Abstract
Myocardial injury is increased in the aged heart following ischemia and reperfusion (I-R) in both humans and experimental models. Hearts from aged 24 mo. old Fischer 344 rats sustain greater cell death and decreased contractile recovery after I-R compared to 6 mo. adult controls. Cardiac mitochondria incur damage during I-R contributing to cell death. Aged rats have a defect in complex III of the mitochondrial electron transport chain (ETC) localized to the interfibrillar population of cardiac mitochondria (IFM), situated in the interior of the cardiomyocyte among the myofibrils. The defect involves the quinol oxidation site (Qo) and increases the production of reactive oxygen species (ROS) in the baseline state. Ischemia further decreases complex III activity via functional inactivation of the iron-sulfur subunit. We studied the contribution of ischemia-induced defects in complex III to the increased cardiac injury in the aged heart. The reversible blockade of the ETC proximal to complex III during ischemia using amobarbital protects mitochondria against ischemic damage, removing the ischemia component of mitochondrial dysfunction. Reperfusion of the aged heart in the absence of ischemic mitochondrial damage decreases net ROS production from mitochondria and reduces cell death. Thus, even despite the persistence of the age-related defects in electron transport, protection against ischemic damage to mitochondria can reduce injury in the aged heart. The direct therapeutic targeting of mitochondria protects against ischemic damage and decreases cardiac injury during reperfusion in the high risk elderly heart.
Keywords: ischemia, cytochrome c, myocardial infarction, aging, electron transport chain, reactive oxygen species
INTRODUCTION
The aged heart sustains greater injury during ischemia and reperfusion in experimental animal models 1 as well as in elderly patients.2 Myocardial cell death is increased during ischemia and reperfusion in the aged heart compared to adult hearts. Furthermore, the activation of cytoprotective signal transduction systems to protect the aged heart, either via preconditioning before ischemia3 or postconditioning at the onset of reperfusion3; 4 are far less effective in the elderly heart compared to the young heart. Thus, the aged heart suffers not only greater cell death, but has a decreased innate capacity for cytoprotection. New, direct therapeutic approaches to decrease ischemia-reperfusion injury in the aged heart are needed.
Cardiac mitochondria contribute a critical role in the genesis of injury during ischemia and reperfusion,5 and exist in two populations: subsarcolemmal mitochondria (SSM), located beneath the plasma membrane, and interfibrillar mitochondria (IFM) present between the myofibrils.6 Aging decreases oxidative phosphorylation and the activity of complexes III and IV only in interfibrillar mitochondria (IFM) that reside among the myofibrils, whereas subsarcolemmal mitochondria (SSM), located beneath the plasma membrane, remain unaltered.7; 8 The peptide subunit composition of complexes III7; 9 and IV10 remains preserved in IFM during aging. The aging-defect in complex IV is due to the altered inner membrane lipid environment.10; 11 The defect in complex III is within the ubiquinol binding site of the cytochrome b subunit and increases the production of reactive oxygen species even in the baseline state.12
Ischemia damages the mitochondrial electron transport chain in both the adult and aged heart, leading to decreased complex III activity,7 diminished cytochrome c content,7; 13 and reduced cytochrome oxidase activity.7 Ischemia inactivates the iron-sulfur peptide subunit in complex III.7 Ischemia-mediated damage of the electron transport chain persists during reperfusion.14; 15 The presence of damaged mitochondria during reperfusion decreases energy production,5; 16 increases the generation of reactive oxygen species (ROS),17 and favors the release of cytochrome c from mitochondria to trigger myocyte cell death during reperfusion.5
We propose that both age-related and ischemia-induced defects in mitochondria contribute to the enhanced age-induced damage in the elderly heart evident following ischemia and reperfusion. Both aging and ischemia lead to defects within the distal electron transport chain. We tested the hypothesis that blockade of electron flow into the distal electron transport chain during the period of ischemia would attenuate mitochondrial damage and subsequent cardiac injury in the aged heart.
The electron transport chain itself is the source of ischemic damage to mitochondria that leads to mitochondrial-derived injury during reperfusion in the adult heart.15; 18 The blockade of electron transport during ischemia protects cardiac mitochondria15; 19 indicating that the electron transport chain itself contributes to the mitochondrial chain damage. The reversible blockade of electron transport before ischemia with amobarbital protects mitochondria and decreases myocardial injury during reperfusion in the adult heart, supporting that ischemic mitochondrial damage contributes to myocardial injury during reperfusion.15; 19
Amobarbital (AMO) is a short-acting barbiturate that reversibly inhibits at the rotenone-site of complex I.20 Administration of AMO (2.5 mM) before ischemia resulted in an significant increase of NADH fluorescence in buffer perfused hearts, supporting that AMO blocks complex I in situ.21 In AMO-treated hearts, cardiac contractility recovered within 30 seconds of reperfusion21, indicating that AMO is a reversible complex I inhibitor and can wash out efficiently within a half minute of perfusion. Amobarbital (2.5 mM) given immediately before ischemia preserves oxidative phosphorylation in the isolated adult rat heart,15 performed as a preliminary dose-ranging study for the current investigation. In the present study, we tested if reversible blockade of electron transport with amobarbital before ischemia could attenuate the ischemic component of damage present in the aged heart. More importantly, the impact of potential mitochondrial protection was assessed to evaluate if a decrease in ischemic mitochondrial damage would translate into reduced myocardial injury following ischemia and reperfusion in the aged heart. Despite the presence of age-related defects in electron transport, the reversible blockade of electron flow during ischemia does indeed protect the aged heart. Thus, the aged heart can still be protected despite pre-existing aging-induced defects in respiration. The direct modulation of mitochondrial electron transport during ischemia can attenuate the damage from age-related respiratory defects and provides a key option to protect the high risk aged heart during ischemia and reperfusion.
