Abstract
Background and Purpose
We previously demonstrated that radiation may arrest tumor cells at G2 phase, which in turn prevents the cytotoxicity of antimicrotubule drugs and results in antagonistic interaction between these two modalities. Herein we tested whether G2 abrogators would attenuate the above antagonistic interaction and improve the therapeutic efficacy of combination therapy between radiation and antimicrotubule drugs.
Materials and Methods
Breast cancer BCap37 and epidermoid carcinoma KB cell lines were administered with radiation, UCN-01 (a model drug of G2 abrogator), paclitaxel or vincristine, alone or in combinations. The antitumor activities of single and combined treatments were analyzed by a series of cytotoxic, apoptosis, cell cycle, morphological and biochemical assays.
Results
UCN-01 significantly enhanced the cytotoxicity of radiation, antimitotic drugs, and their combined treatments in vitro. Further investigations demonstrated that UCN-01 attenuated radiation-induced G2 arrest, and subsequently repressed the inhibitory effect of radiation on drug-induced mitotic arrest and apoptosis.
Conclusions
This is the first report demonstrating that G2 checkpoint abrogation represses the inhibitory effect of radiation on antimicrotubule drugs, which may be implicated in cancer combination therapy. Considering that G2 abrogators are under extensive evaluation for cancer treatment, our findings provide valuable information for this class of promising compounds.
Keywords: radiation therapy, antimicrotubule drugs, UCN-01, breast cancer, apoptosis, cell cycle arrest
Introduction
Taxanes such as paclitaxel, and vinca alkaloids such as vincristine, have been widely used for the treatment of a variety of tumors including breast, head and neck and other solid tumors [1]. Previous studies demonstrated that the antitumor effects of taxanes and vinca alkaloids mainly result from interference with the normal function of microtubules and the blockage of cell cycle progression in late G2-M phase. Moreover, these drugs also possess cell-killing activity by induction of apoptosis [2]. Combination therapy with multiple drugs or modalities is a common practice in cancer treatment. Ideally, the combination therapy with different agents or modalities would increase the therapeutic efficacy, via additive or synergistic effects. As cells at the G2-M phase were considered to be exquisitely radiosensitive [3], it was theorized that the combination of antimitotic agents with radiation therapy would behave synergistically owing to the ability of these drugs to arrest cells at the G2-M phase. However, the accumulating data do not always support this hypothesis. Studies in a variety of preclinical and clinical models have produced contradictory results [4–11], e.g. some investigations indicate the lack of cooperation between paclitaxel and radiation [6–8, 11].
We recently found that exposure to radiation caused cell cycle arrest at G2 phase in BCap37 and KB tumor cells, which subsequently prevented the cytotoxicity of paclitaxel and vinca alkaloids on both mitotic arrest and apoptosis [7, 8]. Eventually, radiation-induced G2 arrest caused cell cycle-dependent antagonistic interaction between antimicrotubule drugs and radiation therapy [7, 8]. To resolve this clinically relevant problem, we hypothesized that G2 abrogator may abolish the above G2 arrest and thereby repress the inhibitory effect of radiation on antimicrotubule drugs. To test this hypothesis, we introduced UCN-01 (7-hydroxystaurosporine), a model drug of G2 abrogater [12], into the combination therapy between radiation and antimitotic drugs. The antitumor activities of single and combined treatments, especially the potential influence of UCN-01 on chemoradiotherapy were analyzed by a series of assays including cytotoxic, morphological and biochemical examinations. For the first time, we demonstrated that G2 abrogator could abate the above cell cycle-dependent antagonistic interaction between drugs and radiation, and subsequently enhanced the therapeutic effect of combination therapy.
Methods and Materials
Cell culture and drugs
The human breast cancer BCap37 and epidermoid carcinoma KB cell lines were cultured in RPMI1640 medium with 10% fetal bovine serum [7, 8]. Paclitaxel (T), vincristine (V) and UCN-01 (U) were purchased from Sigma Chemical Co. and dissolved in DMSO. Drugs were stored at −20ºC and diluted in culture medium right before use. In the following description, the numbers after R, U, V and T indicate the dosages tested (Gy for radiation; nM for paclitaxel, vincristine and UCN-01).
