Abstract
The amino acid sequence of the UL31 protein (UL31P) of equine herpesvirus 1 (EHV-1) has homology to that of the ICP8 of herpes simplex virus type 1 (HSV-1). Here we show that the UL31 gene is synergistically trans-activated by the IEP and the UL5P (EICP27). Detection of the UL31 RNA transcript and the UL31P in EHV-1-infected cells at 6 h post infection (hpi) as well as metabolic inhibition assays indicated that UL31 is an early gene. The UL31P preferentially bound to single-stranded DNA over double-stranded DNA in gel shift assays. Subcellular localization of the green fluorescent protein (GFP)-UL31 fusion proteins revealed that the C-terminal 32 amino acid residues of the UL31P are responsible for the nuclear localization. These findings may contribute to defining the role of the UL31P single-stranded DNA-binding protein in EHV-1 DNA replication.
Keywords: Equine herpesvirus 1, UL31 protein of EHV-1, Single-stranded DNA-binding protein of EHV-1, EHV-1 DNA replication, Nuclear localization signal
Introduction
Equine herpesvirus 1 (EHV-1) is a member of the Alphaherpesvirinae subfamily along with varicella-zoster virus (VZV), pseudorabies virus (PRV), bovine herpesvirus 1 (BHV-1), and herpes simplex virus type 1 (HSV-1) (Roizman and Pellet, 2001). As a major pathogen of horses, EHV-1 causes respiratory disease, neurological disorders, and abortion in pregnant mares (Allen and Bryans, 1986; Crabb and Studdert, 1995; O’Callaghan and Osterrieder, 2008). During a lytic infection, EHV-1 gene expression is controlled by viral negative and positive regulatory molecules and is divided into immediate-early (IE), early, and late stages (Ahn et al., 2007, 2010, 2011; Caughman et al., 1985; Charvat et al., 2011; Gray et al., 1987a, 1987b; Holden et al., 1995; Kim et al., 1997, 1999, 2006, 2011; Smith et al., 1992; Zhao et al., 1995). The sole IE gene is expressed first followed by expression of early genes, and then late genes are turned on after viral DNA replication (O’Callaghan and Osterrieder, 2008).
Extensive studies of HSV-1 DNA replication showed that seven viral proteins are essential for HSV-1 DNA replication (Challberg, 1986; Conley et al., 1981; Gao et al., 1993): a HSV-1 single-stranded DNA-binding protein (ICP8), a DNA polymerase (UL30P), a DNA polymerase accessory protein (UL42P), an origin-binding protein (UL9P), and a helicase-primase complex (UL5P, UL8P, and UL52P). Among the seven DNA replication-associated proteins, the roles of single-stranded DNA-binding protein are quite interesting. HSV-1 ICP8 has multi-functions in viral DNA replication. It preferentially binds to single-stranded DNA (Gourves et al., 2000; Lee and Knipe, 1985; Ruyechan, 1983, 1988), mediates strand exchange, inhibits single-stranded DNA digestion, and stimulates double-stranded DNA digestion (Boehmer and Lehman, 1993; Bortner et al., 1993; Dutch and Lehman, 1993; Reuven and Weller, 2005). Furthermore, ICP8 has been reported to modulate the activity of the viral polymerase (UL30P) (Chiou et al., 1985; Hernandez and Lehman, 1990; O’Donnell et al., 1987) and to promote the helicase activity of the viral helicase-primase complex (UL5P/UL8P/UL52P) (Falkenberg et al., 1998; Hamatake et al., 1997). It has been proposed that the C-terminal α-helix is required for the localization of ICP8 to prereplicative sites by binding to viral or cellular factors that target or retain ICP8 at specific intranuclear sites (Taylor and Knipe, 2003). The VZV counterpart of single-stranded DNA-binding protein is encoded by VZV open reading frame (ORF) 29 and is expressed in human ganglia during latency (Kinchington et al., 1988; Lungu et al., 1998; Roberts et al., 1985). It appears in the nucleus during lytic infection but in the cytoplasm of infected neurons during latency (Lungu et al., 1998). In addition, ORF29P influences expression of some viral genes that are regulated by an IE ORF62 protein (ORF62P) (Boucaud et al., 1998; Cohen et al., 2007).
It has been previously demonstrated that the putative catalytic subunit (pORF30) and accessory protein (pORF18) of EHV-1 DNA polymerase are the counterparts of HSV-1 UL30 and UL42, respectively (Allen et al., 1977; Loregian et al., 2006). Activity of ORF30P as a DNA polymerase is stimulated by the ORF18P as an accessory subunit of the viral DNA polymerase. The amino acid sequence of EHV-1 UL31P (previously designated as ICP130) revealed its homology with HSV-1 ICP8 and VZV ORF29P, suggesting that the UL31P may have similar properties of HSV-1 ICP8 or VZV ORF29P in EHV-1 DNA replication. A previous study reported that EHV-1 UL31 nuclear protein bound to both single- and double-stranded DNA when using nuclear cell extracts of EHV-1 infected RK13 cells in an in vitro interaction assay (Lewis et al., 1995). However, it is still unknown how UL31 gene is regulated, at which stage the UL31 gene is expressed, which region of the UL31P is responsible for its nuclear localization, and whether UL31P harbors the independent capability to bind to both single- and double-stranded DNAs. In this study, we showed that the EHV-1 UL31 gene is synergistically trans-activated by the IEP and the UL5P (EICP27). Metabolic inhibition assays revealed that UL31 is an early gene. Moreover, the UL31P harbors a nuclear localization signal sequence at the extreme 32 residues of the C-terminus and preferentially binds to single-stranded DNA over double-stranded DNA in gel shift assays.
Results
The UL31 gene trans-activated by the IEP encodes ~120 kDa UL31 protein
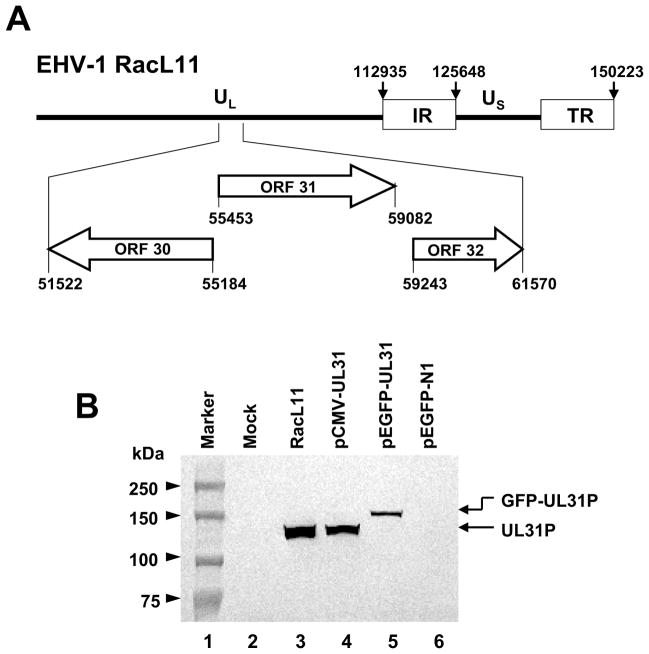
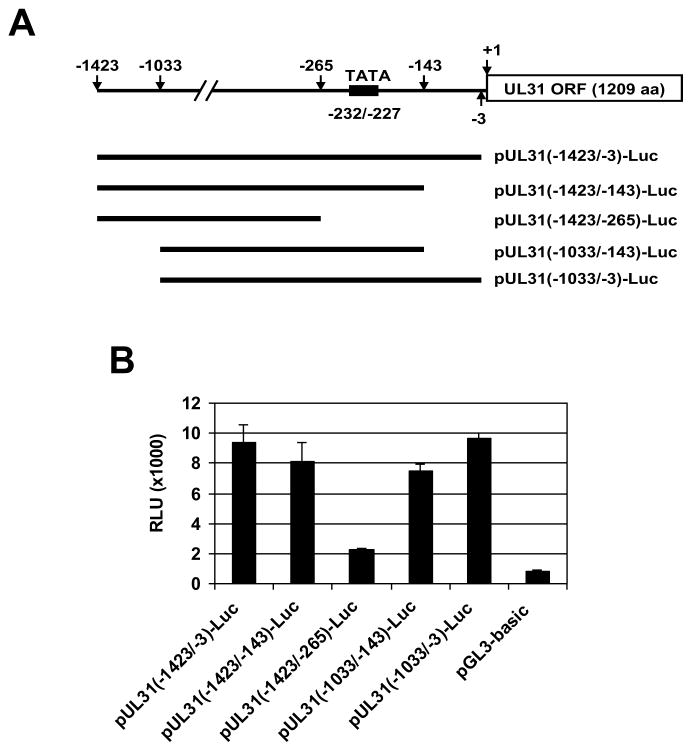
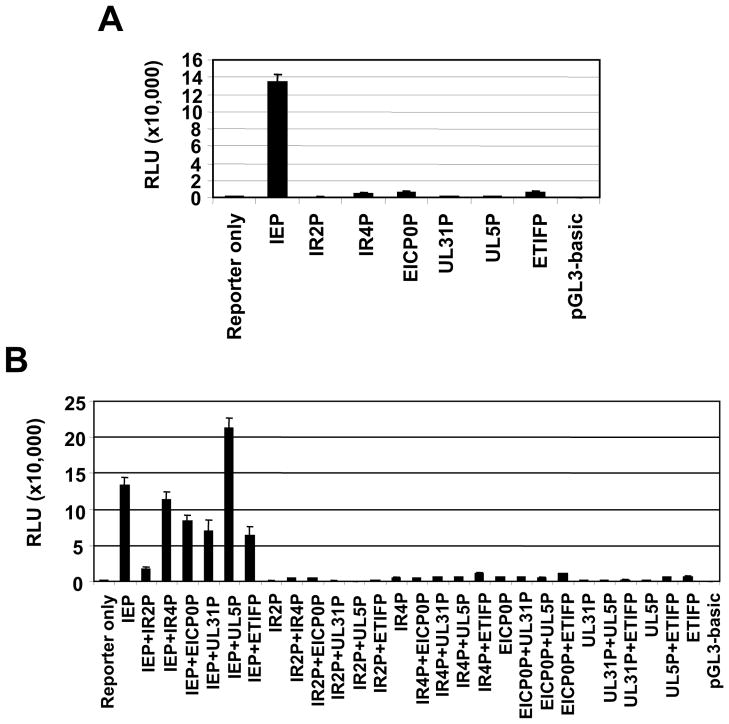
