Abstract
The aged immune system, typically hyporesponsive to infection and vaccination, can be hyperresponsive in the context of inflammatory pathology. Here we review current work examining the mechanisms behind the amplified inflammatory profile of aged adaptive immunity, and the reciprocal relationship between chronic inflammation and immune aging. Aged hematopoietic stem cells are driven to differentiate following accumulated DNA damage, thus depleting the stem cell pool and increasing the number of damaged effector cells in the circulation. Chronic DNA damage responses in lymphocytes as well as senescent cells of other lineages initiate the production of inflammatory mediators. In addition, aged lymphocytes become less reliant on specific antigen for stimulation and more prone to activation through innate receptors. When these lymphocytes are exposed to inflammatory signals produced by senescent tissues, the bias toward inflammation exacerbates destruction without necessarily improving immunity.
Introduction
The aging of the immune system comes with an apparent paradox. Despite a decrease in immune responsiveness to infection and vaccination [1], and even a reduction in infection-associated immunopathology [2,3], the elderly individual experiences an increase in systemic inflammation, which can aggravate degenerative diseases, and is at increased risk of autoimmune disease. Some autoimmune diseases, such as giant cell arteritis and polymyalgia rheumatica, are typical diseases of the elderly [4]. Others, such as rheumatoid arthritis (RA), increase in incidence with age and peak when immune competence is already on the decline and the control of exogenous or latent infections is impaired [5]. In addition to classical autoimmune diseases, many age-associated pathologies are associated with inflammation. Even more importantly, inflammation in longitudinal studies has a negative impact on healthy aging. In the Cardiovascular Healthy AllStar Study, approximately one-fifth of all participants had a doubling in serum IL-6 over a ten-year period. This doubling was associated with a higher risk of physical and cognitive impairment as well as with an increase in adverse cardiovascular events and mortality [6]. These and other studies [7] are consistent with the interpretation that increased inflammation is an age-associated primary event that sets the stage for later development of age-associated diseases. In the classical paradigm, the source of inflammatory mediators is the innate immune system. Stimulation of innate immunity may be a consequence of poor containment by, and increased bacterial translocation from, the gastrointestinal system as has been implicated for the increased inflammatory response in HIV-infected patients [8,9]. Alternatively, the gradual dysfunction of adaptive immunity may increase the infectious load from exogenous and latent infections, thus increasing immune exposure to pathogen-associated ligands. In addition to these more traditional mechanisms, there is increasing evidence that at least two age-related pathway modifications contribute to increased inflammatory responses during aging: changes in immune cell generation and homeostasis, and cell-intrinsic mechanisms associated with cellular senescence and chronic DNA damage responses [10].
Age-associated remodeling of lineage differentiation
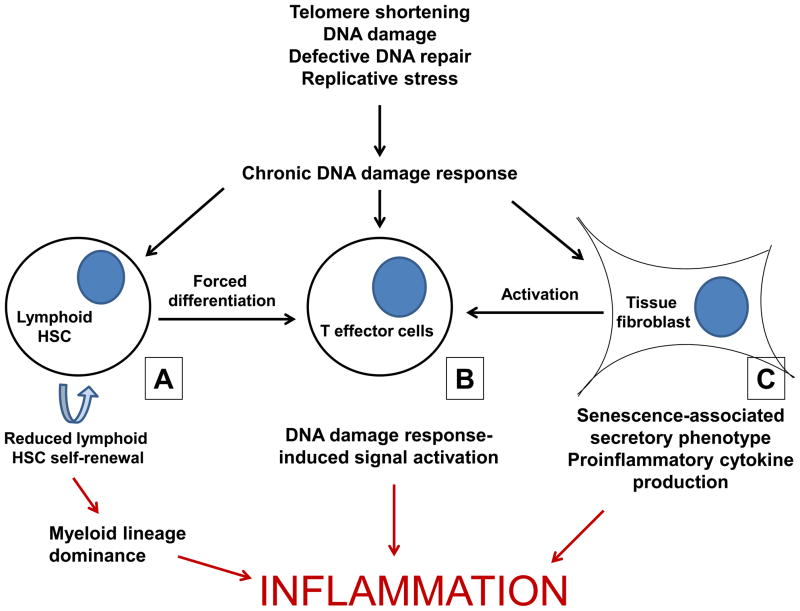
Since immune cell generation is central to immune function, aging of hematopoietic stem cells (HSCs) is intimately involved with age-related immune pathophysiology. Whilst maintaining pluripotency into advanced age, there is growing evidence that HSCs not only lose regenerative capacity, but also have altered lineage potential. The lymphoid lineage commitment dominant in children and young adults is replaced by preferential myeloid differentiation in the elderly [11,12]. In a phenotypic study of human bone marrow, the proportion of multipotent CD34+CD38− cells was shown to increase in elderly. Myeloid progenitor cells (defined as CD45RA negative) persist at the same level, while the frequency of early or committed B lymphoid progenitor cells (defined by the expression of CD45RA, CD38, CD10 and CD19) decreases with age [13]. Alterations in lineage-specific programs, as well as a clonal dominance of myeloid-biased HSC, are thought to provide the underlying mechanisms [14,15]. The resulting