METHODS
Preparation of rat hearts for perfusion
The Animal Care and Use Committees of the Louis Stokes Cleveland VA Medical Center and Case Western Reserve University approved the protocol. Male Fisher 344 24 months rats (410 – 440 g) were anesthetized with pentobarbital sodium (100 mg/kg i.p.) and anti-coagulated with heparin (1000 IU/kg i.p.). Hearts were excised and perfused retrograde via the aorta with modified Krebs-Henseleit (K-H) buffer oxygenated with 95% O2/5% CO2 as previously described.13 Left ventricular developed pressure (LVDP) was measured with a balloon inserted into left ventricle. In the untreated ischemia-reperfusion group (IR), hearts initially were perfused for 15 min. followed by 25 min. global ischemia (37°C) and 30 min. reperfusion. In the amobarbital-treated group (AMO), hearts followed the same perfusion protocol except that amobarbital (2.5 mM) was perfused in identical K-H buffer for one min. immediately before ischemia.13 Hearts in the time control group (TC) were perfused for 71 min without any treatment. Hearts were paced at 300 beats per min during the 15 min. equilibration period and after 10 min. reperfusion. During amobarbital perfusion, pacing was stopped. The release of lactate dehydrogenase (LDH) from myocardium was assayed in coronary effluent by the fluorometric measurement of NADH consumption during the conversion of lactate to pyruvate.15
Isolation of subsarcolemmal (SSM) and interfibrillar (IFM) mitochondria
At the end of perfusion, hearts were removed from the cannula and placed into buffer A [(mM) 100 KCl, 50 MOPS, 1 EGTA [ethylene glycol-bis (β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid], 5 MgSO4•7 H2O, and 1 ATP; pH 7.4] at 4°C. Cardiac mitochondria were isolated using the procedure of Palmer22 except that trypsin was used as the protease.13 Cardiac tissue was finely minced and placed in buffer A containing 0.2% bovine serum album and homogenized with a polytron tissue processor (Brinkman Instruments, Westbury, NY) for 2.5 s at a rheostat setting of 6.0. The polytron homogenate was centrifuged at 500 g, the supernatant saved for isolation of SSM, and the pellet washed. The combined supernatants were centrifuged at 3,000 g to sediment SSM. IFM were isolated by incubation of skinned myofibers, obtained following polytron treatment, with 5mg/g (wet weight) trypsin for 10 min at 4 °C. SSM and IFM were washed twice and then suspended in KME (80 mM KCl, 50 mM MOPS, and 0.5 mM EGTA). Mitochondrial protein concentration was measured by the Lowry method, using bovine serum album as a standard.23
Mitochondrial oxidative phosphorylation
Oxygen consumption by mitochondria was measured using a Clark-type oxygen electrode at 30°C.13 Mitochondria were incubated in 80 mM KCl, 50 mM MOPS, 1 mM EGTA, 5 mM KH2PO4, and 1 mg defatted, dialyzed bovine serum albumin/ml BSA at pH 7.4. Glutamate (complex I substrate, 20 mM), succinate (complex II substrate, 20 mM), duroquinol (complex III substrate, 5 mM), and TMPD-ascorbate (complex IV substrate, 10 mM) were used and state 3 (0.2 mM ADP-stimulated), state 4 (ADP-limited) respiration, respiratory control ratio, maximal ADP-stimulated respiration (2 mM ADP), and the ADP/O ratio were determined. Rotenone (5 µM) was used with non-complex I substrates. Mitochondria always were used within 6 hr. after isolation from tissue. Endogenous substrates were depleted by addition of 0.1 mM ADP when glutamate was the substrate.
