Abstract
Background
Experimental changes in resting cerebral blood flow (CBF) affect task-related blood oxygenation level dependent (BOLD) responses. Since patients with schizophrenia have been shown to have abnormal resting CBF, we sought to determine whether differences between patients and healthy controls in resting CBF contributed to group differences in BOLD response.
Methods
BOLD images were acquired in nineteen patients and twenty healthy controls looking at photographs of faces, and resting CBF was measured by arterial spinning labeling. Resting CBF was then used to adjust group differences in task-related BOLD signal increases in linear models.
Results
Patients had different resting CBF from healthy controls in right basal ganglion and bilateral thalami. Associations between resting CBF and delta BOLD were evident in bilateral prefrontal areas, visual processing areas and right fusiform gyrus. Other areas showed significant three-way interactions among group, delta BOLD and resting CBF. Incorporating resting CBF when modeling group differences in BOLD responses identified areas of group differences in task-related delta BOLD response that were not evident in simple group contrasts. These were in right inferior frontal cortex, left insula, left middle frontal cortex and bilateral frontal poles.
Conclusion
Adjusting for inter-group differences in resting CBF altered inter-group differences in task-related BOLD response in some areas, suggesting that assessing resting CBF in task-related BOLD studies could increase sensitivity and validity. In multiple regions, the relationship between resting CBF and task-related signal increases in BOLD, differed between patients and controls, providing new evidence of possible metabolic and/or vascular pathology.
Keywords: schizophrenia, resting cerebral blood flow, fMRI, Arterial spin labeling, emotion, face processing
1. Introduction
When cerebral blood flow (CBF) of the baseline or resting condition is changed pharmacologically, for example by sevoflurance or acetazolamide, the blood oxygenation level dependent (BOLD) response associated with a task is changed (Qiu et al., 2008; Yamauchi et al., 2003). The possibility that inter-group differences in BOLD response are related to differences in resting CBF is relevant to schizophrenia research since patients with schizophrenia are known to differ from healthy controls in resting CBF in frontal and other areas(Berman et al., 1986; Hoshi et al., 2006; Kanahara et al., 2009; Weinberger and Berman, 1988; Weinberger et al., 1986). Hence, it is important to clarify whether apparent abnormalities in task-related BOLD responses in schizophrenia are associated with and potentially secondary to abnormalities in resting CBF rather than in abnormalities in task-related neural activation per se.
In the present study, we compared resting CBF as measured by arterial spin labeling (ASL) and task-related change in the BOLD signal in patients with schizophrenia and healthy controls with the primary goal of determining whether patient abnormalities in task-related changes in BOLD signal are in the same places as patient-control differences in resting CBF. The absence of such an association would mean that the extensive work being done using BOLD to identify patient abnormalities in task related brain activations can probably be viewed as unconfounded by patient-control differences in resting CBF, whether such differences arise from illness or its treatment (e.g., medications). On the other hand, if there is an association between group differences in resting CBF and BOLD, then it will be important for studies to measure resting CBF and use it as a covariant when evaluating BOLD differences. Secondary goals were to examine patient-control differences in resting CBF itself, and in the relationship between resting CBF and BOLD independent of group differences in resting CBF or BOLD themselves.
2. Methods
2.1. Participants
Nineteen patients meeting DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, fourth edition) diagnostic criteria for schizophrenia or schizoaffective disorder and twenty healthy controls participated in the study (Table 1). Patients were clinically stable outpatients (no hospitalizations, homelessness or substance abuse in the last six months) and taking medication. Chlorpromazine equivalent doses (Andreasen et al., 2010) are indicated in Table 1. All participants had no history of epilepsy, neurological disease, brain injury, or developmental disability, and were right-handed (Wexler and Halwes, 1983) native English speakers with normal or corrected to normal vision. All gave written informed consent following procedures approved by the Institutional Review Boards of Yale University and were drug free as confirmed by urine toxicology before imaging.
Table 1.
