containing phycoerythrin-conjugated syndecan-4 antibody. After 1 hour, BMDMs were washed twice and analyzed using the Guava System (Millipore, Billerica, MA).
Statistical Analysis
Comparisons between multiple groups were performed using one-way ANOVA with Bonferroni's multiple comparison test or the Kruskal-Wallis test and Dunn's multiple comparison test. Student's t test and the Mann-Whitney U test where used for comparisons between two groups. For all analyses, P ≤ 0.05 was accepted as significant. The values shown are means ± SEM.
Results
Measurement of mRNA for HSPGs in Whole Lung Homogenates
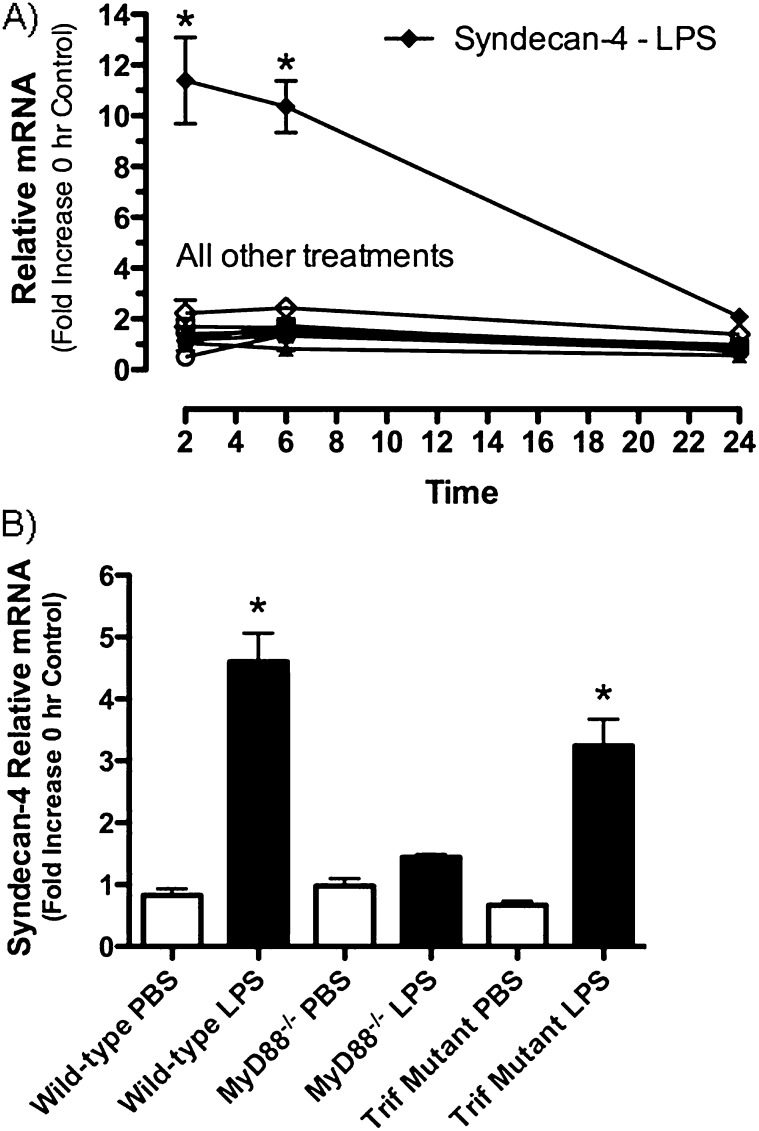
To characterize changes in the expression of mRNA for the HSPGs perlecan, syndecan-1, syndecan-2, and syndecan-4, mice were treated with LPS, and quantitative real-time PCR was performed using mRNA collected from whole lung homogenates. This work showed that, of the four HSPGs studied, only syndecan-4 mRNA was significantly increased after intratracheal LPS (11.4 ± 1.7-fold and 10.4 ± 1.7-fold at 2 and 6 h, respectively) when compared with control mice (Figure 1A). These findings show that among the HSPGs studied, syndecan-4 is rapidly and selectively up-regulated in response to LPS.
Figure 1.
Changes in the relative amounts of mRNA for the heparan sulfate proteoglycans syndecan-1, -2, and -4 and perlecan, were determined using mRNA collected from whole lung homogenates and quantitative real-time PCR. Comparison of mRNA recovered from lungs of mice treated with PBS (open symbols) and LPS (closed symbols) were made at 2, 6, and 24 hours (A). Expression of syndecan-4 in whole lung homogenates collected from C57BL/6 (WT), MyD88 knockout (MyD88 KO), and Trif mutant mice (Trif mut) treated with PBS (open bars) or LPS (closed bars) for 2 hours (B). Values are the mean ± SEM with a minimum n = 3 for each group studied. The expression of mRNA for each proteoglycan studied is expressed as a relative fold increase in mRNA over the 0-hour control group. *Groups that are significantly different (P ≤ 0.05) when mice treated with PBS and LPS were compared. The Mann-Whitney U-test was used for comparisons between the two treatment groups (A) and the Kruskal-Wallis test with Dunn's multiple comparison test was performed when multiple comparisons were made (B).
We next investigated the signaling pathways responsible for the increased expression of syndecan-4. The binding of LPS to the TLR4/MD2/CD14 complex results in signaling through two adaptor molecules, MyD88 and Trif (34). To evaluate the roles of these pathways in regulating the expression of syndecan-4 in lungs, mRNA was obtained from whole lung homogenates collected from C57BL/6 (i.e., wild-type controls), MyD88-null (MyD88−/−) mice, and Trif mutant mice. Mice were killed 2 hours after treatment with LPS to minimize the chance that cytokines or growth factors produced in response to the LPS would indirectly increase the expression of syndecan-4 mRNA. The results showed that syndecan-4 mRNA was significantly increased in the wild-type and Trif mutant mice treated with LPS but not in the mice lacking MyD88 (Figure 1B). Thus, the increased expression of syndecan-4 in response to LPS was mediated via MyD88-dependent signaling pathways.
