Abstract
Apolipoprotein A-I (apoA-I) is a key component of high-density lipoproteins that mediates reverse cholesterol transport from cells and reduces vascular inflammation. We investigated whether endogenous apoA-I modulates ovalbumin (OVA)–induced airway inflammation in mice. We found that apoA-I expression was significantly reduced in the lungs of OVA-challenged, compared with saline-challenged, wild-type (WT) mice. Next, to investigate the role of endogenous apoA-I in the pathogenesis of OVA-induced airway inflammation, WT and apoA-I−/− mice were sensitized by intraperitoneal injections of OVA and aluminum hydroxide, followed by multiple nasal OVA challenges for 4 weeks. OVA-challenged apoA-I−/− mice exhibited a phenotype of increased airway neutrophils compared with WT mice, which could be rescued by an administration of a 5A apoA-I mimetic peptide. Multiple pathways promoted neutrophilic inflammation in OVA-challenged apoA-I−/− mice, including the up-regulated expression of (1) proinflammatory cytokines (IL-17A and TNF-α), (2) CXC chemokines (CXCL5), (3) vascular adhesion molecules (i.e., vascular cell adhesion molecule–1), and (4) granulocyte colony–stimulating factors (G-CSF). Because concentrations of G-CSF in bronchoalveolar lavage fluid (BALF) were markedly increased in OVA-challenged apoA-I−/− mice, we hypothesized that enhanced G-CSF expression may represent the predominant pathway mediating increased neutrophilic inflammation. This was confirmed by the intranasal administration of a neutralizing anti–G-CSF antibody, which significantly reduced BALF neutrophilia by 72% in OVA-challenged apoA-I−/− mice, compared with mice that received a control antibody. We conclude that endogenous apoA-I negatively regulates OVA-induced neutrophilic airway inflammation, primarily via a G-CSF–dependent mechanism. Furthermore, these findings suggest that apoA-I may play an important role in modulating the severity of neutrophilic airway inflammation in asthma.
Keywords: airway inflammation, ovalbumin, neutrophil, apolipoprotein A-I, G-CSF
Clinical Relevance
This study shows that endogenous apolipoprotein A-I negatively regulates ovalbumin-induced neutrophilic airway inflammation primarily via a granulocyte colony–stimulating factor–dependent mechanism. These findings suggest that apolipoprotein A-I may play an important role in modulating the severity of neutrophilic airway inflammation in asthma.
Asthma is a common disorder that affects approximately 300 million individuals worldwide (1). Asthma, which is manifested by airway inflammation, airway remodeling, and airway hyperreactivity, has a complex and heterogeneous pathogenesis that involves both innate and adaptive immune responses (2). Multiple cell types are involved in the pathogenesis of asthma, including dendritic cells, T cells, epithelial cells, mast cells, basophils, macrophages, and neutrophils. Furthermore, distinct inflammatory asthma phenotypes have been identified and are characterized by a predominance of eosinophils and neutrophils or the absence inflammatory cells (i.e., pauci-granulocytic) (3).
Apolipoproteins are key constituents of lipoprotein particles that primarily mediate lipid transport. Recent studies, however, suggest that apolipoprotein E (apoE) and apolipoprotein A-I (apoA-I) may also modulate the pathogenesis of asthma. For example, apoE, which is expressed by alveolar macrophages in the lung, was shown to negatively regulate airway hyperreactivity (AHR) and goblet-cell hyperplasia in a murine model of house dust mite (HDM)–induced asthma via an interaction with low-density lipoprotein (LDL) receptors that are expressed by ciliated airway epithelial cells (4). Furthermore, administration of an apoE mimetic peptide that corresponded to the LDL receptor–binding domain of the holo-apoE protein attenuated the induction of HDM-induced airway inflammation, AHR, and goblet-cell hyperplasia. Apolipoprotein A-I is the major protein constituent of high-density lipoproteins (HDLs) that promotes reverse cholesterol transport out of cells via binding to the ATP-binding cassette (ABC) transporter A1 (ABCA1) (5, 6). Based on the anti-inflammatory properties of apoA-I in vascular inflammation, we previously hypothesized that apoA-I might be used as a pharmacologic agent to attenuate the induction of asthma (7). We showed that the systemic administration of a 5A apoA-I mimetic peptide reduced airway inflammation, airway remodeling, and airway hyperreactivity in a murine model of HDM-induced asthma (8). In particular, HDM-challenged mice that received the 5A apoA-I mimetic peptide demonstrated significant reductions in the number of bronchoalveolar lavage fluid neutrophils, lymphocytes, and eosinophils. Similarly, the intranasal administration of D-4F, another apoA-I mimetic peptide, was also shown to reduce airway eosinophilia and airway resistance in ovalbumin (OVA)–challenged mice (9).
Although these studies provide evidence to suggest that the pharmacologic administration of apoA-I mimetic peptides may represent a novel treatment approach that attenuates the manifestations of asthma, the role of endogenous apoA-I in the pathogenesis of asthma is not known. Here, we show that apoA-I is expressed in the lung, where it functions as a negative regulator of neutrophilic airway inflammation in OVA-challenged mice via the suppression of multiple pathways that include proinflammatory cytokines (IL-17A and TNF-α), NF-κB signaling, chemokines (i.e., C-X-C motif ligand [CXCL]5), vascular adhesion molecules (i.e., vascular cell adhesion molecule–1; VCAM-1), and granulocyte colony–stimulating factor (G-CSF). Furthermore, we show that the ability of apoA-I to suppress OVA-induced airway inflammation is mediated primarily via a G-CSF–dependent mechanism. These results suggest that apoA-I in the lung may play an important role in modulating the pathogenesis and severity of neutrophilic airway inflammation in asthma.
Marterials and Methods
More detailed methods are provided in the online supplement.
Model of OVA Challenge
Six-to-8-week-old C57BL/6 mice and apoA-1−/− mice were obtained from Jackson Laboratory (Bar Harbor, ME). On Days 1 and 8, mice were sensitized with an intraperitoneal injection of 50 μg OVA (Grade V) plus 0.4 mg of aluminum hydroxide (alum) (Sigma-Aldrich, St. Louis, MO). Mice received daily intranasal challenges with 150 μg OVA in 10 μl saline, 5 days per week, on Days 15–42. Control mice were sensitized and challenged with saline. For apoA-I reconstitution experiments, 10 mg/kg of the 5A apoA-I mimetic peptide or an irrelevant scrambled apolipoprotein E control peptide were nasally administered daily, 5 days per week on Days 15–42, 30 minutes after the OVA challenge (4, 8). For G-CSF inhibition experiments, 5 μg of a neutralizing, anti-mouse G-CSF antibody or control antibody (R&D Systems, Minneapolis, MN) were nasally administered 3 days per week during Days 15–42. Endpoints were analyzed 24 hours after the final challenge. For the single OVA challenge experiment, mice that had not been sensitized to OVA + alum received one intranasal administration of 150 μg OVA in 10 μl saline, and bronchoalveolar lavage (BAL) was performed 24 hours later. Experiments were approved by the Animal Care and Use Committee of the National Heart, Lung, and Blood Institute.
