Summary
The commensal bacteria normally resident in the gastrointestinal tract represent an enormous pool of foreign antigen within the body. Although mechanical barriers limit entry of bacteria into the host, recent data suggest that T cells routinely interact with commensal bacteria using both antigen-specific and non-specific receptors. Depending on the bacterial species, either regulatory or effector T cell responses can be generated. For example, segmented filamentous bacteria (SFB) favor effector Th17 responses whereas Bacteroides fragilis and certain Clostridium species favor Foxp3+ regulatory T (Treg) cell responses. Thus, in contrast with the notion that only tolerogenic responses are required to self, gut homeostasis may require both tolerance and immunity to various constituents of the commensal microbiota.
Introduction
The process of lymphocyte maturation generates a diverse array of receptors capable of recognizing a wide variety of antigens. However, a consequence of this diversity is that self-antigens will inevitably be recognized and cause autoimmunity, necessitating the need for tolerance to self. For T cells, this is primarily accomplished by the deletion of self-reactive cells or their selection into the suppressive regulatory T (Treg) cell subset during T cell development in the thymus [1,2]. However, it has been unclear how tolerance is established to antigens that are not present in the thymus. In particular, the commensal bacteria resident in the gut represent a large, ever-present source of foreign antigens, with over 1000 species encoding 100-times as many genes as the human genome [3]. Although most of these antigens are sequestered in the lumen under normal conditions due to the mucous layer, IgA, and anti-microbial peptides near the mucosal barrier [4–6], some antigens cross this barrier and are presented to the immune system. In the small intestine (SI), antigens may be actively transported across the barrier by M cells in the Peyer’s Patches (PP) [7], dendritic cell extensions into the lumen [8], or through channels in close proximity to goblet cells [9]. Presentation of antigen to naïve T cells then initiates peripheral T cell differentiation. In the colon, the processes that allow bacterial antigen presentation are currently unknown. Here, we review the recent studies of T cell interactions with commensal bacteria which show that some species can induce peripheral regulatory T cell selection and tolerance, whereas others appear to elicit effector T cell responses and immunity.
Treg cells are important for gut tolerance
One suggestion that the immune system is normally tolerant to gut microbial antigens comes from clinical experience with human inflammatory bowel disease (IBD), which afflicts ~ 1 in 200 people with symptoms such as bloody diarrhea and abdominal pain, causing substantial morbidity and mortality [10]. Even though the gut is a common route of pathogen entry, an infectious etiology has not been identified. Moreover, treatment of IBD commonly utilizes immunosuppression, which would be predicted to exacerbate an infection [11]. As immunosuppression is normally used for treating autoimmune disease, this suggests that IBD results from a breakdown in immune tolerance in the gut [10–13].
The notion that T cells are actively tolerant to commensal bacteria was suggested two decades ago by the classic studies of Powrie et al. Using an adoptive transfer model, it was shown that the normal CD4+ T cell population contains naive T cells marked by CD45RBhi that can cause colitis [14], but are normally held in check by another CD4+ T cell subset now known to be Treg cells [12]. The inflammatory responses were dependent on the presence of commensal bacteria, as transfer into germ-free (GF) hosts did not lead to pathology [15]. Thus, the presence of Treg cells normally prevents inappropriate T cell responses to commensal bacteria that can cause an inflammatory colitis.
Initial studies using GF mice suggested, however, that commensal bacteria were not essential for the generation of a protective Treg cell population. It was observed that Treg cells are readily found in the gut of GF mice [16–18], suggesting that commensal bacteria are not required for Treg cell generation. Moreover, the Treg cells from GF mice were able to protect against colitis in the aforementioned Powrie adoptive transfer model [17,19], although they were quantitatively less effective than Treg cells from conventionally housed (conv.) mice. Thus, these data suggested that Treg cells are required for colonic tolerance to commensal bacteria, but that Treg cell generation and function can occur independently of commensal bacteria.
