Highlights
► Recent work on sequencing intestinal viromes. ► Different methods of viral detection by host cells. ► Type I and III interferon responses to viral infections. ► Different effects of bacterial–viral interactions on host phenotypes.
Abstract
This review explores the recent advances that have been made in our understanding of host viral interactions in the intestine. Technical advances have allowed the initial definition of intestinal viromes in a number of species including humans. Important advances in our knowledge of the host response to viral infection have shown that interferon lambda has a role that is unique from type I interferons in the intestine. Lastly, our understanding of virally induced phenotypes has expanded through new studies that show bacteria can play an important role in the outcome of viral infection in the intestine.
Current Opinion in Immunology 2012, 24:405–410
This review comes from a themed issue on Host pathogens
Edited by Anne O’Garra and Eric Pamer
For a complete overview see the Issue and the Editorial
Available online 22nd May 2012
0952-7915/$ – see front matter, © 2012 Elsevier Ltd. All rights reserved.
Introduction
The gastrointestinal tract is densely colonized by commensal bacteria and represents a primary site of interaction between the host and microbes. The host immune system has evolved mechanisms to tolerate these commensal organisms while at the same time providing protection for the host from pathogens. Therefore, not surprisingly, extensive crosstalk exists between the host and the luminal environment of the intestine. In addition to the widely recognized role of commensal bacteria, other types of microbes including viruses participate in this crosstalk. Recent work has begun to define the intestinal virome and to determine its functional interactions with other microbes and the host in health and various disease states.
Intestinal virome
Interest in the intestinal virome has grown out of studies of the intestinal microbiome. Sequence based methods of discovery have dominated the recent literature. Viral discovery has been enabled partly by advances in the DNA sequencing technology and bioinformatic analysis. Some technical challenges still exist including methods of sample preparation, selection of the sequencing method and informatics analysis. For collections, many investigators attempt to enrich for viral-like particles through filtration and DNase/RNase treatment. However, this method clearly only creates partial enrichment as there are still ample contaminating host and bacterial reads. Some groups have used nucleic acid extraction kits for RNA/DNA while others have focused on DNA extraction. Multiple sequencing modalities have been used including shotgun 454 and Illumina sequencing [1, 2, 3••, 4•].
One limitation is that the analysis of sequence reads has been done using programs that rely on BLAST searches of existing databases. The challenges include sifting through the sequences obtained to remove those encoding mammalian, bacterial, protozoal and fungal DNA. Using this technique, viral sequences can only be identified if they have some degree of homology to a sequence in the database that is used for comparison. In addition, there is no one database that currently is universally employed, further enhancing the difficulty in comparing studies. The other major challenge is that platforms with greater numbers of reads (that typical cost less per read) create shorter reads that are much more challenging to analyze.
The initial studies published by several groups investigated the composition of fecal viromes in different hosts. Analysis of samples from 105 wild rodents in the United States revealed many previously unreported viral species [1], highlighting the limited knowledge in this area. Interestingly, the viruses could be classified in three major groups including mammalian viruses (Circoviridae, Picobirnaviridae, Picornaviridae, Astroviridae, Parvoviridae, Papillomaviridae, Adenoviridae, and Coronaviridae), insect viruses (Densoviridae, Iridoviridae, Polydnaviridae, Dicistroviriade, Bromoviridae, and Virgaviridae), and plant viruses (Nanoviridae, Geminiviridae, Phycodnaviridae, Secoviridae, Partitiviridae, Tymoviridae, Alphaflexiviridae, and Tombusviridae). Similarly, Ge et al. examined viral like particles present in bat fecal samples collected in China [2]. Among the eukaryotic viruses, the most frequent reads were related to vertebrate viruses (coronaviruses, parvoviruses), insect viruses (densoviruses, dicistroviruses), and plant viruses (tobamoviruses). One interpretation of these data is that the substantial contribution of insect and plant viruses in these fecal viromes reflects the largely insect and plant diets of these animals. Whether these viruses affect the physiology of the rodent host has yet to be determined.
Recent work by Reyes et al. studied the intestinal virome in humans and found it is largely composed of bacteriophages [3••]. While intestinal bacterial communities show significant similarities between genetically related individuals, human fecal viromes were found to be unique between individuals regardless of relatedness. In addition, >95% of virotypes found in an individual were retained over the 1 year period surveyed, illustrating the stability of the virome. Interestingly, analysis of the viral sequences revealed that ∼82% of the reads did not match to any known virus. In addition, bacterial and archaeal species have a shared protection mechanism against infections by bacteriophages [5, 6].
