Abstract
BACKGROUND
This study examined the effects of brief daily yogic meditation on mental health, cognitive functioning, and immune cell telomerase activity in family dementia caregivers with mild depressive symptoms.
METHODS
Thirty-nine family dementia caregivers (mean age 60.3 years old (SD=10.2)) were randomized to practicing Kirtan Kriya or listening to relaxation music for 12 minutes per day for eight weeks. The severity of depressive symptoms, mental and cognitive functioning were assessed at baseline and follow-up. Telomerase activity in peripheral blood mononuclear cells (PMBC) was examined in peripheral PBMC pre- and post-intervention.
RESULTS
The meditation group showed significantly lower levels of depressive symptoms and greater improvement in mental health and cognitive functioning compared to the relaxation group. In the meditation group, 65.2% showed 50% improvement on the Hamilton Depression Rating scale and 52% of the participants showed 50% improvement on the Mental Health Composite Summary score (MCS) of the SF-36 scale; compared to 31.2% and 19% respectively in the relaxation group (pp<0.05). The meditation group showed 43% improvement in telomerase activity compared to 3.7% in the relaxation group (p=0.05).
CONCLUSION
This pilot study found that brief daily meditation practices by family dementia caregivers can lead to improved mental and cognitive functioning, and lower levels of depressive symptoms. This improvement is accompanied by an increase in telomerase activity suggesting improvement in stress-induced cellular aging. These results need to be confirmed in a larger sample.
Keywords: Dementia caregiver, stress, depression, resilience, cognition, Kirtan Kriya, yoga, meditation, relaxation, telomerase, NFkB
The prevalence of dementia and the number of family caregivers will increase dramatically in the next two decades adding to the cost of clinical care and burden as the US aging population increases. Currently, at least five million Americans provide care for someone with dementia (Schulz & Martire, 2004). The detrimental impact to personal, social, and health outcomes of informal dementia caregiving has been well documented in recent years (Lavretsky, 2005; Lavretsky et al., 2010).
Chronic stress places caregivers at a higher risk for developing depression. On average, the incidence and prevalence of clinical depression in family dementia caregivers approaches 50% (Lavretsky, 2005). Caregivers are twice as likely to report high levels of emotional distress (Lavretsky, 2005). The trend of impaired resilience to stress with advancing age, indicates an increased rate of cardiovascular disease and mortality. Although the relation between mental and physical health have been previously documented, the mechanistic links are beginning to be understood at the cellular level (G. Miller et al., 2009). Telomere length has recently been proposed as a useful ‘psychobiomarker’ linking chronic psychological stress and diseases in aging (E. S. Epel et al., 2006). A telomere is a region of repetitive DNA sequences at the end of a chromosome, which protects the end of the chromosome from deterioration. Shortened telomere length and reduced telomerase (the cellular enzyme primarily responsible for telomere length and maintenance) are associated with premature mortality and predict a host of health risks and diseases (J. Lin, Epel, E.S., Blackburn, E.H., 2009), which may be regulated in part by psychological stress (E. Epel et al., 2009; E. S. Epel et al., 2004; Ornish et al., 2008). Over the long term, high telomerase likely promotes improvement in telomere maintenance and immune cell longevity(Jacobs et al., 2010). Although the link between replicative senescence and exhaustion that control T-cell proliferative activity and function is still not clear (Akbar & Henson, 2011), the change in these parameters in the context of randomized mind-body interventions is of interest. A recent study (Jacobs et al., 2010) showed that meditation and positive psychological change was associated with higher levels of telomerase activity.
Many of the existing psychosocial interventions for caregiver stress and depression show only modest benefits in caregiver outcomes (Lavretsky, 2005). In our recent study of dementia caregiver depression, we demonstrated greater improvement in depression and resilience with the standard antidepressant treatment using escitalopram compared to a placebo (Lavretsky et al., 2010). However, many caregivers may be opposed to the use of medication because of the associated cost and drug side-effects, and prefer to use complementary and alternative medicine for stress reduction. These considerations motivated us to test a brief mind-body intervention for stress reduction. We performed a randomized pilot study of Kundalini yoga Kirtan Kriya meditation compared to passive relaxation with listening to instrumental music for 12 minutes per day for eight weeks. The relaxation control group was selected to test whether relaxation response was responsible for the effect of Kirtan Kriya on distress and cognition in caregivers, in which case both groups would have similar outcomes. Two prior studies indicated changes in the brain blood flow with Kirtan Kriya (Khalsa et al., 2009; Newberg et al., 2010). However, it is unclear whether there are any other mechanisms that contribute to the meditation response compared to relaxation.