Specific commensal bacterial species influence the colonic Treg cell population
Contrary to these initial data from GF mice, recent studies suggest that commensal bacteria play an important role in shaping the colonic Treg cell population. For example, it was observed in some labs that the frequency of colonic Treg cells was 2–3 fold higher in mice with commensal bacteria than in GF mice [17,18,20,21]. A study of the constituents of the commensal microbiota revealed that certain Clostridium species, but not Lactobacillus or Bacteroides species, are sufficient to increase the frequency of Foxp3+ Treg cells in the colon when transferred into GF mice [20]. In particular, Clostridium species from phylogenetic clusters IV and XIV were the most effective in generating a high frequency of Treg cells in the CD4+ T cell population. Although much remains to be learned regarding the microbial species that affect the colonic Treg cell population, it is clear that some species are preferentially able to increase the Treg cell population in the colon.
In addition to the ability of Clostridium species to enhance the frequency of Treg cells in the colon, it was observed that commensal bacteria affected Treg cell expression of Helios [20], an Ikaros family transcription factor that has been proposed as a marker for thymically derived Treg cells [22]. Most Treg cells in the colons of GF mice were Helioshi, whereas Treg cells in conv. mice were mostly Helioslo [20,23]. Thus, these data implied that the colons of GF mice contain mostly Treg cells of thymic origin, whereas the commensal microbiota in conv. mice robustly triggers the peripheral differentiation of induced Treg (iTreg) cells that preferentially survive or expand at the expense of thymically-derived Treg cells.
Induction of commensal-bacteria specific iTreg cells
Although the use of Helios as a thymic Treg cell marker is still controversial [24–26], independent confirmation for the hypothesis that commensal bacteria induce iTreg cell generation came from an analysis of the colonic Treg TCR repertoire. This study utilized a fixed TCRβ model to analyze TCRs at the individual TCR level [23], and found that colonic Treg cells use TCRs that are different from those used by Treg cells in other secondary lymphoid tissues, suggesting that colonic Treg cells recognize local antigens. Functional in vitro assays using colonic Treg TCR expressing hybridoma cells revealed that a large fraction of the common Treg TCRs reacted to either colonic contents or bacterial isolates, implying that the majority of colonic Treg cells recognize commensal bacteria. Although the specificity of most Treg TCRs analyzed was not mapped to an individual bacterial species, these data suggested that many, and perhaps the majority, of Treg TCRs could recognize commensal bacteria.
Analysis of this set of colonic Treg TCRs in vivo revealed that they were unable to facilitate thymic Treg cell differentiation. Further analysis of two of these TCRs showed that they facilitate peripheral Treg cell induction in response to the commensal microbiota [23]. Thus, consistent with the Helios results discussed above, these data suggested that most colonic Treg cells differentiate from naive T cells in the periphery, rather than derive from nTreg cells selected in the thymus.
The notion that peripheral Treg cell induction occurs locally in the gut is also supported by other studies. For example, TGFβ, an important factor for inducing Foxp3 expression in naive T cells [27], is enriched in the gut. One potential contributor to this is bacterial-derived metallo-matrix proteases, which can facilitate the conversion of latent TGFβ to its active form, promoting peripheral Treg cell induction [20]. CD103+ dendritic cells in the mesenteric lymph node, but not splenic dendritic cells, have been shown to facilitate the process of peripheral conversion in the gut, in part mediated by the synthesis of retinoic acid [28–31]. However, recent studies have also suggested a pro-inflammatory effect of retinoic acid [32,33], implying that it has a context dependent role in immunity and tolerance.
The observation that thymus-derived Treg cells markedly decrease in proportion to iTreg cells in the presence of commensal bacteria poses an interesting question regarding the competitive fitness of these two Treg cell subsets. One possibility is that bacterial products may favor the preferential expansion or survival of iTreg cells. This may occur via stimulation of innate immune cells leading to the release of cytokines, or via stimulation of T cells themselves. A well-studied example of this type of response is polysaccharide A (PSA) from B. fragilis [34,35], which has recently been shown to activate Treg cells via TLR2 and facilitate the production of IL-10 [36]. A TLR-mediated mechanism for increasing the frequency of iTreg cells, however, has not yet been described. Another possibility is that bacterial products or their antigens may indirectly facilitate iTreg cell development or expansion via the stimulation of effector T cells to secrete IL-2, a cytokine important for peripheral Treg cell proliferation and survival [37–41]. Whether this could increase the frequency of Treg cells to a substantial degree without overt pathology being observed is unclear. Moreover, it is unknown whether these mechanisms affect Helios expression. Yet, it seems likely that these mechanisms will contribute at least in part towards generating an iTreg cell population specific to commensal bacteria.