As the intestinal microbiome can alter its bacterial composition in response to diet changes [7], there is similar interest in the effect of diet of the intestinal virome. Minot et al. investigated the changes in human intestinal viromes in response to a controlled feeding regimen [4•]. Consistent with the results seen by Reyes et al., they found robust intrapersonal stability and interpersonal variation, with a high representation of bacteriophages. Furthermore, this interpersonal variation was present even among individuals on the same diet, suggesting that the vast majority of phage populations are not acquired via a daily transient means such as food intake. Interestingly, the proportions of pre-existing viral populations were found to be significantly changed after alteration of diet. Thus, the virome composition does respond to diet through as yet unclear mechanisms.
For humans, it is estimated that healthy individuals are chronically infected with 8–12 different known viruses [8]. Recently, Li et al. investigated the presence of bacterial and viral elements in the plasma of HIV/AIDS patients compared to healthy controls [9]. It is thought that in HIV infection, impaired integrity of the intestinal epithelial barrier allows for increased bacterial translocation resulting in chronic immune activation [10]. Recently, Nazli et al. showed that interaction of HIV envelope protein (gp120) with intestinal epithelial cells in vitro can lead to upregulation of inflammatory cytokines, increased barrier permeability, and increased bacterial translocation [11]. Furthermore, there may be a defect in the clearance of bacteria in infected patients, which may further contribute to the immune activation seen in these patients [12].
The HIV-associated plasma microbiome was mainly composed of bacteria from the orders Pseudomonadales, Lactobacillus, Burkholderiales, Bacillales, and Enterobacteriales. Supporting the idea of bacterial translocation across the intestinal epithelial barrier, many of these microbes could be found in the normal human gut. Analysis of the microbiome of healthy individuals showed fewer bacterial DNA sequences in the plasma, with the most common orders being Clostridiales, Verrucomicrobiales, and Burkholderiales.
The plasma virome of HIV/AIDS patients and normal individuals showed many similarities. However, the striking difference was the large proportion of bacteriophages (84.51% of total plasma viral groups) found in HIV/AIDS plasma, which was completely lacking in normal human plasma. In addition to phages, HIV/AIDS plasma also contained endogenous retroviruses, non-human viruses, as well as novel viruses. The most abundant viruses detected in normal human plasma consisted of Anelloviruses.
Detection of viruses
Host cells express a variety of pattern recognition receptors (PRRs) that aid in the detection of viruses, including Toll-like receptors (TLRs), retinoic acid inducible gene I (RIG-I)-like receptors (RLRs), and nucleotide binding domain and leucine-rich repeat containing receptors (NLRs). Signaling through these different PRRs ultimately results in the production of antiviral proteins, most notably type I interferons, which are important for the control of virally infected cells.
TLRs
Many TLRs have been shown to play a role in viral detection. TLR2 and TLR4 can detect viral proteins, whereas TLR3, TLR7, TLR8, and TLR9 have been shown to detect viral nucleic acids [13, 14, 15]. Importantly for antiviral functions, some of these TLRs are able to induce the expression of type I IFNs via the activation of interferon regulatory factors 3/7 (IRF3/7) [16].
TLR3 has been shown to play an important role in viral detection in the intestine. TLR3 is localized to endosomes and recognizes double-stranded RNA (dsRNA). It signals via the adaptor protein TRIF, leading to the activation of IRF3 and NFκB, and ultimately the production of type I interferons and pro-inflammatory cytokines. Recently, Omagari et al. have shown that TLR3 stimulation of intestinal epithelial HT-29 cells led to the expression of human beta defensin 2 (hBD-2) in an NFκB-dependent manner [17].
RLRs
The major members of the RIG-I-like receptor family include RIG-I and MDA5 (melanoma differentiation factor-5) [18, 19]. These RLRs contain RNA helicase domains as well as caspase activation and recruitment domains (CARDs). RIG-I recognizes RNA 5′-triphosphate residues, whereas MDA5 binds to long dsRNA structures. Recognition of viral RNA by these RLRs leads to CARD-mediated signaling via MAVS (mitochondrial antiviral signaling protein) leading to the activation of IRF3, and resulting in the production of type I IFNs.