Measurement of the Innate Immune Response in Mice Deficient in Syndecan-4
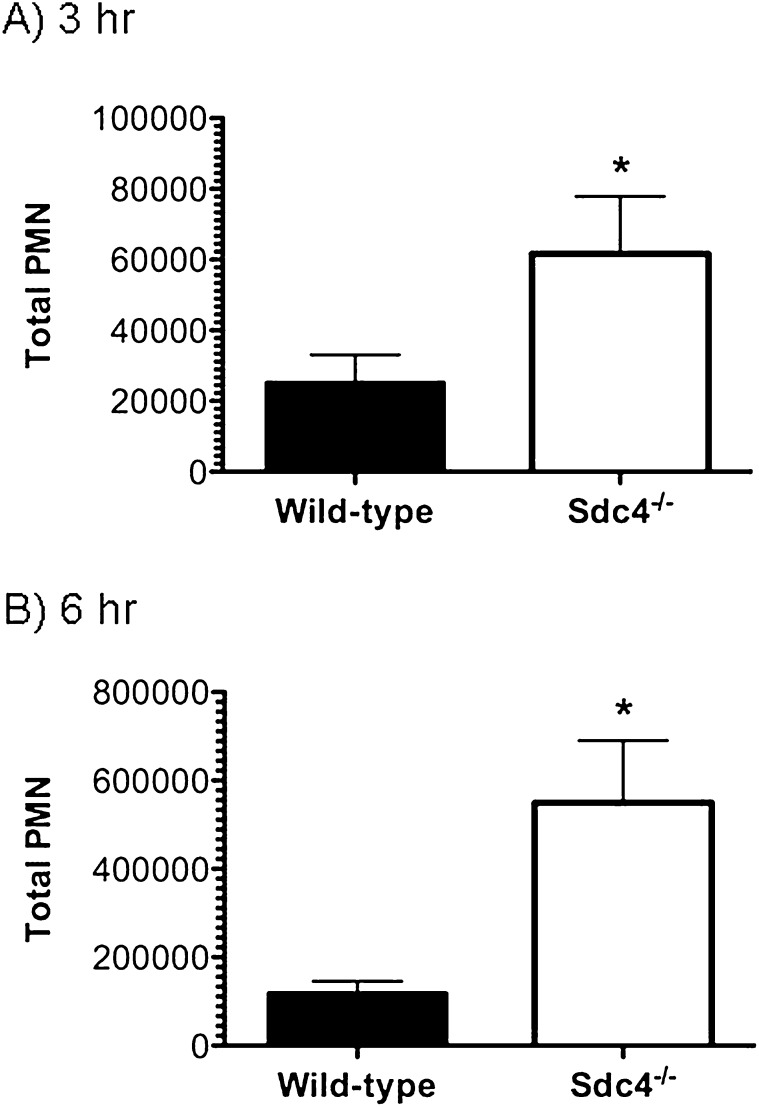
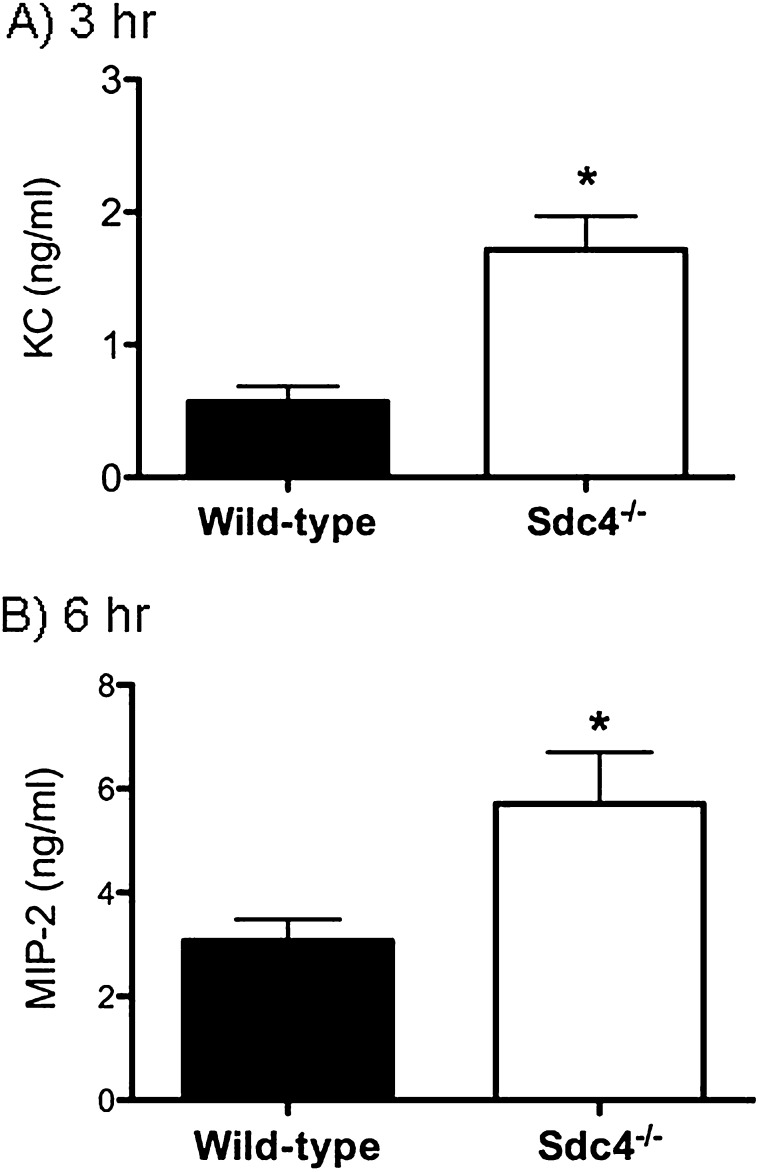
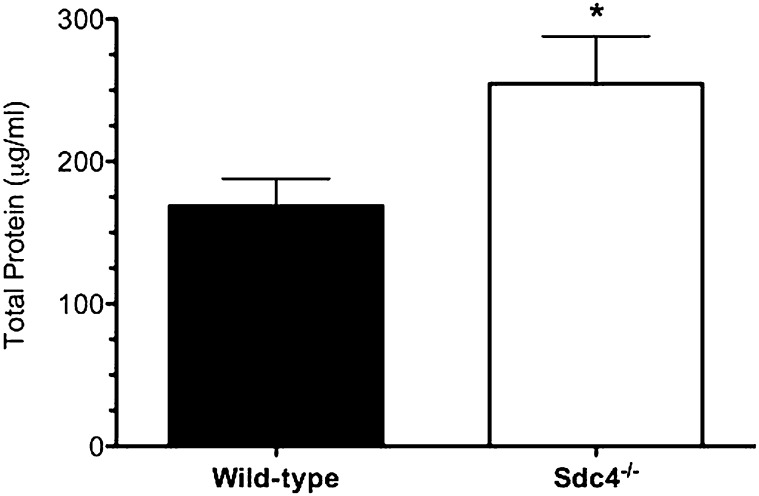
To identify the role of syndecan-4 in modulating the pulmonary recruitment of neutrophils and the development of lung injury, wild-type mice and mice deficient in syndecan-4 (Sdc4−/− mice) were studied 3 and 6 hours after treatment with intratracheal LPS. We recovered significantly more neutrophils and greater amounts of the neutrophil chemotactic factors KC and MIP-2 from the airspaces of the Sdc4−/− lungs at 3 and 6 hours compared with wild-type control mice (Figures 2 and 3). Consistent with enhanced neutrophil influx leading to more injury, Sdc4−/− mice had a significant increase in total protein in the BAL fluid (Figure 4). These findings indicate that syndecan-4 plays an important role in the innate immune response to LPS by limiting the inflammatory response and lung injury in mice treated with LPS.
Figure 2.
The total number of neutrophils recovered in bronchoalveolar lavage fluid collected from wild-type and syndecan-4 knockout (Sdc4−/−) mice treated with LPS (1 μg/g) and followed for 3 hours (A) and 6 hours (B) was determined. Values are the mean ± SEM with n = 8 to 10 mice per group for the 3-hour study and n = 11 mice per group for the 6-hour study. The y axis differs on the graphs displaying the results for the 3- and 6-hour studies. *Significantly different using the Mann-Whitney U test and a P ≤ 0.05.
Figure 3.
The amount of two neutrophil chemotactic factors, KC (A) and MIP-2 (B), was measured in the bronchoalveolar lavage fluid collected from wild-type and syndecan-4 knockout (Sdc4−/−) mice treated with LPS (1 μg/g) and followed for 6 hours. Values are the mean ± SEM with n = 8 to 10 mice per group for the 3-hour study and n = 11 mice per group for the 6-hour study. *Significantly different using the Mann-Whitney U test and (P ≤ 0.05).
Figure 4.
The amount of total protein in the bronchoalveolar lavage fluid collected from wild-type and syndecan-4 knockout (Sdc4−/−) mice treated with LPS (1 μg/g) was measured at 6 hours. Values are the mean ± SEM with n = 11 mice per group. *Significantly different using the Mann-Whitney's U test and (P ≤ 0.05).