We hypothesized that Kirtan Kriya practice will result in improved levels of primary (i.e., mental health and depression) and secondary outcomes (i.e., cognition) compared to passivelistening-to-music relaxation in an eight week intervention study. We also anticipated that meditation would increase telomerase activity as compared to the relaxation based on prior findings that caregiver stress is associated with cellular senescence (E. Epel et al., 2009), and that a meditation intervention can increase telomerase levels (Jacobs et al., 2010). The primary outcome was identified as response with 50% improvement in the Hamilton Depression Rating Scale score (HAMD) (Hamilton, 1960) and in the Mental Health composite summary score (MCS) of the Medical Outcomes Study Short Form 36-Item Health Survey (SF-36) (Ware & Sherbourne, 1992), an instrument that measures mental and physical functioning.
Methods
Over a period of 12 months, we screened a total of 69 family caregivers and recruited 49 family caregivers (45–91 years of age, 2 men, 36 adult children, 13 spouses) who were taking care of their relatives with dementia. After completing a full description of study to the subjects, written informed consent was obtained in accordance with the procedures set by the UCLA Institutional Review Board.
Potential subjects were required to be adult or elderly caregivers of patients with dementia being evaluated by the geriatric psychiatry and memory clinics. In addition, the subjects were identified by the patient or clinical staff as the primary source of assistance and/or support, and were in contact with the dementia patient at least three days per week. First, subjects were screened using the Structured Clinical Interview for the DSM-IVR (SCID) was administered by a single rater (HL) to exclude those with major depressive disorder because of the restriction on psychotropic drug use or any other treatment for depression(Spitzer et al., 1992). The Hamilton Rating Scale for Depression (HAM-D-24 item) was used to rule out major depression that would require other treatment modalities (Hamilton, 1960). Those who had scores between 5 and 17 signifying mild-to-moderate levels of depressive symptoms were invited to participate in the trial. Lastly, a score of 26 or higher on the Folstein Mini-mental State Examination (MMSE) was required for inclusion (Folstein et al., 1975). Exclusion criteria were: 1) A history of any other psychiatric illness; 2) alcohol and/or substance abuse or dependence; 3) severe or acute medical illness; 4) acute suicidal or violent behavior; and 5) any other CNS disease or dementia.
Procedures
All subjects were randomized using a computer-generated randomization table by a masked statistician to participate in either Kirtan Kriya practice or a relaxation practice for 12 minutes per day for eight weeks. The protocol for Kirtan Kriya is standard for the Kundalini Yoga practice as taught by Yogi Bhajan that was utilized in the previous studies in older adults (Newberg et al., 2010). We assessed the severity of depressive symptoms, mental and physical functioning and cognition at baseline, at the end of the eight-week study, or upon early termination. In addition to the treatment protocol, all caregivers also received psychoeducation about the course and prognosis of dementia and about caregiver health, which was intended to address identified shortcomings in the care of their care-recipients.
Intervention protocol
We provided samples of both the meditation and relaxation CDs during screening visits to assess if subjects were amenable to the interventions. Only subjects who were comfortable with either intervention and agreed to participate were recruited.
Meditation group procedures
Meditation was introduced to caregivers during their baseline visit. A brief 12 minute yogic practice included an ancient chanting meditation, Kirtan Kriya, which was performed every day at the same time of the day for a total of eight weeks, based on a utilized and previously tested protocol with recorded CD (Newberg et al., 2010). The meditation involved repetitive finger movements or mudras, as well as chanting of the mantra “Saa, Taa, Naa, Maa,” meaning “Birth, Life, Death, and Rebirth” that are chanted first aloud, then in a whisper, and silently for the total of 11 minutes with 1 minute allocated to “tuning in” at the beginning and the final deep breathing relaxation accompanied by the visualization of light ("Alzheimer's research & prevention foundation: Arpf research: Kirtan kriya", 2011).
Relaxation group procedures
Participants in the relaxation group were asked to relax in a quiet place with their eyes closed while listening to the instrumental music on the relaxation CD for 12 minutes every day at the same time for eight weeks. The music was provided to the subjects on a CD along with instructions to find a quiet place without distractions and close their eyes and try to relax. The choice of control was motivated by the intention to test whether relaxation response was solely responsible for the effect of meditation. Because Kirtan Kriya involved melodic chanting, we chose to control with relaxation while listening to instrumental music. Compliance and satisfaction with either intervention were monitored at each visit by direct interview and review of daily diaries documenting the length of and adherence to the practice.