In the context of viral infections in the intestine, RIG-I and MDA5 have been shown to play an important role in the innate immune response against rotavirus, a dsRNA virus that can directly infect intestinal epithelial cells and lead to severe diarrhea. Work performed by Broquet et al. showed that infection intestinal epithelial cells with rotavirus led to the upregulation of RIG-I/MDA5 expression, but not TLR3 or PKR [20•]. In addition, rotavirus infection led to the expression and secretion of IFNβ in a RIG-I/MDA5/MAVS-dependent manner, both in vitro and in vivo. Furthermore, MAVS−/− mice (but not TRIF−/− mice) also showed increased rotavirus titers in IECs 4 days post infection along with increased viral shedding in feces. Together these data show the important role of the RIG-I/MDA-5/MAVS signaling pathway in the detection and control of rotavirus in the intestinal epithelium.
NLRs
Of the NLR family, cytosolic NOD1 and NOD2 have been the best characterized in the intestine, and are known to recognize different peptidoglycan components of bacterial cell walls; NOD1 recognizes g-d-glutamyl-mesodiaminopimelic acid (iE-DAP), and NOD2 binds muramyl dipeptide (MDP) [21]. Detection of these ligands by NOD1 and NOD2 leads to activation of the NFκB and MAPK pathways, ultimately resulting in production of pro-inflammatory cytokines and antimicrobial peptides.
Though the interaction of NOD2 and bacterial products has been known for some time, recent work has shown the involvement of NOD2 in the detection of viral products as well. Using several different cell lines, Sabbah et al. showed that treatment with synthetic ssRNA or infection with different ssRNA viruses can lead to increased expression and activation of NOD2, resulting in IFNβ production via MAVS and IRF3 [22]. This Type I IFN response was abrogated in cells lacking NOD2. Interestingly, this effect was greater at earlier time points compared to late. It is thought that other PRRs such as RIG-I may activate the IRF3-IFN pathway at the later time points, whereas NOD2 may act in an earlier role. In addition, the authors showed that NOD2 was able to associate with viral RNA but not control host RNA in an immunoprecipitation assay. Nod2 was also found to interact with MAVS via the leucine-rich-region of the nucleotide binding domain. Finally, Nod2 −/− mice failed to efficiently produce interferon after infection, which corresponded with increased viral titers and enhanced susceptibility to virus-induced pathogenesis.
Recent work by Morosky et al. has shown a potential interaction between RIG-I and NOD2 [23•]. Using different deletion mutants, the CARDs of RIG-I were shown to be crucial for this interaction. The CARDs of NOD2 alone were able to bind to RIG-I, however expression of NOD2 mutants lacking the CARDs were still able to bind to the full length RIG-I protein, suggesting that there are other sites that can interact with RIG-I. Overexpression of these proteins showed that NOD2 and RIG-I negatively regulated each other.
Response to viruses
Type I interferons
Type I interferons are an important part of the host immune response against viral infections. This family of cytokines is composed of 16 members (12 IFNα subtypes, IFNβ, IFNɛ, IFNκ, and IFNω) and signal through a common heterodimeric receptor (IFNαβ receptor or IFNAR) [24]. Not only can type I IFNs be produced by almost every cell type, but the receptor is expressed ubiquitously as well. Receptor binding leads to phosphorylation of Janus kinase 1 (JAK1) and non-receptor tyrosine kinase 2 (TYK2), resulting in activation of several signal transducer and activator of transcription (STAT) family members. In the case of STAT1 and STAT2 activation, IRF9 is recruited to form the transcription factor complex IFN-stimulated gene factor 3 (ISGF3) [24, 25]. ISGF3 translocates into the nucleus, binds to IFN-stimulated response elements (ISREs) leading to transcription of IFN-stimulated genes (ISGs) that play an important antiviral role.
Type III interferons
Type III IFNs were identified in 2003 by two independent groups [26, 27]. This family of cytokines consists of IFN-λ1 (IL-29), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B). Although distantly related to type I IFNs (15–20% amino acid identity), type III IFNs were also shown to be induced by viral infection and exhibited antiviral activity [28]. The IFN-λs signal through a heterodimeric receptor complex (IL-10Rβ and IL-28Rα) that is distinct from the type I IFN receptor complex, but both have similar intracellular signaling pathways (see above) resulting in functionally similar biological activities. As a result, signaling via type III IFNs leads to a type I IFN-like gene expression profile. Early experiments showed that many cell types could express both Type I and Type III IFNs in response to viral infection or TLR stimulation. However, a major difference was found to lie in the cell type able to respond to these different IFNs. While most nucleated cells are able to response to type I IFNs, the type III IFN receptor complex expressing cells are limited to a smaller subset including pDCs, keratinocytes, and epithelial cells of the respiratory, reproductive and gastrointestinal tracts. In addition, initial studies seem to indicate that most of the functions elicited by IFN-λs were redundant to the type I IFN responses, and IFN-λs alone were not sufficient to protect against systemic virus infections.