Drugs and radiation treatment
Cells were exposed to single treatment of paclitaxel or vincristine, UCN-01, radiation, or their various combinations. We designated the time point at which the cells were exposed to antimicrotubule drug as 0 h. The radiated groups were subjected to a single dose of radiation using the model 143 irradiator (JL Shepherd & Associates) [7, 8]. Radiation and antimicrotubule drugs were administered simultaneously, while UCN-01 was administered 6 h prior to radiation.
MTT assay
Aliquots of the cell suspension were evenly distributed into 96-well culture plates. After overnight incubation, cells were treated with designated regimes. At the end of each time point, the plates were centrifuged to save all the cells. The remaining steps were performed as described previously [7, 8].
Clonogenic assay
Cells were plated on six-well plates with 200–10,000 cells/well based upon the dosage of radiation (2, 4, 8 and 10 Gy), paclitaxel (2, 10 and 50 nM) and UCN-01 (100 nM). After plating, tumor cells were incubated for 8 h before they were exposed to designated regimes. After 48 h treatment, the plates were centrifuged to save all the cells and washed with fresh medium before further incubation. The remaining experimental steps and the calculation of surviving fractions were performed as described previously [7, 8].
Detection of internucleosomal DNA fragmentation
Cells were harvested and suspended in lysis solution for 30 min on ice. Crude DNA samples were extracted with phenol/chloroform/isoamyl alcohol (25:24:1). The detailed experiments for detection of internucleosomal DNA cleavage were performed as described previously [7, 8]. DNA samples were analyzed by electrophoresis in agarose slab gel containing ethidium bromide, and visualized under ultraviolet illumination.
Morphological examination
Cells were cultured in 6-cm dishes and treated with designated regimes. After 24 h of treatment, cells were examined and photographed using a phase-contrast Olympus microscope. Mitotically arrested cells were characterized by bright, rounded features and may be detached from the bottom of the culture dish [7, 8].
Flow cytometric analysis
Cell sample preparation was performed according to the method described previously [7, 8]. Briefly, at the designated time point, cells were harvested and washed with PBS followed by fixation in 70% ethanol. Cells were then incubated in PBS containing RNase and PI before flow cytometry analysis. Cell cycle distribution and DNA content were determined using a Coulter Epics V instrument (Beckman Coulter) with an argon laser set to excite at 488 nm.
Western blotting
Cellular protein was isolated with protein extraction buffer (Beyotime). Equal amounts of proteins were fractionated on SDS-PAGE gels and transferred to PVDF membranes. The membranes were incubated with anti-IκBα (Santa Cruz, 1:500), Bcl-2 (DAKO, 1:500), Cdc2 (Sigma, 1:500), Cyclin B1 (Santa Cruz, 1:200) and Cdc25C (Santa Cruz, 1:200) primary antibodies, respectively. After washing with PBS containing Tween-20, the membranes were incubated with corresponding secondary antibodies followed by chemiluminescent staining using ECL system. β-actin (Sigma, 1:200) was blotted as a control.
Statistical analysis
Data are presented as means±S.D. for each group. The data within each group are normally distributed and have homoscedasticity, therefore comparisons of multiple means were analyzed by one-way ANOVA (analysis of variance). Subsequently, SNK-q test were further used for comparisons between two groups, especially those with and without UCN-01 (e.g. T vs UT, R vs UR, TR vs UTR). Differences were considered statistically significant at a level of p<0.05.