A final possibility is that the commensal microbiota may markedly alter the antigenic landscape in the colon by supplanting the presentation of self-ligands typically recognized by thymic Treg cells with bacterial antigens. This would be consistent with previous studies showing that the Treg TCR repertoire varies by anatomic location [23], suggesting that the Treg cell population is heavily shaped by the local antigenic milieu. Thus, we speculate that the high proportion of foreign versus self-antigens facilitates the maintenance of the commensal bacteria-specific iTreg cell population.
Do Treg cells need to recognize commensal bacteria to prevent colitis?
The observation that Treg cells from GF mice can protect in the adoptive transfer model of colitis, albeit less effectively than those from conv. mice, suggests that commensal bacteria-induced iTreg cell generation may not be required for colonic tolerance [19]. However, several recent studies argue that this is not the case. First, it was observed that colonic Treg TCRs could induce colitis in lymphopenic hosts if expressed on effector T cells [23], suggesting that peripheral conversion prevents cells from differentiating into effector cells. Second, it was shown that adoptive transfer of Treg cells limited the spontaneous immunopathology in Foxp3-deficient mice, consistent with a previous report [42], but were unable to prevent the development of colitis [25]. Co-transfer of both naïve Foxp3− and Foxp3+ Treg cells were required to cure Foxp3-deficient hosts and enable them to breed, suggesting that the provisioning of additional TCR repertoire diversity into the Treg cell population via the peripheral conversion of naïve T cells was required for colonic tolerance. Finally, a study of mice deficient in peripheral, but not thymic, Treg cell differentiation found that they developed a Th2-mediated colitis with time [43]. The specific blockade in iTreg cell generation resulted from the deletion of the conserved-nucleotide sequence 1 (CNS1) portion of the Foxp3 locus [44]. Thus, these studies suggest that peripheral generation of Treg cells in response to commensal bacteria is important for maintaining colonic tolerance.
Although these recent reports seem at odds with earlier data on GF Treg cells in the transfer model, there may be some potential explanations. One possibility is that thymic Treg cells do provide a measure of tolerance in the colon. There is still a sizable fraction of Helioshi cells in the colons of conv. mice (~ 20–25%) [20,23]. Consistent with this observation, TCR repertoire studies showed that a minority of colonic Treg cells utilize TCRs that are also found on thymic Treg cells [23]. Another possibility is that the use of splenic Treg cells in the transfer model may limit the impact of commensal bacteria on that Treg cell population. The frequency of splenic Treg cells that are Helioshi (i.e. thymic) is essentially unchanged in the presence of commensal microbiota [20,23]. Moreover, splenic Treg cells utilize TCRs that are mostly different from colonic Treg cells [23]. We would therefore predict that there is a higher degree of similarity in the splenic Treg TCR repertoires between GF and conv. mice, whereas the colonic repertoires would be quite different. If these arguments are correct, this would suggest that Treg cells in this transfer model of colitis may act primarily to limit the pro-inflammatory effects of lymphopenia [45], presumably in the lymph node, rather than directly controlling immune responses to commensal bacteria in the colon in an antigen specific fashion. However, limiting the pro-inflammatory signals during lymphopenia may allow the transferred CD45RBhi T cells to differentiate into iTreg cells, rather than effector cells, in response to commensal bacterial antigens.
Effector T cell responses to commensal bacteria
As mentioned above, the classic adoptive transfer studies showed that CD4+ T cells can induce immunopathology in the colon, but were held in check by Treg cells [12]. The notion that effector T cells can respond to commensal bacteria was supported by a series of studies showing that effector T cells in a spontaneous model of colitis often recognized a flagellin epitope [46]. Moreover, the response of TCR transgenic cells to this antigen in lymphopenic hosts causes colitis and is skewed towards effector T cell differentiation (Th1 and Th17), rather than Treg cell generation [46,47].
Another example of commensal bacteria initiating effector T cell responses came from the serendipitous observation that mice from different vendors had vastly varying frequency of Th17 cells [48]. It was subsequently shown that the development of Th17 cells was dependent on the presence of segmented filamentous bacteria (SFB) [49,50], although the antigen specificity of the Th17 cells has not yet been reported. Thus, these data suggest that SFB, perhaps due to its ability to attach to the epithelium [49], has a unique ability to induce Th17 responses under normal homeostatic conditions.