The UL31 ORF located within the EHV-1 UL genomic region encodes a UL31 protein (UL31P) of 1209 amino acids (Fig. 1A) (Telford et al., 1992). The UL31 gene of EHV-1 RacL11 strain was PCR-amplified, cloned, and then sequenced (Kim et al., unpublished data). Comparisons of the DNA sequences of the Ab4p and RacL11 UL31 genes showed seven nucleotide differences (99.8% identity) that resulted in three amino acid differences (99.8% identity). In order to perform functional studies, we cloned and expressed UL31P in RK13 cells as an intact protein as well as a recombinant green fluorescent protein (GFP) fusion protein. An ~120 kDa UL31P was detected in RK13 cells infected with EHV-1 (Fig. 1B, lane 3) or transfected with the plasmid expressing the UL31 ORF (Fig. 1B, lane 4), but not in mock-infected cells (Fig. 1B, lane 2). Moreover, anti-UL31P monoclonal antibody YC3 was also able to detect an ~147 kDa GFP-UL31P fusion protein in cells transfected with the pEGFP-UL31 plasmid (Fig. 1B, lane 5), but not with the pEGFP-N1 control plasmid (Fig. 1B, lane 6). These findings indicated that the UL31 gene is expressed as an ~120 kDa protein. Next, luciferase reporter assays were performed to examine how the UL31 gene is regulated. As shown in Fig. 2, the different regions of the UL31 ORF upstream sequence were cloned into the pGL3-Basic plasmid [pUL31(-1423/-3)-Luc, pUL31(-1423/-143)-Luc, pUL31(-1423/-265)-Luc, pUL31(-1033/-143)-Luc, and pUL31(-1033/-3)-Luc]. Reporter plasmids were co-transfected into RK13 cells without effector plasmids, and promoter activities were determined. The maximal luciferase activity was shown from pUL31(-1033/-3)-Luc (Fig. 2B, bar 5) so that this plasmid was used for following luciferase assays. Interestingly, pUL31(-1423/-265)-Luc lacking a tentative TATA box sequences [UL31(-232/-227)] showed very low promoter activity (Fig. 2B, bar 3), suggesting that a TATA box sequence (TATAAT) is required for full activation of the UL31 gene. To determine which EHV-1 regulatory protein trans-activates the UL31 gene, further promoter assays were performed using pUL31(-1033/-3)-Luc and effector vectors expressing each of the EHV-1 regulatory proteins. The UL31 gene was trans-activated by the IEP, but not independently by any other EHV-1 regulatory protein (Fig. 3A). Further, additional assays revealed that the IEP synergistically trans-activates the UL31 gene with the UL5P, a homolog of HSV-1 ICP27 (Fig. 3B), whereas IR2P, a negative EHV-1 regulatory molecule (Kim et al., 2006, 2011), interfered with trans-activation of the IEP (Fig. 3B).
Fig. 1.
Location of UL31 gene in EHV-1 genome and detection of UL31 protein. (A) Location of UL31 gene in EHV-1 genome. Gene location was based on previously published Ab4 EHV-1 sequence (Telford et al., 1992). (B) Detection of UL31P from RK13 cells infected with EHV-1 and transfected with plasmid expressing UL31P or GFP-UL31P.
Fig. 2.
The UL31 promoter region cloned into luciferase reporter vector and UL31 promoter activity. (A) The UL31 promoter region cloned into luciferase reporter vector. The number indicates nucleotide distance from UL31 ORF first nucleotide (+ 1). The tentative TATA box sequence is located on UL31(-232/-227). Different regions of UL31 promoter were cloned into luciferase reporter vector as described in Materials and methods. (B) The UL31 promoter activities were examined without effector plasmid as described in Materials and methods. Promoter activity is expressed as relative luciferase units (RLU). Each experiment was carried out in triplicate. Error bars represent the mean ± standard deviation (SD) of three independent experiments.
Fig. 3.
The trans-activation of the UL31 promoter by a single or double effector vectors. Reporter plasmid [pUL31(-1033/-3)-Luc], and effector plasmids (pSVIE, pSVIR2, pCMV-IR4 (EICP22), pSVEICP0, pSVUL5 (EICP27), pCMV-ETIF, and pCMV-UL31) were used for promoter assays. Relative luciferase units (RLU). Error bars indicate the mean ± SD. (A) UL31 promoter activity by a single effector plasmid. (B) UL31 promoter activity by double effector plasmids.
The UL31 is an early gene
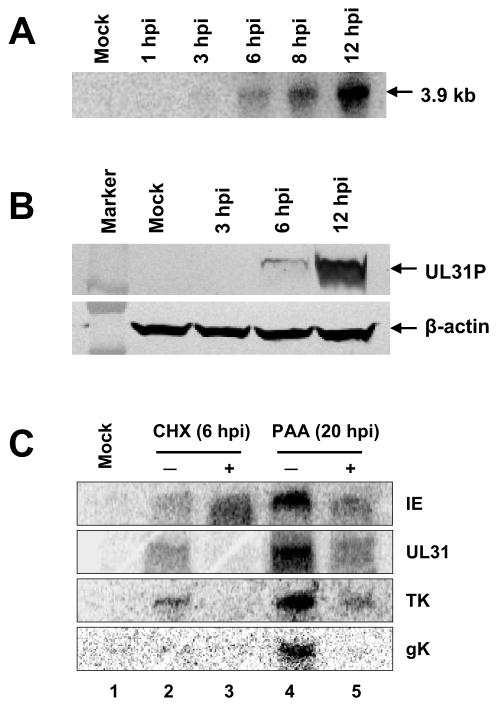
Next, to determine in which stage of the EHV-1 replication cycle the UL31 gene is expressed, the synthesis of the UL31 transcript and UL31 protein was examined at various times after infection. The UL31 transcript was not detected at the immediate-early time of either 1 or 3 h post infection (hpi; Fig. 4A, lanes 2 and 3, respectively) or in mock-infected cells (Fig. 4A, lane 1), but was first detected at 6 hpi and increased significantly in amount at late times of infection at 8 and 12 hpi (Fig. 4A, lanes 5 and 6, respectively). Similarly, the UL31 protein was first detected in small amounts at 6 hpi and was produced in large amounts at 12 hpi (Fig. 4B, lanes 4 and 5, respectively). These findings suggested that UL31 is an early gene. To confirm the assignment of UL31 as a member of the early class, assays employing metabolic inhibitors were performed by using cycloheximide (CHX) to block protein synthesis and phosphonoacetic acid (PAA) to inhibit EHV-1 DNA synthesis. The EHV-1 IE, early thymidine kinase (TK), and late glycoprotein K (gK) genes were used as positive controls. As expected, the IE transcript was expressed in uninhibited and CHX-treated infected RK13 cells (Fig. 4C, IE lanes 2 and 3, respectively). The TK transcript was detected in both uninhibited cells and in PAA-treated cells (Fig. 4C, TK, lanes 2, 4, and 5, respectively) but not in CHX-treated cells even at 6 hpi (Fig. 4C, TK, lane 3) as expected for a gene of the early class. Expression of the gK transcript was not detected at 6 hpi in either uninhibited or CHX-treated infected cells (Fig. 4C, gK, lanes 2 and 3, respectively) and also was not detected at 20 hpi in PAA-treated cells (Fig. 4C, gK, lane 5), confirming that gK is a true late gene as its transcription requires the initiation of viral DNA synthesis. In the case of the UL31 gene, the results were identical to those of the TK gene in that synthesis of the UL31 transcript was readily detected at 6 hpi in uninhibited infected cells and at 20 hpi in PAA-treated cells (Fig. 4C, UL31 lanes 2 and 5, respectively), but was inhibited in CHX-treated cells. Thus, the results of all experiments in Figure 4 clearly demonstrate that EHV-1 UL31 is an early gene.