Assessment instruments
Several instruments were used to assess psychological distress and coping. Mental Health composite score (MCS score) was assessed using the Medical Outcomes Study Short Form 36-Item Health Survey (SF-36) (Ware & Sherbourne, 1992), an instrument that measures health-related quality-of-life, mental, physical, and social functioning. The Hamilton Rating Scale for Depression (HRSD-24 item) (Hamilton, 1960) was used to quantify mood symptoms.
The remaining instruments were used to compare groups at baseline. Cognitive performance was measured by the Mini-Mental State Examination (MMSE) (Folstein et al., 1975). The Cumulative Illness Rating Scale (CIRS) (M. D. Miller et al., 1992) was used for rating global chronic medical illness burden.
Cognitive functioning
We administered a brief neuropsychological assessment battery that measured the domains likely to show impairment in geriatric depression. The battery was comprised of the following instruments: California Verbal Learning Test II (CVLT II) (Delis et al., 2000); to test verbal memory, Trailmaking A (Reitan, 1958) to test attention and speeded information processing, and Trailmaking B to test executive function.
Blood sample collection, PBMC isolation, and telomerase extract preparation
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized whole blood by density gradient separation (Ficoll-paque plus), then washed three times in phosphatebuffered saline (PBS). Live cells were counted by Trypan Blue dye exclusion using a hemocytometer. Extracts corresponding to 5,000 live cells/μL were made, based on the protocol provided in the TRAPeze telomerase detection kit (Chemicon, Temecula, CA). The extracts were frozen and stored at −80 °C, then shipped on dry ice to the UCSF Blackburn Laboratory for analyses.
Telomeric repeat amplification protocol
Quantification of telomerase activity was measured from the extract using the telomeric repeat amplification protocol (TRAP) as previously described with a commercial kit according to the kit manual (TRAPeze®, Chemicon, Temecula, CA). Between 2000 and 10000 cells were used for TRAP reactions to ensure that the assay was in linear range for each sample (J. Lin, Epel, E.S., Blackburn, E.H., 2009). The PCR program used was: 94 °C for 2 minutes; 94 °C for 30 seconds, 59 °C for 30 seconds for 30 cycles. The products were fractionated on a 10% polyacrylamide-8 M urea sequencing gel and scanned on a STORM 860 molecular imager (GE Healthcare, Piscataway, NJ). The 293T cell line was used as a positive telomerase activity control and reference standard. Telomerase activity was quantified using the software ImageQuant 5.2 (GE Healthcare, Piscataway, NJ) as previously described (J. Lin et al., 2010) which reports activity per 10,000 cells.
Statistical analysis
All data were entered into the database at the time of their collection. Participants in the two treatment groups were compared on all demographic and clinical measures at baseline to assess the success of the randomization procedures using chi-square tests for the categorical measures and two-sided t-tests for the continuous measures.
Mental health, the primary outcome, was compared using two measures – the Mental Health Scale of the SF-36 and HAM-D. Subjects who improved by fifty percent or more in the Mental Health Scale of the SF-36 and HAM-D were classified as responders. Fisher’s exact test was used to test response in the meditation group compared to the relaxation group. Cognition, the secondary outcome measure, was analyzed using a multivariate analysis of covariance (MANCOVA; controlling for age) with the Mini Mental State Examination score, Trailmaking A (time), Trailmaking B (time) and CVLT (long delay cued recall) as the dependent variables. If the MANCOVA was significant, ANCOVAs were performed to determine which, if any, individual measures contributed to the significant difference. As exploratory studies, changes in telomerase levels in the two groups were analyzed using a separate ANCOVA, controlling for age. We also explored the association between changes in telomerase with changes in depression and health functioning using Pearson correlation coefficients. Significance was set at p≤ 0.05. Two-sided p-values are reported for all baseline comparisons, and in keeping with the directional hypotheses of the study, one-sided p-values are reported for all the univariate tests pertaining to change scores.