preferential myeloid differentiation results in a bias towards the innate immune system and increased inflammatory activity. A new study now links changes in differentiation potential directly to chronic DNA damage responses. The authors examined HSC differentiation in a setting of telomerase deficiency to mimic age-related DNA damage. They identified the basic leucine zipper transcription factor, ATF-like (BATF) as central for limiting the self-renewal of lymphoid-biased HSCs and inducing cell differentiation in response to DNA damage [16**]. In the accompanying editorial, Mandal and Rossi postulate that the combination of compulsory lymphoid differentiation and diminished self-renewal of lymphoid precursor cells eventually leads to a loss of pluripotency and dominance of myeloid lineage HSCs [17]. Indeed, accumulation of DNA damage and increased expression of BATF are features of HSC aging in humans [16**,18]. Figure 1A shows the ways in which the skewing of the HSC pool combines with other inflammatory processes, all involving DNA damage, to exacerbate inflammation.
Figure 1. DNA damage responses (DDR) are induced by many different age-associated DNA damage events and promotes inflammation through different mechanisms.
A) Accumulated DNA damage in lymphoid hematopoietic stem cells (HSCs) induces differentiation into mature lymphocytes and leads to an imbalance in the stem cell pool, which becomes dominated by myeloid precursors. The reduction in lymphoid HSCs, combined with thymic involution and reduced bone marrow output, leads to a reliance on innate immunity favouring broad inflammatory responses. B) The lymphocytes differentiating from damaged HSCs may harbor unfavorable mutations that then integrate into the adaptive repertoire. In addition, DDR induced by age-associated nontelomeric DNA damage or telomeric erosions can contribute to the activation of T effector cell populations and production of inflammatory mediators. C) Cellular senescence induced by DNA damage has also been shown to induce the production of inflammatory cytokines by a variety of cell lineages not directly related to the immune system, a process coined as senescence-associated secretory pattern (SASP). In addition to directly causing pathology, this inflammatory tissue environment can attract and activate lymphocytes in a bystander fashion, even in the absence of specific antigen stimulation.
Age-associated alterations in subset composition and mature lymphocyte function
In addition to a reduced number of lymphoid precursor cells and the oligoclonal expansion of myeloid precursor cells, molecular defects in the lymphoid lineage have been identified that correlate with the age-associated defective differentiation of mature lymphocytes. A critical event for B cells appears to be the loss of the B lineage-specific effector molecules EBF and PAX5. Transduction of lymphoid precursor cells from old mice with a constitutively active form of STAT5 restored expression of both EBF and PAX5 and increased B cell potential [19].
The decline in B lineage commitment favors innate immunity, and could itself have consequences for inflammation. A bias towards autoreactivity in the formation of the B or T cell repertoire may additionally tilt the balance. Cancro et al have provided evidence for the hypothesis that central and peripheral selection checkpoints involved in B cell differentiation are governed by competition for limited cellular growth and survival factors, such as B Lymphocyte Stimulator (BLyS) [20,21]. If survival factors are no longer limiting, either owing to a decreased number of precursor cells or following increased production of these factors as a compensatory homeostatic mechanism, B cell development is biased toward survival and the generation of a more autoimmune repertoire [22]. A similar risk may apply to thymic function in old age, in particular under conditions of thymic stimulation. Medullary islet complexity and tissue-restricted antigen expression in the thymus decrease with age and are not restored by induced thymic regrowth. Similarly, signaling abnormalities in the WNT pathway remain defective. Thymic restoration, therefore, does not reverse age-associated adaptive immune deterioration. Degenerative changes persist, any restored function is very transient and, in addition, may be associated with the selection of a more autoreactive repertoire [23**].
