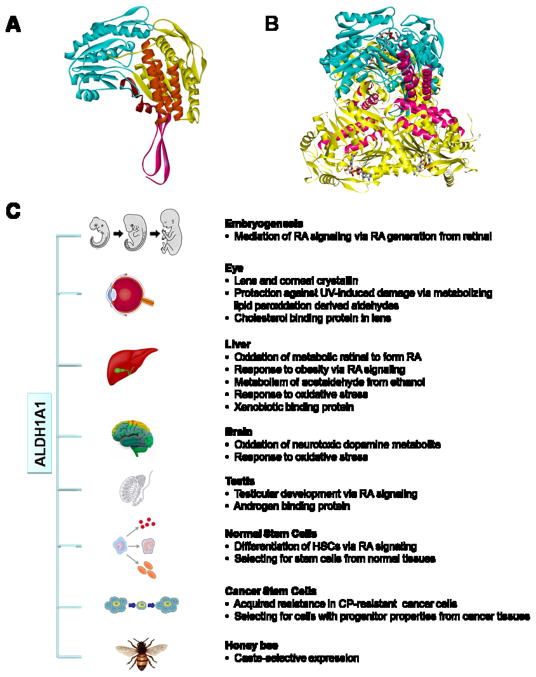
1. Structure
Aldehyde dehydrogenase 1A1 (ALDH1A1) belongs to a superfamily of enzymes, most of which catalyze the NAD(P)+-dependent irreversible oxidation of a wide variety of endogenous and exogenous aldehyde substrates to their corresponding acids (Marchitti et al., 2008). ALDH1A1 is well conserved across species, showing 90% identity in amino acid sequence with mammals and 80% with other species, including chicken, frog and fish. In all of these species, the essential residues for NAD+-binding (Lys-192, Gly-245, Gly-250, Glu-399 and Phe-401; numbering based on human ALDH1A1) and for catalytic activity (Cys-302 and Glu-268) are strictly conserved. Structurally, mammalian ALDH1A1 is a homotetramer with a 55 kDa subunit. Each monomer (Fig. 1A) is composed of an βαβ N-terminal nucleotide-binding-domain (yellow), a βαβ catalytic domain (blue) and a C-terminal small β-sheet oligomerization domain (pink). The dimer is formed through the contacts of monomer α-helices and β-sheet extension between the catalytic and oligomerization domains. In the tetrameric holoenzyme (Fig. 1B), the active sites reside at the base of a hydrophobic tunnel penetrating from the surface of the enzyme and they are close to the tetrameric interface and opposite to the cofactor binding sites, which is beneficial to catalysis. Interestingly, the COILS program (http://www.ch.embnet.org/software/COILS_form.html) predicts three coiled-coil motifs in human ALDH1A1 protein sequence at 81–99, 114–144, and 172–183 residues (Fig. 1A, orange), which reside in the nucleotide-binding-domain. The coiled-coil domain is a highly stable oligomerization motif found in diverse proteins functioning in gene regulation, cell communication, membrane fusion and drug extrusion.
Fig. 1. Protein structure and function of ALDH1A1.
(A) Homology model of human ALDH1A1 monomer showing NAD binding domain (yellow), catalytic domain (blue), oligomerization domain (pink) and three predicated coiled-coil motifs (orange) inside the NAD binding domain. (B) Superimposition of human ALDH1A1 monomer (blue) onto sheep liver ALDH1A1 trimer (yellow), with coiled coil motifs highlighted in pink. Bound NAD for each monomer is shown as stick figure. (C) Physiological and toxicological roles of of ALDH1A1. ALDH1A1 is catalytically active towards the oxidation of wide varieties of aldehyde substrates derived from endogenous or exogenous precursors. In addition, ALDH1A1 is capable of non-catalytic interactions with chemically-diverse compounds. Through its metabolic and non-metabolic properties, ALDH1A1 is involved in multiple physiological and toxicological processes (details are presented in the text). ALDH1A1, aldehyde dehydrogenase 1A1. RA, retinoic acid. DOPAL, 3,4-dihydroxyphenylacetaldehyde. HSCs, hematopoietic stem cells. CP, cyclophosphamide.