BAL and Lung Histopathologic Examination
BAL cell counts were performed using Diff-Quik–stained cytospin slides (Siemens Healthcare Diagnostics, Deerfield, IL) (4, 8). Lungs were inflated to 25 cm H2O, fixed in formalin, dehydrated through gradient ethanol, and embedded in paraffin before the cutting of 5-μm sagittal sections. The quantification of goblet-cell hyperplasia was performed as previously described (4, 8).
Quantitative RT-PCR
Total RNA was isolated using the mirVana kit from lungs frozen in RNAlater (Ambion, Austin, TX). DNase-treated RNA was reverse transcribed using the High-Capacity cDNA Reverse Transcription kit and amplified using the TaqMan Universal PCR Master Mix, FAM dye-labeled TaqMan MGB probes, and a 7500 Real Time PCR System (Applied Biosystems, Foster City, CA). Gene expression was quantified relative to 18S ribosomal RNA, using the control sample as a calibrator to calculate the difference in cycle threshold values.
Western Blotting
Western blots were performed as previously described (10). Lung proteins (12.5 μg) or BAL fluid (BALF) (30 μl) were separated by SDS-PAGE, using 10% Bis-Tris Nupage gels (Invitrogen, Carlsbad, CA). The antibodies against apoA-I, VCAM-1, and albumin were obtained from Abcam (Cambridge, MA), the anti-IκBα and anti–phospho-IκBα antibodies were obtained from Cell Signaling (Danvers, MA), and the anti–β-actin antibody was obtained from Sigma-Aldrich. Membranes were stripped using a Re-Blot recycling kit (Chemicon International, Temecula, CA). Densitometry was performed using NIH ImageJ software (National Institutes of Health, Bethesda, MD).
ELISA
The quantity of protein in BALF was measured using kits from R&D Systems. The lower limits of detection of the assays were 5.4 pg/ml for IL-17A, 5.9 pg/ml for TNF-α, 4.7 pg/ml for IFN-γ, 15.6 pg/ml for CXCL5, and 7 pg/ml for G-CSF.
Statistical Analysis
Results are presented as means ± SEM. One-way ANOVA with the Bonferroni multiple comparison test (GraphPad Prism, version 5.0a; GraphPad Software, Inc., La Jolla, CA) was used. P < 0.05 was considered significant.
Results
Ovalbumin Challenge Down-Regulates Lung Apolipoprotein A-I Expression
Wild-type (WT) C57BL/6 mice and apoA-I−/− mice were sensitized to OVA (50 μg) complexed with alum (0.4 mg) by intraperitoneal injection on Days 1 and 8, followed by nasal OVA (150 μg) challenges, 5 days per week for 4 weeks, beginning on Day 15 and ending on Day 42. As shown in Figure 1, the quantity of apoA-I protein present in BALF from OVA-challenged WT mice was significantly reduced, compared with that in saline-challenged WT mice. Lung mRNA concentrations of apoA-I were also significantly reduced in OVA-challenged WT mice, whereas neither apoA-I mRNA nor protein was detected in apoA-I−/− mice.
Figure 1.
Apolipoprotein A-I (apoA-I) expression is attenuated in the lungs of ovalbumin-challenged mice. (A) The amount of apoA-I protein present in bronchoalveolar lavage fluid (BALF) from wild-type (WT) C57BL/6 mice and apoA-I−/− mice that had been challenged with nasal saline or ovalbumin (OVA) was assessed by Western blotting. Thirty microliters of BALF were loaded per lane. The amount of albumin present in BALF is shown as a control for the equivalency of protein loading. A representative blot is shown from six replicate experiments. (B) The relative expression of apoA-I compared with that of albumin was quantified by densitometry (*P < 0.01, n = 6, WT + saline versus WT + OVA). (C) The quantification of lung mRNA concentrations for apoA-I using quantitative RT-PCR is presented as relative mRNA expression (*P < 0.001, n = 6, WT + saline versus WT + OVA).
Endogenous Apolipoprotein A-I Negatively Regulates Neutrophilic Airway Inflammation in OVA-Challenged Mice
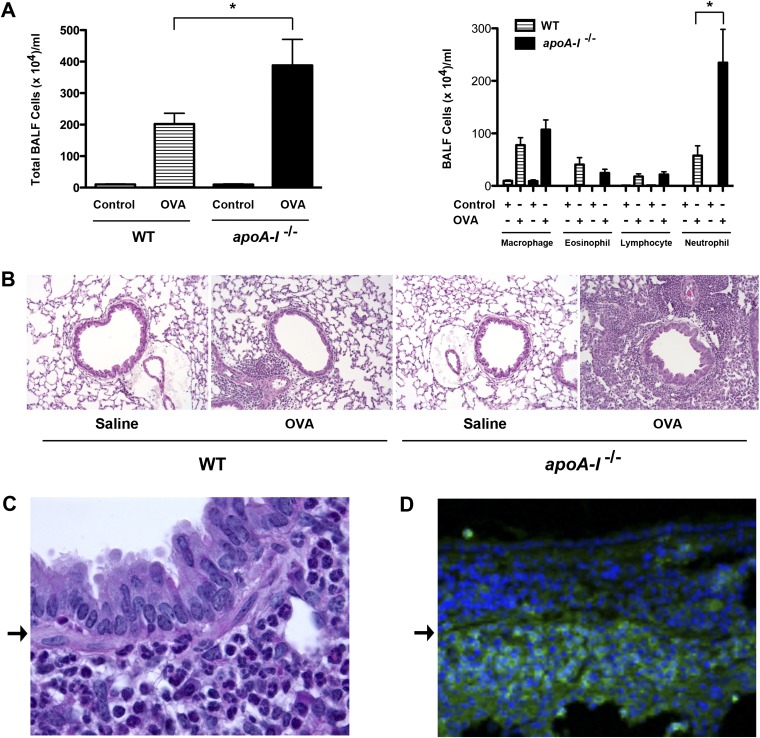
Experiments were then performed to characterize the role of endogenous apoA-I in the modulation of airway inflammation, IgE production, goblet-cell hyperplasia, and AHR, which are key pathogenic manifestations of allergic asthma. As shown in Figure 2A, both the total number of BALF inflammatory cells and the number of BALF neutrophils were increased in OVA-challenged apoA-I−/− mice, compared with OVA-challenged WT mice. Although the numbers of BALF eosinophils, lymphocytes, and macrophages were increased after OVA challenge, no differences were evident between apoA-I−/− and WT mice. Consistent with the results of the BALF cell counts, lung histologic sections (Figure 2B) demonstrated an increase in peribronchial inflammatory cells, which primarily comprised neutrophils located in the submucosa (Figure 2C). These submucosal inflammatory cells reacted with an anti–Gr-1 (Ly-6G) antibody, as shown by confocal immunofluorescence microscopy in Figure 2D, thus confirming the identity of the infiltrating inflammatory cells as neutrophils (11).
Figure 2.