Results
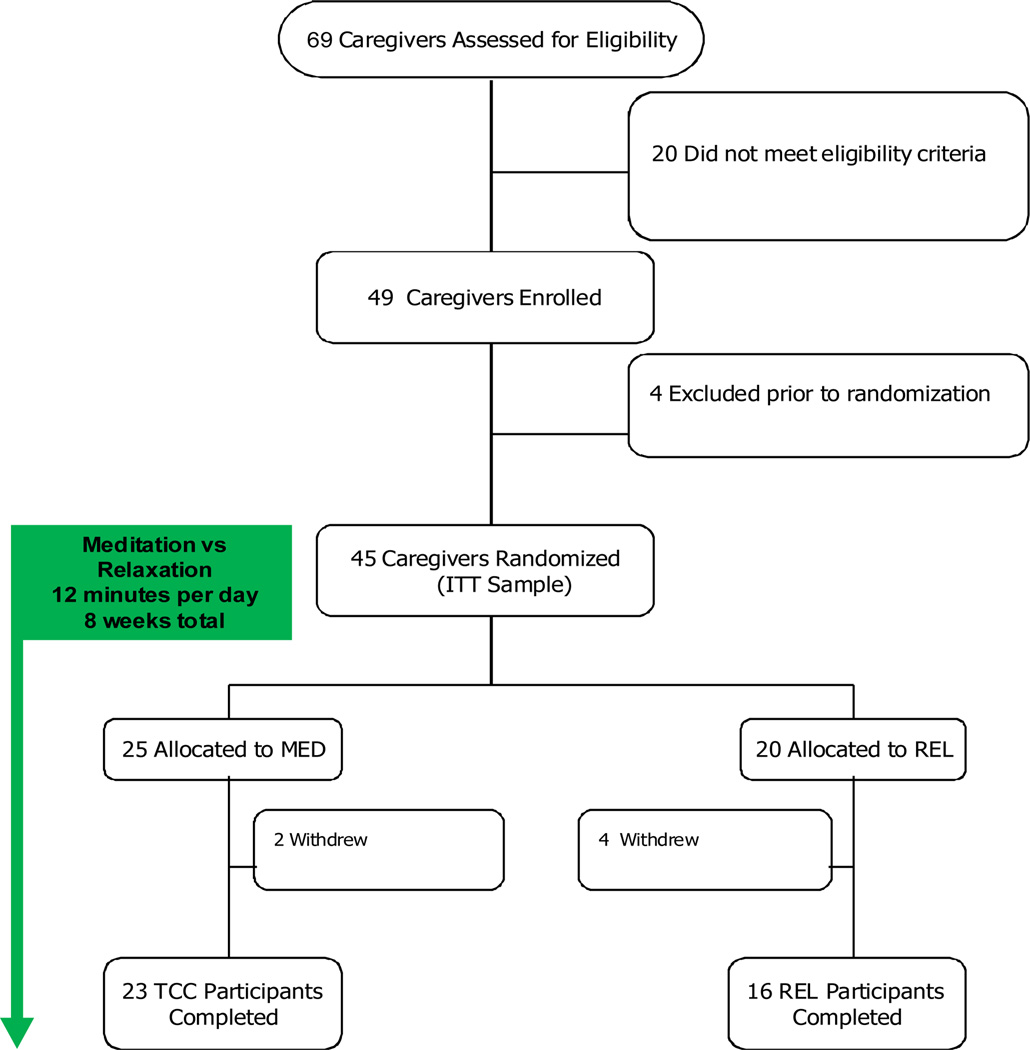
Of 49 recruited subjects, 45 were randomized, and 39 (80%) completed the intervention: 23 subjects in the meditation group, and 16 subjects in the relaxation group. Total of 10 subjects (20%) dropped out: four dropped out prior to randomization, and post-randomization, four dropped out from the relaxation group and two from the meditation group. Dropouts in either group occurred due to lack of interest in the intervention or inability to commit to the study schedule (Figure 1), and did not differ from the participants on any characteristics. Table 1 presents the baseline demographic and clinical characteristics of the completers in the two subject groups.
Figure 1.
Participant Flow and Distribution of Subjects in the Study.
Table 1.
Comparison of the baseline demographic, clinical, and biological characteristics in the two treatment groups.