Citrate synthase enzyme activities
Citrate synthase activity was measured in detergent-solubilized, isolated SSM and IFM using previously described methods.24
Detection of H2O2 production
H2O2 production from intact mitochondria was measured using the oxidation of the fluorogenic indicator amplex red in the presence of horseradish peroxidase (HRP).25
Measurement of cytochrome content
Cytochrome contents were determined in mitochondria solubilized in 2% deoxycholate in 10 mM sodium phosphate buffer using the difference of sodium dithionite reduced and air-oxidized spectra.7
Statistical Analysis
26 Data are expressed as the mean ± standard error of the mean. Differences among groups were compared by one-way analysis of variance with post-hoc comparisons performed using the Student-Newman-Keuls test of multiple comparisons. A paired t-test was used to analyze the difference between SSM and corresponding IFM in oxidative phosphorylation and H2O2 generation experiments. A difference of p<0.05 was considered significant.
RESULTS
Reversible blockade of electron transport during ischemia decreases myocardial injury measured following reperfusion
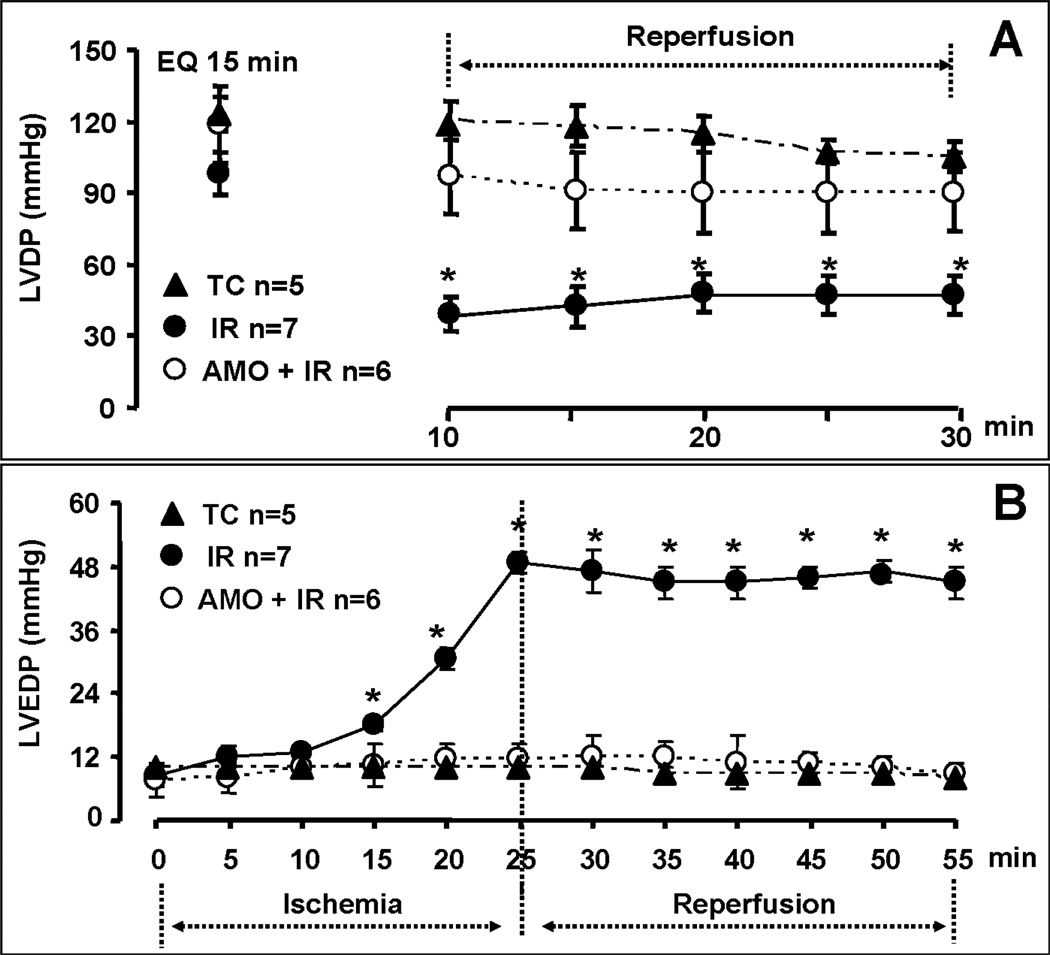
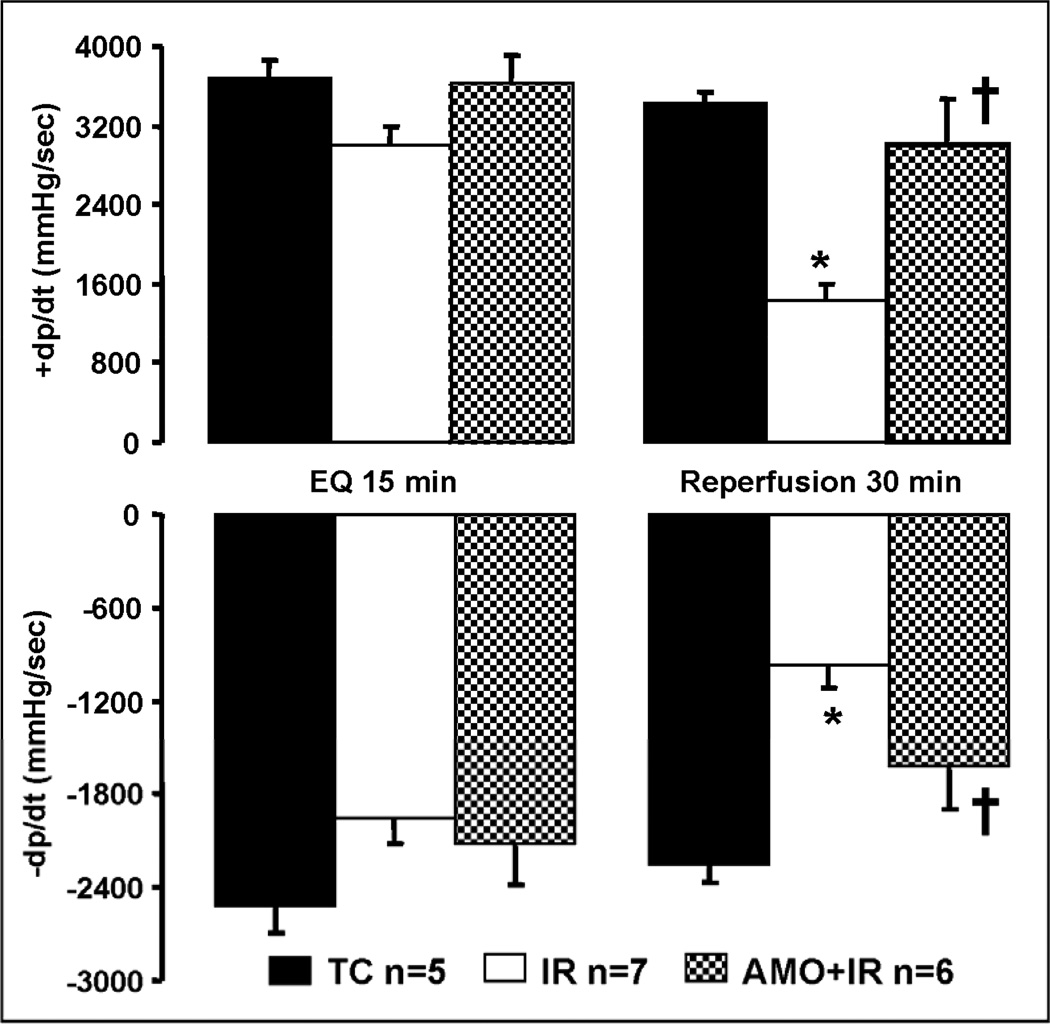
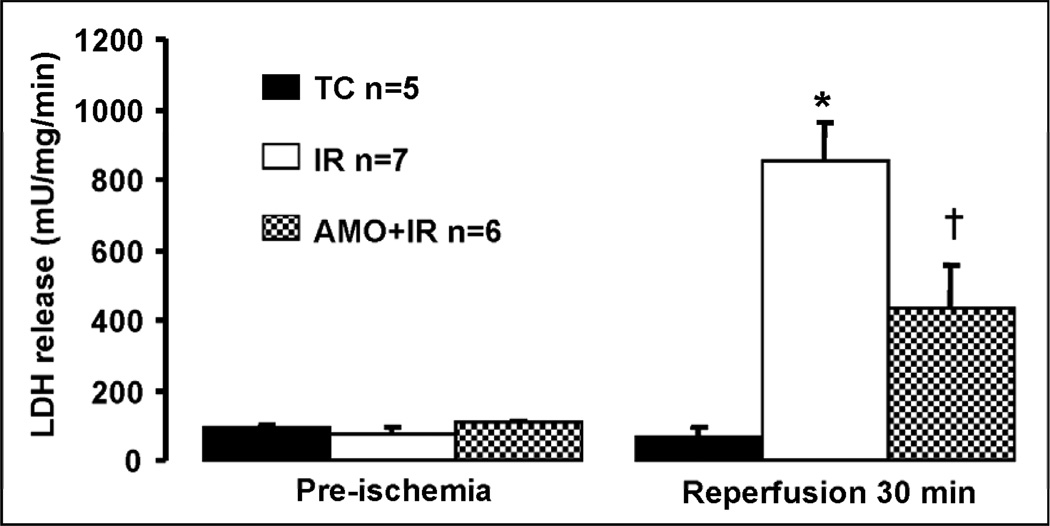
There were no differences in left ventricle developed pressure (LVDP) and diastolic pressure between groups (TC, IR, AMO) at the end of the equilibration perfusion (15 min.) immediately before the administration of amobarbital or vehicle (Figure 1, Panel A & B). Ischemia markedly increased diastolic pressure in untreated hearts, whereas blockade of electron transport with amobarbital prevented this increase (Figure 1, Panel B). The recovery of left ventricular systolic function (Figure 1&2, Panel A: LVDP and dp/dtmax) and diastolic function (Figure 1&2, Panel B: diastolic pressure and dp/dtmin) during reperfusion were substantially improved by reversible blockade of electron transport during ischemia. Reversible blockade of electron transport during ischemia significantly attenuated myocardial injury as shown by the decreased LDH release during reperfusion (Figure 3).
Figure 1. Hemodynamic performance during ischemia-reperfusion (IR) with and without amobarbital (AMO) treatment.
AMO treatment immediately before ischemia improved the recovery of myocardial contractile function during reperfusion shown by the improvement in left ventricular developed pressure (LVDP, Panel A). AMO also prevented ischemic contracture during 25 min. of ischemia and decreased diastolic pressure during reperfusion (Panel B). Data are expressed as mean ± SEM; * p<0.05 vs. non-ischemic time control (TC); † p<0.05 vs. untreated ischemia and reperfusion (IR).
Figure 2. Left ventricular contractile function during ischemia-reperfusion.
AMO treatment immediately before ischemia improved the recovery of myocardial contractility during reperfusion shown by the improvement in +dp/dt compared to untreated ischemia-reperfusion hearts (Panel A). AMO also significantly improved myocardial relaxation during reperfusion with improved −dp/dt (Panel B). Data are expressed as mean ± SEM; * p<0.05 vs. non-ischemic time control (TC); † p<0.05 vs. untreated ischemia and reperfusion.