While these age-related changes in the central selection mechanisms of T and B cells may favor classical autoreactivity and possibly autoimmunity, further deficiencies in peripheral selection mechanisms may account for increased inflammatory responses irrespective of antigen recognition. Recently, an age-associated B cell (ABC) subset has been described, that can comprise up to 30% of the mature B cell pool in aged mice. These cells are not reliant on BLyS for survival, but continue to express receptors for this cytokine, potentially sequestering survival signals from other mature B cells in situations of limiting BLyS concentrations. ABCs are unique in that they are refractory to B cell receptor stimulation, and responsive to signals from Toll-like receptors (TLR) 7 and 9. In addition to their ability to produce cytokines and immunoglobulins, these B cells are very effective antigen presenters and favor polarization to a Th17 profile [24*]. Aged mice also accumulate a population of B7-DC (PD-L2)+ B cells that facilitate the induction of a Th1, as well as a Th17, response [25]. These populations, therefore, not only combine features of the innate and the adaptive immune system, but also induce inflammatory T cell responses. A similar concept of sharing features of innate and adaptive immunity has been proposed for T cells, in that T cell effector populations acquire regulatory elements that are more typically expressed by NK cells [26]. In contrast to the B cell population described by Cancro et al, which is exhausted but able to respond to TLR stimulation and induce inflammatory T cells, the T cell effector populations that accumulate with age remain responsive to antigenic stimulation. In humans, these cells are predominantly specific for cytomegalovirus (CMV). In a rhesus macaque model, clonally expanded CMV-specific CD8 T cells remain fully functional and may, therefore, contribute to inflammation directly rather than indirectly through diminished control of CMV infection [27]. Inflammatory markers in the context of chronic CMV infection are a predictor for age-associated morbidity and mortality [7]. In contrast to clonally exhausted T cell populations, where repeated antigenic stimulation drives differentiation [28], a rapid response to IL-12 with increased expression of T-bet is important for the expansion of the LCMV-specific T cell effector population in chronically-infected mice [29], again consistent with the theme that responsiveness to innate stimuli changes with age and that these cells commit to a more inflammatory lineage.
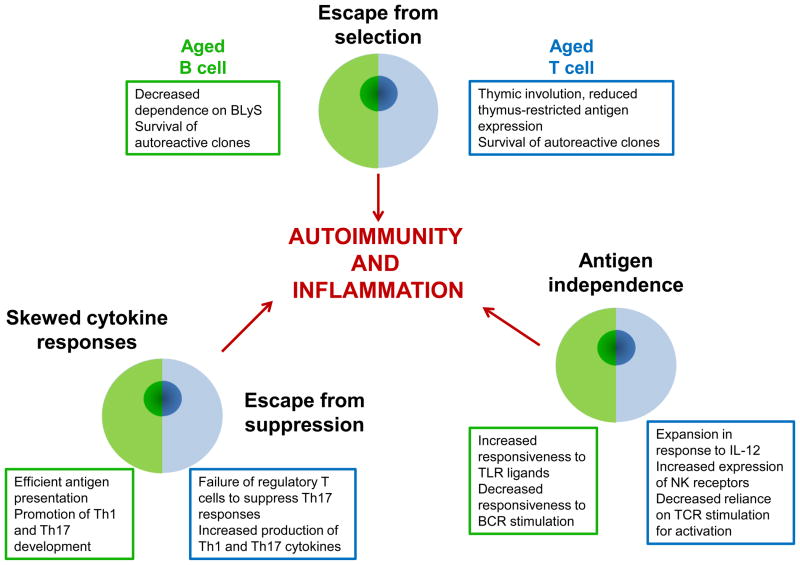
Whether defects in regulatory T cells (Treg) develop with age and contribute to the imbalance in favor of autoimmunity and inflammatory cytokine production, remains an attractive but contentious model. While frequencies of CD4+ Tregs and their ability to suppress T cell proliferation or interferon production are not impaired in most studies, Sun et al recently describe an inability of these cells to contain IL-17 production in a colitis model in old mice [30]. Similarly, a regulatory CD8+ T cell population that is derived from naïve CD8+ T cells and continues to express CD28 and CCR7 has been found to decline with age in both function and frequency and is decreased in patients with rheumatoid arthritis [31]. Figure 2 summarizes the alterations to lymphocytes that occur with aging. The changes to B and T lymphocytes have certain parallels that lead to an overall move towards antigen-independence and the promotion of adaptive inflammation.