However, a recent study shows a primary role for type III IFNs against viral infections in the intestinal epithelial cells [29••]. After oral rotavirus infection, both Type I and Type III IFNs were expressed by the intestinal epithelial cells, as well as downstream target genes. However, mice lacking IL-28Rα (but not IFNAR1) lacked expression of downstream IFN response genes in the epithelial cells. These mice also showed increased viral antigen in the epithelial cells, increased viral antigen in feces, and resulted in more severe pathology. In addition, these authors show that the treatment of suckling mice with IFN-λ before oral infection repressed rotavirus replication in gut, whereas treatment of type I IFN was not effective. Together these data show an important non-redundant role for type III IFNs in the intestine that is distinct from type I IFNs.
Modulation of viral phenotypes by interaction with bacteria
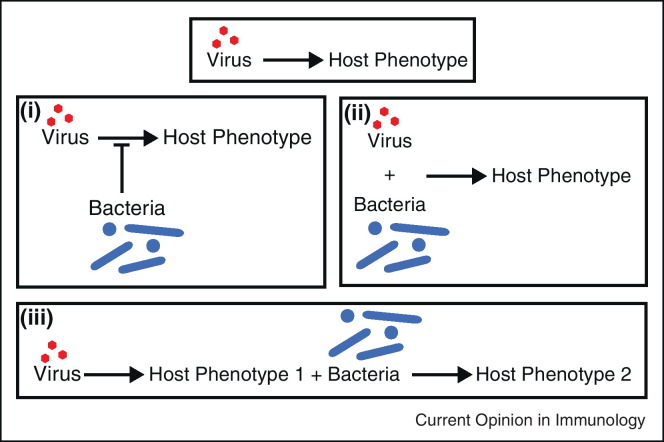
Viruses interact with the host to produce phenotypes. A new and important area of interest is how this basic interaction is modulated by bacteria. These studies all have a link to the intestine. We have categorized interactions described in the recent literature into three basic types (Figure 1 ).
Figure 1.
Variations on virally induced host phenotypes. Viruses can interact directly with the host to produce a specific phenotype. However, additional interactions with commensal bacteria in the intestine can modulate outcomes as well. These bacteria may act to inhibit viral interaction and infection (scenario I). Conversely, viruses and bacteria may work in concert to produce phenotypes (scenario II). In a third scenario, a virally induced phenotype may allow for bacteria to lead to an additional phenotype.
Bacteria antagonize virus induced phenotypes
Not all interactions between bacteria and viruses benefit the virus. Recent studies show that intestinal bacteria can act antagonistically to viral infection. For example, soluble factors derived from Bacteroides thetaiotaomicron and Lactobacillus casei block rotavirus infection of intestinal epithelial cells in vitro [30•]. The proposed mechanism of action was that these bacteria increased the activity of enzymes that can modify host cell glycans and prevent rotavirus attachment to the host cell surface. This scenario highlights an ability of commensal bacteria to potentially protect the host from viral infections.
Germ free mice are well known to be more susceptible to influenza than conventionally raised mice [31]. Ichinohe et al. recently determined a mechanism by which the gut microbiota regulates immune defense against influenza virus infections in the respiratory tract [32••]. Conventionally raised mice were treated with an antibiotic cocktail for 4 weeks before intranasal challenge with a sublethal dose of A/PR8 influenza virus. These antibiotic-treated mice showed decreased titers of virus-specific antibody, impaired CD4/CD8 T cell responses, and increased pulmonary viral titers at day 9 post infection. Interestingly, administration of TLR ligands locally (intranasal) and distally (intrarectal) at the time of influenza infection were both able to restore the antibody and T cell responses in the antibiotic-treated mice. In addition, the antibiotic-treated mice showed reduced pro-IL-1β and pro-IL-18 expression as well as decreased IL-1β secretion after infection with the virus. Taken together, these data support the idea that the commensal microbiota provides a critical first signal needed for expression of the pro forms of these cytokines at steady state, thereby priming the system for a second signal (virus infection), leading to activation of the inflammasome and maturation/release of these cytokines.