2. Function
ALDH1A1, a cytosolic protein, is ubiquitously expressed in many tissues where it serves both metabolic and non-metabolic functions (Fig. 1B). ALDH1A1 has been identified as a crystallin in the cornea and lens of several animal species (Lassen et al., 2008). Its distribution between the cornea and lens varies between species. In humans, ALDH1A1 is roughly evenly distributed, representing 2 – 3% of the soluble proteins in these tissues. It is predominantly found in the lens of mice and in the cornea of rabbits and certain types of fish (www.aldh.org). In still other species, other members of the ALDH1 family serve as lens crystallin, such as ALDH1C1 and ALDH1C2 (ω-crystallin) in octopus and squid. In ocular tissues, ALDH1A1 may play a key metabolic role in protecting the eye from UV-induced damage by degrading toxic aldehydes produced during lipid peroxidation. However, the levels of ALDH1A1 (and other ALDH1 isoforms) in lens and cornea exceed those predicted to be needed for such metabolism. This has led to the proposal that this protein possesses additional roles and serves as an example of “gene-sharing” described by Joram Piatigorky (reviewed in Lassen et al. 2008). In this respect, ALDH1A1 may function as a structural element in the lens and cornea. Indeed, ALDH1A1 has been shown to contribute substantially to the transparency and refractory aspects of the cornea in the rabbit (Lassen et al., 2008). Studies demonstrate that (i) injury-induced corneal haze is associated with the loss of ALDH1A1 expression in the corneal stromal keratocytes, and (ii) the development of postnatal corneal transparency is linked with stromal keratocyte quiescence and increased ALDH1A1 expression. ALDH1A1 protein has also been found in the developing and adult retina of the mouse, rabbit and chicken. Given that Aldh1a1(−/−) knockout mice exhibit normal retinal development, the functional role of ALDH1A1 in the retina remains to be elucidated. However, we believe that ALDH1A1 may serve to protect the retina from oxidative damage.
ALDH1A1 is best known as an enzyme that catalyzes the oxidation of the retinol (vitamin A) metabolite, retinal (retinaldehyde), to retinoic acid (RA). It has high affinity for the oxidation of both all-trans- and 9-cis-retinal, exhibiting a Km in the lower micromolar range (Marchitti et al., 2008). RA acts as a ligand for the heterodimer of the nuclear RA receptor and the retinoic X receptor, which binds to the RA response elements in downstream target genes to activate gene expression. The observation that the expression of ALDH1A1 (and other retinal-oxidizing ALDH isozymes) is regulated throughout the development of certain tissues supports the notion that these ALDH enzymes play important roles during embryonic development via their effect on RA signaling. In addition to RA biosynthesis, ALDH1A1 also exerts a cellular protective role by metabolizing highly reactive aldehydes. ALDH1A1 is one of the major enzymes involved in ethanol metabolism by eliminating its toxic metabolite acetaldehyde. ALDH1A1 also detoxifies 4-hydroxynonenal and malondialdehyde, two major products of lipid peroxidation generated during oxidative stress. In dopaminergic neurons, ALDH1A1 may be involved in neuroprotection by oxidizing 3,4-dihydroxyphenylacetaldehyde, a toxic metabolite of dopamine. Interestingly, ALDH1A1 is highly expressed in queen honey bees but not in worker honey bees. Such selective expression of ALDH1A1 may be associated with caste-selective status of metabolism and antioxidant response. Aside from its metabolic properties, ALDH1A1 has been shown to be capable of non-catalytic interactions with chemically-diverse compounds, including endobiotics (androgen, thyroid hormone and cholesterol) and xenobiotics (flavopiridol, daunorubicin and quinolone). The physiological/toxicological role of such interactions remains unclear (Marchitti et al., 2008); however, the coiled-coil motif in ALDH1A1 may facilitate such interactions.