Measurement of Syndecan-4 mRNA in Macrophages and Epithelial Cells Treated with LPS
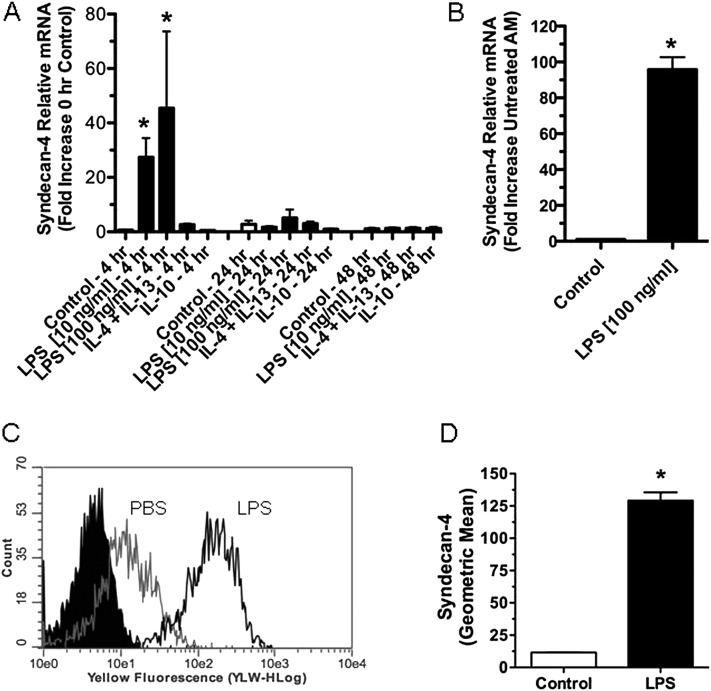
We assessed which cells in the lungs were responsible for the increased expression of syndecan-4. Immunohistochemistry of tissues from Sdc4−/− mice with commercial antibodies demonstrated that none was specific for this antigen (data not shown). Thus, we used cell culture models to identify the cell types in lungs that might be responsible for the increased expression of syndecan-4. Macrophages and lung epithelial cells were chosen for these studies because they are the first cells exposed to an airway challenge of LPS and because both cell types express the TLR4/MD2/CD14 complex required to recognize and respond to LPS (35–38). For studies where mRNA was collected to measure alterations in syndecan-4 expression in macrophages, BMDMs were used for most experiments because of the numbers of cells required to make direct comparisons among the different treatments, which included two concentrations of LPS (10 and 100 ng/ml). The combination of IL-4/IL-13 or IL-10 alone was used for 4, 24, and 48 hours. LPS was used to differentiate cells into classically activated macrophages (M1), whereas IL-4/IL-13 in combination or IL-10 alone was used to differentiate these cells into alternatively activated macrophages (M2a and M2c, respectively) (39–41). The expression of syndecan-4 mRNA by macrophages was significantly increased after treatment with LPS but not with IL-4/IL-13 or IL-10 (Figure 5A), suggesting that this HSPG is a new marker of M1 macrophages. Compared with 0-hour control macrophages, syndecan-4 mRNA increased 41 ± 21-fold at 4 hours with 10 ng/ml LPS and increased 45 ± 28-fold with 100 ng/ml LPS. By 24 hours, the amount of syndecan-4 mRNA expression in macrophages treated with LPS had returned to control levels. In a limited number of experiments, murine alveolar macrophages were studied to confirm that these macrophages also increased the expression of syndecan-4 mRNA after treatment with LPS. These studies show that alveolar macrophages treated with LPS (100 ng/ml) for 4 hours had a 96.8 ± 6.8-fold increase in syndecan-4 mRNA (Figure 5B).