Fig. 4.
Detection of the UL31 transcript and the UL31P. Western blot and northern blot analysis were performed as described in Materials and methods. (A) Detection of the UL31 transcript from EHV-1-infected RK13 cells at indicated time points. (B) Detection of the UL31P from EHV-1-infected RK13 cells at different time points. Western blot was performed using an anti-UL31 monoclonal antibody YC3 (1:1,000) and an anti-β-actin monoclonal antibody (1:5,000) as an internal control. (C) Detection of EHV-1 representative gene and UL31 gene transcripts from EHV-1-infected RK13 cells in the presence of protein synthesis inhibitor CHX or viral DNA synthesis inhibitor PAA.
The UL31P binds to a single-stranded DNA in a sequence-independent manner
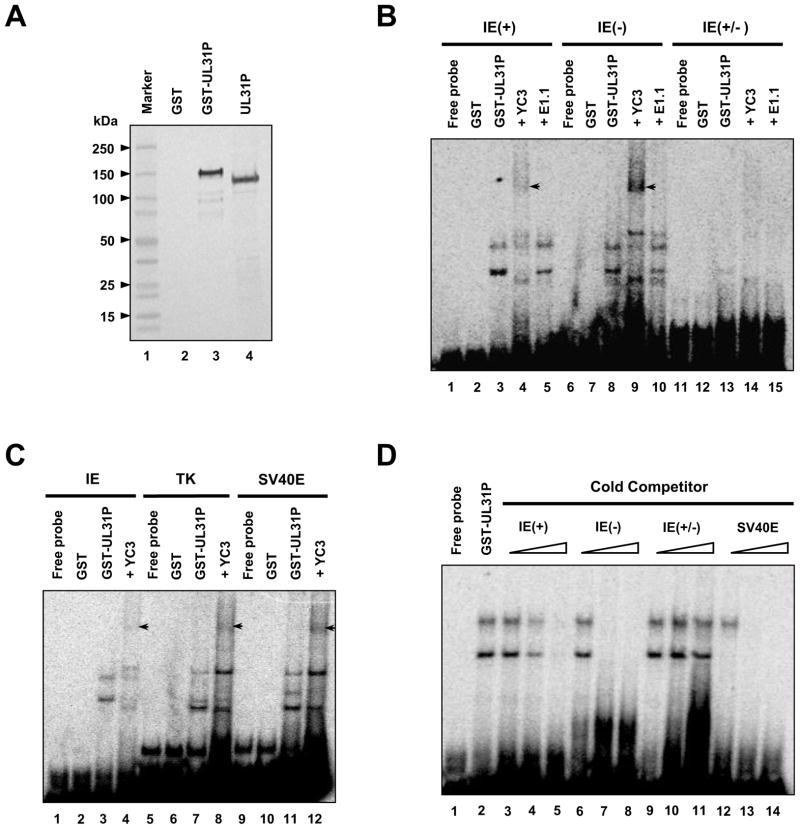
According to previous studies (Lewis et al., 1995), the UL31P (previously designated ICP130) in nuclear extracts obtained from EHV-1-infected RK13 cells bound to both single- and double-stranded DNAs. However, the amino acid sequence homology of UL31P to the HSV-1 single-stranded DNA binding protein (ICP8) suggests the possibility that the UL31P may have a binding specificity to single-stranded DNA and that the UL31P indirectly binds to double-stranded DNA by nuclear cell extracts obtained from EHV-1-infected cells. To examine this possibility, we purified full-length UL31P as a glutathione S-transferase (GST)-UL31P fusion protein and employed it in DNA-binding assays with a radiolabeled single- and double-stranded DNA probe as described in Materials and methods. Purified GST-UL31 fusion protein (~150 kDa) was detected by western blot using an anti-UL31P monoclonal antibody (Fig. 5A, lane 3). The purified GST-UL31P bound to single-stranded DNA probes and yielded a pattern with multiple bands (Fig. 5B, lanes 3 and 8), but not to double-stranded DNA (Fig. 5B, lane 13), whereas GST protein did not bind to either a single-stranded or double-stranded DNA (Fig. 5B, lanes 2, 7, and 12). Moreover, the addition of anti-UL31P monoclonal antibody YC3 led to a supershift of the single-stranded DNA probe (Fig. 5B, lanes 4 and 9). However, the DNA-protein complex was not supershifted by the addition of EHV-1 IE region 2-specific monoclonal antibody E1.1 (Fig. 5B, lanes 5 and 10). These results indicate that the UL31P binds to a single-stranded DNA.
Fig. 5.
Interaction of the UL31P with DNA. Gel shift and supershift assays were performed by using radiolabeled single- and double-stranded DNA probes, GST-UL31P fusion protein, and anti-UL31P antibody YC3 as described in Materials and methods. (A) Western blot analysis of the purified GST-UL31 fusion protein with an anti-UL31 monoclonal antibody YC3. (B) Interaction of the UL31P with single-stranded DNA. The GST protein and purified GST-UL31P were mixed with 32P-labeled two single-stranded DNA probes 46-mer EHV-1 IE (+) oligonucleotide (positions −18 to −63 nt relative to the transcription start site, lanes 1–5) and its complementary oligonucleotide (lanes 6–10), and 46-bp double-stranded IE(+/−) DNA (lanes 11–15) in the presence or absence of an anti-UL31 antibody YC3 and EHV-1 IE region 2-specific monoclonal antibody E1.1. The complexes supershifted with YC3 monoclonal antibody are indicated by arrowheads. (C) Gel shift assay using three different sequence oligonucleotide probes. The GST-UL31P was mixed with radiolabeled 46-mer EHV-1 IE(+) oligonucleotide (lanes 3 and 4), 60-mer EHV-1 TK promoter sequence oligonucleotide (positions −290 to −349 nt relative to the translational start site of TK ORF, lanes 7 and 8), or 60-mer SV40 early promoter sequence oligonucleotide (positions −10 to −69 nt relative to the transcription start site, lanes 11 and 12). The complexes supershifted with YC3 monoclonal antibody are indicated by arrowheads. (D) Competition assay. The GST-UL31P was mixed with radiolabeled IE(+) DNA in the presence or absence of increasing amounts of 46-mer IE(+) oligonucleotide (lanes 3–5), 46-mer IE(−) oligonucleotide (lanes 6–8), 46-bp IE(+/−) double-stranded DNA (lanes 9–11), or 60-mer SV40E(+) oligonucleotide competitor (lanes 12–14).
To determine whether the interaction of UL31P with a single-stranded DNA is sequence-specific, three different sequence DNA probes were employed in gel shift assays: a 46-mer oligonulceotide corresponding to nucleotides (nt) -18 to -63 of the EHV-1 IE gene promoter (Fig. 5C, lanes 1–4), a 60-mer spanning the EHV-1 early TK gene promoter (positions −290 to −349 nt relative to the translational start codon of TK ORF; Fig. 5C, lanes 5–8), and a 60-mer spanning the simian virus 40 (SV40) early promoter (positions −10 to −69 nt relative to the transcription start site; Fig. 5C, lanes 9–12). The GST-UL31P was able to bind to all three single-stranded DNA probes with multiple bands (Fig. 5C, lanes 3, 7, and 11), suggesting that the DNA-binding ability of the UL31P is not dependent on DNA sequence. The presence of multiple bands in gel shift assays may be due to the cooperative binding of the UL31P to single-stranded DNA as shown in binding of HSV-1 ICP8 (EHV-1 UL31P homolog) to single-stranded DNA (Dudas and Ruyechan, 1998; Mapelli et al., 2000; Ruyechan, 1983) and interaction of single-stranded DNA with the ICP130 in nuclear extracts obtained from EHV-1-infected cells (Lewis et al., 1995). To further confirm the binding specificity of the UL31P to the single-stranded DNA, competition experiments were performed using approximately 1-, 10-, and 100-fold molar excesses of unlabeled competitors (Fig. 5D). The formation of the GST-UL31P-IE(+) DNA complex was inhibited in the presence of increasing amounts of three single-stranded DNA competitors IE(+), IE(−), and SV40E(+), respectively (Fig. 5D, lanes 4–5, 7–8, and 12–14). However, the double-stranded DNA competitor IE(+/−) did not inhibit the protein-DNA complexes in the presence of increasing amounts of the competitor (Fig. 5D, lanes 9–11). These observations suggest that the UL31P has binding specificity for a single-stranded DNA.