| Meditation (N=23) Mean (SD) or N (%) |
Relaxation (N=16) Mean (SD) or N (%) |
t/Chi-square | p-value | |
|---|---|---|---|---|
| Age in years | 60.5 (28.2) | 60.6 (12.5) | 0.03 | 0.9 |
| Male gender* | 0 (0%) | 2 (13%) | 3.0 | 0.08 |
| Education, years | 16.1 (2.1) | 15.1 (2.8) | 1.2 | 0.2 |
| Months of depression | 45.1 (35.4) | 39 (21.2) | 0.6 | 0.5 |
| Years of caregiving | 4.7 (2.4) | 4.2 (2.9) | 0.6 | 0.6 |
| Hours of care per week | 47.8 (35.8) | 63.3 (36.2) | −1.3 | 0.2 |
|
Medical burden CIRS |
3.0 (2.3) | 4.6 (3.1) | −1.8 | 0.08 |
| CVRF | 5.2 (3.7) | 7.4 (6.4) | −1.4 | 0.2 |
|
Mental Health HAM-D |
11.9 (4.1) | 11.4 (4.0) | 0.4 | 0.7 |
| MCS of SF-36 | 34.4(10.2) | 37.3 (11.0) | −0.8 | 0.4 |
|
Cognition MMSE |
29.45 (1.0) | 29.6 (0.6) | −0.3 | 0.6 |
| Trails A time | 35.3 (15.4) | 51.2 (41.1) | −1.7 | 0.1 |
| Trails B time | 76.3 (36.7) | 97.8 (58.4) | −1.4 | 0.2 |
| CVLT Long delay cued recall | 12.9 (2.7) | 11.3 (2.8) | 1.7 | 0.1 |
|
Biological activity Telomerase, unit/10,000 |
2.7 (1.3) | 3.0 (1.3) | −0.6 | 0.6 |
chi-square statistics (df=1) reported for gender ratio; t-statistics (df=37) for all other variables; two-sided p-values reported for all variables
SF-36 MCS – Mental Health composite summary scores
MMSE – Mini-Mental State Examination scale
HAM-D – Hamilton Depression Rating Scale
CIRS – Cumulative Illness Rating Scale
CVRF – Cerebrovascular Risk Factors scale
Trail A – time in sec
Trail B – time in sec
The groups did not differ on any of the baseline characteristics. The groups did not differ either on the time spent in support or the psychoeducation activities. Age was the only demographic variable that correlated with the outcome measures (age was associated with cognitive measures at baseline, p <.001-.0001); hence, age was used as a covariate in the analyses.
Mental Health
As indexed by a response of 50% or greater improvement on the Mental Health Summary (MCS) score of the SF-36, 12 subjects (52.2%) responded in the meditation group, while 3 subjects (18.7%) responded in the relaxation group (p = .02, one-tailed Fisher's exact test). Similarly, for HAM-D, 15 subjects (65.2%) responded in the meditation group, while 5 subjects (31.2%) responded in the relaxation group (p = .03, one-tailed Fisher's exact test).
Cognition
The MANCOVA showed significant group differences in change of cognition (F(4,33) = 3.82, p = .01). Individually, the MMSE total and Trailmaking B (time) showed significant improvement in the meditation group compared to the relaxation group (Table 2).
Table 2.
Comparison of the change scores in clinical outcomes and biological measures in the two treatment groups.
| Change scores | Meditation (N=23) Mean (SD) |
Relaxation (N=16) Mean (SD) |
F (1,36)* |
p-value* | Effect size Cohen’s d |
|---|---|---|---|---|---|
|
Mental health HAM-D |
−7.4 (3.7) | −5.3 (4.5) | 2.43 | 0.06 | 0.51 |
| MCS of SF-36 | 12.8 (9.7) | 6.3 (10.6) | 3.87 | 0.03 | 0.64 |
| Role emotional scale | 33.3 (30.2) | −0.01 (47.2) | 7.08 | 0.005 | 0.84 |
| Energy scale | 19.6 (20.6) | 5.0 (16.7) | 5.68 | 0.01 | 0.78 |
|
Cognition MMSE |
0.2 (0.7) | −0.9(1.2) | 13.79 | 0.0003 | 1.12 |
| Trails A time | −3.6 (10.4) | −1.3 (18.7) | 0.32 | 0.29 | 0.15 |
| Trails B time | −11.2 (19.7) | 9.9 (30.5) | 7.46 | 0.005 | 0.82 |
| CVLT long delay cued recall | −0.6 (2.4) | −0.8 (2.5) | 0.10 | 0.38 | 0.08 |
|
Biological activity Telomerase, units/10,000 |
0.9 (2.5) | −0.2 (1.2) | 2.75 | 0.05 | 0.56 |
F-statistics (df=1,33 for telomerase only) and one-sided p-values are reported for all variables
SF-36 MCS– Mental Health composite summary scores
MMSE – Mini-Mental State Examination scale
HAM-D – Hamilton Depression Rating Scale
Trail A – time in sec
Trail B – time in sec
Telomerase Activity
The meditation group showed 43.3% improvement in telomerase activity compared to 3.7% improvement in the relaxation group (Figure 2). ANCOVA controlling for age revealed a significant increase in telomerase activity from baseline to post-intervention in the meditation group versus the relaxation group (F(1,33) = 2.75, p=0.05; Table 2).