Neutrophilic airway inflammation is exacerbated in OVA-challenged apoA-I−/− mice. (A) Number of total BALF inflammatory cells (left) and inflammatory cell types (right) (n = 9–10 mice, *P < 0.01, apoA-I−/− + OVA versus WT + OVA). Data shown are representative of two experiments that produced similar results. (B) Representative histologic sections of lungs from WT and apoA-I−/− mice that had been challenged with saline or OVA, and were stained with hematoxylin and eosin (×200). (C) A representative higher magnification image (×1,000) of a hematoxylin and eosin–stained histologic section of a bronchus from an OVA-challenged apoA-I−/− mouse that demonstrated a submucosal infiltration comprised primarily of neutrophils. (D) Confocal immunofluorescence microscopy image (×400) of a bronchus from an OVA-challenged apoA-I−/− mouse that was reacted with an anti–Gr-1 antibody and a secondary antibody conjugated with Alexa Fluor 488 (green) that demonstrated a submucosal infiltration comprised primarily of neutrophils. The bronchus in D has a similar orientation to that of the bronchus in C, with the airway lumen located at the top of the image and the basement membrane denoted by an arrow.
Next, experiments were performed to demonstrate that the increase in BAL neutrophils did not reflect an isolated innate immune response to the low concentrations of LPS present in the OVA, but instead reflected an adaptive immune response to the OVA. The quantity of LPS present in the OVA preparation was measured by a limulus amebocyte assay, and was found to correspond to 0.006 μg of LPS per challenge. This is a low amount that was previously associated with the development of Th2 responses in experimental models of murine asthma, characterized by increased numbers of BALF macrophages, lymphocytes, neutrophils, and eosinophils (12, 13). Furthermore, the administration of higher doses of LPS to the lung was previously demonstrated to result in a significant influx of neutrophils at 24 hours (14–16). Therefore, WT and apoA-I−/− mice received a single intranasal challenge of OVA or saline, and BALF was collected after 24 hours to assess whether the small amount of LPS present in the OVA preparation could induce pulmonary neutrophilia. As shown in Figure 3A, no significant increase was evident in the total number of BALF cells or the numbers of neutrophils, macrophages, eosinophils, or lymphocytes in either OVA-challenged WT or apoA-I−/− mice, compared with mice that received a saline challenge.
Figure 3.
Modulation of BALF neutrophilia by a single OVA challenge and administration of the 5A apoA-I mimetic peptide in OVA-challenged apoA-I−/− mice. (A) Number of total inflammatory cells and inflammatory cell types in BALF 24 hours after a single OVA challenge (n = 3 mice). (B) Number of total inflammatory cells and inflammatory cell types in BALF from mice that had received an intranasal administration of either the 5A apolipoprotein A-I mimetic peptide (5A) or an irrelevant control peptide (control), concurrent with multiple daily saline or OVA challenges, 5 days per week for 4 weeks, after intraperitoneal sensitization with OVA plus aluminum hydroxide (n = 10 mice, *P < 0.01). NS, no significance.
Apolipoprotein A-I reconstitution experiments were performed to confirm that the phenotype of increased neutrophilic airway inflammation in the OVA-challenged apoA-I−/− mice was causally mediated by the absence of apoA-I expression. To address this question, apoA-I−/− mice received either the 5A apoA-I mimetic peptide or an irrelevant control peptide via an intranasal route daily, 5 days per week for 4 weeks, 30 minutes after daily OVA challenges. As shown in Figure 3B, the number of total BALF cells and BALF neutrophils recovered from OVA-challenged apoA-I−/− mice that received the 5A apoA-I mimetic peptide was reduced, compared with those that received the scrambled control peptide. Furthermore, total BALF cells and BALF neutrophils were increased to the same extent in OVA-challenged apoA-I−/− mice that received the 5A apoA-I mimetic peptide as OVA-challenged WT mice. This demonstrates that administration of the 5A apoA-I mimetic peptide can rescue the phenotype of increased neutrophilic airway inflammation in OVA-challenged apoA-I−/− mice. This finding is consistent with the conclusion that endogenous apoA-I functions to attenuate excessive OVA-induced airway neutrophilia in WT mice.
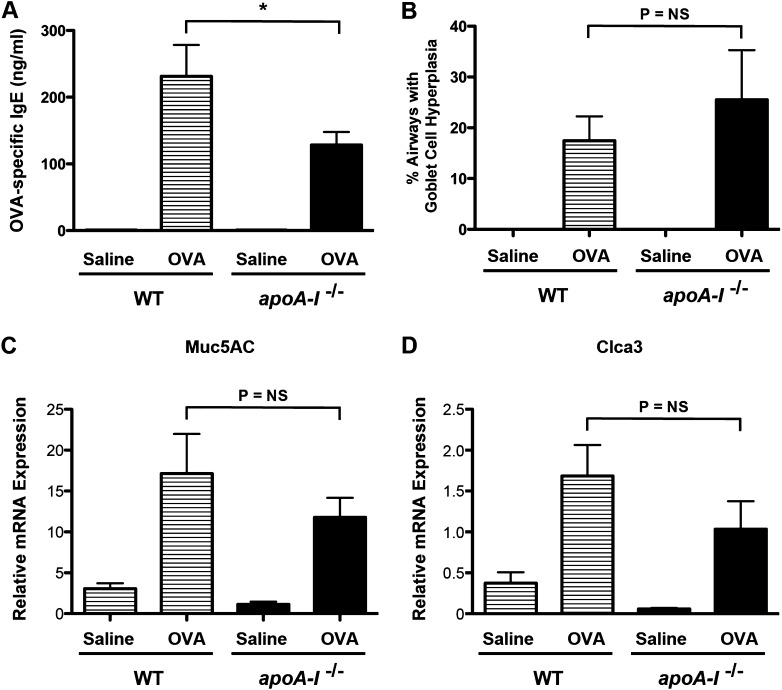
To further confirm that multiple OVA challenges induced an adaptive rather than an innate immune response, serum concentrations of OVA-specific IgE were measured. As shown in Figure 4A, increases in OVA-specific serum IgE concentrations were observed in OVA-challenged, but not saline-challenged, WT and apoA-I−/− mice. Furthermore, serum concentrations of OVA-specific IgE were significantly reduced in OVA-challenged apoA-I−/− mice, compared with OVA-challenged WT mice. However, no difference was evident in goblet-cell hyperplasia or mRNA concentrations of mucin 5AC or chloride channel calcium activated 3 between OVA-challenged apoA-I−/− mice and WT mice (Figures 4B–4D). Neither WT nor apoA-I−/− mice demonstrated AHR in response to OVA challenge (data not shown), which may reflect the C57BL/6 genetic background that has been associated with a reduced susceptibility to the development of AHR (17). Taken together, these data demonstrate that apoA-I−/− mice have a phenotype of enhanced neutrophilic airway inflammation and reduced serum IgE concentrations in response to OVA challenge.
Figure 4.
Attenuation of OVA-specific IgE in OVA-challenged apoA-I−/− mice. (A) Quantification of serum OVA-specific IgE (n = 9–10 mice, *P < 0.01, apoA-I−/− + OVA versus WT + OVA). (B) Goblet-cell hyperplasia is presented as the percentage of airways containing periodic acid–Schiff–positive cells (n = 5 mice, *P = NS, apoA-I−/− + OVA versus WT + OVA). We inspected 33.7 ± 1.9 airways in each mouse. Pooled data from two independent experiments are shown. (C and D) Quantification of lung mRNA concentrations for mucin 5AC and chloride channel calcium activated 3 (n = 6 mice, *P = NS, apoA-I−/− + OVA versus WT + OVA).