Results
Effect of UCN-01 on the cell-killing activity of radiation
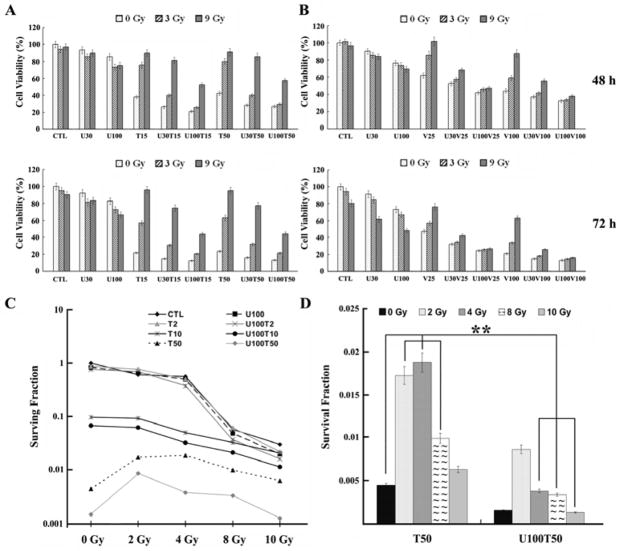
First, MTT assays were carried out in BCap37 and KB cells. The results depicted in Fig. 1A and B showed that UCN-01 had little cytotoxicity at the concentrations tested (30 and 100 nM). When BCap37 cells were exposed to R3 or R9 for 72 h, the cell viabilities were around 95.0% and 90.0%, respectively. However, when cells were co-treated with UCN-01 and radiation, particularly U100, the cell viabilities were significantly decreased (95.0% vs 72.6%, 90.0% vs 66.4%, respectively, p<0.01). Similar results were obtained in KB cells (p<0.01, data not shown). This radiosensitization effect of UCN-01 was further confirmed by clonogenic assay when comparing the surviving fractions of R8 with and without combination of U100 (48±2.71‰ vs 57.33±2.9‰, p<0.05) and those of R10 with and without U100 (19.73±1.5‰ vs 30.13 ±1.61‰, p<0.01).
Fig. 1. Overall cytotoxicities of radiation, antimicrotubule drugs, UCN-01 and their combinations.
A-B, MTT assays; C-D, clonogenic assays. Data presented are means±SD, based on three independent experiments in BCap37 cells. CTL, Control; U, UCN-01; T, paclitaxel; V, vincristine. The numbers after U, T and V indicate the concentrations tested (nM). U100 abolished the antagonistic interaction between T50 and its combinations with 4–10 Gy of radiation (**).
Effect of UCN-01 on the antitumor activity of antimicrotubule drugs
As shown in Fig. 1A, cell survivals after exposure to 15 and 50 nM of paclitaxel for 48 h were around 40% in BCap37 cells. However, when T15 and T50 were combined with U100, the cell survivals were significantly decreased (38.0% vs 21.1%, 42.6% vs 26.7%, respectively, p<0.01). Moreover, cell survivals after the treatment of 25 and 100 nM of vincristine for 48 h were 62.0% and 44.2%, respectively (Fig. 1B). These rates decreased to 42.2% and 32.3%, respectively, when cells were co-treated with U100 (p<0.01). Data obtained in clonogenic assay further confirmed these results as the surviving fractions induced by U100T10 and U100T50 were significantly lower than those induced by T10 and T50 alone (Fig. 1C and D, p<0.001). Similar results were obtained in KB cells (data not shown). These results indicate that UCN-01 could significantly enhance the in vitro cytoxicity of paclitaxel and vincristine in BCap37 and KB cells.