The UL31P harbors a nuclear localization signal in the extreme 32 residues of the C-terminus
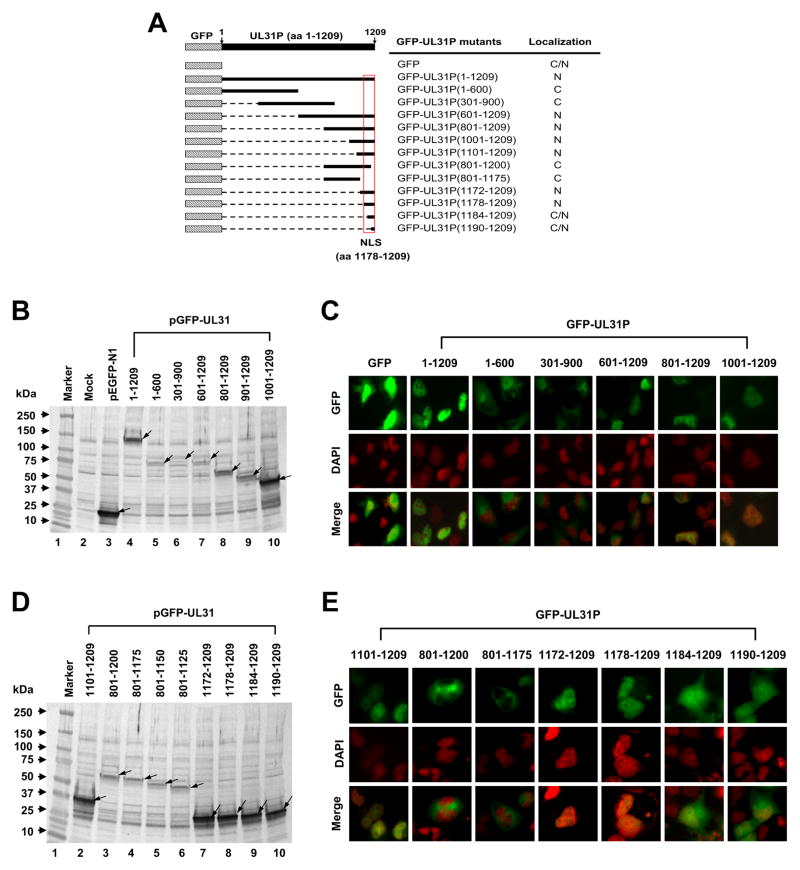
Since the UL31P plays an essential role in viral DNA replication and is a DNA-binding protein, it may harbor nuclear localization signal sequences. To identify a sequence of the UL31P responsible for nuclear localization, the plasmids of pGFP-UL31 encoding different regions of the UL31 protein (Fig. 6A) were transfected into RK13 cells, and the distribution of the green fluorescent protein (GFP) was determined under fluorescence microscopy as described in Materials and methods. The protein expression level of all GFP-UL31 constructs was confirmed by western blot using an anti-rabbit polyclonal GFP antibody (Fig. 6B and D). As shown in Fig. 6C, the full-length GFP-UL31P(1-1209) and GFP-UL31P(601-1209) were localized in the nucleus, but the control GFP was distributed in both cytoplasm and nucleus. GFP-UL31P(1-600) and GFP-UL31P(301-900) were distributed in cytoplasm, suggesting that the nuclear localization signal (NLS) sequence is located within residues 601 to 1209 of the UL31P. The UL31P(601-1209) was further dissected to identify the NLS sequence (Fig. 6A). The GFP-UL31P(801-1209) and GFP-UL31P(1001-1209) fusion proteins (Fig. 6C) as well as GFP-UL31P(1101-1209) (Fig. 6E) were localized in the nucleus, indicating that the NLS sequence of the UL31P is located within the C-terminal 109 amino acid residues. To define the essential sequences of UL31P NLS, N-terminal sequences of UL31P(1101-1209) and C-terminal sequences of UL31P(801-1209) were deleted as described in Materials and Methods. The deletion of C-terminal 9 and 34 residues of the UL31P(801-1209) resulted in the failure of nuclear localization of the protein (Fig. 6E, 801–1200 and 801–1175), but only UL31P(801-1209) containing the intact C-terminal sequence showed nuclear localization of GFP (Fig. 6C). Furthermore, N-terminal sequence of the UL31P(1101-1209) was deleted to determine the minimal NSL sequence required for its nuclear localization. Deletion of N-terminal 71 and 77 amino acid residues of the UL31P(1101-1209) [GFP-UL31P(1172-1209) and GFP-UL31P(1178-1209)] did not affect the nuclear localization of GFP-UL31P (Fig. 6E). However, deletion of the 83 and 89 amino acid residues [GFP-UL31P(1184-1209) and GFP-UL31P(1190-1209)] led to GFP distribution in both cytoplasm and nucleus (Fig. 6E), indicating that the minimal NLS sequence of the UL31P is located within the C-terminal 32 residues (aa 1178 to 1209).
Fig. 6.
Mapping of the nuclear localization signal (NLS) of EHV-1 UL31P. Construction of GFP-UL31 fusion genes and fluorescence assays were performed as described in Materials and methods. The numbers within UL31 parentheses indicate amino acid sequences of UL31P. (A) The schematic illustrates the amino acid sequence regions of UL31P fused to the GFP and the subcellular localization of the GFP-UL31 fusion protein; N for nuclear, C for cytoplasmic, and C/N for both cytoplasmic and nuclear. (B and D) Expression of GFP-UL31 fusion proteins. The GFP fusion proteins containing truncations of UL31 ORF were detected from whole-cell lysates of transfected RK13 cells at 24 h post transfection by using an anti-rabbit GFP antibody. Arrows, bands corresponding to GFP and GFP-UL31 fusion proteins. (C and E) Localization of GFP-UL31 fusion proteins. Top, middle, and bottom panels indicate distribution of GFP-UL31 fusion protein, RK13 cell nucleus stained by DAPI, and merge of GFP and nucleus, respectively.
To further determine the functional relevance of the NLS of UL31P homologs, we compared the C-terminal 50 amino acid residues of EHV-1 UL31 ORF harboring its NLS sequence with those of UL31P homologs encoded by three other alphaherpesviruses. Multiple sequence alignment showed that the extreme C-terminus of EHV-1 UL31P has approximately 42.6% and 44% amino acid identity with that of HSV-1 ICP8 and PRV UL29, respectively, but exhibited only 10% homology with that of VZV ORF29 that contains the NLS domain within its N-terminus (Fig. 7). The minimal NLS sequence of the UL31P is similar to that of HSV-1 ICP8.
Fig. 7.
Alignment of the C-terminal 50 amino acids of EHV-1 UL31P with those of alphaherpesvirus homologues. The amino acid sequences of EHV-1 UL31 ORF and its homologs were aligned by use of CLUSTALW2 multiple sequence alignment program (http://www.ebi.ac.uk/Tools/es/cgi-bin/clustalw2). EHV-1 UL31 (Ab4p strain, accession number YP_053076; RacL11 strain, Kim et al., unpublished data); HSV-1 ICP8 (accession number BAE78520); PRV UL29 (accession number YP_068332); VZV ORF29 (accession number ABW06912). The numbers indicate the amino acid position relative to the N-terminus for each UL31P homologues. The NLS sequences of EHV-1 UL31P and HSV-1 ICP8 are underlined.
Discussion
The 78 genes of EHV-1 are regulated by EHV-1 regulatory molecules (O’Callaghan and Osterrieder, 2008) at immediate-early, early, and late stages (Caughman et al., 1985; Gray et al., 1987a). After the expression of immediate-early and early genes, viral DNA replication occurs followed by the expression of the late genes (O’Callaghan and Osterrieder, 2008). Elucidating the functions of replication-associated proteins is a key to understanding the mechanisms of DNA replication and the pathogenesis of viral diseases. There is little known about viral proteins associated with EHV-1 replication except that ORF30P and ORF18P were previously identified as a DNA polymerase and its accessory subunit, respectively (Loregian et al., 2006). Considering the diverse role of HSV-1 ICP8 and VZV ORF29P in the replication of HSV-1 and VZV, respectively, it is still necessary to further characterize the UL31 gene and the UL31P for a better understanding of EHV-1 replication.