Figure 2.
Change in Telomerase Levels in Meditation and Relaxation Groups
Significant correlations were found between increased telomerase activity, a decrease in levels of depression (r=−0.33; p=0.05), and mental health score of SF-36 (r = 0.44; p = .01; Figure 3) in the full sample. The association of increased telomerase activity with an improved mental health score was significant only in the meditation group (r=0.59; p=0.01); this finding was not significant in the relaxation group.
Discussion
Our study is the first to investigate the efficacy of daily practice of Kirtan Kriya to improve: 1) depressive symptoms; 2) mental health; 3) cognition; and 4) telomerase activity in family dementia caregivers with mild depressive symptoms in a randomized controlled study. One prior pilot study used yogic meditation and mindfulness to improve coping, but in comparison to our study, no biological or cognitive correlates of stress reduction were reported. (Oken et al., 2010). Despite the fact that caregivers in our study had higher levels of distress and mild depressive symptoms compared to other studies of caregiver stress (Roth et al., 2003; Teri et al., 1992) we found an improvement across measures of mental health and cognitive functioning, psychological distress, and telomerase activity in caregivers performing daily Kirtan Kriya compared to the relaxation group.
Multiple psychosocial intervention studies aimed at decreasing the burden of caregiving have shown that they can enhance mental health. The effects of psychosocial interventions vary greatly (Sorensen et al., 2002), and rarely include biological measures of stress-response (Wisniewski et al., 2003). In the recent meta-analysis, Pinquart and Sorensen (2006) integrated the results of 127 intervention studies with dementia caregivers published or presented between 1982 and 2005 (Pinquart & Sorensen, 2006) and demonstrated small effects on caregiver burden, depression, and subjective well-being. Given the magnitude of the caregiver burden, it is surprising that very few interventions translated into clinical practice. The financial burden of complex psychosocial interventions in the community is one of many explanations for the lack of translation into community practice. Our study suggests a low-cost behavioral intervention that can enhance coping and quality of life of the caregivers.
A few relatively small pilot studies evaluated the effects of meditation on caregiver stress with reported improvement in coping. In a study similar to ours, Oken et al (2010) reported the results of a pilot randomized trial of a Mindfulness-Based Cognitive Therapy program in the two active comparison groups and a respite-only group serving as a pragmatic control (Oken et al., 2010). The investigators reported a significant effect on levels of distress, with both active interventions showing improvement compared with the respite-only group. Franco et al (2010) reported the effects of a mindfulness development meditation program on psychological discomfort and burden in 36 family dementia caregivers (Franco et al., 2010). Oman et al (2008) reported positive effects in self-efficacy assessment by professional caregivers after spiritual wisdom-based mindfulness training (Oman et al., 2008). Finally, Waelde et al (2004) reported the effects of a six-session manualized yoga-meditation program in twelve older female dementia family caregivers (Waelde et al., 2004) with significant reductions in depression and anxiety and improvements in perceived self-efficacy. To summarize, all of the recent pilot studies were more limited in assessing multifaceted outcomes of mind-body interventions on caregiver stress, and did not report the biological and cognitive correlates of stress reduction in dementia caregivers compared to our study.
Very few studies evaluated cognitive impairment among caregivers and converged to suggest that chronic stress of caregiving can have a negative effect on cognition. Stress and sleep deprivation can impair cognitive abilities, ranging from attention to implicit and procedural learning and episodic memory (Lupien et al., 2005) that may reduce the caregivers’ ability to monitor their performance (Mackenzie et al., 2007). Our study found beneficial effects of meditation on the global measure (MMSE) and on measures of attention and executive function (Trails B) compared to the effects of relaxation. In support of cognitive changes with meditation, there is an increasing literature of structural and functional neuroimaging identifying regional changes with meditation. (Davidson, 2011; Holzel et al., 2011a; Holzel et al., 2011b; Kerr et al., 2011; Lazar et al., 2000; Lazar et al., 2005; Luders et al., 2011; Luders et al., 2009; Lutz et al., 2009; Slagter et al., 2009)
Cellular mechanisms of mind-body interventions are only beginning to be understood (G. Miller et al., 2009). The relationship between telomere-dependent senescence, telomerase and stress remains a subject of debate (13). It has been observed that psychological stress can boost inflammation and oxidative stress (E. S. Epel et al., 2004), as well as accelerate telomere attrition. Chronic stress has been linked to telomere length and telomerase activity (E. S. Epel et al., 2004). Shortened telomere length and reduced telomerase predict a host of health risks and diseases (J. Lin, Epel, E.S., Blackburn, E.H., 2009), and new findings suggest they may be regulated in part by psychological stress, stress appraisals, and well-being (E. Epel et al., 2009; E. S. Epel et al., 2004). Jacobs et al (2010) reported increased telomerase activity in 30 meditation retreat participants with a six-hour daily meditation practice for three months compared to 30 wait-list control participants matched by age, sex, and prior meditation experience (Jacobs et al., 2010). Our study is the first to indicate changes in telomerase levels in distressed caregivers with Kirtan Kriya meditation.