UCN-01 enhances the overall cytotoxicity and apoptosis induced by combination of radiation and antimicrotubule drugs
Consistent with our previous reports [7, 8], we observed significant antagonistic interaction between radiation and paclitaxel/vincristine. For instance, the cell survivals were 42.6%, 80.0% and 91.0%, respectively, when BCap37 cells were treated with T50, T50R3, T50R9 (Fig. 1A). Moreover, radiation significantly repressed paclitaxel and vincristine-induced apoptosis, as demonstrated by DNA fragmentation and flow cytometric assays (lane 12 vs 13, 14 in supplementary Fig.S1, lane 1 vs 2, 3 in Fig. 2D). However, when cells were co-treated with UCN-01, the inhibitory effect of radiation on paclitaxel was significantly decreased. In the presence of U100, the cell viability of T50R3 decreased from 80.0% to 29.2%, and that of T50R9 decreased from 91% to 57.3% (Fig. 1A and B, 48 h, p<0.001). Similarly, co-treatment with U100 almost completely abolished the antagonistic interaction between radiation and vincristine. For instance, the cell survivals after 72 h treatment of V100, V100R3 and V100R9 were 20.8%, 33.9% and 62.9%, respectively. When combined with U100, these rates decreased to 13.3%, 14.5% and 15.9% (Fig. 1B), respectively. These data indicate that UCN-01 dramatically increased the antitumor activity of chemoradiotherapy, with combined efficacy greater than any of the single modality. Clonogenic assay further confirmed that UCN-01 significantly increased the cell-killing activity of combined treatments between 2–10 Gy of radiation and 2–50 nM of paclitaxel (Fig. 1C, p>0.05 for T2R2 vs U100T2R2, all others p<0.05). As included in Fig. 1C and emphasized in Fig. 1D (**), UCN-01 even completely abolished the antagonistic interaction between T50 and its combinations with 4–10 Gy of radiation. Furthermore, UCN-01 significantly enhanced the apoptosis induced by combination of radiation and paclitaxel/vincristine (lane 14 vs 20 in Supplementary Fig. S1, lane 2 vs 6, 7 in Fig. 2D).
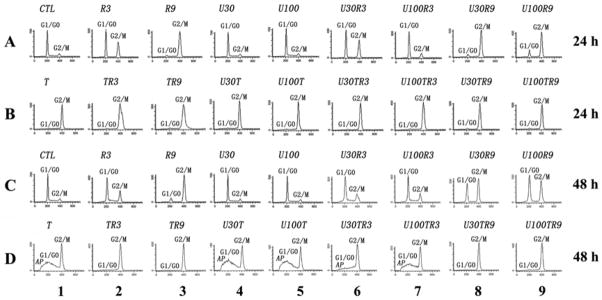
Fig. 2. Cell cycle distribution and induction of apoptosis.
BCap37 cells exposed to designated treatments were prepared for flow cytometric analysis. The peaks corresponding to G1/G0 and G2/M phases of the cell cycle are indicated. The sub-G0/G1 peaks labeled as AP represent the population of apoptotic cells. CTL, control; R, radiation; U, UCN-01; T, 50 nM of paclitaxel. The numbers after R, U and T indicate the dosages tested (Gy for radiation; nM for drugs).
UCN-01 attenuates G2 arrest and promotes mitotic arrest in tumor cells co-treated with radiation and antimicrotubule drugs
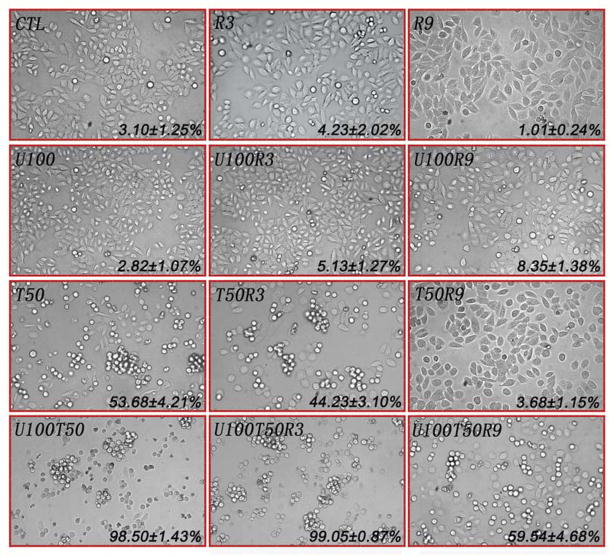