Promoter assays indicated that the UL31 gene is trans-activated only by the IEP in synergistic fashion with the UL5P (EICP27). In addition, detection of the UL31 RNA transcript and the UL31P at the early stage of viral infection as well as metabolic inhibition assays revealed that the UL31 gene, like the EHV-1 representative early gene TK, is an early gene. We also observed that a consensus IEP-binding site (IEBS; 5′-ATCGT-3′) is located at 455-bp upstream of a tentative TATA box within the UL31 promoter region. This consensus IEBS may be a cis-acting element required for IEP-mediated trans-activation of the UL31 gene although we did not provide any evidence of the interaction of the IEP with the IEBS sequence in this study.
Although previous studies (Lewis et al., 1995) reported that the UL31P (previously designated ICP130) binds to both single- and double-stranded DNAs in an in vitro interaction assay using EHV-1-infected cell nuclear extract, sequence analysis of EHV-1 UL31P showed a homology to single-stranded DNA-binding proteins, such as HSV-1 ICP8 and VZV ORF29P that have multiple functions in DNA replication and gene regulation (Cohen et al., 2007; Cohrs et al., 2002; Reuven et al., 2003; Reuven and Weller, 2005; Stallings et al., 2006). These observations imply that the certain protein(s) in EHV-1-infected nuclear cell extract may be involved in binding of the UL31P to the double-stranded DNA. To examine this possibility, we employed purified GST-UL31P fusion protein for gel shift assays. Similar to HSV-1 ICP8, which binds to a single-stranded DNA (Gourves et al., 2000), the UL31P preferentially bound to a single-stranded DNA in a sequence-independent manner. This result supports that the UL31P is a single-stranded DNA-binding protein, and also suggests that the binding of UL31P to double-stranded DNA may be due to the additional viral and/or cellular protein(s).
HSV-1 ICP8 and VZV ORF29P that harbor DNA-binding activity are localized in the nucleus (Cohrs et al., 2002; Taylor and Knipe, 2003). It was also shown that the UL31P, a single-stranded DNA-binding protein, is localized in the nucleus, suggesting that the UL31P harbors nuclear localization signal sequence. Dissection of the UL31P fused to the GFP revealed that the essential NLS sequence of UL31P is located at the C-terminus of the protein and the extreme 32 amino acid residues (aa 1178 to 1209) of UL31P is responsible for its nuclear localization. According to previous results, HSV-1 ICP8, like UL31P, harbors a NLS located in the C-terminal 28 amino acid residues (Gao and Knipe, 1992), whereas VZV ORF29P contains noncanonical NLS sequence located in amino acids 9 to 154 of N-terminus (Stallings and Silverstein, 2005). Multiple sequence alignments exhibited that ICP8 and UL31P share 52.6% overall amino acid identity and 51.7% amino acid identity in the minimal NLS domain, implying that the nuclear import mechanism of EHV-1 UL31P may be similar to that of HSV-1 ICP8.
Overall, characterization of the UL31P as a single-stranded DNA protein suggests that UL31P may play an important role in the DNA replicative complex. Considering multiple functions of the single-stranded DNA-binding proteins of other herpes viruses, the UL31P may have additional role(s) in EHV-1 DNA replication. Our findings such as the regulation of the early UL31 gene, NLS localization of the UL31P, and a single-stranded DNA-binding function of the UL31P would give an insight into further studies for elucidating the mechanism of the UL31P in viral DNA replication.
Materials and methods
Cell and Virus
Rabbit kidney RK13 cells were maintained with Eagle’s minimal essential medium supplemented with 100 units/ml of penicillin, 100 μg/ml of streptomycin, nonessential amino acids, and 5% fetal bovine serum. Pathogenic RacL11 was used as wild-type EHV-1.
Construction of the plasmids
PCR products were amplified using a pRacL11 EHV-1 BAC template, Accuprime pfx polymerase (Invitrogen, Carlsbad, CA), and appropriate primers. PCR products were digested with appropriate enzymes and cloned into the appropriate vector using standard methods (Sambrook et al., 1989), and were confirmed by sequence analysis. Primer design was based on the published sequences (Telford et al., 1992). The UL31 promoter regions were PCR amplified as shown in Fig. 2A. PCR products of UL31(-1423/-3), UL31(-1423/-143), UL31(-1423/-265), UL31(-1033/-143), and UL31(-1033/-3) were digested with XhoI and HindIII, and cloned into pGL3-Basic (Promega, Madison, WI) digested with XhoI and HindIII [named pUL31(-1423/-3)-Luc, pUL31(-1423/-143)-Luc, pUL31(-1423/-265)-Luc, pUL31(-1033/-143)-Luc, and pUL31(-1033/-3)-Luc, respectively].
The UL31ORF PCR product was digested with EcoRI and NotI, and cloned into pEGFP-N1 (BD Bioscience, San Jose, CA) digested with EcoRI and NotI (named pCMV-UL31). To create a SpeI enzyme site on pEGFP-N1 plasmid, the UL31(607-889) PCR product was digested with BsrGI and NotI, and cloned into pEGFP-N1 (Clonetech, Mountain View, CA) (named pEGFP-N1SpeI). To construct the GFP-UL31 fusion gene containing different regions of the UL31 ORF, PCR products of UL31(aa 1-1209), UL31(aa 1-600), UL31(aa 601-1209), UL31(aa 301-900), UL31(aa 801-1209), UL31(aa 901-1209), UL31(aa 1001-1209), UL31(aa 1101-1209), UL31(aa 801-1200), UL31(aa 801-1175), UL31(aa 801-1150), and UL31(aa 801-1125) were digested with SpeI and NotI, and cloned into pEGFP-N1SpeI digested with SpeI and NotI [named pGFP-UL31P(1-1209), pGFP-UL31P(1-600), pGFP-UL31P(601-1209), pGFP-UL31P(301-900), pGFP-UL31P(801-1209), pGFP-UL31P(901-1209), pGFP-UL31P(1001-1209), pGFP-UL31P(1101-1209), pGFP-UL31P(801-1200), pGFP-UL31P(801-1175), pGFP-UL31P(801-1150), and pGFP-UL31P(801-1125), respectively].
Next PCR amplifications were performed using a pGFP-UL31P(1101-1209) as a template. PCR products of UL31(aa 1202-1209), UL31(aa 1196-1209), UL31(aa 1190-1209), UL31(aa 1184-1209), UL31(aa 1178-1209) and UL31(aa 1172-1209) were digested with SpeI and AflII, and cloned into pEGFP-NSpeI digested with SpeI and AflII [named pGFP-UL31P(1202-1209), pGFP-UL31P(1196-1209), pGFP-UL31P(1190-1209), pGFP-UL31P(1184-1209), pGFP-UL31P(1178-1209), and pGFP-UL31P(1172-1209), respectively]. To construct GST-UL31 fusion protein, PCR product of UL31 ORF was digested with EcoRI and NotI, and cloned into pGEX-4T-1 (GE Healthcare, Piscataway, NJ) digested with EcoRI and NotI (named pGST-UL31).
Luciferase reporter assay
Promoter activity was examined as described in previous studies (Kim et al., 2006). Briefly, 70% confluent RK13 cells were prepared in 24-well plates and 0.1 pM of reporter vector and 0.3 pM of effector vector were used for cotransfection. Reporter vectors were pUL31(-1423/-3)-Luc, pUL31(-1423/-143)-Luc, pUL31(-1423/-265)-Luc, pUL31(-1033/-143)-Luc, and pUL31(-1033/-3)-Luc. The EHV-1 regulatory protein-expressing vectors such as pSVIE, pSVIR2, pSVEICP0, pCMV-IR4 (EICP22), pSVUL5 (EICP27), and pCMV-ETIF (Ahn et al., 2007) as well as pCMV-UL31 were used as effector vectors. Six microlitters of lipofectin (Invitrogen) was mixed with 300 μl of Opti-MEM medium (Gibco, BRL, Gaithersburg, MD), and incubated for 45 min at room temperature. Reporter plasmid with or without effector plasmid(s) were mixed with 300 μl of Opti-MEM medium (Gibco, BRL), and total DNAs were adjusted to the same amount with the pSVSPORT1 DNA (Gibco, BRL). The mixture was combined and incubated at room temperature for 15 min. One-third of the total mixture was transferred into each of three wells of the RK13 cells. At 5 h post transfection (hpt), normal growth medium was added, and luciferase activities were measured at 48 hpt by using a luciferase assay kit (Promega) and a POLARstar OPTIMA plate reader (BMG LABTECH Inc., Burham, NC) according to the manufacturer’s directions.