Figure 2. Age-associated changes to B and T lymphocytes.
Aging of the immune system is associated with changes to B and T lymphocytes. These changes have different underlying mechanisms but related downstream consequences. T and B cells experience reduced selection pressure, owing to changes in primary lymphoid tissues and changes in survival requirements. Both cell types become less reliant on exposure to specific antigen through the BCR and TCR for activation, and more responsive to innate/antigen-independent signals, such as TLR ligands and cytokines. Additionally, there is cross talk between populations. Aged B cells are effective antigen presenters, and preferentially induce Th1 and Th17 responses in T cells. In parallel, aged regulatory T cells fail to suppress Th17 responses, with the effect that proinflammatory responses are promoted.
DNA damage responses and inflammation
Chronic DNA damage responses are apparent in hematopoietic lineages in older people, where they are explained only in part by telomeric dysfunction. Rübe et al showed recently that aged HSCs have an increased frequency of γ-H2AX foci, most of which do not colocalize with telomeres. These DNA damage-associated foci are only partly a consequence of noxious molecules such as oxygen radicals, and the major contributing factor appears to be individual differences in DNA repair suggesting that other, cell-intrinsic mechanisms are involved [18]. An increase in frequency of double-strand breaks is also found in peripheral T cells during aging. Of note, DNA damage in memory T cells far exceeds that found in naïve T cells, at any age [32]. Inefficient DNA repair is one of the major driving forces in the accelerated immune aging seen in patients with rheumatoid arthritis [33]. In these patients, at least two repair mechanisms are defective. Cells express reduced telomerase activity which causes telomeric erosion in all hematopoietic lineages ranging from stem cells to mature naïve and memory T cells [34]. In addition, expression and function of members of the ATM pathway are reduced, leading to increased nontelomeric DNA damage [35]. The resulting chronic DNA damage responses mostly involve the activation of DNA-PKcs. DNA-PKcs activity influences intracellular signaling pathways through at least two mechanisms. It activates the inflammasome and increases NFκB activity; and it activates the stress kinase JNK pathway [32,36]. Both pathways can contribute to the production of inflammatory cytokines (Figure 1).
DNA damage may also be linked to the increased inflammatory responses seen with age. Increased DNA damage and cell death provide a source of cell-free DNA in the plasma which, in a prospectively followed cohort of nonagenarians, has been shown to correlate with inflammatory biomarkers including C-reactive protein and indolamine-2,3-dioxygenase enzyme activity. In this study, cell-free DNA concentrations also independently predicted subsequent coronary artery events and all cause mortality [37]. Whether cell-free DNA in the plasma is a consequence of inflammation or whether the increased circulating DNA fragments provide a stimulatory signal, for example through the stimulation of TLRs, is unclear. Increased TLR stimulation has been implicated in a number of autoimmune diseases in the elderly, in particular polymyalgia rheumatica and giant cell arteritis [38–40].
Chronic DNA damage responses play a major role in regulating intrinsic cell activation and cell differentiation pathways. As mentioned above, increased DNA damage induces a differentiation program in HSCs and may, therefore, shift the balance of immune subpopulations towards a more inflammatory setting. More directly, DNA damage responses have been shown to be important in the production of inflammatory mediators following cellular senescence [41]. Campisi and colleagues have shown that senescent cells are not only characterized by their irreversible growth arrest, but also by the secretion of a number of inflammatory mediators, coined the senescence-associated secretory phenotype (SASP) [42]. Obviously this phenotype is not specific for immune cells, largely expanding the potential source of inflammatory mediators. DNA damage response signaling involving the DNA repair molecules ATM, NBS1 and CHK2 is essential, but not sufficient for the production of inflammatory cytokines, possibly because of the regulatory activity of p53. In typical senescent cells, the negative regulation of p53 is overcome by the activation of p38 MAPK [43], which increases NFκB transcriptional activity, consistent with the notion that the p53 and NFκB pathways reciprocally cross-regulate [44]. Selected components of the SASP, including the production of several proinflammatory cytokines, are sensitive to the suppressive action of glucocorticoids without reversion of senescence-associated growth arrest [45*] suggesting that steroid treatment could reduce tissue inflammation in the elderly.