OVA-Induced IL-17A and TNF-α Expression and NF-κB Activation Are Increased in the Lungs of apoA-I−/− Mice
Next, we investigated the mechanisms mediating the enhanced neutrophilic airway inflammation in OVA-challenged apoA-I−/− mice. First, we examined whether the expression of Th1, Th2, and Th17 cytokines were modified. As shown in Figure 5, lung mRNA concentrations of IFN-γ and IL-17A were significantly increased in OVA-challenged apoA-I−/− mice, compared with OVA-challenged wild-type mice, whereas mRNA concentrations of IL-4, IL-5, and IL-13 were not increased. BALF IL-17A protein concentrations were also significantly increased in OVA-challenged apoA-I−/− mice, whereas BALF concentrations of IFN-γ were not detectable. In addition, both the percentage and mean fluorescence intensity of CD3+/CD4+/IL-17A+ T cells present in BALF were increased in OVA-challenged apoA-I−/− mice, compared with OVA-challenged WT mice. Taken together, these results are consistent with the conclusion that that apoA-I suppresses OVA-induced increases in IL-17A expression by Th17 T cells as part of the mechanism by which neutrophilic airway inflammation may be attenuated.
Figure 5.
Enhanced expression of mRNA encoding IL-17A in the lungs of OVA-challenged apoA-I−/− mice. (A–E) The quantification of lung mRNA concentrations for Th1 (A, IFN-γ), Th2 (B, IL-4; C, IL-5; and D, IL-13), and Th17 (E, IL-17A) cytokines was performed using quantitative RT-PCR, and is presented as relative mRNA expression (n = 6 mice, *P < 0.05, OVA-challenged apoA-I−/− mice versus OVA-challenged WT mice). (F) The quantification of IL-17A protein concentrations in BALF was performed by ELISA (n = 9 mice, *P < 0.01, OVA-challenged apoA-I−/− mice versus OVA-challenged WT mice). Data are representative of two experiments that produced similar results. (G and H) The percentage (G) and mean fluorescence intensity (MFI) (H) of CD3+/CD4+/IL-17A+ cells present in BALF from OVA-challenged WT and apoA-I−/− mice (n = 6 mice, *P < 0.05).
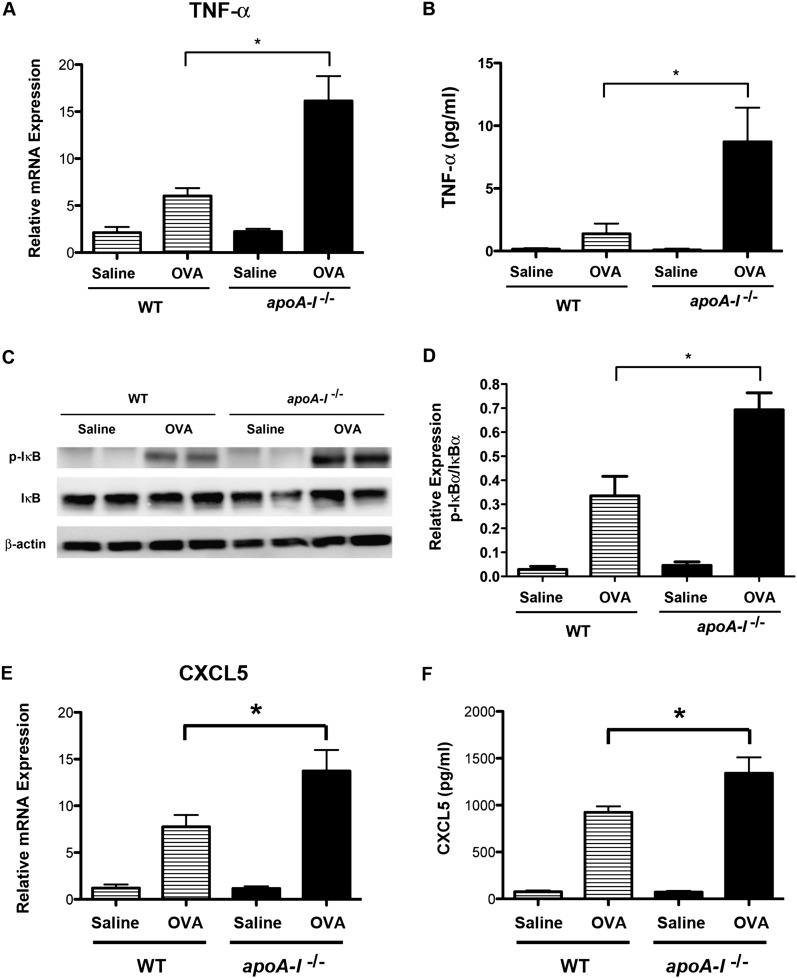
IL-17A and TNF-α were recently shown to mediate airway neutrophilia synergistically via the enhanced expression of CXCL5 (18). Furthermore, tumor necrosis factor mediates its proinflammatory effects in part via the enhanced expression of VCAM-1 and G-CSF, which are TNF-α–responsive genes (19). Therefore, we investigated whether TNF-α expression might be up-regulated in the lungs of OVA-challenged apoA-I−/− mice and thereby participate in the mechanism by which airway neutrophilia is increased. Compared with OVA-challenged WT mice, lung mRNA concentrations of TNF-α were significantly increased in OVA-challenged apoA-I−/− mice (Figure 6A). Similarly, TNF-α protein concentrations in BALF were increased in OVA-challenged apoA-I−/− mice, compared with OVA-challenged WT mice (Figure 6B). Furthermore, phospho-IκBα concentrations were significantly increased in OVA-challenged apoA-I−/− mice, which is consistent with an enhanced activation of NF-κB signaling pathways (Figures 6C and 6D). This suggests that increases in TNF-α expression and NF-κB signaling may participate in the mechanism by which airway neutrophilia is exacerbated in OVA-challenged apoA-I−/− mice.
Figure 6.
Enhanced expression of TNF-α and C-X-C motif ligand (CXCL)5 and increased activation of NF-κB in the lungs of OVA-challenged apoA-I−/− mice. (A) The quantification of lung mRNA concentrations for TNF-α was performed using quantitative RT-PCR, and is presented as relative mRNA expression (n = 6 mice, *P < 0.001, OVA-challenged apoA-I−/− mice versus OVA-challenged WT mice). (B) The quantification of TNF-α protein concentrations in BALF was performed by ELISA (n = 8 mice, *P < 0.01, OVA-challenged apoA-I−/− mice versus OVA-challenged WT mice). (C) Western blots of lung proteins were reacted with antibodies directed against phospho-IκBα, IκBα, or β-actin. Lung proteins from two individual mice are shown. The amount of β-actin present is shown as a control for the equivalency of protein loading. A representative blot is shown from four replicate experiments. (D) The ratio of phospho-IκBα to IκBα protein was quantified by densitometry (*P < 0.01, n = 4, OVA-challenged apoA-I−/− mice versus OVA-challenged WT mice). (E) The quantification of lung mRNA concentrations for CXCL5 was performed using quantitative RT-PCR, and is presented as relative mRNA expression (n = 6 mice, *P < 0.05, OVA-challenged apoA-I−/− mice versus OVA-challenged WT mice). (F) The quantification of CXCL5 protein concentrations in BALF was performed by ELISA (n = 9–10 mice, *P < 0.01, OVA-challenged apoA-I−/− mice versus OVA-challenged WT mice). Data are representative of two experiments that produced similar results.