We previously demonstrated that although significantly elevated G2-M peaks were detected by flow cytometric assay, radiation actually arrests cells at the G2 phase in BCap37 and KB cells [7, 8]. Herein, we observed that U100 significantly reduced the G2 arrest induced by R3 and R9 (lane 2 vs 7, lane 3 vs 9 in Fig. 2A, 2C), which was further confirmed by microscope examination, as UCN-01 significantly restored the cell morphology and increased the percentage of mitotic cells after radiation treatment (R9 vs U100R9, p<0.001, see both morphological and quantitative results in Fig. 3). Consistent with our earlier reports [7, 8], the number of mitotic cells induced by paclitaxel were significantly decreased when cells were co-treated with radiation (p<0.05 and p<0.001, respectively, for T50 vs T50R3 and T50 vs T50R9, Fig. 3). However, UCN-01 not only significantly promoted paclitaxel-induced mitotic arrest (p<0.01 for T50 vs U100T50), but also abrogated radiation-induced G2 arrest and thereby significantly promoted mitotic arrest in cells co-treated with radiation and paclitaxel (both p<0.001 for T50R3 vs U100T50R3, T50R9 vs U100T50R9). Similar phenomenon was observed in KB cells as well as when both cell lines exposed to vincristine (data not shown). These data coincides with the above findings that UCN-01 significantly enhanced the antitumor activity and apoptosis induced by radiation, antimicrotubule drugs, and their combination therapy.
Fig. 3. Morphological examination with light microscopy and quantitative analysis of mitotic arrest.
After exposure to designated regimes for 24 h, BCap37 cells were examined using a phase contrast microscopy. Cells that appear bright, rounded and detached from the dish are mitotic cells. The data (Mean±S.D.) marked in each photo indicate the ratios of mitotic cells in corresponding groups. CTL, control; R3 and R9, 3 and 9 Gy of radiation, respectively; U100, 100 nM of UCN-01; T50, 50 nM of paclitaxel. Magnification, ×40
Exploration of regulatory proteins involved in UCN-01-mediated enhancement of combination therapy
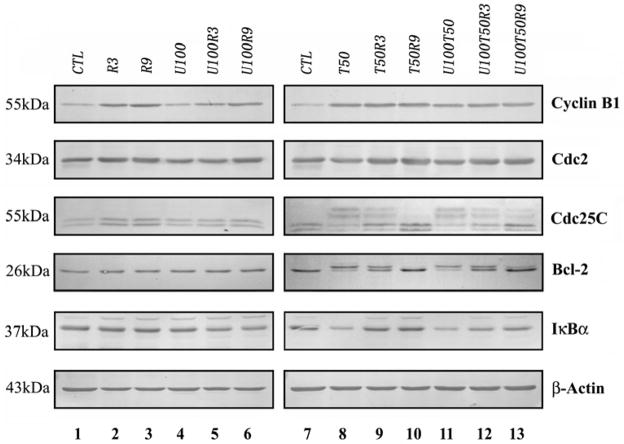
To investigate the possible mechanisms, we examined several regulatory proteins associated with the G2-M phase of the cell cycle and drug-induced apoptosis. The results depicted in Fig. 4 showed that compared with the control and UCN-01-treated groups, Cyclin B1 were significantly up-regulated after exposure to radiation or paclitaxel alone, as well as combinations containing either radiation or paclitaxel. Interestingly, the addition of UCN-01 induced less up-regulation of Cyclin B1 in comparison with radiation alone (lane 5 vs 2, lane 6 vs 3). Consistent with previous reports, paclitaxel induced Cdc2 mobility shift and Cdc25C hyperphosphorylation, with or without the presence of UCN-01 (corresponding lanes 8 and 11), which are indicative for activation of mitosis [13, 14]. Moreover, paclitaxel caused IκBα degradation and Bcl-2 phosphorylation (lane 8), which play important roles in the induction of mitotic arrest and apoptosis [7, 8]. However, radiation significantly inhibited paclitaxel-induced above changes on Cdc2, Cdc25, IκBα and Bcl-2 (corresponding lane 8 vs 9/10). Interestingly, the addition of UCN-01 re-sensitized tumor cells to the above changes induced by paclitaxel in the presence of radiation (e.g. lane 9 vs 12 and lane 10 vs 13 for IκBα).