The SASP, by increasing the inflammatory environment in tissues, may provide further impetus for the activation of adaptive immunity and the perpetuation of inflammation by the stimulation of activated lymphocytes, and potentially by bystander activation of nonspecific lymphocytes [46–48*]. As DNA damage accumulates in tissues, lymphocytes and lymphocyte progenitors, this bias toward activation and inflammation is exaggerated (Figure 1).
Conclusions
As we age, our immune system ages with us and it is increasingly clear that changes to adaptive immunity result in decreased responsiveness in some situations (infection) and increased responsiveness in others (inflammation). Recent studies suggest that this apparent paradox is the result of changes to a number of basic mechanisms that are compounded with increasing age: increased DNA damage and decreased telomere length in HSCs resulting in chronic DNA repair responses; decreased reliance of adaptive immunity on specific antigen stimuli and increased responsiveness to nonantigenic stimuli; and increased production of inflammatory mediators by senescent cells from multiple cell types (SASP). In combination, these processes conspire to cause chronic inflammation which, once established promotes its own persistence and, even in the absence of overt autoimmunity, is associated with adverse events in age.
Highlights.
The aging immune system is more prone to inflammation but less protective.
DNA damage reduces stem cell renewal, depleting lymphoid and promoting myeloid progenitors.
Poor DNA repair perpetuates damage and alters mature lymphocyte function.
Aged T and B lymphocytes are less antigen-dependent and more inflammatory.
Cytokine production by senescent cells exacerbates inflammation.
Acknowledgments
This work was supported by grants from the National Institutes of Health (U19 AI 57266 and U19 AI090019 to JJG, and R01 AR42527, R01 EY11916, R01 AI44142 and P01 HL058000 toCMW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, Pawelec G. The unmet need in the elderly: How immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine. 2012;30:2060–2067. doi: 10.1016/j.vaccine.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smallman-Raynor M, Cliff AD. Avian influenza A (H5N1) age distribution in humans. Emerg Infect Dis. 2007;13:510–512. doi: 10.3201/eid1303.060849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liem NT, Tung CV, Hien ND, Hien TT, Chau NQ, Long HT, Hien NT, Mai le Q, Taylor WR, Wertheim H, et al. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004–2006. Clin Infect Dis. 2009;48:1639–1646. doi: 10.1086/599031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan SV, Liao YJ, Kim JW, Goronzy JJ, Weyand CM. Giant cell arteritis: immune and vascular aging as disease risk factors. Arthritis Res Ther. 2011;13:231. doi: 10.1186/ar3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goronzy JJ, Shao L, Weyand CM. Immune aging and rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36:297–310. doi: 10.1016/j.rdc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenny NS, French B, Arnold AM, Strotmeyer ES, Cushman M, Chaves PH, Ding J, Fried LP, Kritchevsky SB, Rifkin DE, et al. Long-term Assessment of Inflammation and Healthy Aging in Late Life: The Cardiovascular Health Study All Stars. J Gerontol A Biol Sci Med Sci. 2012 doi: 10.1093/gerona/glr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One. 2011;6:e16103. doi: 10.1371/journal.pone.0016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 9.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol life Sci. doi: 10.1007/s00018-012-0970-0. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111:5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller-Sieburg C, Sieburg HB, Bernitz JM, Cattarossi G. Stem cell heterogeneity: implications for aging and regenerative medicine. Blood. 2012 doi: 10.1182/blood-2011-12-376749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuranda K, Vargaftig J, de la Rochere P, Dosquet C, Charron D, Bardin F, Tonnelle C, Bonnet D, Goodhardt M. Age-related changes in human hematopoietic stem/progenitor cells. Aging Cell. 2011;10:542–546. doi: 10.1111/j.1474-9726.2011.00675.x. [DOI] [PubMed] [Google Scholar]