Up-Regulated Expression of CXCL5, VCAM-1, and G-CSF in the Lungs of OVA-Challenged apoA-I−/− Mice
Experiments were then performed to investigate whether pathways that mediate airway neutrophilia downstream of IL-17A and TNF are attenuated by apoA-I. First, the expressions of CXC chemokines that mediate neutrophil chemotaxis were assessed. As shown in Figures 6E and 6F, lung mRNA and BALF protein concentrations of CXCL5, a chemokine that promotes neutrophil chemotaxis in the mouse, were increased in OVA-challenged apoA-I−/− mice, compared with OVA-challenged WT mice. In contrast, BALF protein concentrations of CXCL1 and CXCL2 were no different between OVA-challenged apoA-I−/− mice and OVA-challenged WT mice (Figure E1 in the online supplement). Taken together, this shows that endogenous apoA-I negatively regulates OVA-induced increases in expression of a chemokine pathway involving CXCL5 that is known to promote neutrophil chemotaxis.
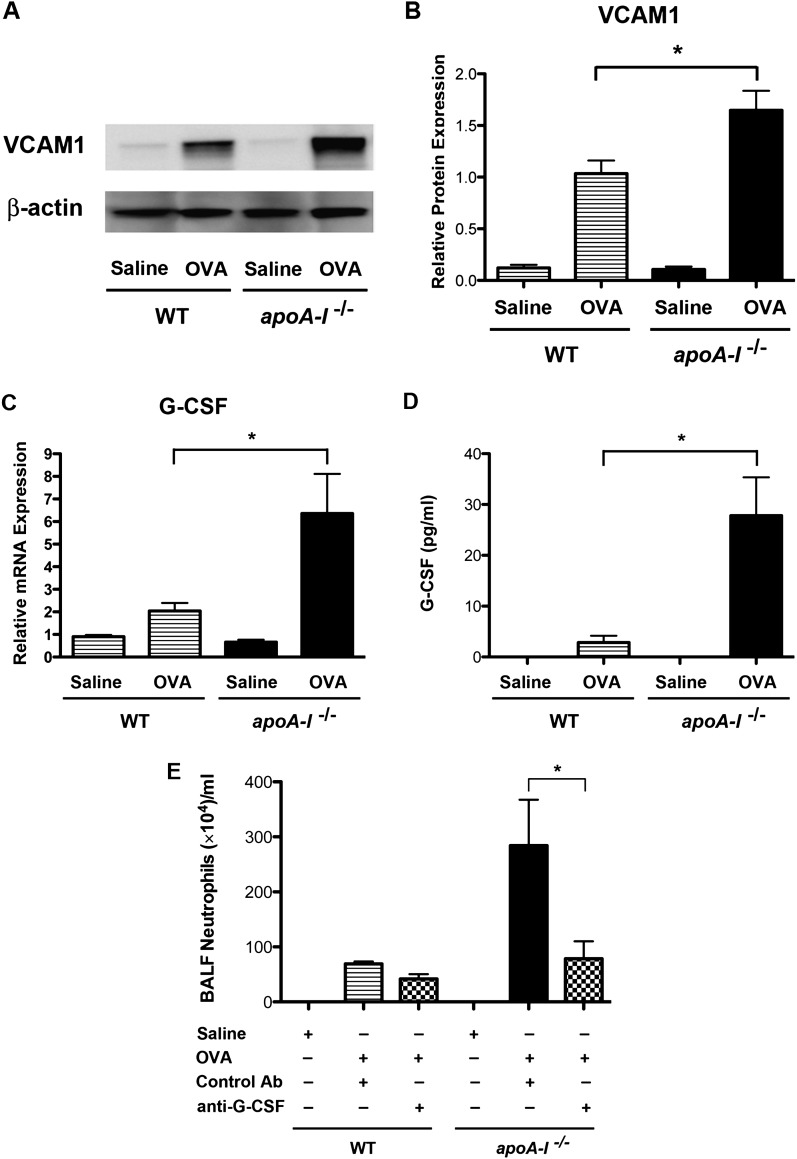
Next, we examined the effects of apoA-I on cell surface adhesion molecules that mediate leukocyte–endothelial cell interactions. Lung mRNA concentrations of VCAM-1 were significantly increased in OVA-challenged apoA-I−/− mice compared with OVA-challenged WT mice, whereas lung mRNA concentrations of intercellular adhesion molecule–1 (ICAM-1) were not altered (Figure E2). As shown in Figures 7A and 7B, the quantity of VCAM-1 protein present in the lungs of OVA-challenged apoA-I−/− mice was significantly increased. This suggests that OVA-induced increases in VCAM-1 protein expression may contribute to enhanced neutrophil transendothelial migration within the lung vasculature.
Figure 7.
The role of vascular cell adhesion molecule–1 (VCAM-1) and granulocyte colony–stimulating factor (G-CSF) in OVA-induced BALF neutrophilia in apoA-I−/− mice. (A) Western blots of lung proteins were reacted with antibodies directed against VCAM-1 or β-actin. We loaded 12.5 μg of protein per lane. The amount of β-actin present is shown as a control for the equivalency of protein loading. A representative blot is shown from four replicate experiments. (B) The relative expression of VCAM-1 compared with that of albumin was quantified by densitometry (*P < 0.01, n = 4, OVA-challenged apoA-I−/− mice versus OVA-challenged WT mice). (C) The quantification of lung mRNA concentrations for G-CSF was performed, using quantitative RT-PCR presented as relative mRNA expression (n = 6 mice, *P < 0.001, OVA-challenged apoA-I−/− mice versus OVA-challenged WT mice). (D) The quantification of G-CSF concentrations in BALF was performed (n = 9–10 mice, *P < 0.01, OVA-challenged apoA-I−/− mice versus OVA-challenged WT mice). (E) WT and apoA-I−/− mice were challenged with saline or OVA, concurrent with a nasal administration of either a neutralizing anti–G-CSF antibody or a control immunoglobulin. Numbers of BALF neutrophils are shown (n = 4–5 mice, *P < 0.001). Data are representative of two experiments that produced similar results.
Because the pathways that mediate neutrophil chemotaxis and vascular adhesion are up-regulated in the lungs of OVA-challenged apoA-I−/− mice, we assessed whether factors that promote the survival of recruited neutrophils are modified by apoA-I. After neutrophils have been recruited to the lung, survival factors, such as G-CSF, promote cell survival and thereby attenuate clearance from sites of inflammation (20). We found that lung mRNA concentrations of G-CSF (Figure 7C), but not of granulocyte–macrophage colony–stimulating factor (data not shown), were elevated in the lungs of OVA-challenged apoA-I−/− mice compared with OVA-challenged WT mice. Similarly, the quantity of G-CSF protein present in BALF was significantly increased in OVA-challenged apoA-I−/− mice (Figure 7D).