Although it appears that many constituents of the commensal microbiota favor iTreg cell differentiation, it is unclear why SFB and other bacteria induce Th17 cells. One possibility is that the array of TLR ligands is different on these bacteria. For example, they may include bacterial products such as the TLR5 ligand flagellin, which can stimulate Th17 cell differentiation in vitro [51]. Another possibility is that bacteria-induced apoptosis leads to a unique combination of TLR and apoptotic signals that favor TGFβ and IL-6 production, resulting in Th17 development [52]. Whether this apoptotic mechanism occurs with commensal bacteria, however, is not clear. Future studies will be required to determine why certain bacteria induce effector T cell, and not iTreg cell, differentiation, and establish whether this induction is only for the bacteria-specific T cells, or creates a cytokine environment that skews T cell differentiation to the effector cell lineage, potentially including T cells recognizing commensal bacteria that normally elicit iTreg cell responses.
Does gut homeostasis require both effector and regulatory T cell responses?
One intriguing issue is that all of these T cell differentiation events, both pro- and anti-inflammatory, normally occur in every individual. Whereas humans routinely encounter new bacteria, mice in SPF facilities should not, implying that the process of effector and iTreg cell selection is somehow compartmentalized. Consistent with this hypothesis, little overlap was observed between the effector and Treg TCR repertoires at steady state [23], suggesting that different antigen specificities result in different T cell fates. One possibility is that effector and Treg cell differentiation are temporally separated, such that when IL-6 is high, Th17 cells are generated, and when it is low, Treg cells are generated [53]. Another possibility is that effector and Treg cell differentiation is segregated spatially due to different APCs or lymphoid structures. This could be related to the APC subset that picks up the bacteria, or the array of TLR ligands on the bacteria. A final variable may be the amount of antigen [54]. Future studies will be required to determine whether effector and Treg cell differentiation can occur simultaneously in different compartments.
Although it is clear that effector T cells can be generated in response to commensal bacteria, their function is not obvious. One possibility is that they are essentially unwanted byproducts of the diverse TCR repertoire, such as autoimmune effector cells. On the other hand, effector T cells to commensal bacteria may be beneficial. For example, it was shown that mice with SFB show greater resistance to Citrobacter rodentium infection [49]. The Th17 response induced by SFB may come at the cost of decreased tolerance, as it was associated with a decrease in Treg cell frequencies [48] that may have affected systemic tolerance in a TCR transgenic autoimmune arthritis model [55]. Another example is that T cell deficient mice can develop colitis caused by the commensal microbiota. As these bacteria do not cause pathology in normal mice, this suggests that a lack of T cell effector function may lead to opportunistic infection by certain commensal bacterial species that are currently unknown. SFB and the bacterial species that cause opportunistic infections may have features of pathogenic organisms which trigger an effector T cell response. Thus, we speculate that immune homeostasis to commensal bacteria is achieved by a mixture of immunity and tolerance (Fig. 1).
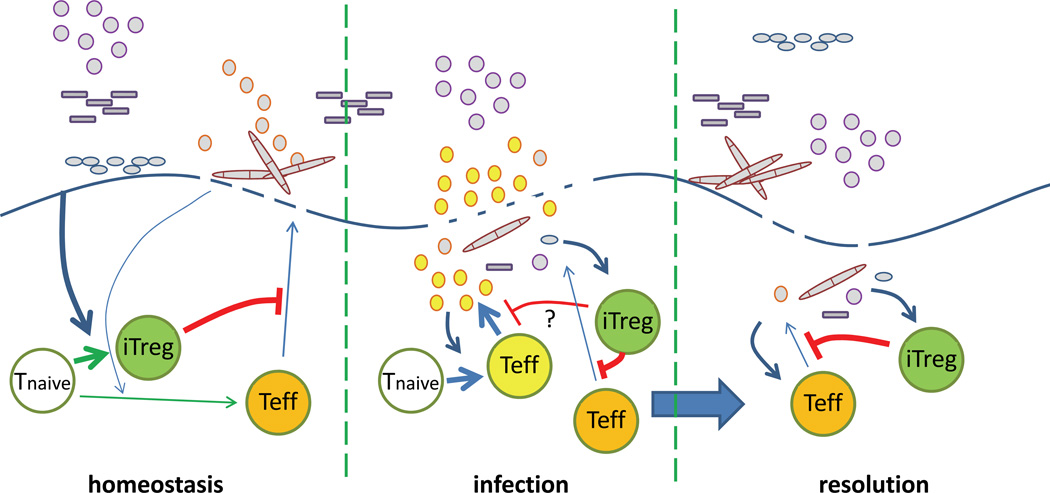
Figure 1. A hypothetical model of T cell-mediated gut homeostasis.