Western and northern blot analyses
90% confluent RK13 cells were infected with EHV-1 at a multiplicity of infection (moi) of 5 and transfected with pCMV-UL31, pEGFP-UL31 or pEGFP-N1 plasmid using a Lipofectin (Invitrogen) according to the manufacturer’s directions. After 24 h incubation, total cell extracts were prepared from virus-infected or plasmid-transfected RK13 cells and applied to western blot analyses. To determine the expression level of the UL31P at indicated times post infection, RK13 cells were infected with RacL11 at an moi of 5. Whole cell lysates were separated by a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to a nitrocellulose membrane (Ambion, Austin, TX). The UL31P was detected by using an anti-UL31P monoclonal antibody YC3 (a gift from Dr. Caughman, Medical College of Georgia) as primary antibodies and an anti-mouse IgG[Fc]-alkaline phosphatase conjugate (Promega) as a secondary antibody. In addition, the GFP-UL31 fusion proteins were detected with an anti-rabbit polyclonal GFP antibody (Santa Cruz). Protein was visualized by incubating the membrane containing blotted proteins in the AP conjugate substrate (AP conjugate substrate kit, Bio-Rad Laboratories) according to manufacturer’s directions.
To detect the UL31, gK, TK, and IE transcripts, total RNA was isolated from mock- and virus-infected cells by using a RNeasy Mini kit (Qiagen, Valencia, CA). Total RNA was separated on 3 or 5% denaturing acrylamide gel (8M Urea) and transferred to a nylon membrane (Ambion) by using a semi-dry electroblotter (Bio-Rad Laboratories). The PCR amplicons of UL31 gene (primers, 5′-ctc gcg caa gcc agc ccc tgc tca-3′/5′-gaa ctg ggc cag ctc ccc gat gtt-3′), TK gene (primers, 5′-gcc ggt cag gcg gca agt aac aat agt tag-3′/5′-gag ggt gct gct cat gag ggg tat tag aga-3′), gK gene (primers, 5′-tta gga cga gaa gga ctc gcc caa taa gcc-3′/5′-ctt atg ccg cgt tta cca tct ggt ata ccc-3′), and IE gene (primers, 5′-ctc tac gac ttc atc gag agc aac gac ttc-3′/5′-tcg ggc ctc tcc agc gtc ttg gcc aga ttg-3′) were end-labeled with [γ-32P]dATP (New England Nuclear Corporation, Boston, MA) by T4 polynucleotide kinase (NEB, Beverly, MA) according to the manufacturer’s directions, respectively. The radiolabeled probe was denatured by adding a 1/10 volume of 3 M NaOH for 10 min at room temperature and then neutralized by adding an equal volume of 1 M Tris-HCl (pH 7). Prehybridization, hybridization and washing were performed using the NorthernMax Kit (Ambion) followed by autoradiography using a phosphor image screen and the molecular imager FX system (Bio-Rad Laboratories).
Metabolic inhibition assays
Metabolic inhibition assays were performed using virus-infected RK13 cells as previously described (Gray et al., 1987b; Zhou et al., 1997). Briefly, 85% confluent RK13 cells were infected with EHV-1 at an moi of 5 and incubated at 37°C in the presence or absence of the protein synthesis inhibitor cycloheximide (CHX, 100 μg/ml; Sigma-Aldrich Corp. St. Louis, MO) for 6 h, or in the presence of the viral DNA replication inhibitor phosphonoacetic acid (PAA, 200 μg/ml; Sigma-Aldrich Corp.) for 20 h followed by total RNA isolation using a RNeasy Mini Kit (Qiagen, Valencia, CA). Presence of RNA transcripts were determined by northern blot assays using total RNA and probes specific to each of IE, UL31, gK and TK RNA transcripts.
Gel shift assay
The DNA-binding assay and purification of glutathione S-transferase-UL31 fusion protein (GST-UL31P) were conducted as previously described (Kim et al., 1995). A 46-mer DNA oligonucleotide and its complement spanning the EHV-1 IE promoter region (positions −18 to −63 nt relative to the transcription start site, 5′-cca cta ggg gga agg caa aac tcc ctc gta gta gta taa agc acc t-3′ and 5′-agg tgc ttt ata cta cga ggg agt ttt gcc ttc ccc cta gtg g-3′), a 60-mer spanning EHV-1 thymidine kinase promoter region (positions −290 to −349 nt relative to the translational start site of TK ORF, 5′-tac tcg cgg tgt tca tat ttt tgg aaa agc gac acg ttt tta gct cta tta gga tgc aca-3′), and a 60-mer spanning SV40 early promoter (positions -10 to -69 nt relative to the transcription start site, 5′-agt tcc gcc cat tct ccg ccc cat cgc tga cta att ttt ttt att tat gca gag gcc gag-3) were used in gel shift assays. Each oligonucleotide and its complement were annealed and either end-labeled with [γ-32P]dATP (New England Nuclear Corporation) and T4 polynucleotide kinase (Promega) according to the manufacturer’s directions. The DNA-binding reactions were carried out in a total volume of 20 μl containing approximately 1 ng of radiolabeled single- or double-stranded DNA probes (2×105 cpm/ng), 0.1 μg of poly(dI-dC) as a nonspecific competitor, binding buffer (20 mM HEPES-KOH [pH 7.9], 0.5 mM dithiothreitol, 10% glycerol, 0.1 mM EDTA, 0.025% NP-40, 25 mM KCl, 2 mM MgCl2), and 100 ng of indicated protein. The DNA binding reaction mixtures were incubated at room temperature for 20 min. In the supershift assay, an anti-UL31P monoclonal antibody YC3 or EHV-1 IE region 2-specific monoclonal antibody E1.1 was added to the indicated reactions, and the reactions were incubated for another 20 min at room temperature. For the competition assay, 1-, 10-, 100-fold molar excess of unlabeled DNA competitor were added to the binding reaction. After the reaction, 5 μl of loading buffer (200 mM HEPES-KOH [pH 7.9], 50% [vol/vol] glycerol, 0.02% bromphenol blue) was added, and the reaction samples were separated by electrophoresis using a 3.5% polyacrylamide gel with 0.5× Tris–borate–EDTA running buffer and 2.5% glycerol [vol/vol] for 2.5 h at 200 V followed by autoradiography using a phosphorimage screen and molecular imager FX system (Bio-Rad Laboratories).
Direct fluorescence microscopy
70% confluent RK13 cells on cover slips were transfected with GFP-UL31 fusion protein expressing plasmids or pEGFP-N1 control plasmid using a MATra A reagent (IBA Biotechnology, Goettingen) according to manufacturer’s instructions. Briefly, three micrograms of plasmid DNA was diluted to 200 μl of complete media (Mediatech, Inc., Manassas, VA) and mixed with 3 μl of MATra A reagent, and the mixture was incubated at room temperature for 20 min. Cells were washed with complete media once, and one milliliter of complete media was added to DNA-MATra A mixture. After the addition of 1.2 ml of the mixture to each well, the plate was incubated on the magnet plate for 15 min. The mixtures were removed and replaced with 3 ml of normal growth media followed by 24 h incubation at 37° C in a humidified CO2 incubator. The cells were washed twice with PBS at 24 hpt, fixed with 4% paraformaldehyde for 15 min at room temperature, washed, and permeabilized with 0.2% Triton X-100 in PBS for 2 min followed by three PBS washes. Mounting solution containing DAPI, Gold Antifade (Invitrogen), was applied to the RK13 cells, and the location of GFP within the cell was examined by a fluorescence microscopy (Leica DMBI 6000 microscope).
Table 1.