Fig. 4. Western blot analysis.
Total proteins were extracted from BCap37 cells treated with designated regimes for 24 h. Equal amounts of proteins were fractionated on SDS-PAGE gel and transferred to PVDF membranes. The membranes were blotted with indicated antibodies. β-actin was blotted as a control. CTL, control; R3 and R9, 3 and 9 Gy of radiation, respectively; U100, 100 nM of UCN-01; T50, 50 nM of paclitaxel.
Discussion
The purpose of using different drugs or modalities in combination is to achieve therapeutic effects greater than those provided by a single drug or modality. The combination therapy between antimicrotubule drugs and radiation is under extensive investigations on the rationale that these drugs are able to arrest cells at the most radiosensitive G2-M phase of the cell cycle [3]. However, some reports indicate that paclitaxel combined with radiation might not result in any synergistic or additive effects [6–8, 11]. Moreover, both Grau and Osmak reported significant therapeutic loss when combining vincristine with radiation [9, 10]. On the other hand, relapse to cancer treatment is often caused by a subpopulation of drug resistant cancer cells. Drug resistance may arise from multiple mechanisms, e.g. P-glycoprotein, steroid hormones/hormone receptors, or treatment-induced cell cycle arrest which protects cancer cells from other phase-specific agents. Our recent studies indicate that the combination of radiation therapy with antimicrotubule drugs may result in the cell cycle-dependent antagonistic interaction on the antitumor activity [7, 8]. These findings have raised a clinically relevant question as to how the combination of radiotherapy with antimicrotubule drugs can really benefit the therapeutics efficacy. Herein, we investigated whether G2 abrogators may attenuate or abolish the reported G2 arrest-mediated antagonistic interaction, and thereby improve the therapeutic efficacy of the combination therapy.
It was revealed that many cancer cells have defective G1 checkpoint mechanisms and that cancer cells depend on G2 checkpoint far more than normal cells. These findings have given rise to the concept of “cell cycle G2 checkpoint abrogation” as a tactic for the development of cancer cell specific medicines [15]. Among the available candidates of therapeutic G2 abrogators, UCN-01 is most well-known and clinically advanced and was therefore used to test our hypothesis [12, 16]. Cytotoxic analysis demonstrated that at a concentration-dependent manner, UCN-01 significantly sensitized both BCap37 and KB cell lines to radiation. These results were consistent with previously reports that UCN-01, similar to caffeine and staurosporine, could enhance radiation cytotoxicity in other cell lines. Although this increase of cytotoxicity involves multiple mechanisms, the ability of UCN-01 to abrogate G2 checkpoint is believed to be a major pathway. By flow cytometric assay and bright-field microscope examination, we indeed observed that at a concentration-dependent manner, UCN-01 could attenuate the G2 arrest induced by radiation (Figs. 3 and 4). Moreover, the significant increase of Cyclin B1 coincides with radiation-induced G2 arrest because Cyclin B1 accumulates at this phase. However, UCN-01 significantly reduced the level of Cyclin B1 upregulation, providing additional molecular evidence on the attenuation of radiation-induced G2 arrest by UCN-01. Moreover, UCN-01 was shown to enhance the cytotoxicity of some DNA-damaging agents and antimetabolites in preclinical models, e.g. cisplatin and mytomycin C [12, 16]. Herein, for the first time, we demonstrated that at a dose-dependent manner, UCN-01 significantly enhanced the antitumor activity of paclitaxel and vincristine in BCap37 and KB cell lines (Figs. 1 and 2). Further investigations indicated that the combination of UCN-01 promoted mitotic arrest and apoptosis induced by these drugs (Figs. 3 and 4). These findings may explain in part the potentiation of the antitumor activities of antimitotic drugs by UCN-01.