In comparison to previous reports, our pilot results are striking given a brief daily Kirtan Kriya practice can lead to improvement in mental health, cognition, and telomerase activity over the course of eight weeks. Observed close relationships between changes in telomerase levels, levels of depression, and anxiety and distress are novel and deserve further examination. Controlling for relaxation by the virtue of having the relaxation control group, gave us an opportunity to separate the effects of relaxation on outcomes from other meditation-specific effects. Some of the observed effects could be due to the relaxation response in both groups (e.g., improved depression), while the effects on cognitive and mental functioning and telomerase activity (E. S. Epel et al., 2004) were specific to the Kirtan Kriya. Because Kirtan Kriya had several elements of using chanting, mudras and visualization, there was a “brain fitness” effect in addition to stress-reduction that contributed to the overall effect of the meditation. We will confirm this potential mechanism in the follow up neuroimaging study of Kirtan Kriya.
One might consider the characteristics of our samples as the limitations of the study that includes a mixed and relatively small sample size of adult children and spousal caregivers. On the other hand, this sample is representative of community caregivers. Other limitations of our study include the relatively large number of assessment instruments given the relatively small pilot sample that may introduce the possibility of finding a significant difference by chance alone, especially with respect to telomerase levels. Although not statistically significant, the relaxation group had higher medical burden compared to the meditation group that may have contributed to the groups differences in outcomes. Nevertheless, we are encouraged by the overall pattern of improvement in distress, cognition, and inflammatory biomarkers; as well as an observed close relationship between changes in these variables. Our approach, may offer valuable and cost-effective prevention strategy of treating depressive symptoms in a high-risk population for major depression. Cultural acceptability of a particular mind-body technique to an individual is a limitation among older participants, and has to be done with cultural sensitivity. Our results will need to be replicated in a larger sample to document the effects of mind-body approaches on stress reduction and depression prevention in this difficult-to-manage population.
Acknowledgments
This work was supported by the Alzheimer’s Research and Prevention Foundation grant; and the NIH grants MH077650, MH086481 and AT003480 to Dr Lavretsky, and NIH grants T32-MH19925, HL 079955, AG 026364, CA 10014152, CA116778, RR00827, P30-AG028748, the UCLA Cousins Center at the Semel Institute for Neurosciences, and the UCLA Older Americans Independence Center Inflammatory Biology Core to Dr. Irwin, the Bernard and Barbro Fund to Dr. Blackburn.
References
- Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11(4):289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- Alzheimer's research & prevention foundation: Arpf research: Kirtan kriya. 2011 http://www.alzheimersprevention.org/kirtan_kriya.htm.