Primers used to construct UL31 promoter-luciferase reporter plasmids and full-length and truncated UL31 protein-expressing plasmids
| Plasmid | Primer name (sequence [5′-3′]) |
|---|---|
| pUL31(-1423/-3)-Luc | UL31-F1 (ggctcgagaggtcctcttggttagttgc) |
| UL31-R4 (ccaagctttgtttacgaaactcggcccg) | |
| pUL31(-1423/-143)-Luc | UL31-F1/UL31-R3 (gggaagcttgttggcgggcagacttta) |
| pUL31(-1423/-265)-Luc | UL31-F1/UL31-R2 (tttaagcttacagacatggcggcgcgcga) |
| pUL31(-1033/-143)-Luc | UL31-F2 (ggctcgagtagtacagcgtcgtgtggca)/UL31-R3 |
| pUL31(-1033/-3)-Luc | UL31-F2/UL31-R4 |
| pCMV-UL31 | UL31ORF-F (ccgaattcgccaccatggagtctgcgcccaag) UL31ORF-R (gtttgcggccgcttagagcatgtcaaaggtgagc) |
| pEGFP-N1SpeI | SpeI-linker-F (tttgtacattactagttgctcagcccccaccagc) SpeI-linker-R (gtttgcggccgctcaggttaataaagtttatggc) |
| pGFP-UL31P(1-1209) | GFP-UL31-F1 (ttactagtatggagtctgcgcccaagacagtgag)/UL31ORF-R |
| pGFP-UL31P(1-600) | GFP-UL31-F1/UL31P-600-R (gtttgcggccgcttacgtttccttgtcaatgagt) |
| pGFP-UL31P(601-1209) | SpeI601-F (ttactagtctcgcgcaagccagcccc)/UL31ORF-R |
| pGFP-UL31P(301-900) | SpeI301-F (ttactagtcaaggggatggcagcgga) 900-R (gtttgcggccgcttagaactgggccagctccccgat) |
| pGFP-UL31P(801-1209) | SpeI801-F (ttactagtgccggcatggccagcgcctatcgcag)/UL31ORF-R |
| pGFP-UL31P(901-1209) | SpeI901-F (ttactagttactttgccaacctggtgctcaaata)/UL31ORF-R |
| pGFP-UL31P(1001-1209) | SpeI1001-F (ttactagtgtagacgacgagtttttggcggcgag)/UL31ORF-R |
| pGFP-UL31P(1101-1209) | SpeI1101-F (ttactagtgcaggaaacaatcgcgtgtttcaggc)/UL31ORF-R |
| pGFP-UL31P(801-1200) | SpeI801-F/1200-R (gtttgcggccgcttatttctccgggggggcaccc) |
| pGFP-UL31P(801-1175) | SpeI801-F/1175-R (gtttgcggccgcttacatggccaggccaccgtta) |
| pGFP-UL31P(801-1150) | SpeI801-F/1150-R (gtttgcggccgcttatccctcgccggcagctacc) |
| pGFP-UL31P(801-1125) | SpeI801-F/1125-R (gtttgcggccgcttagctccactctccaaattga) |
| pGFP-UL31P(1202-1209) | SpeI1202-F (ttactagttcggggctcacctttgacatgctc) AflII-R (ttacgccttaagatacattgatgagtttggac) |
| pGFP-UL31P(1196-1209) | SpeI1196-F (ttactagtgcccccccggagaaaaagtcgggg)/AflII-R |
| pGFP-UL31P(1190-1209) | SpeI1190-F (ttactagtatcctgtttgacatgggtgccccc)/AflII-R |
| pGFP-UL31P(1184-1209) | SpeI1184-F (ttactagtcggtccctaccggatgatatcctg)/AflII-R |
| pGFP-UL31P(1178-1209) | SpeI1178-F (ttactagtgcccctgctggacaaaaacggtcc)/AflII-R |
| pGFP-UL31P(1172-1209) | SpeI1172-F (ttactagtggcctggccatggcagctgcccct)/AflII-R |
| pGST-UL31 | GEX-UL31-F (ttgaattcatggagtctgcgcccaagacagtgag)/UL31ORF-R |
Research Highlights.
We determined the molecular properties of the EHV-1 UL31 gene.
The UL31 gene is an early gene that is synergistically trans-activated by the IEP and the UL5P.
The UL31P is a single-stranded DNA-binding protein.
The UL31P has a nuclear localization signal sequence at the C-terminal 32 amino acid residues.
Acknowledgments
We thank Mrs. Suzanne Zavecz and LaShunta Barrow for excellent technical assistance. This research was supported by Agriculture and Food Research Initiative Competitive Grant 2008-35204-04438 from the USDA National Institute of Food and Agriculture, and by COBRE grant GM103433 from the National Institute of General Medical Sciences of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn BC, Breitenbach JE, Kim SK, O’Callaghan DJ. The equine herpesvirus-1 IR3 gene that lies antisense to the sole immediate-early (IE) gene is trans-activated by the IE protein, and is poorly expressed to a protein. Virology. 2007;363:15–25. doi: 10.1016/j.virol.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn BC, Kim S, Zhang Y, Charvat RA, O’Callaghan DJ. The early UL3 gene of equine herpesvirus-1 encodes a tegument protein not essential for replication or virulence in the mouse. Virology. 2011;420:20–31. doi: 10.1016/j.virol.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn BC, Zhang Y, O’Callaghan DJ. The equine herpesvirus-1 (EHV-1) IR3 transcript downregulates expression of the IE gene and the absence of IR3 gene expression alters EHV-1 biological properties and virulence. Virology. 2010;402:327–337. doi: 10.1016/j.virol.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GP, Bryans JT. Molecular epizootiology, pathogenesis, and prophylaxis of equine herpesvirus-1 infections. Prog Vet Microbiol Immunol. 1986;2:78–144. [PubMed] [Google Scholar]
- Allen GP, O’Callaghan DJ, Randall CC. Purification and characterization of equine herpesvirus-induced DNA polymerase. Virology. 1977;76:395–408. doi: 10.1016/0042-6822(77)90311-7. [DOI] [PubMed] [Google Scholar]
- Boehmer PE, Lehman IR. Herpes simplex virus type 1 ICP8: helix-destabilizing properties. J Virol. 1993;67:711–715. doi: 10.1128/jvi.67.2.711-715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortner C, Hernandez TR, Lehman IR, Griffith J. Herpes simplex virus 1 single-strand DNA-binding protein (ICP8) will promote homologous pairing and strand transfer. J Mol Biol. 1993;231:241–250. doi: 10.1006/jmbi.1993.1279. [DOI] [PubMed] [Google Scholar]
- Boucaud D, Yoshitake H, Hay J, Ruyechan W. The varicella-zoster virus (VZV) open-reading frame 29 protein acts as a modulator of a late VZV gene promoter. J Infect Dis. 1998;178:S34–38. doi: 10.1086/514276. [DOI] [PubMed] [Google Scholar]
- Caughman GB, Staczek J, O’Callaghan DJ. Equine herpesvirus type 1 infected cell polypeptides: evidence for immediate early/early/late regulation of viral gene expression. Virology. 1985;145:49–61. doi: 10.1016/0042-6822(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Challberg MD. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci U S A. 1986;83:9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charvat RA, Breitenbach JE, Ahn B, Zhang Y, O’Callaghan DJ. The UL4 protein of equine herpesvirus 1 is not essential for replication or pathogenesis and inhibits gene expression controlled by viral and heterologous promoters. Virology. 2011;412:366–377. doi: 10.1016/j.virol.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou HC, Weller SK, Coen DM. Mutations in the herpes simplex virus major DNA-binding protein gene leading to altered sensitivity to DNA polymerase inhibitors. Virology. 1985;145:213–226. doi: 10.1016/0042-6822(85)90155-2. [DOI] [PubMed] [Google Scholar]
- Cohen JI, Krogmann T, Pesnicak L, Ali MA. Absence or overexpression of the Varicella-Zoster Virus (VZV) ORF29 latency-associated protein impairs late gene expression and reduces VZV latency in a rodent model. J Virol. 2007;81:1586–1591. doi: 10.1128/JVI.01220-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs RJ, Wischer J, Essman C, Gilden DH. Characterization of varicella-zoster virus gene 21 and 29 proteins in infected cells. J Virol. 2002;76:7228–7238. doi: 10.1128/JVI.76.14.7228-7238.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley AJ, Knipe DM, Jones PC, Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981;37:191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb BS, Studdert MJ. Equine herpesviruses 4 (equine rhinopneumonitis virus) and 1 (equine abortion virus) Adv Virus Res. 1995;45:153–190. doi: 10.1016/s0065-3527(08)60060-3. [DOI] [PubMed] [Google Scholar]
- Dudas KC, Ruyechan WT. Identification of a region of the herpes simplex virus single-stranded DNA-binding protein involved in cooperative binding. J Virol. 1998;72:257–265. doi: 10.1128/jvi.72.1.257-265.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutch RE, Lehman IR. Renaturation of complementary DNA strands by herpes simplex virus type 1 ICP8. J Virol. 1993;67:6945–6949. doi: 10.1128/jvi.67.12.6945-6949.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg M, Elias P, Lehman IR. The herpes simplex virus type 1 helicase-primase. Analysis of helicase activity. J Biol Chem. 1998;273:32154–32157. doi: 10.1074/jbc.273.48.32154. [DOI] [PubMed] [Google Scholar]
- Gao M, DiTusa SF, Cordingley MG. The C-terminal third of UL42, a HSV-1 DNA replication protein, is dispensable for viral growth. Virology. 1993;194:647–653. doi: 10.1006/viro.1993.1304. [DOI] [PubMed] [Google Scholar]