Most importantly, we investigated whether UCN-01 could repress the inhibitory effect of radiation on antimicrotubule drugs. Interestingly, although a significant antagonistic interaction between radiation and paclitaxel or vincristine was observed as expected, the inhibitory effect of radiation on the overall cytotoxicity of these drugs was significantly repressed by the addition of UCN-01 (Fig. 1). Further investigations demonstrated that UCN-01 significantly enhanced the mitotic arrest and apoptosis induced by the combination of radiation and antimicrotubule drugs (Figs. 2–4). Moreover, it has been shown that Cdc2 mobility shift, Cdc25C hyperphosphorylation, IκBα degradation and Bcl-2 phosphorylation are important events during paclitaxel-induced mitotic arrest and apoptosis. However, radiation significantly inhibited these molecular changes induced by paclitaxel (Fig. 4). Encouragingly, UCN-01 re-sensitized tumor cells to the above changes caused by paclitaxel in the presence of radiation (Fig. 4). All these findings support our hypothesis that G2 abrogators might attenuate the G2 arrest-mediated antagonistic interaction between radiation and antimicrotubule drugs, and thereby increase the therapeutic efficacy of the combination therapy. As we know, this is the first report investigating the potential impact of G2 abrogator on chemoradiotherapy (see supplementary Fig. S2). In this study, UCN-01 was tested at non- or low-toxic dosage (cell survival >80% at 72 h), and we expect that higher concentrations of UCN-01 may further increase the therapeutic efficacy of the above treatments (clinically relevant concentration: 50–300nM). In fact, the idea of adding a checkpoint abrogator to a combination therapy which involves DNA damaging agent and mitosis specific drug has been suggested in two recent reports, where combinations of doxorobucin+UCN-01+paclitaxel and doxorubicin+Go6976+paclitaxel were investigated respectively [17, 18]. Together with previous reports from our laboratory and others, our findings have suggested the potential use of G2 abrogators in combination therapy between radiation and chemotherapy for cancer treatment. Considering that G2 abrogators are currently under extensive evaluation for cancer treatment, both as a single agent and in combinations [16], our findings may provide valuable information for this class of promising compounds.
Supplementary Material
Acknowledgments
We would thank Dr. Donghai Jiang for his kindly technical help. This work is financially supported by NIH CA92880 (to W. Fan), NSFC Grants (21104065 to M. Sui, 81071880 and 30973456 to W. Fan) and funding support from Hangzhou’s New Drug Harbor for R&D platform for Antibodies and Targeted Therapeutics (to W. Fan and M. Sui).
Footnotes
Conflict of Interest Statement
The authors declare that they have no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Choe KS, Salama JK, Stenson KM, et al. Adjuvant chemotherapy prior to postoperative concurrent chemoradiotherapy for locoregionally advanced head and neck cancer. Radiother Oncol. 2010;97:318–321. doi: 10.1016/j.radonc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Fan W. Possible mechanisms of paclitaxel-induced apoptosis. Biochem Pharmacol. 1999;57:1215–1221. doi: 10.1016/s0006-2952(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 3.Terasima T, Tolmach LJ. Variations in several responses of HeLa cells to x-irradiation during the division cycle. Biophys J. 1963;3:11–33. doi: 10.1016/s0006-3495(63)86801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milas L, Hunter NR, Mason KA, Kurdoglu B, Peters LJ. Enhancement of tumor radioresponse of a murine mammary carcinoma by paclitaxel. Cancer Res. 1994;54:3506–3510. [PubMed] [Google Scholar]