Figure 3. Amobarbital treatment decreases myocardial injury during reperfusion.
LDH release into coronary effluent was measured as an index of myocardial injury. Baseline release of LDH during the equilibration period was minimal in all groups as expected (Pre-ischemia value). Ischemia-reperfusion led to marked increase in LDH release during the 30 min reperfusion period as expected. In contrast, amobarbital treatment immediately before ischemia significantly decreased LDH release during reperfusion, indicating less myocardial injury. Data are expressed as mean ± SEM; * p<0.05 vs. non-ischemic time control (TC); † p<0.05 vs. untreated ischemia and reperfusion.
Reversible blockade of electron transport during ischemia preserves mitochondrial function following reperfusion
The protein yield of SSM and IFM was not altered by ischemia-reperfusion or amobarbital treatment (Table 1). The activity of citrate synthase, a mitochondrial matrix marker enzyme, was also similar in all groups, confirming a similar relative purity of mitochondrial isolates in all groups (Table 1). In time control and amobarbital treated hearts, the rate of oxidative phosphorylation in IFM is higher than that in corresponding SSM, consistent with our previous findings (Table 2 and Figure 4) 10.
Table 1.
Mitochondrial protein yield and citrate synthase activity in SSM and IFM following ischemia and reperfusion
| SSM | IFM | |||||
|---|---|---|---|---|---|---|
| TC | IR | AMO | TC | IR | AMO | |
| Protein | 11.6±0.6 | 10.4 ± 0.4 | 10.5 ± 0.5 | 5.3± 0.5 | 5.2± 0.4 | 7.6±0.3 |
| CS | 2800±822 | 3194 ± 172 | 2731±233 | 3151±1225 | 3387±116 | 2953±292 |
Mean ± SEM. Ischemia and reperfusion does not change the protein yield (mg/g tissue) and the activity of citrate synthase (CS). TC, time control; IR, ischemia-reperfusion; AMO, amobarbital.
Table 2.
Oxidative phosphorylation in SSM and IFM with glutamate as complex I substrate
| TC | IR | AMO | TC | IR | AMO | |
|---|---|---|---|---|---|---|
| SSM | IFM | |||||
| State 3 | 229 ± 22 | 132 ± 13* | 244 ± 9† | 288 ± 21‡ | 149 ± 17* | 270 ± 14†‡ |
| State 4 | 27 ± 4 | 41 ± 5* | 40 ± 4* | 37 ± 7 | 46 ± 6 | 42 ± 3 |
| RCR | 9.1 ± 1.3 | 3.5 ± 0.4* | 6.6 ± 0.9† | 8.8 ± 1.1 | 3.5 ± 0.5* | 6.8 ± 0.8† |
| DNP | 233 ± 21 | 154 ± 21* | 269 ± 14† | 294 ± 32‡ | 159 ± 26* | 286 ± 25†‡ |
Mean ± SEM;
p<0.05 vs. TC-(time control, n=5);
p<0.05 vs. IR (untreated, n=7);
p<0.05 vs. corresponding SSM, AMO (amobarbital, n=6); DNP (0.2 mM), dinitrophenol; TC, time control; IR, ischemia-reperfusion; AMO, amobarbital.
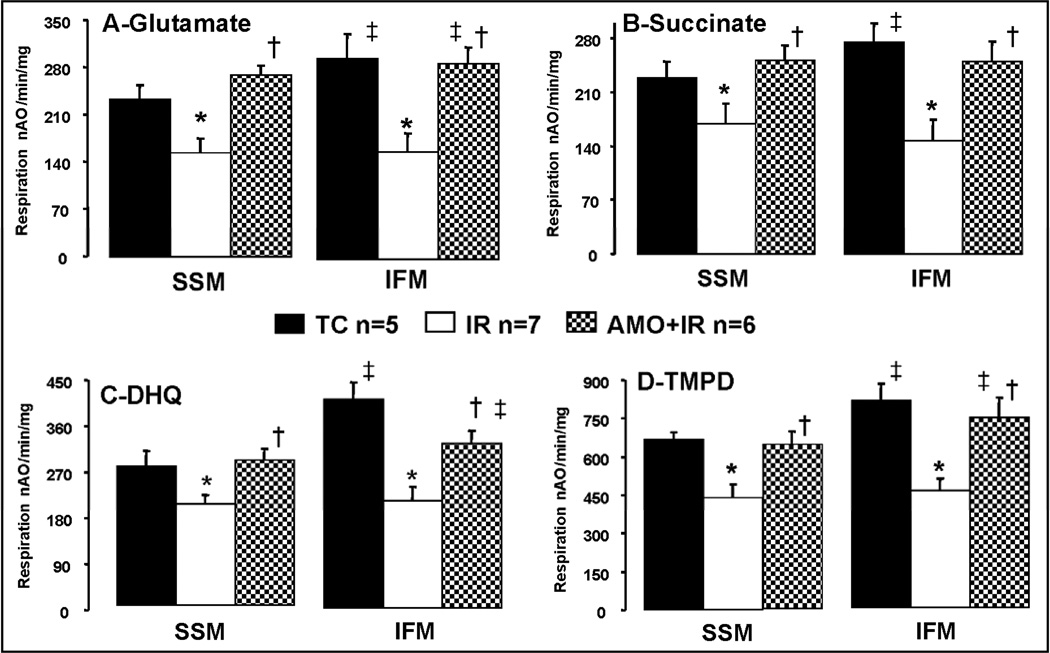
Figure 4. Maximal rates of state 3 (ADP-stimulated) respiration in subsarcolemmal (SSM) and interfibrillar (IFM) cardiac mitochondria following ischemia-reperfusion.
Cardiac ischemia-reperfusion decreased the ADP stimulated respiration in both SSM and IFM oxidizing glutamate (Panel A), succinate (Panel B), DHQ (Panel C), and TMPD-ascorbate (Panel D) as complex I, complex II, complex III, and complex IV substrates, respectively. Oxidative phosphorylation was significantly improved in mitochondria isolated from amobarbital treated hearts following ischemia and reperfusion compared to untreated hearts. Data are expressed as mean ± SEM; * p<0.05 vs. time control; † p<0.05 vs. untreated ischemia and reperfusion; ‡ p<0.05 vs. corresponding SSM; nAO: natom oxygen.
With glutamate as complex I substrate, ischemia-reperfusion decreased the rate of ADP-stimulated state 3 and dinitrophenol-uncoupled respiration in both SSM and IFM compared to the time control group (Table 2, Figure 4 Panel A). Amobarbital treatment only during ischemia markedly improved state 3 and uncoupled respiration measured following 30 min. reperfusion compared to the untreated ischemia-reperfusion group (Table 2). Ischemia-reperfusion slightly increased the rate of ADP-limited state 4 respiration compared to time control in SSM (Table 2). The preserved rate of state 3 respiration improved the coupling of respiration, reflected in the respiratory control ratio (Table 2).