Figure 5.
(A) Quantitative real-time PCR showing the relative expression for syndecan-4 mRNA collected from bone marrow–derived macrophages (BMDMs) treated in vitro with RPMI media (control), two concentrations of the M1 agonist LPS (10 and 100 ng/ml), the M2a agonists IL-4/IL-13 (10 ng/ml), or the M2c agonist IL-10 for up to 48 hours (n = 4–13). (B) Quantitative real-time PCR showing the relative expression for syndecan-4 mRNA collected from alveolar macrophages treated with RPMI media (control) or LPS (100 ng/ml) for 4 hours (n = 4). (C and D) A representative histogram (C) and the geometric mean of syndecan-4 expression (D) on BMDMs measured with flow cytometry. Flow cytometry was performed 3 hours after the treatment of mouse BMDMs with RPMI or LPS (10 ng/ml) (n = 3). Values are the mean ± SEM. *Groups different from the control mice (P ≤ 0.05) using the Kruskal-Wallis test with Dunn's multiple comparison test (A) and the Mann-Whitney U test (B and D).
We next used flow cytometry to measure the amount of syndecan-4 present on the surface of BMDMs. Consistent with the increased expression of syndecan-4 mRNA (Figure 5A), macrophages treated with LPS had a significant increase in the amount of syndecan-4 measured on their cell surface (Figures 5C and 5D). Control macrophages had a mean fluorescence of 11.56 ± 0.19, whereas cells treated with LPS had a mean fluorescence of 129.2 ± 6.38. Confirmatory experiments using antibodies for syndecan-4 and the macrophage marker F4/80 showed that the syndecan-4–positive cells were macrophages (data not shown).
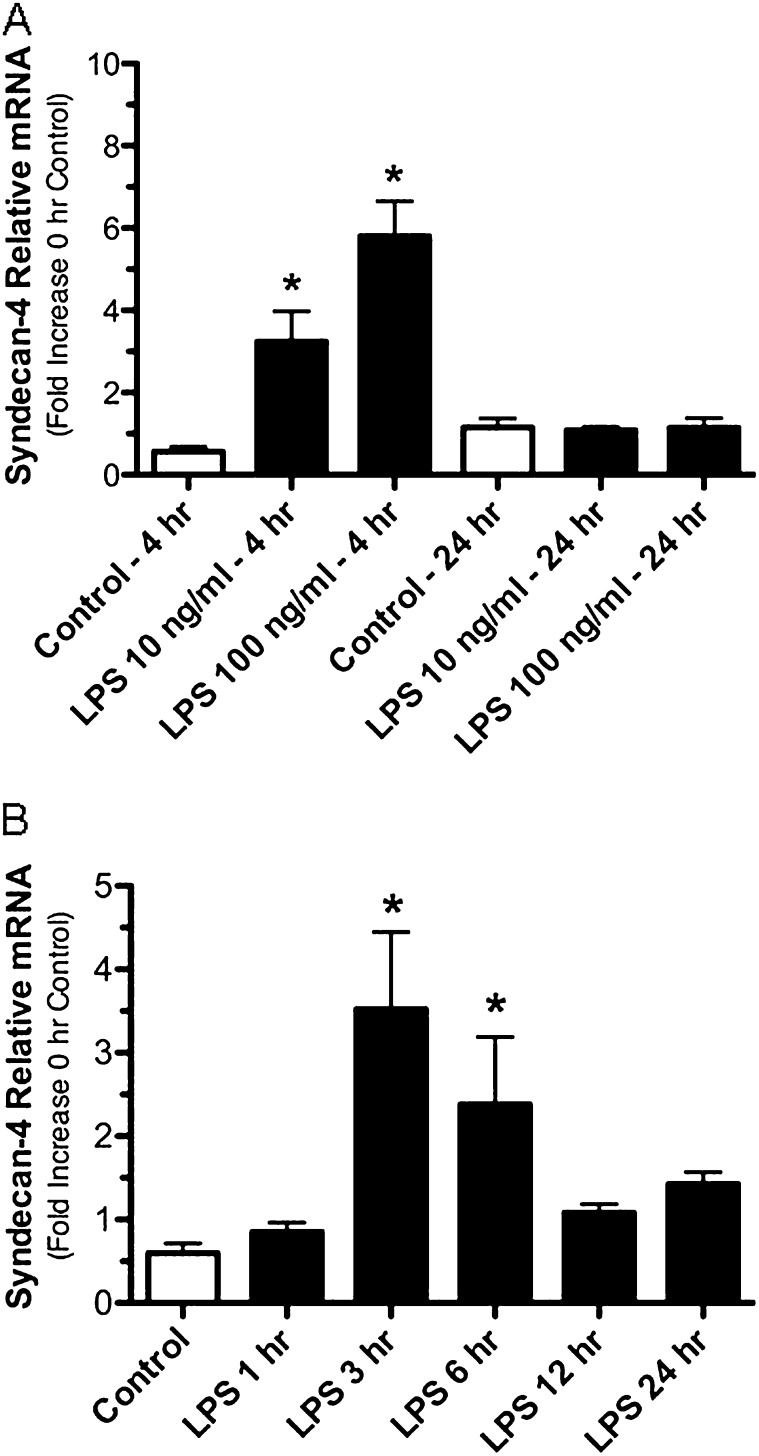
We also found that syndecan-4 expression by epithelial cells was increased by LPS (Figure 6) but not to the degree seen in macrophages. Murine tracheal epithelial cells grown at an air–liquid interface and treated with LPS at concentrations of 10 and 100 ng/ml for 4 hours had a 3.2 ± 0.74-fold and a 5.8 ± 0.85-fold increase in syndecan-4 mRNA, respectively (Figure 6A). In comparison when the human bronchial epithelial cell line BEAS-2B cells were treated with LPS (1,000 ng/ml), there was a 5.87 ± 1.53-fold and 3.94 ± 1.35-fold increase in syndecan-4 mRNA after treatment for 3 and 6 hours, respectively. Taken together, these findings show that LPS causes a rapid up-regulation of syndecan-4 mRNA in macrophages and epithelial cells. In addition, we show that the rapid increase in the expression of syndecan-4 is a characteristic of classically activated macrophages.
Figure 6.
Quantitative real time PCR showing the relative expression for syndecan-4 mRNA collected from murine tracheal air–liquid interface cell cultures treated with LPS at 10 and 100 ng/ml (A) and BEAS-2B cells treated with LPS at 1,000 ng/ml (B) for the specified times. For all cultures, n = 3; for BEAS-2B cells, n = 4. Values are the mean ± SEM. *Groups different from the controls using the Kruskal-Wallis test with Dunn's multiple comparison test with P ≤ 0.05.
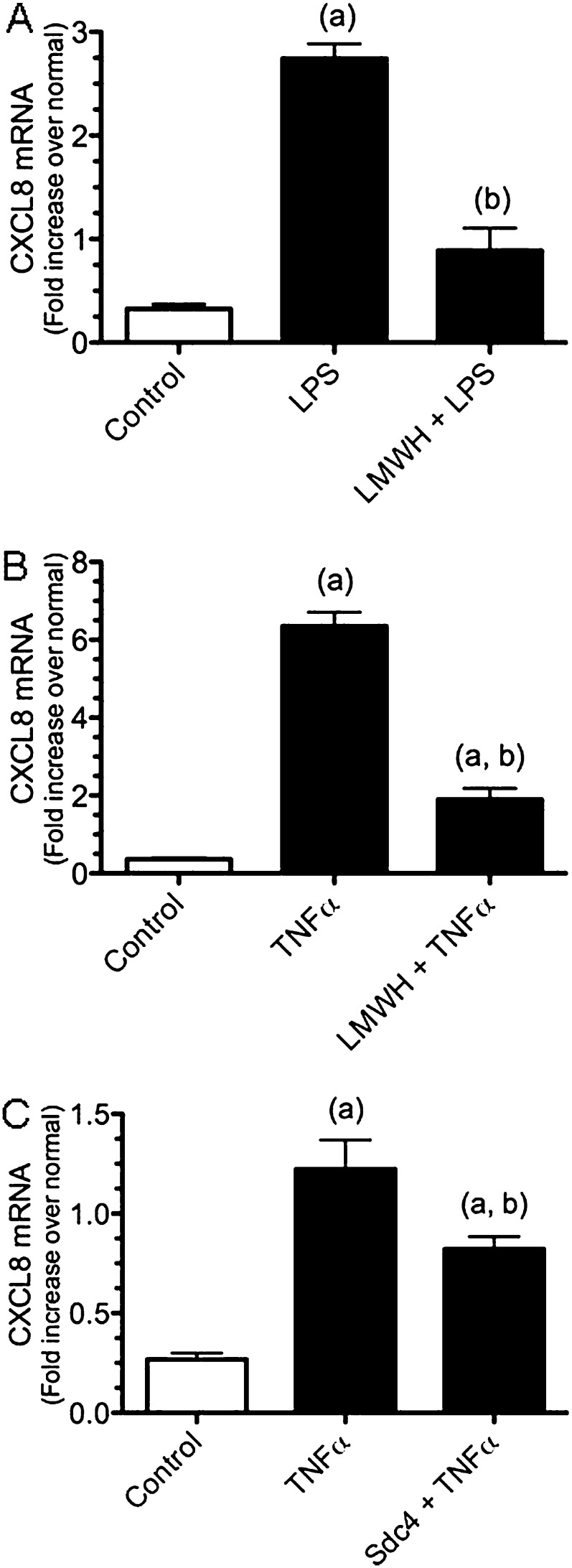
Effect of Low-Molecular-Weight Heparin or Syndecan-4 on CXCL8 Expression in BEAS-2B Cells
We assessed if soluble forms of heparin or syndecan-4 alter the inflammatory response of BEAS-2B cells treated with LPS or TNF-α, a heparin-binding proinflammatory cytokine. Heparin was used for these studies because it is readily available and because it has a similar, though more sulfated, GAG backbone to heparan sulfate (18). BEAS-2B cells treated with LPS alone had significantly increased CXCL8 mRNA production compared with control cells (no LPS) (8.5 ± 0.45-fold increase versus control cells). However, when BEAS-2B cells were pretreated with low-molecular-weight heparin (LMWH) and then treated with LPS, these cells expressed significantly less CXCL8 mRNA (2.8 ± 0.67 for cells) as compared with cells treated with LPS alone (Figure 7A).