Neutralization of G-CSF Attenuates OVA-Induced Increases in BALF Neutrophilia in apoA-I−/− Mice
Lastly, we hypothesized that G-CSF might play a key functional role in mediating the enhanced airway neutrophilia in apoA-I−/− mice, based on the marked increase in G-CSF protein concentrations in BALF from OVA-challenged apoA-I−/− mice compared with OVA-challenged WT mice. To investigate the role of G-CSF in mediating enhanced airway neutrophilia in apoA-I−/− mice, a neutralizing anti–G-CSF antibody or control antibody was nasally administered concurrent with the multiple nasal OVA challenges. As shown in Figure 7E, the number of neutrophils recovered in BALF was significantly reduced by 72% in OVA-challenged apoA-I−/− mice that received the neutralizing anti–G-CSF antibody, compared with OVA-challenged apoA-I−/− mice that received the control antibody. This demonstrates that the ability of apoA-I to negatively regulate OVA-induced airway neutrophilia occurs primarily via a G-CSF–dependent mechanism.
Discussion
Apolipoprotein A-I is a major component of HDLs that mediate reverse cholesterol transport out of cells and also have anti-inflammatory, antioxidant, antithrombotic, and antifibrotic properties (5, 6). Consistent with this, the administration of 5A and D-4F apoA-I mimetic peptides was shown to inhibit airway inflammation, AHR, and goblet-cell hyperplasia in experimental murine models of asthma (8, 9). Furthermore, the delivery of apoA-I or apoA-I mimetic peptides was found to exert beneficial effects in other experimental models of lung disease. For example, the administration of purified human apoA-I decreased the number of macrophages, neutrophils, and lymphocytes recovered from BALF in a murine model of bleomycin-induced pulmonary lung inflammation and fibrosis, and also abrogated bleomycin-induced collagen deposition (21). The administration of purified human apoA-I was reported to diminish the acute lung injury caused by LPS or lipoteichoic acid (22, 23). Similarly, the administration of D-4F apoA-I mimetic peptide in a murine model of influenza infection reduced lung viral titers and prevented arterial macrophage trafficking (24). Experiments using type II pneumocytes infected with influenza A suggested that the effect of D-4F was mediated by a reduction in proinflammatory oxidized phospholipid secretion, caspase-3 and caspase-9 activation, IL-6 production, and viral replication (25).
Although the pharmacologic administration of apoA-I mimetic peptides can be used to attenuate the manifestations of asthma in murine models, the role of endogenous apoA-I in asthma was not known (8, 9). Here, we investigated whether manifestations of experimental asthma are modified in apoA-I−/− mice. We used a murine model of chronic allergen exposure that induced increases in BALF eosinophils, lymphocytes, and neutrophils, as well as in goblet-cell hyperplasia and OVA-specific IgE. OVA-challenged WT mice demonstrated a decrease in expression of apoA-I in the lung at both the mRNA and protein levels that correlated with a decrease in apoA-I protein in BALF. Interestingly, a recent proteomic analysis of BALF proteins also found that concentrations of apoA-I were decreased in patients with asthma compared with patients without asthma (26). Furthermore, apoA-I was reported to be expressed in the lung by epithelial cells and macrophages (21). We confirmed by confocal immunofluorescence microscopy that apoA-I is expressed by alveolar epithelial cells. However, apoA-I did not colocalize with CD68+ alveolar macrophages (Figure E3). It is also important to point out that plasma may represent an additional source of apoA-I in the lung, especially in the setting of inflammatory lung diseases, which can be associated with microvascular injury and the leakage of plasma proteins into the lung.
Next, we assessed whether the manifestations of asthma were modulated by apoA-I. We found that the lungs of OVA-challenged apoA-I−/− mice displayed a marked increase in the number of BALF neutrophils compared with wild-type mice, which suggests that endogenous apoA-I plays an important role in suppressing neutrophilic airway inflammation in asthma. Consistent with this conclusion, we showed that the phenotype of increased neutrophilic airway inflammation in OVA-challenged apoA-I−/− mice could be rescued by the administration of the 5A apoA-I mimetic peptide (8). Airway neutrophilia was shown to correlate with the severity of airflow obstruction in asthma, as well as with disease exacerbations, thus suggesting a causal relationship (27–32). Furthermore, patients with neutrophil-predominant asthma phenotypes demonstrate limited responsiveness to treatment with inhaled corticosteroids and would benefit from the development of alternative treatment approaches (33). Therefore, the identification of new mechanisms that modulate the pathogenesis of neutrophilic airway inflammation could represent a significant advance for patients with neutrophil-predominant forms of asthma.
We showed that endogenous apoA-I attenuated multiple pathways that participate in neutrophilic airway inflammation in OVA-challenged mice. First, mRNA and BALF protein concentrations of IL-17A were increased in the lungs of OVA-challenged apoA-I−/− mice. The expression of IL-17 is associated with neutrophilia, AHR, and disease severity in patients with asthma (2, 34–37). IL-17 induces neutrophilic airway inflammation via the generation of CXC chemokines (CXCL1, CXCL2, and CXCL5) and granulocyte colony-stimulating factors (G-CSF). Furthermore, OVA was shown to induce neutrophilic airway inflammation via an IL-17–dependent pathway involving CXCL5 (epithelial cell–derived neutrophil-activating peptide–78; ENA-78) and CXCR2 (38). It was recently demonstrated, however, that the intratracheal instillation of IL-17A alone is not sufficient to induce increases in BALF concentrations of CXCL5, G-CSF, or BAL neutrophils, but instead requires a synergistic effect with TNF-α (18). Consistent with this, we found that OVA-challenged apoA-I−/− mice also demonstrated significant increases in lung mRNA and BALF concentrations of TNF-α, as well as NF-κB activation.
The role of CXC chemokines and their receptors in mediating the increase in airway neutrophilia in OVA-challenged apoA-I−/− mice was also investigated. Patients with severe exacerbations of asthma exhibited increases in bronchial mucosal neutrophils, CXCL5, CXCL8, CXCR1, and CXCR2 (39). Although CXCL8 (IL-8) is a key chemokine mediating neutrophil chemotaxis in humans, it is not present in mice (40). Therefore, other chemokines, such as CXCL1 (growth-related oncogene α and keratinocyte-derived chemokine), CXCL2 (macrophage inflammatory protein [MIP]2α), CXCL3 (MIP2β), and CXCL5 (ENA-78), were shown to mediate neutrophil chemotaxis in mice (38, 40). We found that BALF concentrations of CXCL5, but not of CXCL1 or CXCL2, were increased in OVA-challenged apoA-I−/− mice compared with OVA-challenged WT mice. Taken together, these results indicate that endogenous apoA-I functions to attenuate OVA-induced neutrophilic airway inflammation by the down-regulation of multiple pathways that recruit neutrophils to the lung, which include proinflammatory cytokines (IL-17 and TNF-α) and chemokines (CXCL5) (40).
The transendothelial migration of neutrophils that are recruited to sites of inflammation requires cellular adhesion to activated vascular endothelial cells (41). VCAM-1 plays an important role in both the adhesion and transendothelial migration of neutrophils via interactions with integrins such as α9β1, expressed in human neutrophils, and α4β1, expressed in murine and rat neutrophils (41–44). Furthermore, IL-17A and TNF-α, which are up-regulated in OVA-challenged apoA-I−/− mice, are important stimuli for the expression of both VCAM-1 and G-CSF (41, 44). In addition, VCAM-1 expression in vascular endothelial cells in the setting of OVA challenge was shown to be CXCR2-dependent (45). Here, we show that VCAM-1 expression was significantly increased in the lungs of OVA-challenged apoA-I−/− mice that demonstrated enhanced expression of IL-17A and TNF-α. The expression of G-CSF was also significantly up-regulated in the lungs of OVA-challenged apoA-I−/− mice. Although G-CSF contributes to granulocytopoiesis in bone marrow, the extent of tissue neutrophilia is regulated by the rate of cellular apoptosis (41). G-CSF can enhance tissue neutrophilia by preventing apoptosis and thereby prolonging neutrophil survival in the lung (46). Taken together, these data suggest that the mechanism by which apoA-I attenuates OVA-induced neutrophilic airway inflammation also involves the down-regulation of VCAM-1 and G-CSF expression in the lung.