Ischemia-reperfusion decreased the rate of oxidative phosphorylation with succinate (complex II), duroquinol (complex III), and complex IV substrates in both SSM and IFM, whereas amobarbital treatment preserved oxidative phosphorylation in the two populations of mitochondria with all substrates (Figure 4, Panel B, C, and D). Thus, the reversible blockade of proximal electron transport only during ischemia prevented damage to distal part of electron transport chain during the subsequent reperfusion period.
Reversible blockade of electron transport during ischemia preserves cytochrome c content following reperfusion
Ischemia-reperfusion caused cytochrome c loss from both SSM and IFM (Table 3). Blockade of electron transport with amobarbital before ischemia preserved cytochrome c content in both SSM and IFM that were isolated at the end of reperfusion (Table 3). There were no differences in contents of cytochromes c1, b, and aa3 between time control, ischemia-reperfusion, and amobarbital treated ischemia-reperfusion groups (Table 3).
Table 3.
Cytochrome contents in SSM and IFM following ischemia and reperfusion
| SSM | IFM | |||||
|---|---|---|---|---|---|---|
| TC | IR | AMO | TC | IR | AMO | |
| c | 0.294±0.016 | 0.249±.024* | 0.327±0.009 | 0.353±0.041 | 0.208±0.030* | 0.355±0.250† |
| c1 | 0.169±0.012 | 0.177±0.140 | 0.217±0.007 | 0.115±0.044 | 0.068±0.035 | 0.141±0.039 |
| b | 0.235±0.014 | 0.275±0.010 | 0.219±0.016 | 0.301±0.103 | 0.382±0.058 | 0.279±0.017 |
| aa3 | 0.645±0.054 | 0.696±0.039 | 0.549±0.058 | 0.893±0.286 | 1.070±0.201 | 0.790±0.097 |
Mean ± SEM;
p<0.05 vs. time control (n=5);
p<0.05 vs. untreated (IR, n=7); amobarbital (AMO, n=6); TC, time control; IR, ischemia-reperfusion; AMO, amobarbital.
Reversible blockade of electron transport during ischemia decreases net H2O2 production from SSM and IFM following reperfusion
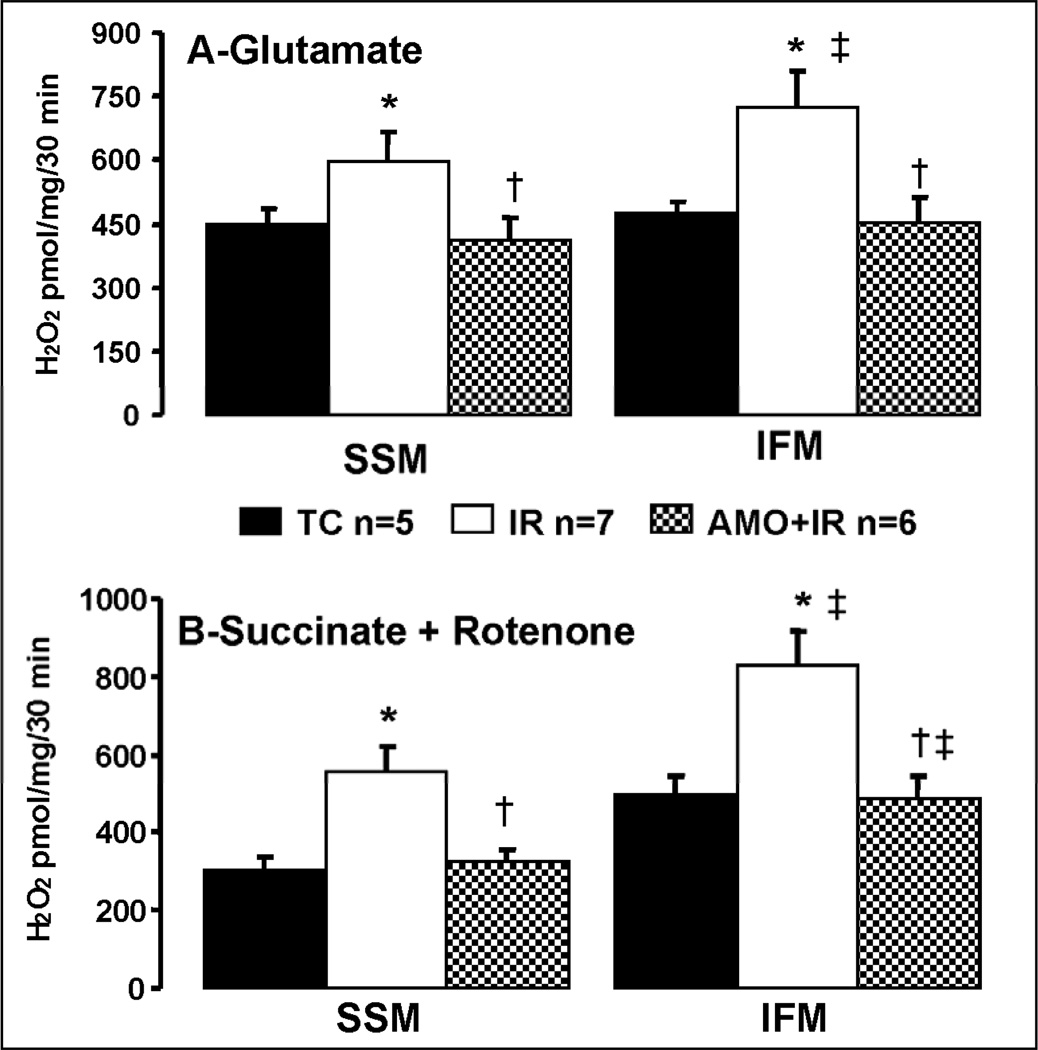
Ischemia-reperfusion damaged the mitochondrial electron transport chain and increased the net release of H2O2 from isolated, intact mitochondria compared to time control with glutamate as complex I substrate (Figure 5, Panel A). With succinate as the complex II substrate in the presence of rotenone to prevent reverse electron flow, net H2O2 release was also markedly increased in SSM and IFM following ischemia-reperfusion (Figure 5, Panel B). Amobarbital inhibition protected the electron transport chain and decreased net H2O2 release in both SSM and IFM in the presence of complex I and complex II substrates (Figure 5), suggesting that the damaged electron transport chain is the source for the increased H2O2 generation. Ischemia-damaged IFM generated more H2O2 compared to their corresponding SSM when glutamate and succinate + rotenone were used as complex I and complex II substrates (Figure 5). Thus, the superimposed damage in aged IFM may augment myocardial injury by increasing ROS generation.
Figure 5.