In recent years, ALDH1A1 has been proposed to be a specific marker of stem cells, both normal and cancer–associated (Marchitti et al., 2008). The ALDH1A1 gene is highly expressed in primary human hematopoietic stem cells (HSCs) and these cells are rich in ALDH activity. Latter studies have provided several lines of evidence supporting a critical role for ALDH1A1-mediated RA-signaling in regulating HSC self-renewal and differentiation. More recently, ALDH activity has been widely used to identify and isolate cancer stem cells (CSCs) from tumors of various tissue origins. With the use of Aldefluor®, a commercial metabolism-based assay, a subpopulation of cells possessing high ALDH activity (ALDH+ cells) can be sorted from normal or tumor tissues. These ALDH+ cells exhibit profound tumorigenic potential. The molecular basis of the selective expression of ALDH1A1 in CSCs is not completely understood. Functionally, it is possible that a cytoprotective effect mediated by ALDH1A1 is a key determinant of cell survival and drug resistance of CSCs (Marchitti et al., 2008).
3. Disease involvement
The Aldh1a1−/− knockout transgenic mouse strain has been developed and used by multiple investigators to study pathophysiologies associated with ALDH1A1 deficiency (Fan et al., 2003). A protective role of ALDH1A1 in the eye is supported by the observation that Aldh1a1−/− knockout mice develop cataracts at 6–9 months of age and are sensitive to UV-induced cataract formation (Lassen et al., 2007). Despite of a robust expression of ALDH1A1 and ALDH3A1 in the wild-type mouse cornea, existing studies report no aberrant corneal phenotypes in either single (Aldh1a1−/− or Aldh3a1−/−) or the double (Aldh1a1−/−/Aldh3a1−/−) knockout models (Lassen et al., 2007). Livers from Aldh1a1−/− knockout mice display reduced RA synthesis and increased serum retinal levels following retinol treatment (Fan et al., 2003), indicating a key role of liver ALDH1A1 in oxidizing retinal. Interestingly, Aldh1a1−/− knockout mice are protected against diet-induced obesity and insulin resistance, suggesting that ALDH1A1 may regulate the metabolic response to high-fat diet through an action involving retinal metabolism (Marchitti et al., 2008). In the human population, polymorphic variants of ALDH1A1 resulting in low enzyme activity have been reported to be associated with alcohol sensitivity in Caucasians (Marchitti et al., 2008). Finally, immunohistochemical studies have revealed increased expression of ALDH1A1 protein in human epithelial cancers; such expression appears to be an indicator of poor prognosis and correlates with the histological grade of the tumor (Marchitti et al., 2008).
4. Future studies
ALDH1A1 is an interesting and enigmatic molecule. It possesses important physiological and pathophysiological roles in the body, some of which involve its metabolic activity while others its non-metabolic functions. Given the variety of cells and tissues expressing ALDH1A1, clear elucidation of its function and the molecular mechanisms governing its expression will invariably provide valuable insights into cellular function in health and disease and potential therapeutic targets. For example, what role does ALDH1A1 play in stem cell function? Could it be a target for the treatment of cancer? In the eye, could up-regulation of ALDH1A1 expression or activity prevent or treat cataracts? Given that proteins possessing coiled-coils have been used in therapeutic applications for the development of anticancer drugs and vaccines, could the coiled-coil motif of ALDH1A1 be engineered for selective drug delivery? As we begin to address these questions and learn more about ALDH1A1, it is anticipated that it will become less enigmatic but certainly remain an interesting molecule.
Acknowledgments
We thank Philip Reigan for his help on the molecular modeling of human ALDH1A1. This work was supported by NIH grant EY017963. We apologize to authors whose work cannot be cited due to limits for reference citation.
References
- Fan X, Molotkov A, Manabe S, Donmoyer CM, Deltour L, Foglio MH, Cuenca AE, Blaner WS, Lipton SA, Duester G. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol Cell Biol. 2003;23:4637–4648. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen N, Bateman JB, Estey T, Kuszak JR, Nees DW, Piatigorsky J, Duester G, Day BJ, Huang J, Hines LM, Vasiliou V. Multiple and additive functions of ALDH3A1 and ALDH1A1: cataract phenotype and ocular oxidative damage in Aldh3a1(−/−)/Aldh1a1(−/−) knock-out mice. Journal of Biological Chemistry. 2007;282:25668–25676. doi: 10.1074/jbc.M702076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen N, Black WJ, Estey T, Vasiliou V. The role of corneal crystallins in the cellular defense mechanisms against oxidative stress. Semin Cell Dev Biol. 2008;19:100–112. doi: 10.1016/j.semcdb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4:697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]