(Left) During homeostasis, commensal bacteria primarily induce iTreg cell differentiation (green cell), although some bacteria (e.g. SFB) may elicit effector T (Teff) cell responses (orange). iTreg cells would then inhibit effector cell responses to commensal bacteria, as well as inhibit effector T cell differentiation. This may limit immunopathology and permit healing of transient breaks in the mucosal barrier. It is unclear whether iTreg cells block effector responses to bacteria which the Treg cells do not recognize. We hypothesize that the effector cells generated during normal conditions may serve to keep certain commensal bacterial species from causing pathology. (Middle) During infection, the influx of pathogenic bacteria induces differentiation of antigen-specific effector T cells (yellow). Pre-existing Treg cells specific to commensal bacteria may suppress effector responses to commensal bacteria (orange cell) to prevent additional immunopathology. Whether commensal bacteria-specific iTreg cells also suppress the pathogen-specific response is unclear. The inflammatory response to pathogen may also inhibit iTreg cell differentiation (not depicted). (Right) During resolution, iTreg cells limit excessive pathology to the commensal bacteria that pass through the healing mucosal barrier.
Concluding remarks
Our understanding of immune homeostasis with commensal bacteria has substantially increased during the past several years, but much remains to be learned. For example, the relationship between the various effector (Th1, Th17) and regulatory (Treg, Tr1) T cell subsets still needs to be clarified, in terms of antigen-specificity, development, and function. For example, IL-10 producing Foxp3− cells (e.g. Tr1) may be an important contributor to colonic tolerance [56], as they are enhanced when iTreg cell generation is inhibited by CNS1-deficiency in the Foxp3 locus [44]. Although this may suggest that Tr1 cells are developmentally related to Treg cells, an alternative possibility is that IL-10 gene induction does not require Foxp3 expression.
Another question is to understand the mechanisms that discriminate between pathogenic and commensal bacteria. Pathogens may co-opt the gut tolerance mechanisms for commensal bacteria to facilitate their survival [36]. Infections may also skew T cell development from iTreg to effector in response to new commensal bacteria species, potentially breaking tolerance and facilitating IBD [57].
Finally, it would be useful to translate these findings to facilitate treatment of IBD. One approach would be to alter the patient’s microbiota to bacteria facilitate iTreg cell induction. Another possibility would be ex vivo expansion and re-infusion of Treg cells, a strategy being attempted for the treatment of autoimmune diabetes [58]. Specifically expanding Treg cells that recognize commensal microbiota may facilitate Treg cell localization to the gut and enhance suppressive activity. Thus, understanding the mechanisms of immune homeostasis to commensal bacteria may provide insight into the pathogenesis and treatment of inflammatory bowel disease.
Highlights.