Figure 7.
(A and B) Pretreatment of BEAS-2B cells with RPMI media or low-molecular-weight heparin (LMWH) (10 μg/ml) for 1 hour followed by treatment with media, LPS (A), or TNF-α (B) for 3 hours. (C) Pretreatment of BEAS-2B cells with RPMI media or syndecan-4 (2.5 μg/ml) for 1 hour followed by treatment with media or TNF-α for 3 hours. Values are the mean ± SEM with n = 6. An (a) shows groups that are different from the control group, and a (b) shows differences between LPS or TNF-α treatment groups using the Kruskal-Wallis test with Dunn's multiple comparison test with P ≤ 0.05.
To determine if pretreatment with heparin would affect the activation of epithelial cells stimulated with the heparin-binding cytokine TNF-α (42, 43), BEAS-2B cells were pretreated with RPMI or LMWH and then treated with TNF-α. These studies showed that the preincubation of epithelial cells with LMWH significantly decreased the TNF-α induced up-regulation of CXCL8 mRNA expression (Figure 7B). Finally, to determine if syndecan-4 would affect the activation of epithelial cells stimulated with TNF-α, BEAS-2B cells were preincubated with human recombinant syndecan-4 (2.5 μg/ml) before treatment with TNF-α. The treatment of epithelial cells with recombinant syndecan-4 significantly inhibited the TNF-α induced up-regulation of CXCL8 mRNA in BEAS-2B cells (Figure 7C). As was observed with the LMWH, syndecan-4 did not completely abolish the TNF-α activation of BEAS-2B cells. These studies showed that pretreatment with heparin and HS are able to decrease the inflammatory response of epithelial cells subsequently treated with LPS and TNF-α in vitro.
Discussion
The purpose of this work was to identify changes in the composition of HSPGs in mice exposed to gram-negative bacterial products and to determine how these changes alter pulmonary inflammation. To reduce animal-to-animal variability resulting from live bacteria, mice were treated with intratracheal LPS, a component of the cell wall of gram-negative bacteria. The treatment of mice with intratracheal LPS resulted in a rapid increase in syndecan-4 mRNA in lungs that was regulated through MyD88-dependent signaling. Work performed using Sdc4–/– mice showed that the lack of this HSPG resulted in a significant increase in pulmonary inflammation and injury after treatment with LPS. Finally, experiments performed in vitro showed that macrophages and epithelial cells increase the expression of syndecan-4 in response to treatment with LPS and that pretreatment with heparin and syndecan-4 decreased the expression of CXCL8 mRNA in a human epithelial cell line treated with LPS and TNF-α. These studies show that syndecan-4 plays a key role in regulating the early innate immune response to lung infection.
Little is known about how the composition of HSPG changes during gram-negative pneumonia or about the signaling pathways responsible for these changes. Of the four HSPG examined, only the expression of syndecan-4 increased in mice treated with LPS. The rapid increase in syndecan-4 mRNA in lungs, which occurs in a similar time frame to the increased expression of proinflammatory cytokines and chemokines (44), suggests that the expression of this HSPG in mice exposed to intratracheal LPS is regulated directly through TLR4. To better characterize the signaling pathways responsible for the increased expression of syndecan-4, studies were performed in mice deficient in MyD88 or Triff. The inability of the MyD88−/− mice to increase syndecan-4 mRNA in response to LPS shows that the recruitment of MyD88 to TLR4 mediates signal transduction to downstream signaling components, such as NF-κB, which modulates syndecan-4 production (Figure 1B). This extends the previous work of Smith and colleagues who found that signaling through NF-κB was responsible for the increased expression of syndecan-4 in a gastric epithelial cell line treated with TLR2 and TLR4 agonists in vitro (45).
MyD88 is an adaptor molecular common to all TLRs (except for TLR3- and for IL-1R1) (3). Therefore, it is likely that syndecan-4 will be increased in a number of infectious and noninfectious lung diseases, with the common link being activation of MyD88 signaling pathways. For example, IL-1R1/MyD88 signaling and the inflammasome are essential in the development of pulmonary inflammation and fibrosis in bleomycin- induced lung injury (46), suggesting that IL-1R1/MyD88 signaling may be responsible for the increased expression of syndecan-4 reported in mice treated with bleomycin (47). These findings suggest an important role for syndecan-4 in regulating the inflammatory response in lungs after the activation of MyD88 signaling pathways.
To determine the role of syndecan-4 in the innate immune response to LPS, we studied mice lacking syndecan-4. This work showed that Sdc4−/− mice had increased pulmonary inflammation and lung injury after treatment with LPS. These results are consistent with previous reports, such as the work of Ishiguro and colleagues, who found that the injection of LPS into the peritoneum of Sdc4−/− mice resulted in increased mortality (48). In a more recent study, Jiang and colleagues showed that Sdc4−/− mice treated with bleomycin had increased pulmonary fibrosis (47). In addition, studies performed in vitro with BEAS-2B cells showed that pretreatment with heparin or syndecan-4 decreased the expression of CXCL8 mRNA when the BEAS-2B cells were subsequently treated with LPS or TNF-α (Figure 7). Taken together, this body of work shows that syndecan-4 regulates the inflammatory response and suggests that syndecan-4 may play a key role in preventing the adverse consequences of tissue inflammation, such as lung injury, pulmonary fibrosis, and the systemic inflammatory response.