- 14.Beerman I, Bhattacharya D, Zandi S, Sigvardsson M, Weissman IL, Bryder D, Rossi DJ. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci U S A. 2011;108:20012–20017. doi: 10.1073/pnas.1116110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Wang J, Sun Q, Morita Y, Jiang H, Gross A, Lechel A, Hildner K, Guachalla LM, Gompf A, Hartmann D, et al. A Differentiation Checkpoint Limits Hematopoietic Stem Cell Self-Renewal in Response to DNA Damage. Cell. 2012;148:1001–1014. doi: 10.1016/j.cell.2012.01.040. The authors use an RNAi screen to identify the transcription factor BATF as a key regulator of HSC differentiation and describe its role in the skewing of the stem cell pool to favour myeloid precursors. [DOI] [PubMed] [Google Scholar]
- 17.Mandal PK, Rossi DJ. DNA-Damage-Induced Differentiation in Hematopoietic Stem Cells. Cell. 2012;148:847–848. doi: 10.1016/j.cell.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Rube CE, Fricke A, Widmann TA, Furst T, Madry H, Pfreundschuh M, Rube C. Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One. 2011;6:e17487. doi: 10.1371/journal.pone.0017487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lescale C, Dias S, Maes J, Cumano A, Szabo P, Charron D, Weksler ME, Dosquet C, Vieira P, Goodhardt M. Reduced EBF expression underlies loss of B-cell potential of hematopoietic progenitors with age. Aging Cell. 2010;9:410–419. doi: 10.1111/j.1474-9726.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- 20.Scholz JL, Crowley JE, Tomayko MM, Steinel N, O’Neill PJ, Quinn WJ, 3rd, Goenka R, Miller JP, Cho YH, Long V, et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A. 2008;105:15517–15522. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, 3rd, Brezski RJ, Treml LS, Jordan KA, Monroe JG, et al. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholz JL, Cancro MP. Resolve, revise, and relax: The 3 Rs of B cell repertoire adjustment. Immunol Lett. 2012 doi: 10.1016/j.imlet.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **23.Griffith AV, Fallahi M, Venables T, Petrie HT. Persistent degenerative changes in thymic organ function revealed by an inducible model of organ regrowth. Aging Cell. 2012;11:169–177. doi: 10.1111/j.1474-9726.2011.00773.x. The authors use an in vivo system in combination with computational modelling to describe, in detail, the changes to thymic stromal cells with age and to show that effective thymic T cell generation is not restored by regrowth in aged mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **24.Hao Y, O’Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. 2011;118:1294–1304. doi: 10.1182/blood-2011-01-330530. This paper describes a novel age-associated B cell subset which can be activated independently of antigen and promotes inflammatory T cell responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomihara K, Shin T, Hurez VJ, Yagita H, Pardoll DM, Zhang B, Curiel TJ. Aging-associated B7-DC+ B cells enhance anti-tumor immunity via Th1 and Th17 induction. Aging Cell. 2012;11:128–138. doi: 10.1111/j.1474-9726.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warrington KJ, Takemura S, Goronzy JJ, Weyand CM. CD4+,CD28− T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 2001;44:13–20. doi: 10.1002/1529-0131(200101)44:1<13::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Cicin-Sain L, Sylwester AW, Hagen SI, Siess DC, Currier N, Legasse AW, Fischer MB, Koudelka CW, Axthelm MK, Nikolich-Zugich J, Picker LJ. Cytomegalovirus-specific T cell immunity is maintained in immunosenescent rhesus macaques. J Immunol. 2011;187:1722–1732. doi: 10.4049/jimmunol.1100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bucks CM, Norton JA, Boesteanu AC, Mueller YM, Katsikis PD. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J Immunol. 2009;182:6697–6708. doi: 10.4049/jimmunol.0800997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L, Hurez V, Thibodeaux SR, Kious MJ, Liu A, Lin P, Murthy K, Pandeswara S, Shin T, Curiel TJ. Aged regulatory T cells protect from autoimmune inflammation despite reduced STAT3 activation and decreased constraint of IL-17 producing T cells. Aging Cell. 2012 doi: 10.1111/j.1474-9726.2012.00812.x. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M, Jagger A, Konya C, Goronzy JJ, Weyand CM. CD8+CCR7+Foxp3+ T cells with immunosuppressive properties: a novel subset of inducible human regulatory T cells. J Immunol. doi: 10.4049/jimmunol.1200122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao L, Goronzy JJ, Weyand CM. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med. 2010;2:415–427. doi: 10.1002/emmm.201000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hohensinner PJ, Goronzy JJ, Weyand CM. Telomere dysfunction, autoimmunity and aging. Aging Dis. 2011;2:524–537. [PMC free article] [PubMed] [Google Scholar]
- 34.Fujii H, Shao L, Colmegna I, Goronzy JJ, Weyand CM. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–1449. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajagopalan S, Moyle MW, Joosten I, Long EO. DNA-PKcs controls an endosomal signaling pathway for a proinflammatory response by natural killer cells. Sci Signal. 2010;3:ra14. doi: 10.1126/scisignal.2000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jylhava J, Jylha M, Lehtimaki T, Hervonen A, Hurme M. Circulating cell-free DNA is associated with mortality and inflammatory markers in nonagenarians: The Vitality 90+ Study. Exp Gerontol. 2012 doi: 10.1016/j.exger.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Alvarez Rodriguez L, Lopez-Hoyos M, Mata C, Fontalba A, Calvo Alen J, Marin MJ, Fernandez-Luna JL, Aguero Balbin J, Aranzamendi Zaldunbide M, Blanco R, Martinez-Taboada VM. Expression and function of toll-like receptors in peripheral blood mononuclear cells of patients with polymyalgia rheumatica and giant cell arteritis. Ann Rheum Dis. 2011;70:1677–1683. doi: 10.1136/ard.2010.140194. [DOI] [PubMed] [Google Scholar]
- 39.Deng J, Ma-Krupa W, Gewirtz AT, Younge BR, Goronzy JJ, Weyand CM. Toll-like receptors 4 and 5 induce distinct types of vasculitis. Circ Res. 2009;104:488–495. doi: 10.1161/CIRCRESAHA.108.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freund A, Patil CK, Campisi J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. Embo J. 2011;30:1536–1548. doi: 10.1038/emboj.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gudkov AV, Gurova KV, Komarova EA. Inflammation and p53: A Tale of Two Stresses. Genes Cancer. 2011;2:503–516. doi: 10.1177/1947601911409747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45.Laberge RM, Zhou L, Sarantos MR, Rodier F, Freund A, de Keizer PL, Liu S, Demaria M, Cong YS, Kapahi P, et al. Glucocorticoids Suppress Selected Components of the Senescence-Associated Secretory Phenotype. Aging Cell. 2012 doi: 10.1111/j.1474-9726.2012.00818.x. This paper provides evidence to suggest that corticosteroid treatment may reduce the senescence-associated secretory phenotype, thus ameliorating age-assoicated inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maroof A, Beattie L, Kirby A, Coles M, Kaye PM. Dendritic cells matured by inflammation induce CD86-dependent priming of naive CD8+ T cells in the absence of their cognate peptide antigen. J Immunol. 2009;183:7095–7103. doi: 10.4049/jimmunol.0901330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nogai A, Siffrin V, Bonhagen K, Pfueller CF, Hohnstein T, Volkmer-Engert R, Bruck W, Stadelmann C, Kamradt T. Lipopolysaccharide injection induces relapses of experimental autoimmune encephalomyelitis in nontransgenic mice via bystander activation of autoreactive CD4+ cells. J Immunol. 2005;175:959–966. doi: 10.4049/jimmunol.175.2.959. [DOI] [PubMed] [Google Scholar]
- *48.Bou Ghanem EN, Nelson CC, D’Orazio SE. T cell-intrinsic factors contribute to the differential ability of CD8+ T cells to rapidly secrete IFN-gamma in the absence of antigen. J Immunol. 2011;186:1703–1712. doi: 10.4049/jimmunol.1001960. This study shows that IL-12 signalling is one of the mechanisms underlying the antigen-independent activation of memory and “memory-like” CD8+ T cells in an infectious disease model. [DOI] [PubMed] [Google Scholar]