Tolerance is required to commensal bacteria and depends on regulatory T cells
Regulatory T cells specific to commensal bacteria are generated from naïve T cells in the periphery
Bacterial products can facilitate Treg cell function via the induction of IL-10, an immunosuppressive cytokine
Specific commensal bacterial species induce inflammatory or tolerogenic T cell responses in the gut
Acknowledgements
We would like to thank Takeshi Egawa, Sindhuja Rao, Benjamin Solomon (Wash. U.) for helpful discussions and critical reading of the review. Supported by grants from NIH/NIAID and CCFA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol. 2011;89:45–53. doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 3.Tanoue T, Honda K. Induction of Treg cells in the mouse colonic mucosa: A central mechanism to maintain host-microbiota homeostasis. Semin Immunol. 2012;24:50–57. doi: 10.1016/j.smim.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–19261. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 6.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 8.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 9.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 13.Feng T, Elson CO. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol. 2011;4:15–21. doi: 10.1038/mi.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powrie F, Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990;172:1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahill RJ, Foltz CJ, Fox JG, Dangler CA, Powrie F, Schauer DB. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun. 1997;65:3126–3131. doi: 10.1128/iai.65.8.3126-3131.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Min B, Thornton A, Caucheteux SM, Younes SA, Oh K, Hu-Li J, Paul WE. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur J Immunol. 2007;37:1916–1923. doi: 10.1002/eji.200737236. [DOI] [PubMed] [Google Scholar]
- 17.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36:2336–2346. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 18.Strauch UG, Obermeier F, Grunwald N, Gurster S, Dunger N, Schultz M, Griese DP, Mahler M, Scholmerich J, Rath HC. Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut. 2005;54:1546–1552. doi: 10.1136/gut.2004.059451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh B, Read S, Asseman C, Malmstrom V, Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. Control of intestinal inflammation by regulatory T cells. Immunological Reviews. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 20. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469.. ••This manuscript assesses the ability of various commensal bacterial species to increase the frequency of colonic Treg cells, and shows that only certain species, in particular Clostridium species, have this capacity.
- 21. Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021.. •This data assess the ability of a limited commensal bacterial population to affect effector and Treg cell responses.
- 22.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434.. ••This report examines the TCR repertoire of colonic T cells, and suggests that many colonic Treg TCRs recognize commensal bacteria, and facilitate peripheral, but not thymic, conversion.
- 24.Gottschalk RA, Corse E, Allison JP. Expression of Helios in Peripherally Induced Foxp3+ Regulatory T Cells. J Immunol. 2011 doi: 10.4049/jimmunol.1102964. [DOI] [PubMed] [Google Scholar]
- 25. Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity. 2011;35:109–122. doi: 10.1016/j.immuni.2011.03.029.. •These data showed that conversion of transferred Foxp3− cells was required to prevent spontaneous colitis in Treg cell treated Foxp3-deficient mice, suggesting that peripheral conversion is necessary for gut tolerance.
- 26.Akimova T, Beier UH, Wang L, Levine MH, Hancock WW. Helios expression is a marker of T cell activation and proliferation. PLoS One. 2011;6:e24226. doi: 10.1371/journal.pone.0024226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Jin W, Hardegen N, Lei K-j, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. Journal of Experimental Medicine. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 31.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095.. ••This manuscript showed that polysachharide A from B. fragilis activates TLR2 to enhance Treg cell function and establish tolerance to this commensal bacteria.
- 37.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishio J, Feuerer M, Wong J, Mathis D, Benoist C. Anti-CD3 therapy permits regulatory T cells to surmount T cell receptor-specified peripheral niche constraints. J Exp Med. 2010;207:1879–1889. doi: 10.1084/jem.20100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39:948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 41.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature Immunology. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 42.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 43. Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395–399. doi: 10.1038/nature10772.. •Utlizing CNS1-deficiency in the Foxp3 locus to inhibit iTreg cell generation, they observe a spontaneous Th2-cell mediated colitis, suggesting that peripheral conversion is required for gut homeostasis.
- 44.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010 doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med. 2010;207:1321–1332. doi: 10.1084/jem.20092253.. •This manuscript examines the effector response of TCR transgenic cells to flagellin from commensal bacteria.
- 46.Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, Elson CO. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir Mice: Increased T helper cell type 1 response and ability to transfer disease. Journal of Experimental Medicine. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 48.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033.. ••This paper identified a commensal bacterial organism, SFB, as being the etiologic agent for Th17 cell differentiation in a normal non-pathogenic setting.
- 50. Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020.. •Along with (49), this paper examined the effect of SFB on gut effector and Treg cell responses.
- 51.Uematsu S, Akira S. Immune responses of TLR5(+) lamina propria dendritic cells in enterobacterial infection. J Gastroenterol. 2009;44:803–811. doi: 10.1007/s00535-009-0094-y. [DOI] [PubMed] [Google Scholar]
- 52.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 53.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3(+) and Foxp3(−) precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 57.Hansen R, Thomson JM, El-Omar EM, Hold GL. The role of infection in the aetiology of inflammatory bowel disease. J Gastroenterol. 2010;45:266–276. doi: 10.1007/s00535-009-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]