To identify potential cellular sources for the increased expression of syndecan-4, murine macrophages and a human bronchial epithelial cell line (BEAS-2B cells) were treated with LPS for up to 24 hours. Macrophages and epithelial cells were evaluated because these cells are exposed to the highest concentrations of LPS after an airway challenge. In response to treatment with LPS in vitro, macrophages and epithelial cells showed a rapid and significant increase in the expression of syndecan-4, suggesting that they are in part responsible for the increased expression of syndecan-4 mRNA. Whereas the in vitro studies were performed using macrophages and epithelial cells, other cells, such as fibroblasts and endothelial cells, are potential sources of the increased recovery of syndecan-4 mRNA obtained from whole lungs of mice treated with LPS.
Macrophages are broadly classified into two groups: M1 or classically activated macrophages and M2 or alternatively activated macrophages. To characterize whether the early increase of syndecan-4 was specific for M1 macrophages, cells were treated with LPS to differentiate them to a M1 phenotype or IL-4/IL-13 and IL-10 to differentiate them to a M2 phenotype (39, 40). This work showed that the rapid increase in syndecan-4 expression in macrophages occurs only with LPS treatment, which suggests that syndecan-4 is an early marker of M1 macrophages.
In summary, the results of this study show that syndecan-4 is rapidly increased in response to the intratracheal instillation of LPS with expression kinetics similar to that observed for cytokines and chemokines. These studies also show an increased pulmonary inflammatory response and lung injury in syndecan-4–deficient mice treated with intratracheal LPS. Finally, pretreatment of lung epithelial cells with heparin or syndecan-4 inhibits the proinflammatory properties of LPS and TNF-α. We conclude that the increased expression of syndecan-4 in lungs of mice exposed to intratracheal LPS is an important mechanism that regulates neutrophil recruitment and limits the extent of pulmonary inflammation and lung injury. This suggests that the development of therapeutic strategies to capitalize on the protective effects of syndecan-4 may provide treatments to minimize the adverse effects of tissue inflammation.
Supplementary Material
Acknowledgments
The authors thank Timothy Birkland, Ph.D., Kathleen R. Braun, Gina Kiske. and Vivian Lee for excellent technical expertise and assistance.
Footnotes
This work was supported by National Institutes of Health grants HL098067, GM37696, RR030249, HL082658, HL081764, and DK089507 and by the Medical Research Service at the Department of Veterans Affairs and the Diffuse Lung Diseases Research Group from the Ministry of Health, Labor and Welfare, Japan.
Originally Published in Press as DOI: 10.1165/rcmb.2011-0294OC on March 15, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med 2008;358:716–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010;11:373–384 [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805–820 [DOI] [PubMed] [Google Scholar]
- 4.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L379–L399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. Distinct roles of pattern recognition receptors CD14 and Toll-like receptor 4 in acute lung injury. Infect Immun 2005;73:1754–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poltorak A, Smirnova I, Xiaolong H, Liu M, Huffel C, Birdwell D, Alejos E, Silva M, Du X, Thompson P, et al. Genetic and physical mapping of the Lps locus: identification of the Toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis 1998;240:340–355 [DOI] [PubMed] [Google Scholar]
- 7.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol 2007;292:L312–L322 [DOI] [PubMed] [Google Scholar]
- 8.Cai S, Batra S, Shen L, Wakamatsu N, Jeyaseelan S. Both TRIF- and MyD88-dependent signaling contribute to host defense against pulmonary Klebsiella infection. J Immunol 2009;183:6629–6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol 1995;154:335–344 [PubMed] [Google Scholar]
- 10.Gupta S, Feng L, Yoshimura T, Redick J, Fu SM, Rose CE., Jr Intra-alveolar macrophage-inflammatory peptide 2 induces rapid neutrophil localization in the lung. Am J Respir Cell Mol Biol 1996;15:656–663 [DOI] [PubMed] [Google Scholar]
- 11.Balamayooran G, Batra S, Fessler MB, Happel KI, Jeyaseelan S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am J Respir Cell Mol Biol 2010;43:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med 1997;155:1187–1205 [DOI] [PubMed] [Google Scholar]
- 13.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol 2009;40:519–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segel GB, Halterman MW, Lichtman MA. The paradox of the neutrophil's role in tissue injury: a review. J Leukoc Biol 2010;89:359–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esko JD, Lindahl U. Molecular diversity of heparan sulfate. J Clin Invest 2001;108:169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol 2006;6:633–643 [DOI] [PubMed] [Google Scholar]
- 17.Gill S, Wight TN, Frevert CW. Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat Rec (Hoboken) 2010;293:968–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 2007;446:1030–1037 [DOI] [PubMed] [Google Scholar]
- 19.Frevert CW, Kinsella MG, Vathanaprida C, Goodman RB, Baskin DG, Proudfoot A, Wells TN, Wight TN, Martin TR. Binding of interleukin-8 to heparan sulfate and chondroitin sulfate in lung tissue. Am J Respir Cell Mol Biol 2003;28:464–472 [DOI] [PubMed] [Google Scholar]