Although multiple pathways that mediate OVA-induced airway neutrophilia were up-regulated in apoA-I−/− mice, the most marked increase appeared to occur in G-CSF protein concentrations in BALF. Therefore, we hypothesized that the attenuation of G-CSF expression may represent the primary mechanism by which apoA-I negatively modulates OVA-induced neutrophilic airway inflammation. Consistent with this hypothesis, OVA-challenged apoA-I−/− mice that received an intranasal administration of a neutralizing anti–G-CSF antibody demonstrated a significant reduction in airway neutrophilia, compared with OVA-challenged apoA-I−/− mice that received a control antibody. This demonstrates that endogenous apoA-I primarily attenuates OVA-induced airway neutrophilia by suppressing the expression of G-CSF.
Further studies are ongoing to identify the relevant target cells that mediate the effects of apoA-I in the lung. Apolipoprotein A-I and the 5A apoA-I mimetic peptide both induce reverse cholesterol transport out of cells via interactions with the ATP-binding cassette transporter, ABCA1 (5, 7, 47). Thus, it is reasonable to speculate that lung cells that express ABCA1, such as alveolar macrophages, type II pneumocytes, vascular endothelial cells, and the lining epithelium of small bronchioles, mediate the ability of apoA-I to attenuate OVA-induced neutrophilic airway inflammation (48–50). Moreover, apoA-I was reported to bind LPS and directly inactivate its biological activity (51). This raises the additional possibility that the anti-inflammatory mechanism by which apoA-I attenuates neutrophilic airway inflammation could involve the direct binding and sequestration of key proinflammatory molecules or proteins.
In conclusion, we have shown that apoA-I in the lung functions as an endogenous negative regulator of multiple pathways that mediate neutrophilic airway inflammation in OVA-challenged mice. These pathways include the up-regulated expression of (1) proinflammatory cytokines (IL-17A and TNF-α), (2) NF-κB signaling, (3) CXC chemokines that mediate neutrophil recruitment (CXCL5), (4) vascular adhesion molecules (VCAM-1), and (5) colony-stimulating factors (G-CSF). Furthermore, we have shown that the ability of apoA-I to attenuate OVA-induced neutrophilic airway inflammation is mediated primarily via a G-CSF–dependent mechanism. Taken together, these data suggest that endogenous apoA-I may be an important negative modulator of neutrophilic airway inflammation in asthma.
Supplementary Material
Acknowledgments
The authors are extremely appreciative of the staff at the Laboratory of Animal Medicine and Surgery in the National Heart, Lung, and Blood Institute, whose commitment, professional advice, and excellent technical support made this study possible. The authors also thank Drs. Joel Moss and Martha Vaughan for very helpful discussions.
Footnotes
This work was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, and by National Institutes of Health grant 1ZIAHL006053-02 (S.J.L.).
This work was presented in abstract form at the 2011 American Thoracic Society International Conference.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0322OC on March 15, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fanta CH. Asthma. N Engl J Med 2009;360:1002–1014 [DOI] [PubMed] [Google Scholar]
- 2.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol 2010;11:577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet 2006;368:804–813 [DOI] [PubMed] [Google Scholar]
- 4.Yao X, Fredriksson K, Yu ZX, Xu X, Raghavachari N, Keeran KJ, Zywicke GJ, Kwak M, Amar MJ, Remaley AT, et al. Apolipoprotein E negatively regulates house dust mite–induced asthma via a LDL receptor–mediated pathway. Am J Respir Crit Care Med 2010;182:1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol 2011;8:222–232 [DOI] [PubMed] [Google Scholar]
- 6.Osei-Hwedieh DO, Amar M, Sviridov D, Remaley AT. Apolipoprotein mimetic peptides: mechanisms of action as anti-atherogenic agents. Pharmacol Ther 2011;130:83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi AA, Amar M, Shamburek RD, Remaley AT. Apolipoprotein AI mimetic peptides: possible new agents for the treatment of atherosclerosis. Curr Opin Investig Drugs 2007;8:201–212 [PubMed] [Google Scholar]
- 8.Yao X, Dai C, Fredriksson K, Dagur PK, Mccoy JP, Qu X, Yu ZX, Keeran KJ, Zywicke GJ, Amar MJA, et al. 5A, an apolipoprotein A-I mimetic peptide, attenuates the induction of house dust mite–induced asthma. J Immunol 2011;186:576–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nandedkar SD, Weihrauch D, Xu H, Shi Y, Feroah T, Hutchins W, Rickaby DA, Duzgunes N, Hillery CA, Konduri KS, et al. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation and oxidative stress in a murine model of asthma. J Lipid Res 2011;52:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao X, Dai C, Fredriksson K, Lam J, Gao M, Keeran KJ, Nugent GZ, Qu X, Yu ZX, Jeffries N, et al. Human apolipoprotein E genotypes differentially modify house dust mite–induced airway disease in mice. Am J Physiol Lung Cell Mol Physiol 2012;302:L206–L215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su X, Matthay MA, Malik AB. Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing Gram-negative sepsis–induced acute lung inflammatory injury. J Immunol 2010;184:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, Toll-like receptor 4–dependent T helper cell Type 2 responses to inhaled antigen. J Exp Med 2002;196:1645–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YK, Oh SY, Jeon SG, Park HW, Lee SY, Chun EY, Bang B, Lee HS, Oh MH, Kim YS, et al. Airway exposure levels of lipopolysaccharide determine Type 1 versus Type 2 experimental asthma. J Immunol 2007;178:5375–5382 [DOI] [PubMed] [Google Scholar]
- 14.Arndt PG, Young SK, Lieber JG, Fessler MB, Nick JA, Worthen GS. Inhibition of c-Jun N-terminal kinase limits lipopolysaccharide-induced pulmonary neutrophil influx. Am J Respir Crit Care Med 2005;171:978–986 [DOI] [PubMed] [Google Scholar]
- 15.Arndt PG, Young SK, Poch KR, Nick JA, Falk S, Schrier RW, Worthen GS. Systemic inhibition of the angiotensin-converting enzyme limits lipopolysaccharide-induced lung neutrophil recruitment through both bradykinin and angiotensin II–regulated pathways. J Immunol 2006;177:7233–7241 [DOI] [PubMed] [Google Scholar]
- 16.Maris NA, van der Sluijs KF, Florquin S, de Vos AF, Pater JM, Jansen HM, van der Poll T. Salmeterol, a beta2-receptor agonist, attenuates lipopolysaccharide-induced lung inflammation in mice. Am J Physiol Lung Cell Mol Physiol 2004;286:L1122–L1128 [DOI] [PubMed] [Google Scholar]