Virally induced phenotypes that require bacteria
Importantly, interplay between viruses and bacteria occur during infection and disease settings. Mice that are hypomorphic for Atg16L1contain abnormal Paneth cells, a small intestinal epithelial cell type with innate immune function. This finding was significant as patients with inflammatory bowel disease susceptibility mutations for Atg16L1 also have abnormal Paneth cells [33]. More recently, this phenotype was shown to be dependent on a viral trigger [34]. Mice re-derived in an enhanced barrier facility no longer showed Paneth cell abnormalities, but oral inoculation with a persistent strain of murine norovirus (MNV; CR6), led to the appearance of abnormal Paneth cells in Atg16L1 hypomorphic mice by day 7 post-infection. Furthermore, features of dextran sodium sulfate (DSS)-induced damage that were reminiscent of human Crohn's disease were also triggered by MNV CR6. Importantly, the viral trigger for both of these phenotypes was dependent on the commensal microbiota. The specific bacteria that are required to trigger are unknown.
In a second recent example, the intestinal microbiota can play a role in enteric virus replication and transmission. Kuss et al. demonstrated that the commensal bacteria enhanced the ability of two enteric viruses, poliovirus and reovirus, to replicate and infect the host [35••]. Animals treated with antibiotics before inoculation with these viruses were less susceptible to infection and disease. Interestingly, they found that poliovirus was able to bind to bacterial products such as LPS, which allowed for enhanced host cell association and infection.
Kane et al. recently found that the retrovirus, mouse mammary tumor virus (MMTV) also utilized the intestinal microbiota to efficiently infect the host [36••]. Oral inoculation of MMTV in newborn mice induced tolerance and allowed for persistence of the virus. This induction of tolerance required the commensal microbiota. MMTV-bound bacterial LPS was found to trigger TLR4, leading to subsequent IL-6 dependent induction of IL-10. Together, these two papers [35••, 36••] illustrate the ability of different viruses to utilize the commensal bacteria and their products to aid in infection and immune evasion of the host.
Viral infection enhances secondary bacterial infection
Primary viral infections are well known to result in secondary bacterial infections, though the mechanisms are unclear. Recently, Kim et al. showed TRIF-dependent and MAVS-dependent type I IFN production augmented NOD1/NOD2 expression and signaling in mouse bone marrow-derived macrophages, leading to enhanced production of pro-inflammatory cytokines. Oral infection with MNV1 promoted inflammation and lethality in mice superinfected with E. coli [37••]. This effect was attenuated in the absence of NOD1/NOD2 or RIP2. These data show that crosstalk between virally stimulated type I IFNs and NOD1/NOD2 signaling promotes bacteria recognition and may lead to an enhanced response against the secondary bacterial infection.
Conclusions
We anticipate that human viromes for additional disease states will be published in the future. The next challenge will be to determine the role (if any) of the identified viruses in the pathogenesis of a given disease. We anticipate that we will also gain a more detailed understanding of the interaction of bacteria with specific viruses that affect infection phenotypes. The paradigms established in the intestine can then be tested in other systems.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank Scott Handley for comments on the manuscript. This work was funded by R01AI00717231.
References
- 1.Phan T.G., Kapusinszky B., Wang C., Rose R.K., Lipton H.L., Delwart E.L. The fecal viral flora of wild rodents. PLoS Pathog. 2011;7:e1002218. doi: 10.1371/journal.ppat.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge X., Li Y., Yang X., Zhang H., Zhou P., Zhang Y., Shi Z. Metagenomic analysis of viruses from the bat fecal samples reveals many novel viruses in insectivorous bats in China. J Virol. 2012;86:4620–4630. doi: 10.1128/JVI.06671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Reyes A., Haynes M., Hanson N., Angly F.E., Heath A.C., Rohwer F., Gordon J.I. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature. 2010;466:334–338. doi: 10.1038/nature09199. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors sequenced fecal viromes of healthy adult twins and their mothers over a period of one year. Viromes were found to be stable and unique between individuals regardless of genetic relatedness. Bacteriophages were found to make up a large proportion of the intestinal viromes.