Net H2O2 production from cardiac mitochondria following ischemia-reperfusion. The production of H2O2 from subsarcolemmal (SSM) and interfibrillar (IFM) mitochondria following ischemia-reperfusion was markedly increased compared to mitochondria from time control hearts. Glutamate was used as a complex I substrate and succinate (with rotenone) as a complex II substrate. Amobarbital treatment before ischemia significantly decreased H2O2 release from SSM and IFM oxidizing both substrates compared to mitochondria from untreated hearts following ischemia-reperfusion. Data are expressed as mean ± SEM; * p<0.05 vs. time control; † p<0.05 vs. untreated ischemia and reperfusion, ‡ p<0.05 vs. corresponding SSM.
DISCUSSION
Myocardial injury is increased in the aged heart following ischemia-reperfusion.1 Ischemic preconditioning, a powerful endogenous protective mechanism, is unable to protect the aged heart during ischemia-reperfusion.3 In the present study, we found direct, mitochondrial-targeted therapy using the reversible blockade of electron transport during ischemia with amobarbital decreased myocardial injury assessed following reperfusion in aged rat hearts. This protection is related to a decrease in ischemia-mediated damage to the electron transport chain. During reperfusion, the protected mitochondria exhibit improved oxidative phosphorylation, decreased production of reactive oxygen species and improved retention of cytochrome c. These salubrious effects all favor a decrease in myocardial injury during reperfusion. Thus, the direct modulation of mitochondrial respiration protects the aged heart and provides a potential strategy to mitigate aged-enhanced cardiac injury despite the presence of persistent aging-induced defects in mitochondrial respiration.
The aging process decreases mitochondrial respiration.27 Mitochondrial dysfunction is associated with a pro-apoptotic cellular environment in senescent cardiac muscle, especially in IFM in aged Fisher 344 rats.28; 29 The decrease in oxidative phosphorylation in IFM from aged hearts is due to the decreased activities of complex III12 and cytochrome oxidase.28 The age-related defect in complex III in IFM increases ROS generation in the baseline state from complex III 12 leading to increased net production from intact mitochondria.30 These age-related defects in metabolism contribute to the increased injury observed in the aged heart. Acetylcarnitine treatment administered to the animal restores the activities of complex III and complex IV in IFM isolated from the hearts of elderly 24 month F344 rats.31 Hearts from acetylcarnitine-treated 24 month Fischer 344 rats exhibited less LDH release and improved functional recovery following ischemia and reperfusion compared to untreated elderly hearts, supporting that notion that pre-existing age-related defects in complex III contribute to greater cardiac damage following ischemia and reperfusion.31
In the present study, the contribution of mitochondrial damage caused by ischemia to cardiac injury in the aged heart was directly addressed. Could blockade of electron transport during ischemia protect the aged heart that already contained defects in oxidative phosphorylation? The reversible blockade of electron transport only during ischemia using amobarbital protects adult heart mitochondria during ischemia.15 When adult hearts that contain mitochondria lacking ischemic damage are reperfused without additional treatment, mitochondria do not sustain additional damage during reperfusion and myocardial injury is decreased.15 In the present study, amobarbital blockade only during ischemia in the aged heart results in rates of oxidative phosphorylation similar to elderly non-ischemic time controls (Figure 4, Table 2), supporting the notion that mitochondria protected during ischemia do not sustain significant additional damage during reperfusion in the aged heart. In the presence of preserved mitochondrial function during early reperfusion, myocardial injury is decreased, even in the aged heart (Figure 1). Amobarbital treatment before ischemia decreased LDH release about 40% in adult rat hearts following 25 min ischemia and 30 min reperfusion.15 In the current study, amobarbital treatment before ischemia decreased LDH release by approximately 30% in aged hearts during reperfusion. Thus, blockade of electron transport using amobarbital provides a comparable extent of protection with the adult heart during ischemia-reperfusion.
The observation that reversible blockade of respiration during ischemia can decrease myocardial injury in the aged heart suggests that aging-impaired mitochondria can nonetheless still be manipulated at the proximal electron transport chain in order to decrease cardiac injury. In contrast to the direct inhibition of amobarbital, indirect modulation of mitochondrial function by ischemic preconditioning or postconditioning is less effective in the aged heart as an intervention to reduce cardiac injury.32 Thus, direct modulation of mitochondrial respiration could be considered to protect the aged heart despite impaired endogenous protection.
Increased ROS generation from the electron transport chain contributes to cardiac injury.33 Ischemic damage to the electron transport chain increases ROS generation during re-oxygenation.18; 21; 33 Protection of mitochondria during ischemia decreases ROS generation during reperfusion in the adult heart.15 In the current study, amobarbital treatment prevented the ischemic component of damage to the electron transport chain. In the absence of ischemic damage to electron transport, there was less ROS generation during reperfusion, supporting that despite the continued presence of aging defects in electron transport, that prevention of the ischemic component of damage decreased the ROS production during reperfusion.
Cytochrome c contributes a critical role in electron transport under physiological conditions, whereas cytochrome c release from mitochondria triggers programmed cell death.34; 35 Blockade of electron transport at complex I prevents cytochrome c release in adult hearts during ischemia13; 19 with protection that persists into reperfusion.15 Blockade of electron transport only during ischemia preserved cytochrome c content in both SSM and IFM in aged hearts measured after reperfusion, suggesting that electron transport mediated damage during ischemia remains a major contributor to cytochrome c release during reperfusion in the aged heart. Preservation of cytochrome c content is a key mechanism in the protection of myocardium during ischemia and reperfusion.15; 16; 19; 34
Ischemic preconditioning protects mitochondria and decreases myocardial injury in adult hearts.36; 37 Unfortunately, ischemic preconditioning does not protect aged hearts during ischemia and reperfusion as shown by multiple investigators.38 The cardioprotection of preconditioning is mediated via the activation of cytoprotective signaling cascades that converge on mitochondria to attenuate mitochondrial-driven cardiomyocyte injury. Cytoprotective cascades include the activation of PKC epsilon39 to modulate the mitochondrial KATP channel 40 and the activation of the “RISK” kinase pathway that includes Akt to phosphorylate GSK3beta to block mitochondrial permeability transition.41 Although it not entirely clear, it appears that the defect that limits signal transduction mediated cytoprotection lies upstream of the mitochondria.42 The endogenous protective mechanisms can be restored in aged hearts by longer term treatments including caloric restriction,43 an exercise program,44 or a pharmacologic strategy to inhibit protein phosphatase 2A activity.45
Mitochondria in the aged heart remain responsive to direct pharmacologic modulation. In addition to the robust cardioprotection derived from direct targeting of mitochondria to modulate electron transport in the present study, mitochondria from aged hearts remain responsive to direct manipulation of the KATP channel by nicorandil46 or diazoxide treatment.47 Direct modulation of permeability transition pore susceptibility48–50 also limits damage in the aged heart. Alternative approaches to modulate electron transport also exist, including the use of chronic treatment with nitrate to attenuate oxidative damage from mitochondria,51 the use more acutely of nitrite to partially block complex I52 and perhaps hydrogen sulfide generation within the myocardium.53 Thus, when the presence of comorbid conditions such as aging3 or diabetes4 blunt the effectiveness of classic preconditioning, direct targeting of the effector of cardiac injury, the mitochondria, fortunately remains an effective alternative therapeutic strategy.