- 5.Raitanen M, Rantanen V, Kulmala J, et al. Paclitaxel combined with fractionated radiation in vitro: a study with vulvar squamous cell carcinoma cell lines. Int J Cancer. 2002;97:853–857. doi: 10.1002/ijc.10133. [DOI] [PubMed] [Google Scholar]
- 6.Loprevite M, Favoni RE, de Cupis A, et al. Interaction between novel anticancer agents and radiation in non-small cell lung cancer cell lines. Lung Cancer. 2001;33:27–39. doi: 10.1016/s0169-5002(00)00247-6. [DOI] [PubMed] [Google Scholar]
- 7.Sui M, Dziadyk JM, Zhu X, Fan W. Cell cycle-dependent antagonistic interactions between paclitaxel and gamma-radiation in combination therapy. Clin Cancer Res. 2004;10:4848–4857. doi: 10.1158/1078-0432.CCR-03-0707. [DOI] [PubMed] [Google Scholar]
- 8.Sui M, Fan W. Combination of gamma-radiation antagonizes the cytotoxic effects of vincristine and vinblastine on both mitotic arrest and apoptosis. Int J Radiat Oncol Biol Phys. 2005;61:1151–1158. doi: 10.1016/j.ijrobp.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Osmak M. Multifactorial molecular mechanisms are involved in resistance of preirradiated human cervix carcinoma cells to cis-dichlorodiammineplatinum (II) and vincristine. Neoplasma. 1993;40:97–101. [PubMed] [Google Scholar]
- 10.Grau C, Hoyer M, Overgaard J. The in vivo interaction between vincristine and radiation in a C3H mammary carcinoma and the feet of CDF1 mice. Int J Radiat Oncol Biol Phys. 1994;30:1141–1146. doi: 10.1016/0360-3016(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 11.Mason KA, Milas L, Peters LJ. Effect of paclitaxel (taxol) alone and in combination with radiation on the gastrointestinal mucosa. Int J Radiat Oncol Biol Phys. 1995;32:1381–1389. doi: 10.1016/0360-3016(95)00037-Y. [DOI] [PubMed] [Google Scholar]
- 12.Ree AH, Bratland A, Nome RV, Stokke T, Fodstad O, Andersson Y. Inhibitory targeting of checkpoint kinase signaling overrides radiation-induced cell cycle gene regulation: a therapeutic strategy in tumor cell radiosensitization? Radiother Oncol. 2004;72:305–310. doi: 10.1016/j.radonc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Kuo CC, Hsieh HP, Pan WY, et al. BPR0L075, a novel synthetic indole compound with antimitotic activity in human cancer cells, exerts effective antitumoral activity in vivo. Cancer Res. 2004;64:4621–4628. doi: 10.1158/0008-5472.CAN-03-3474. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M, Hofmann HP, Sanders K, Sczakiel G, Beckers TL, Gekeler V. Molecular alterations after Polo-like kinase 1 mRNA suppression versus pharmacologic inhibition in cancer cells. Mol Cancer Ther. 2006;5:809–817. doi: 10.1158/1535-7163.MCT-05-0455. [DOI] [PubMed] [Google Scholar]
- 15.Kawabe T. G2 checkpoint abrogators as anticancer drugs. Mol Cancer Ther. 2004;3:513–519. [PubMed] [Google Scholar]
- 16.Sugiyama K, Shimizu M, Akiyama T, et al. UCN-01 selectively enhances mitomycin C cytotoxicity in p53 defective cells which is mediated through S and/or G(2) checkpoint abrogation. Int J Cancer. 2000;85:703–709. doi: 10.1002/(sici)1097-0215(20000301)85:5<703::aid-ijc17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Blagosklonny MV. Sequential activation and inactivation of G2 checkpoints for selective killing of p53-deficient cells by microtubule-active drugs. Oncogene. 2002;21:6249–6254. doi: 10.1038/sj.onc.1205793. [DOI] [PubMed] [Google Scholar]
- 18.Aaltonen V, Koivunen J, Laato M, Peltonen J. PKC inhibitor Go6976 induces mitosis and enhances doxorubicin-paclitaxel cytotoxicity in urinary bladder carcinoma cells. Cancer Lett. 2007;253:97–107. doi: 10.1016/j.canlet.2007.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.