- 17.De Sanctis GT, Merchant M, Beier DR, Dredge RD, Grobholz JK, Martin TR, Lander ES, Drazen JM. Quantitative locus analysis of airway hyperresponsiveness in A/J and C57BL/6J mice. Nat Genet 1995;11:150–154 [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Mei J, Gonzales L, Yang G, Dai N, Wang P, Zhang P, Favara M, Malcolm KC, Guttentag S, et al. Il-17A and TNF-α exert synergistic effects on expression of CXCL5 by alveolar Type II cells in vivo and in vitro. J Immunol 2011;186:3197–3205 [DOI] [PubMed] [Google Scholar]
- 19.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 1989;59:1203–1211 [DOI] [PubMed] [Google Scholar]
- 20.Eyles JL, Roberts AW, Metcalf D, Wicks IP. Granulocyte colony–stimulating factor and neutrophils: forgotten mediators of inflammatory disease. Nat Clin Pract Rheumatol 2006;2:500–510 [DOI] [PubMed] [Google Scholar]
- 21.Kim TH, Lee YH, Kim KH, Lee SH, Cha JY, Shin EK, Jung S, Jang AS, Park SW, Uh ST, et al. Role of lung apolipoprotein A-I in idiopathic pulmonary fibrosis: antiinflammatory and antifibrotic effect on experimental lung injury and fibrosis. Am J Respir Crit Care Med 2010;182:633–642 [DOI] [PubMed] [Google Scholar]
- 22.Jiao YL, Wu MP. Apolipoprotein A-I diminishes acute lung injury and sepsis in mice induced by lipoteichoic acid. Cytokine 2008;43:83–87 [DOI] [PubMed] [Google Scholar]
- 23.Yan YJ, Li Y, Lou B, Wu MP. Beneficial effects of apoA-I on LPS-induced acute lung injury and endotoxemia in mice. Life Sci 2006;79:210–215 [DOI] [PubMed] [Google Scholar]
- 24.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Garber DW, Fishbein MC, Adhikary L, Nayak DP, Hama S, Navab M, Fogelman AM. Influenza infection promotes macrophage traffic into arteries of mice that is prevented by D-4F, an apolipoprotein A-I mimetic peptide. Circulation 2002;106:1127–1132 [DOI] [PubMed] [Google Scholar]
- 25.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP, Fogelman AM. D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human Type II pneumocytes. Circulation 2004;110:3252–3258 [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Kim KH, Kim JM, Yoon SH, Kim TH, Park SW, Park JS, Uh ST, Lee HS, Kim YH, et al. Relationship between group-specific component protein and the development of asthma. Am J Respir Crit Care Med 2011;184:528–536 [DOI] [PubMed] [Google Scholar]
- 27.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc 2009;6:256–259 [DOI] [PubMed] [Google Scholar]
- 28.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol 1995;95:843–852 [DOI] [PubMed] [Google Scholar]
- 29.Lamblin C, Gosset P, Tillie-Leblond I, Saulnier F, Marquette CH, Wallaert B, Tonnel AB. Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am J Respir Crit Care Med 1998;157:394–402 [DOI] [PubMed] [Google Scholar]
- 30.Ordonez CL, Shaughnessy TE, Matthay MA, Fahy JV. Increased neutrophil numbers and IL-8 levels in airway secretions in acute severe asthma: clinical and biologic significance. Am J Respir Crit Care Med 2000;161:1185–1190 [DOI] [PubMed] [Google Scholar]
- 31.Shaw DE, Berry MA, Hargadon B, McKenna S, Shelley MJ, Green RH, Brightling CE, Wardlaw AJ, Pavord ID. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest 2007;132:1871–1875 [DOI] [PubMed] [Google Scholar]
- 32.Sur S, Crotty TB, Kephart GM, Hyma BA, Colby TV, Reed CE, Hunt LW, Gleich GJ. Sudden-onset fatal asthma: a distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis 1993;148:713–719 [DOI] [PubMed] [Google Scholar]
- 33.Berry M, Morgan A, Shaw DE, Parker D, Green R, Brightling C, Bradding P, Wardlaw AJ, Pavord ID. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007;62:1043–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol 2010;184:1663–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. Il-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res 2006;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med 2003;97:726–733 [DOI] [PubMed] [Google Scholar]
- 37.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008;28:454–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med 2009;180:720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiu Y, Zhu J, Bandi V, Guntupalli KK, Jeffery PK. Bronchial mucosal inflammation and upregulation of CXC chemoattractants and receptors in severe exacerbations of asthma. Thorax 2007;62:475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan X, Patera AC, Pong-Kennedy A, Deno G, Gonsiorek W, Manfra DJ, Vassileva G, Zeng M, Jackson C, Sullivan L, et al. Murine CXCR1 is a functional receptor for GCP-2/CXCL6 and interleukin-8/CXCL8. J Biol Chem 2007;282:11658–11666 [DOI] [PubMed] [Google Scholar]
- 41.Borregaard N. Neutrophils, from marrow to microbes. Immunity 2010;33:657–670 [DOI] [PubMed] [Google Scholar]
- 42.Taooka Y, Chen J, Yednock T, Sheppard D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule–1. J Cell Biol 1999;145:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mambole A, Bigot S, Baruch D, Lesavre P, Halbwachs-Mecarelli L. Human neutrophil integrin alpha9beta1: up-regulation by cell activation and synergy with beta2 integrins during adhesion to endothelium under flow. J Leukoc Biol 2010;88:321–327 [DOI] [PubMed] [Google Scholar]
- 44.Briscoe DM, Cotran RS, Pober JS. Effects of tumor necrosis factor, lipopolysaccharide, and IL-4 on the expression of vascular cell adhesion molecule–1 in vivo: correlation with CD3+ T cell infiltration. J Immunol 1992;149:2954–2960 [PubMed] [Google Scholar]
- 45.Hallgren J, Jones TG, Abonia JP, Xing W, Humbles A, Austen KF, Gurish MF. Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc Natl Acad Sci USA 2007;104:20478–20483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matute-Bello G, Liles WC, Radella F, II, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med 1997;156:1969–1977 [DOI] [PubMed] [Google Scholar]
- 47.Sethi AA, Stonik JA, Thomas F, Demosky SJ, Amar M, Neufeld E, Brewer HB, Davidson WS, D'Souza W, Sviridov D, et al. Asymmetry in the lipid affinity of bihelical amphipathic peptides: a structural determinant for the specificity of ABCA1-dependent cholesterol efflux by peptides. J Biol Chem 2008;283:32273–32282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J Physiol Lung Cell Mol Physiol 2005;289:L980–L989 [DOI] [PubMed] [Google Scholar]
- 49.Wellington CL, Walker EK, Suarez A, Kwok A, Bissada N, Singaraja R, Yang YZ, Zhang LH, James E, Wilson JE, et al. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab Invest 2002;82:273–283 [DOI] [PubMed] [Google Scholar]
- 50.Liao H, Langmann T, Schmitz G, Zhu Y. Native LDL upregulation of ATP-binding cassette transporter–1 in human vascular endothelial cells. Arterioscler Thromb Vasc Biol 2002;22:127–132 [DOI] [PubMed] [Google Scholar]
- 51.Emancipator K, Csako G, Elin RJ. In vitro inactivation of bacterial endotoxin by human lipoproteins and apolipoproteins. Infect Immun 1992;60:596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.