The protected mitochondria during ischemia decrease myocardial injury during reperfusion in aged heart, providing strong support that the resumption of aerobic metabolism by mitochondria with ischemic damage to the electron transport chain augments cardiac injury during reperfusion. If the ischemia-damaged electron transport chain is the cause, then the transient modulation of electron transport at the onset of reperfusion should reduce cardiac injury. Ischemic postconditioning, the use of brief, transient recurrent ischemia during early reperfusion, reduces injury in the adult heart54 but is again ineffective in the elderly heart.3 Since postconditioning and preconditioning and share overlapping mechanisms of protection55 and overlapping extents of cardioprotection,56 it is not surprising that kinase dependent cytoprotection applied at the onset of reperfusion is also ineffective in the aged heart. The brief, transient blockade of electron transport by amobarbital at the onset of reperfusion decreases myocardial injury in adult rat heart15; 57 and rabbit heart.57; 58 Initial results in the aged heart59 suggest that the modulation of electron transport for a brief period at the onset of reperfusion reduces injury consistent with the important principal advanced in the present study.
Thus, even in a setting where the presence of comorbid conditions, in this case aging, enhance myocardial injury2 and reduce the effectiveness of endogenous cytoprotective mechanism,3 transient blockade of electron transport remains an effective intervention to protect mitochondria and myocardium against injury. The robust protection observed by the reversible blockade of electron transport during ischemia highlights the contributions of age-related defects at complex III and complex IV in the distal electron transport chain to cardiac injury in the aged heart. The present study provides key evidence that direct modulation of mitochondrial function despite the presence of important preexisting defects in respiratory function, can nonetheless attenuate damage to mitochondria and translate into myocardial protection, even if activation of upstream signaling systems that converge upon the mitochondria are ineffective. The present study provides potential insight into future strategies for protection of the elderly 3 and possibly diabetic hearts.4
ACKNOWLEDGMENTS
This work was supported by Program Project Grant 2PO1AG15885 from the National Institutes of Health and the Office of Research and Development, Medical Research Service, Department of Veterans Affairs. The assistance of Dr. Shadi Moghaddas and Ms. Sarah Stewart is appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interests: None
REFERENCES
- 1.Lesnefsky EJ, Gallo DS, Ye J, Whittingham TS, Lust WD. Aging increases ischemia-reperfusion injury in the isolated, buffer-perfused heart. J Lab Clin Med. 1994;124:843–851. [PubMed] [Google Scholar]
- 2.Lesnefsky EJ, Lundergan CF, Hodgson JM, et al. Increased left ventricular dysfunction in elderly patients despite successful thrombolysis: the GUSTO-I angiographic experience. J Am Coll Cardiol. 1996;28:331–337. doi: 10.1016/0735-1097(96)00148-9. [DOI] [PubMed] [Google Scholar]
- 3.Vessey DA, Kelley M, Li L, Huang Y. Sphingosine protects aging hearts from ischemia/reperfusion injury: Superiority to sphingosine 1-phosphate and ischemic pre- and post-conditioning. Oxid Med Cell Longev. 2009;2:146–151. doi: 10.4161/oxim.2.3.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downey JM, Cohen MV. Why do we still not have cardioprotective drugs? Circ J. 2009;73:1171–1177. doi: 10.1253/circj.cj-09-0338. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292:C45–C51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 6.Palmer JW, Tandler B, Hoppel CL. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch Biochem Biophys. 1985;236:691–702. doi: 10.1016/0003-9861(85)90675-7. [DOI] [PubMed] [Google Scholar]
- 7.Lesnefsky EJ, Gudz TI, Migita CT, et al. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys. 2001;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- 8.Lesnefsky EJ, Tandler B, Moghaddas S, Hassan MO, Hoppel C. Mitochondrial Electron Transport and Aging in the Heart. In: Mattson MP, editor. Mechanisms of Cardiovascular Aging. First ed. San Diego: Elsevier; 2002. pp. 201–232. [Google Scholar]
- 9.Lesnefsky EJ, Gudz TI, Moghaddas S, et al. Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J Mol Cell Cardiol. 2001;33:37–47. doi: 10.1006/jmcc.2000.1273. [DOI] [PubMed] [Google Scholar]
- 10.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- 11.Lesnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys. 2003;420:287–297. doi: 10.1016/j.abb.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Moghaddas S, Hoppel CL, Lesnefsky EJ. Aging defect at the Qo site of complex III augments oxyradical production in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 2003;414:59–66. doi: 10.1016/s0003-9861(03)00166-8. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Hoppel CL, Lesnefsky EJ. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther. 2006;316:200–207. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- 14.Lesnefsky EJ, Chen Q, Slabe TJ, et al. Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am J Physiol Heart Circ Physiol. 2004;287:H258–H267. doi: 10.1152/ajpheart.00348.2003. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006;319:1405–1412. doi: 10.1124/jpet.106.110262. [DOI] [PubMed] [Google Scholar]
- 16.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 17.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–C466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 19.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during Ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- 20.Chance B, Williams GR, Hollunger G. Inhibition of electron and energy transfer in mitochondria. I. Effects of Amytal, thiopental, rotenone, progesterone, and methylene glycol. J Biol Chem. 1963;238:418–431. [PubMed] [Google Scholar]
- 21.Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008;77:406–415. doi: 10.1016/j.cardiores.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Hoppel CL, Kerr DS, Dahms B, Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest. 1987;80:71–77. doi: 10.1172/JCI113066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: Central role of complex III. J Biol Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 26.Steel R, Torrie J. Principles and procedures of statistics. New York: Mc Graw-Hill; 1960. [Google Scholar]
- 27.Ljubicic V, Menzies KJ, Hood DA. Mitochondrial dysfunction is associated with a pro-apoptotic cellular environment in senescent cardiac muscle. Mech Ageing Dev. 2010;131:79–88. doi: 10.1016/j.mad.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 29.Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–R527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- 30.Sun J, Trumpower BL. Superoxide anion generation by the cytochrome bc1 complex. Arch Biochem Biophys. 2003;419:198–206. doi: 10.1016/j.abb.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 31.Lesnefsky EJ, He D, Moghaddas S, Hoppel CL. Reversal of mitochondrial defects before ischemia protects the aged heart. Faseb J. 2006;20:1543–1545. doi: 10.1096/fj.05-4535fje. [DOI] [PubMed] [Google Scholar]