- 4•.Minot S., Sinha R., Chen J., Li H., Keilbaugh S.A., Wu G.D., Lewis J.D., Bushman F.D. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 2011;21:1616–1625. doi: 10.1101/gr.122705.111. [DOI] [PMC free article] [PubMed] [Google Scholar]; Confirms the trends observed for individual viromes observed in the Reyes et al. paper and studies the effect of diet on the virome.
- 5.Wiedenheft B., Sternberg S.H., Doudna J.A. RNA-guided genetic silencing systems in bacteria and archaea. Nature. 2012;482:331–338. doi: 10.1038/nature10886. [DOI] [PubMed] [Google Scholar]
- 6.Sorek R., Kunin V., Hugenholtz P. CRISPR – a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat Rev Microbiol. 2008;6:181–186. doi: 10.1038/nrmicro1793. [DOI] [PubMed] [Google Scholar]
- 7.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A., Bewtra M., Knights D., Walters W.A., Knight R. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virgin H.W., Wherry E.J., Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Li S.K., Leung R.K., Guo H.X., Wei J.F., Wang J.H., Kwong K.T., Lee S.S., Zhang C., Tsui S.K. Detection and identification of plasma bacterial and viral elements in HIV/AIDS patients in comparison to healthy adults. Clin Microbiol Infect. 2011 doi: 10.1111/j.1469-0691.2011.03690.x. [DOI] [PubMed] [Google Scholar]
- 10.Wardwell L.H., Huttenhower C., Garrett W.S. Current concepts of the intestinal microbiota and the pathogenesis of infection. Curr Infect Dis Rep. 2011;13:28–34. doi: 10.1007/s11908-010-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nazli A., Chan O., Dobson-Belaire W.N., Ouellet M., Tremblay M.J., Gray-Owen S.D., Arsenault A.L., Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofer U., Schlaepfer E., Baenziger S., Nischang M., Regenass S., Schwendener R., Kempf W., Nadal D., Speck R.F. Inadequate clearance of translocated bacterial products in HIV-infected humanized mice. PLoS Pathog. 2010;6:e1000867. doi: 10.1371/journal.ppat.1000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trinchieri G., Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 14.Ishii K.J., Koyama S., Nakagawa A., Coban C., Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008;3:352–363. doi: 10.1016/j.chom.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Kinnebrew M.A., Pamer E.G. Innate immune signaling in defense against intestinal microbes. Immunol Rev. 2012;245:113–131. doi: 10.1111/j.1600-065X.2011.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arpaia N., Barton G.M. Toll-like receptors: key players in antiviral immunity. Curr Opin Virol. 2011;1:447–454. doi: 10.1016/j.coviro.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Omagari D., Takenouchi-Ohkubo N., Endo S., Ishigami T., Sawada A., Moro I., Asano M., Komiyama K. Nuclear factor kappa B plays a pivotal role in polyinosinic-polycytidylic acid-induced expression of human beta-defensin 2 in intestinal epithelial cells. Clin Exp Immunol. 2011;165:85–93. doi: 10.1111/j.1365-2249.2011.04404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichlmair A., Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Fitch P.M., Henderson P., Schwarze J. Respiratory and gastrointestinal epithelial modulation of the immune response during viral infection. Innate Immun. 2011;18:179–189. doi: 10.1177/1753425910391826. [DOI] [PubMed] [Google Scholar]
- 20•.Broquet A.H., Hirata Y., McAllister C.S., Kagnoff M.F. RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium. J Immunol. 2011;186:1618–1626. doi: 10.4049/jimmunol.1002862. [DOI] [PubMed] [Google Scholar]; Rotavirus infection of intestinal epithelial cells leads to the expression and secretion of IFNβ in a RIG-I/MDA5/MAVS-dependent manner both in vitro and in vivo. This response was not dependent on the TLR or PKR pathway, highlighting the specific detection and response of IECs to rotavirus.
- 21.Lecat A., Piette J., Legrand-Poels S. The protein Nod2: an innate receptor more complex than previously assumed. Biochem Pharmacol. 2010;80:2021–2031. doi: 10.1016/j.bcp.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Sabbah A., Chang T.H., Harnack R., Frohlich V., Tominaga K., Dube P.H., Xiang Y., Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10:1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Morosky S.A., Zhu J., Mukherjee A., Sarkar S.N., Coyne C.B. Retinoic acid-induced gene-I (RIG-I) associates with nucleotide-binding oligomerization domain-2 (NOD2) to negatively regulate inflammatory signaling. J Biol Chem. 2011;286:28574–28583. doi: 10.1074/jbc.M111.227942. [DOI] [PMC free article] [PubMed] [Google Scholar]; These authors show RIG-I and NOD2 have the ability to interact and function as negative regulators of each other.