- Gao M, Knipe DM. Distal protein sequences can affect the function of a nuclear localization signal. Mol Cell Biol. 1992;12:1330–1339. doi: 10.1128/mcb.12.3.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourves AS, Tanguy Le Gac N, Villani G, Boehmer PE, Johnson NP. Equilibrium binding of single-stranded DNA with herpes simplex virus type I-coded single-stranded DNA-binding protein, ICP8. J Biol Chem. 2000;275:10864–10869. doi: 10.1074/jbc.275.15.10864. [DOI] [PubMed] [Google Scholar]
- Gray WL, Baumann RP, Robertson AT, Caughman GB, O’Callaghan DJ, Staczek J. Regulation of equine herpesvirus type 1 gene expression: characterization of immediate early, early, and late transcription. Virology. 1987a;158:79–87. doi: 10.1016/0042-6822(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Gray WL, Baumann RP, Robertson AT, O’Callaghan DJ, Staczek J. Characterization and mapping of equine herpesvirus type 1 immediate early, early, and late transcripts. Virus Res. 1987b;8:233–244. doi: 10.1016/0168-1702(87)90018-9. [DOI] [PubMed] [Google Scholar]
- Hamatake RK, Bifano M, Hurlburt WW, Tenney DJ. A functional interaction of ICP8, the herpes simplex virus single-stranded DNA-binding protein, and the helicase-primase complex that is dependent on the presence of the UL8 subunit. J Gen Virol. 1997;78:857–865. doi: 10.1099/0022-1317-78-4-857. [DOI] [PubMed] [Google Scholar]
- Hernandez TR, Lehman IR. Functional interaction between the herpes simplex-1 DNA polymerase and UL42 protein. J Biol Chem. 1990;265:11227–11232. [PubMed] [Google Scholar]
- Holden VR, Zhao Y, Thompson Y, Caughman GB, Smith RH, O’Callaghan DJ. Characterization of the regulatory function of the ICP22 protein of equine herpesvirus type 1. Virology. 1995;210:273–282. doi: 10.1006/viro.1995.1344. [DOI] [PubMed] [Google Scholar]
- Kim SK, Ahn BC, Albrecht RA, O’Callaghan DJ. The unique IR2 protein of equine herpesvirus 1 negatively regulates viral gene expression. J Virol. 2006;80:5041–5049. doi: 10.1128/JVI.80.10.5041-5049.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Bowles DE, O’Callaghan DJ. The gamma2 late glycoprotein K promoter of equine herpesvirus 1 is differentially regulated by the IE and EICP0 proteins. Virology. 1999;256:173–179. doi: 10.1006/viro.1999.9608. [DOI] [PubMed] [Google Scholar]
- Kim SK, Holden VR, O’Callaghan DJ. The ICP22 protein of equine herpesvirus 1 cooperates with the IE protein to regulate viral gene expression. J Virol. 1997;71:1004–1012. doi: 10.1128/jvi.71.2.1004-1012.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Kim S, Dai G, Zhang Y, Ahn BC, O’Callaghan DJ. Identification of functional domains of the IR2 protein of equine herpesvirus 1 required for inhibition of viral gene expression and replication. Virology. 2011;417:430–442. doi: 10.1016/j.virol.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Smith RH, O’Callaghan DJ. Characterization of DNA binding properties of the immediate-early gene product of equine herpesvirus type 1. Virology. 1995;213:46–56. doi: 10.1006/viro.1995.1545. [DOI] [PubMed] [Google Scholar]
- Kinchington PR, Inchauspe G, Subak-Sharpe JH, Robey F, Hay J, Ruyechan WT. Identification and characterization of a varicella-zoster virus DNA-binding protein by using antisera directed against a predicted synthetic oligopeptide. J Virol. 1988;62:802–809. doi: 10.1128/jvi.62.3.802-809.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Knipe DM. An immunoassay for the study of DNA-binding activities of herpes simplex virus protein ICP8. J Virol. 1985;54:731–738. doi: 10.1128/jvi.54.3.731-738.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JB, Thompson YG, Jenkins AC, Caughman GB. Characterization and localization of the equine herpesvirus 1 major DNA binding protein. Virology. 1995;207:380–391. doi: 10.1006/viro.1995.1097. [DOI] [PubMed] [Google Scholar]
- Loregian A, Case A, Cancellotti E, Valente C, Marsden HS, Palu G. Cloning, expression, and functional characterization of the equine herpesvirus 1 DNA polymerase and its accessory subunit. J Virol. 2006;80:6247–6258. doi: 10.1128/JVI.02551-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu O, Panagiotidis CA, Annunziato PW, Gershon AA, Silverstein SJ. Aberrant intracellular localization of Varicella-Zoster virus regulatory proteins during latency. Proc Natl Acad Sci U S A. 1998;95:7080–7085. doi: 10.1073/pnas.95.12.7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli M, Mühleisen M, Persico G, van Der Zandt H, Tucker PA. The 60-residue C-terminal region of the single-stranded DNA binding protein of herpes simplex virus type 1 is required for cooperative DNA binding. J Virol. 2000;74:8812–8822. doi: 10.1128/jvi.74.19.8812-8822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan DJ, Osterrieder N. Herpesviruses of Horses. In: Mahy BWJ, Van Regenmortel MHV, editors. Encyclopedia of Virology. 3. Vol. 2. Elsevier Ltd; Oxford: 2008. pp. 411–420. [Google Scholar]
- O’Donnell ME, Elias P, Funnell BE, Lehman IR. Interaction between the DNA polymerase and single-stranded DNA-binding protein (infected cell protein 8) of herpes simplex virus 1. J Biol Chem. 1987;262:4260–4266. [PubMed] [Google Scholar]
- Reuven NB, Staire AE, Myers RS, Weller SK. The herpes simplex virus type 1 alkaline nuclease and single-stranded DNA binding protein mediate strand exchange in vitro. J Virol. 2003;77:7425–7433. doi: 10.1128/JVI.77.13.7425-7433.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuven NB, Weller SK. Herpes simplex virus type 1 single-strand DNA binding protein ICP8 enhances the nuclease activity of the UL12 alkaline nuclease by increasing its processivity. J Virol. 2005;79:9356–9358. doi: 10.1128/JVI.79.14.9356-9358.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CR, Weir AC, Hay J, Straus SE, Ruyechan WT. DNA-binding proteins present in varicella-zoster virus-infected cells. J Virol. 1985;55:45–53. doi: 10.1128/jvi.55.1.45-53.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B, Pellet PE. The Family of Herpesviridae: a brief introduction. In: Knipe DM, Howley PM, editors. Field Virology. 4. Lippincott Williams and Wilkins; Philadelphia: 2001. pp. 2381–2397. [Google Scholar]
- Ruyechan WT. The major herpes simplex virus DNA-binding protein holds single-stranded DNA in an extended configuration. J Virol. 1983;46:661–666. doi: 10.1128/jvi.46.2.661-666.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan WT. N-ethylmaleimide inhibition of the DNA-binding activity of the herpes simplex virus type 1 major DNA-binding protein. J Virol. 1988;62:810–817. doi: 10.1128/jvi.62.3.810-817.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. In: Molecular Cloning:A Laboratory Mannual. 2. Ford N, Nolan C, Ferguson M, editors. Cold Spring Harbor, NY: Cold Spring harbor Laboratory Press; 1989. [Google Scholar]
- Smith RH, Caughman GB, O’Callaghan DJ. Characterization of the regulatory functions of the equine herpesvirus 1 immediate-early gene product. J Virol. 1992;66:936–945. doi: 10.1128/jvi.66.2.936-945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings CL, Duigou GJ, Gershon AA, Gershon MD, Silverstein SJ. The cellular localization pattern of Varicella-Zoster virus ORF29p is influenced by proteasome-mediated degradation. J Virol. 2006;80:1497–1512. doi: 10.1128/JVI.80.3.1497-1512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings CL, Silverstein S. Dissection of a novel nuclear localization signal in open reading frame 29 of varicella-zoster virus. J Virol. 2005;79:13070–13081. doi: 10.1128/JVI.79.20.13070-13081.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TJ, Knipe DM. C-terminal region of herpes simplex virus ICP8 protein needed for intranuclear localization. Virology. 2003;309:219–231. doi: 10.1016/s0042-6822(03)00108-9. [DOI] [PubMed] [Google Scholar]
- Telford EA, Watson MS, McBride K, Davison AJ. The DNA sequence of equine herpesvirus-1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Holden VR, Smith RH, O’Callaghan DJ. Regulatory function of the equine herpesvirus 1 ICP27 gene product. J Virol. 1995;69:2786–2793. doi: 10.1128/jvi.69.5.2786-2793.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Chandran B, Wood C. Transcriptional patterns of the pCD41 (U27) locus of human herpesvirus 6. J Virol. 1997;71:3420–3430. doi: 10.1128/jvi.71.5.3420-3430.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]