- 20.Tanino Y, Coombe DR, Gill SE, Kett WC, Kajikawa O, Proudfoot AE, Wells TN, Parks WC, Wight TN, Martin TR, et al. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J Immunol 2010;184:2677–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellyard JI, Simson L, Bezos A, Johnston K, Freeman C, Parish CR. Eotaxin selectively binds heparin: an interaction that protects eotaxin from proteolysis and potentiates chemotactic activity in vivo. J Biol Chem 2007;282:15238–15247 [DOI] [PubMed] [Google Scholar]
- 22.Reeves EP, Williamson M, Byrne B, Bergin DA, Smith SG, Greally P, O'Kennedy R, O'Neill SJ, McElvaney NG. IL-8 dictates glycosaminoglycan binding and stability of IL-18 in cystic fibrosis. J Immunol 2010;184:1642–1652 [DOI] [PubMed] [Google Scholar]
- 23.Reeves EP, Williamson M, O'Neill SJ, Greally P, McElvaney NG. Nebulised hypertonic saline decreases interleukin-8 in sputum of patients with cystic fibrosis. Am J Respir Crit Care Med 2011;183:1517–1523 [DOI] [PubMed] [Google Scholar]
- 24.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood 2009;114:3033–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002;111:635–646 [DOI] [PubMed] [Google Scholar]
- 26.Blackwood RA, Cantor JO, Moret J, Mandl I, Turino GM. Glycosaminoglycan synthesis in endotoxin-induced lung injury. Proc Soc Exp Biol Med 1983;174:343–349 [DOI] [PubMed] [Google Scholar]
- 27.Karlinsky JB, Bucay PJ, Ciccolella DE, Crowley MP. Effects of intratracheal endotoxin administration on hamster lung glycosaminoglycans. Am J Physiol 1991;261:L148–L155 [DOI] [PubMed] [Google Scholar]
- 28.Frevert CW, Sannes PL. Matrix proteoglycans as effector molecules for epithelial cell function. Eur Respir Rev 2006;14:137–144 [Google Scholar]
- 29.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med 1996;154:1819–1828 [DOI] [PubMed] [Google Scholar]
- 30.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycans in granulomatous lung diseases. Eur Respir J 1997;10:2731–2737 [DOI] [PubMed] [Google Scholar]
- 31.Tanino Y, Makita H, Miyamoto K, Betsuyaku T, Ohtsuka Y, Nishihira J, Nishimura M. Role of macrophage migration inhibitory factor in bleomycin-induced lung injury and fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2002;283:L156–L162 [DOI] [PubMed] [Google Scholar]
- 32.Smith LS, Gharib SA, Frevert CW, Martin TR. Effects of age on the synergistic interactions between lipopolysaccharide and mechanical ventilation in mice. Am J Respir Cell Mol Biol 2010;43:475–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kassim SY, Gharib SA, Mecham BH, Birkland TP, Parks WC, McGuire JK. Individual matrix metalloproteinases control distinct transcriptional responses in airway epithelial cells infected with Pseudomonas aeruginosa. Infect Immun 2007;75:5640–5650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beutler B, Hoebe K, Georgel P, Tabeta K, Du X. Genetic analysis of innate immunity: identification and function of the TIR adapter proteins. Adv Exp Med Biol 2005;560:29–39 [DOI] [PubMed] [Google Scholar]
- 35.Lin S-M, Frevert C, Kajikawa O, Wurfel MM, Ballman K, Mongovin S, Wong VA, Selk A, Martin TR. Differential regulation of membrane CD14 expression and endotoxin-tolerance in alveolar macrophages. Am J Respir Cell Mol Biol 2004;31:162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kajikawa O, Frevert CW, Lin SM, Goodman RB, Mongovin SM, Wong V, Ballman K, Daubeuf B, Elson G, Martin TR. Gene expression of Toll-like receptor-2, Toll-like receptor-4, and MD2 is differentially regulated in rabbits with Escherichia coli pneumonia. Gene 2005;344:193–202 [DOI] [PubMed] [Google Scholar]
- 37.Daubeuf B, Mathison J, Spiller S, Hugues S, Herren S, Ferlin W, Kosco-Vilbois M, Wagner H, Kirschning CJ, Ulevitch R, et al. TLR4/MD-2 monoclonal antibody therapy affords protection in experimental models of septic shock. J Immunol 2007;179:6107–6114 [DOI] [PubMed] [Google Scholar]
- 38.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 2011;45:189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964 [DOI] [PubMed] [Google Scholar]
- 40.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol 2008;181:3733–3739 [DOI] [PubMed] [Google Scholar]
- 41.Novak R, Dabelic S, Dumic J. Galectin-1 and galectin-3 expression profiles in classically and alternatively activated human macrophages. Biochim Biophys Acta (In press) [DOI] [PubMed] [Google Scholar]
- 42.Axelsson J, Ferreira M, Adolfsson L, McCrea K, Ward R, Larm O. Cytokines in blood from septic patients interact with surface-immobilized heparin. ASAIO J 2010;56:48–51 [DOI] [PubMed] [Google Scholar]
- 43.Kenig M, Gaberc-Porekar V, Fonda I, Menart V. Identification of the heparin-binding domain of TNF-alpha and its use for efficient TNF-alpha purification by heparin-Sepharose affinity chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 2008;867:119–125 [DOI] [PubMed] [Google Scholar]
- 44.Chensue SW, Terebuh PD, Remick DG, Scales WE, Kunkel SL. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia: kinetics, kupffer cell expression, and glucocorticoid effects. Am J Pathol 1991;138:395–402 [PMC free article] [PubMed] [Google Scholar]
- 45.Smith MF, Jr, Novotny J, Carl VS, Comeau LD. Helicobacter pylori and Toll-like receptor agonists induce syndecan-4 expression in an NF-kappaB-dependent manner. Glycobiology 2006;16:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VFJ, Lagente V, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest 2007;117:3786–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang D, Liang J, Campanella GS, Guo R, Yu S, Xie T, Liu N, Jung Y, Homer R, Meltzer EB, et al. Inhibition of pulmonary fibrosis in mice by CXCL10 requires glycosaminoglycan binding and syndecan-4. J Clin Invest 2010;120:2049–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Iwase M, Yoshikai Y, Yanada M, Yamamoto K, Matsushita T, Nishimura M, et al. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J Biol Chem 2001;276:47483–47488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.