- 24.Gonzalez-Navajas J.M., Lee J., David M., Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamming O.J., Gad H.H., Paludan S., Hartmann R. Lambda interferons: new cytokines with old functions. Pharmaceuticals. 2010;3:795–809. doi: 10.3390/ph3040795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheppard P., Kindsvogel W., Xu W., Henderson K., Schlutsmeyer S., Whitmore T.E., Kuestner R., Garrigues U., Birks C., Roraback J. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 27.Kotenko S.V., Gallagher G., Baurin V.V., Lewis-Antes A., Shen M., Shah N.K., Langer J.A., Sheikh F., Dickensheets H., Donnelly R.P. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 28.Kotenko S.V. IFN-lambdas. Curr Opin Immunol. 2011;23:583–590. doi: 10.1016/j.coi.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Pott J., Mahlakoiv T., Mordstein M., Duerr C.U., Michiels T., Stockinger S., Staeheli P., Hornef M.W. IFN-lambda determines the intestinal epithelial antiviral host defense. Proc Natl Acad Sci USA. 2011;108:7944–7949. doi: 10.1073/pnas.1100552108. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the significance of type III interferons in a mucosal setting, and provides an example in which there is a non-redundant role for type III interferons distinct from type I.
- 30•.Varyukhina S., Freitas M., Bardin S., Robillard E., Tavan E., Sapin C., Grill J.P., Trugnan G. Glycan-modifying bacteria-derived soluble factors from Bacteroides thetaiotaomicron and Lactobacillus casei inhibit rotavirus infection in human intestinal cells. Microbes Infect. 2012;14:273–278. doi: 10.1016/j.micinf.2011.10.007. [DOI] [PubMed] [Google Scholar]; Soluble factors made by bacteria can prevent rotavirus attachment and infection of intestinal epithelial cells by modifying host cell surface glycans.
- 31.Dolowy W.C., Muldoon R.L. Studies of germfree animals. I. Response of mice to infection with influenza a virus. Proc Soc Exp Biol Med. 1964;116:365–371. doi: 10.3181/00379727-116-29249. [DOI] [PubMed] [Google Scholar]
- 32••.Ichinohe T., Pang I.K., Kumamoto Y., Peaper D.R., Ho J.H., Murray T.S., Iwasaki A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]; These authors show that the intestinal commensal microbiota can affect the host response to infection with influenza in the lungs. It is thought that the intestinal microbes provide a first signal to prime the immune system for this secondary infection.
- 33.Cadwell K., Liu J.Y., Brown S.L., Miyoshi H., Loh J., Lennerz J.K., Kishi C., Kc W., Carrero J.A., Hunt S. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cadwell K., Patel K.K., Maloney N.S., Liu T.C., Ng A.C., Storer C.E., Head R.D., Xavier R., Stappenbeck T.S., Virgin H.W. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141:1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; The abnormal Paneth cell phenotype seen in mice hypomorphic for IBD-associated gene Atg16L1 requires both the commensal microbiota and a viral trigger. This study highlights the role for host genetics, bacteria, and viruses in disease.
- 35••.Kuss S.K., Best G.T., Etheredge C.A., Pruijssers A.J., Frierson J.M., Hooper L.V., Dermody T.S., Pfeiffer J.K. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334:249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that two enteric viruses require the presence of the intestinal microbiota for maximal infection.
- 36••.Kane M., Case L.K., Kopaskie K., Kozlova A., MacDearmid C., Chervonsky A.V., Golovkina T.V. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334:245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows a mechanism by which a virus can utilize the commensal bacteria to induce tolerance in the host. Mouse mammary tumor virus (MMTV) can bind bacterial LPS and induce tolerance in a TLR4-dependent manner.
- 37••.Kim Y.G., Park J.H., Reimer T., Baker D.P., Kawai T., Kumar H., Akira S., Wobus C., Nunez G. Viral infection augments Nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe. 2011;9:496–507. doi: 10.1016/j.chom.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Virally induced type I interferons can augment NOD1/NOD2 signaling and enhance bacterial recognition.