- 32.Wagner C, Kloeting I, Strasser RH, Weinbrenner C. Cardioprotection by postconditioning is lost in WOKW rats with metabolic syndrome: role of glycogen synthase kinase 3beta. J Cardiovasc Pharmacol. 2008;52:430–437. doi: 10.1097/FJC.0b013e31818c12a7. [DOI] [PubMed] [Google Scholar]
- 33.Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am J Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 34.Borutaite V, Brown GC. Mitochondria in apoptosis of ischemic heart. FEBS Lett. 2003;541:1–5. doi: 10.1016/s0014-5793(03)00278-3. [DOI] [PubMed] [Google Scholar]
- 35.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77:334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 36.Otani H. Ischemic preconditioning: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10:207–247. doi: 10.1089/ars.2007.1679. [DOI] [PubMed] [Google Scholar]
- 37.Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767:1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fenton RA, Dickson EW, Meyer TE, Dobson JG., Jr Aging reduces the cardioprotective effect of ischemic preconditioning in the rat heart. J Mol Cell Cardiol. 2000;32:1371–1375. doi: 10.1006/jmcc.2000.1189. [DOI] [PubMed] [Google Scholar]
- 39.Tomicek NJ, Miller-Lee JL, Hunter JC, Korzick DH. Estrogen Receptor Beta Does Not Influence Ischemic Tolerance in the Aged Female Rat Heart. Cardiovasc Ther. 2011 doi: 10.1111/j.1755-5922.2011.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quinlan CL, Costa AD, Costa CL, Pierre SV, Dos Santos P, Garlid KD. Conditioning the heart induces formation of signalosomes that interact with mitochondria to open mitoKATP channels. Am J Physiol Heart Circ Physiol. 2008;295:H953–H961. doi: 10.1152/ajpheart.00520.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez L, Paillard M, Price M, et al. A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Basic Res Cardiol. 2011 doi: 10.1007/s00395-011-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tani M, Honma Y, Hasegawa H, Tamaki K. Direct activation of mitochondrial KATP channels mimics preconditioning but protein kinase C activation is less effective in middle-aged rat hearts. Cardiovasc Res. 2001;49:56–68. doi: 10.1016/s0008-6363(00)00240-6. [DOI] [PubMed] [Google Scholar]
- 43.Long P, Nguyen Q, Thurow C, Broderick TL. Caloric restriction restores the cardioprotective effect of preconditioning in the rat heart. Mech Ageing Dev. 2002;123:1411–1413. doi: 10.1016/s0047-6374(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 44.Abete P, Testa G, Galizia G, et al. Tandem action of exercise training and food restriction completely preserves ischemic preconditioning in the aging heart. Exp Gerontol. 2005;40:43–50. doi: 10.1016/j.exger.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Fenton RA, Dickson EW, Dobson JG., Jr Inhibition of phosphatase activity enhances preconditioning and limits cell death in the ischemic/reperfused aged rat heart. Life Sci. 2005;77:3375–3388. doi: 10.1016/j.lfs.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 46.Raveaud S, Verdetti J, Faury G. Nicorandil protects ATP-sensitive potassium channels against oxidation-induced dysfunction in cardiomyocytes of aging rats. Biogerontology. 2009;10:537–547. doi: 10.1007/s10522-008-9196-9. [DOI] [PubMed] [Google Scholar]
- 47.Jahangir A, Ozcan C, Holmuhamedov EL, Terzic A. Increased calcium vulnerability of senescent cardiac mitochondria: protective role for a mitochondrial potassium channel opener. Mech Ageing Dev. 2001;122:1073–1086. doi: 10.1016/s0047-6374(01)00242-1. [DOI] [PubMed] [Google Scholar]
- 48.Zhu J, Rebecchi MJ, Glass PS, Brink PR, Liu L. Cardioprotection of the aged rat heart by GSK-3beta inhibitor is attenuated: age-related changes in mitochondrial permeability transition pore modulation. Am J Physiol Heart Circ Physiol. 2011;300:H922–H930. doi: 10.1152/ajpheart.00860.2010. [DOI] [PubMed] [Google Scholar]
- 49.Liu L, Zhu J, Brink PR, Glass PS, Rebecchi MJ. Age-associated differences in the inhibition of mitochondrial permeability transition pore opening by cyclosporine A. Acta Anaesthesiol Scand. 2011;55:622–630. doi: 10.1111/j.1399-6576.2011.02421.x. [DOI] [PubMed] [Google Scholar]
- 50.Korzick DH, Kostyak JC, Hunter JC, Saupe KW. Local delivery of PKCepsilon-activating peptide mimics ischemic preconditioning in aged hearts through GSK-3beta but not F1-ATPase inactivation. Am J Physiol Heart Circ Physiol. 2007;293:H2056–H2063. doi: 10.1152/ajpheart.00403.2007. [DOI] [PubMed] [Google Scholar]
- 51.Zhu SG, Kukreja RC, Das A, Chen Q, Lesnefsky EJ, Xi L. Dietary nitrate supplementation protects against Doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol. 2011;57:2181–2189. doi: 10.1016/j.jacc.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiva S, Sack MN, Greer JJ, et al. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. J Exp Med. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lavu M, Bhushan S, Lefer DJ. Hydrogen sulfide-mediated cardioprotection: mechanisms and therapeutic potential. Clin Sci (Lond) 2011;120:219–229. doi: 10.1042/CS20100462. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka-Esposito C, Chen Q, Moghaddas S, Lesnefsky EJ. Ischemic preconditioning does not protect via blockade of electron transport. J Appl Physiol. 2007;103:623–628. doi: 10.1152/japplphysiol.00943.2006. [DOI] [PubMed] [Google Scholar]
- 55.Murphy E, Steenbergen C. Preconditioning: the mitochondrial connection. Annu Rev Physiol. 2007;69:51–67. doi: 10.1146/annurev.physiol.69.031905.163645. [DOI] [PubMed] [Google Scholar]
- 56.Argaud L, Gateau-Roesch O, Augeul L, et al. Increased mitochondrial calcium coexists with decreased reperfusion injury in postconditioned (but not preconditioned) hearts. Am J Physiol Heart Circ Physiol. 2008;294:H386–H391. doi: 10.1152/ajpheart.01035.2007. [DOI] [PubMed] [Google Scholar]
- 57.Park JW, Chun YS, Kim YH, Kim CH, Kim MS. Ischemic preconditioning reduces Op6 generation and prevents respiratory impairment in the mitochondria of post-ischemic reperfused heart of rat. Life Sci. 1997;60:2207–2219. doi: 10.1016/s0024-3205(97)00236-1. [DOI] [PubMed] [Google Scholar]
- 58.Ambrosio G, Zweier JL, Duilio C, et al. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem. 1993;268:18532–18541. [PubMed] [Google Scholar]
- 59.Chen Q, Ross T, Hu Y, Lesnefsky EJ. Blockade of electron transport at the onset of reperfusion decreases cardiac injury in aged hearts by protecting the inner mitochondrial membrane. J Aging Res. 2012 doi: 10.1155/2012/753949. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]