- Davidson RJ. Empirical explorations of mindfulness: Conceptual and methodological conundrums. Emotion. 2011;10(1):8–11. doi: 10.1037/a0018480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. Cvlt-ii: California verbal learning test, adult version, manual. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Epel E, Daubenmier J, Moskowitz JT, Folkman S, Blackburn E. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 2009;1172:34–53. doi: 10.1111/j.1749-6632.2009.04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Franco C, Sola Mdel M, Justo E. reducing psychological discomfort and overload in alzheimer's family caregivers through a mindfulness meditation program. Rev Esp Geriatr Gerontol. 2010;45(5):252–258. doi: 10.1016/j.regg.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, et al. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2011a;5(1):11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011b;191(1):36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Kerr CE, Jones SR, Wan Q, Pritchett DL, Wasserman RH, Wexler A, et al. Effects of mindfulness meditation training on anticipatory alpha modulation in primary somatosensory cortex. Brain Res Bull. 2011;85(3–4):96–103. doi: 10.1016/j.brainresbull.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Khalsa DS, Amen D, Hanks C, Money N, Newberg A. Cerebral blood flow changes during chanting meditation. Nucl Med Commun. 2009;30(12):956–961. doi: 10.1097/MNM.0b013e32832fa26c. [DOI] [PubMed] [Google Scholar]
- Lavretsky H. Stress and depression in informal dementia caregivers. Health and Aging. 2005;1(1):117–133. [Google Scholar]
- Lavretsky H, Siddarth P, Irwin MR. Improving depression and enhancing resilience in family dementia caregivers: A pilot randomized placebo-controlled trial of escitalopram. Am J Geriatr Psychiatry. 2010;18(2):154–162. doi: 10.1097/JGP.0b013e3181beab1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11(7):1581–1585. [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16(17):1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, et al. Analyses and comparisons of telomerase activity and telomere length in human t and b cells: Insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352(1–2):71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel ES, Blackburn EH. Telomeres, telomerase stress and aging. In: Bernston GG, Cacioppo JT, editors. Handbook of neuroscience for the behavioral sciences. Vol. chapter 65. New Jersey: Wiley; 2009. [Google Scholar]
- Luders E, Clark K, Narr KL, Toga AW. Enhanced brain connectivity in long-term meditation practitioners. Neuroimage. 2011;57(4):1308–1316. doi: 10.1016/j.neuroimage.2011.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: Larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009;45(3):672–678. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30(3):225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Lutz A, Greischar LL, Perlman DM, Davidson RJ. Bold signal in insula is differentially related to cardiac function during compassion meditation in experts vs. Novices. Neuroimage. 2009;47(3):1038–1046. doi: 10.1016/j.neuroimage.2009.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie CS, Smith MC, Hasher L, Leach L, Behl P. Cognitive functioning under stress: Evidence from informal caregivers of palliative patients. J Palliat Med. 2007;10(3):749–758. doi: 10.1089/jpm.2006.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annu Rev Psychol. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the cumulative illness rating scale. Psychiatry Res. 1992;41(3):237–248. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Wintering N, Khalsa DS, Roggenkamp H, Waldman MR. Meditation effects on cognitive function and cerebral blood flow in subjects with memory loss: A preliminary study. J Alzheimers Dis. 2010;20(2):517–526. doi: 10.3233/JAD-2010-1391. [DOI] [PubMed] [Google Scholar]
- Oken BS, Fonareva I, Haas M, Wahbeh H, Lane JB, Zajdel D, et al. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. J Altern Complement Med. 2010;16(10):1031–1038. doi: 10.1089/acm.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oman D, Richards TA, Hedberg J, Thoresen CE. Passage meditation improves caregiving self-efficacy among health professionals: A randomized trial and qualitative assessment. J Health Psychol. 2008;13(8):1119–1135. doi: 10.1177/1359105308095966. [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, et al. Increased telomerase activity and comprehensive lifestyle changes: A pilot study. Lancet Oncol. 2008;9(11):1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sorensen S. Helping caregivers of persons with dementia: Which interventions work and how large are their effects? Int Psychogeriatr. 2006;18(4):577–595. doi: 10.1017/S1041610206003462. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- Roth DL, Burgio LD, Gitlin LN, Gallagher-Thompson D, Coon DW, Belle SH, et al. Psychometric analysis of the revised memory and behavior problems checklist: Factor structure of occurrence and reaction ratings. Psychol Aging. 2003;18(4):906–915. doi: 10.1037/0882-7974.18.4.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Martire LM. Family caregiving of persons with dementia: Prevalence, health effects, and support strategies. Am J Geriatr Psychiatry. 2004;12(3):240–249. [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Nieuwenhuis S, Davidson RJ. Theta phase synchrony and conscious target perception: Impact of intensive mental training. J Cogn Neurosci. 2009;21(8):1536–1549. doi: 10.1162/jocn.2009.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42(3):356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for dsm-iii-r (scid). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: The revised memory and behavior problems checklist. Psychol Aging. 1992;7(4):622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- Waelde LC, Thompson L, Gallagher-Thompson D. A pilot study of a yoga and meditation intervention for dementia caregiver stress. J Clin Psychol. 2004;60(6):677–687. doi: 10.1002/jclp.10259. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The mos 36-item short-form health survey (sf-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- Wisniewski SR, Belle SH, Coon DW, Marcus SM, Ory MG, Burgio LD, et al. The resources for enhancing alzheimer's caregiver health (reach): Project design and baseline characteristics. Psychol Aging. 2003;18(3):375–384. doi: 10.1037/0882-7974.18.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]