General information of participents
| Patients (n=19) | Healthy Controls (n=20) | |
|---|---|---|
| Gender (n,%) | ||
| Male | 11 (57.9%) | 11 (55.0%) |
| Female | 8 (42.1%) | 9 (45.0%) |
| Education years (mean ± SD) | 13. 5 ± 1.8 | 17 ± 1.9 |
| Age at Illness onset (mean ± SD) | 26.0 ± 8.9 | NA |
| Illness Duration, in years (mean ± SD) | 20.5 ± 10.0 | NA |
| Chlorpromazine Equivalents (md/day)* | 622.1 ± 418.0 | NA |
from 17 cases
2.2. Paradigm for BOLD Imaging
Participants viewed 48 photographs of facial displays from the University of Pennsylvania stimulus set, twice each in blocks of happy (H), sad (S) or neutral (N) emotion. Each of the 12 blocks lasted 24 seconds and included 8 faces, each displayed for 2.0 seconds and a fixation cross at the screen center for 1.0 seconds proceeding face stimuli. The twelve blocks ran in the sequence H-S-N-N-S-H-S-N-N-S-H with 12 second baseline blocks of fixation and gray screens following each stimulus block.
2.3. MR Image Acquisitions
Imaging data were collected in the Yale MRI Research Center using a Siemens 3T MRI scanner with twelve channel head coil. BOLD images were acquired by a T2*-weighted gradient echo-planar imaging (EPI) sequence with 1500 ms repetition time (TR). The ASL images were acquired at resting status (black screen without special task). Two-dimension T1-weighted spin-echo images and high-resolution saggittal T1-weighted spin-echo images were acquired for registration. These were also used to identify participants with structural brain abnormalities (none found). Details about sequences and parameters are described in online supplement.
2.4. Data analysis
The BOLD functional data were converted into nifti, and timing and motion corrected in spm5. ASL data were processed in Matlab following the procedure of Qui et al. (Qiu et al., 2010) to calculate CBF. All BOLD and CBF data were registered and re-sampled into 3 mm × 3 mm × 3 mm in Montreal Neurological Institute (MNI) space. Voxel-wise statistical analysis was performed in R(R Development Core Team, 2011). AlphaSim of AFNI package was run to simulate the random field with noise at each given p level to give the cluster-size threshold for partial correction for multiple comparisons.
2.4.1. The group differences of resting CBF and task-related BOLD
Possible signal drift over time and other noise in BOLD data were removed voxel by voxel for each subject individually by extracting signal over time in a large white matter area (bilateral half-ovary areas defined in MNI space). Average percent change in BOLD was then defined by subtracting average signal in baseline blocks from average signal during face viewing, and dividing the difference by the values during baseline blocks. Differences of resting CBF and BOLD response between the two groups were tested using two-sided voxel-based t tests.
2.4.2. Correspondence between resting CBF and task-related BOLD
In order to evaluate the relationships between resting CBF and BOLD signal changes, the relationship between resting CBF and task-related change of BOLD signal was modeled and estimated in linear models across-subjects, and the parameters associated with diagnosis, resting CBF and their interaction, were tested by ANOVAs and t tests. Maps were created showing the distribution of significant parameter values for CBF and for the interaction term.
In preparation for using resting CBF values to refine the BOLD map differences between patients and controls, three models were used to fit the data in voxel-based ANOVA, and the residual sum of squares (RSS) in the models was tested to compare the models and choose the best one to fit the data in each voxel. The first model was the simple model with diagnosis as the only factor (M0); the second model (M1) added resting CBF, and the third model (M2) included the interaction term, resting CBF and diagnosis. More complex models were chosen over simpler models only if the RSS in the more complex model was significantly less than that in the more simple model at p <0.02. Next, the parameter for each item in the chosen model was estimated and statistically evaluated by t test in each voxel.
3. Results
3.1. Behavior data
Patient accuracy was somewhat lower than controls on the gender identification task during viewing of the faces (93% vs. 96%) but the groups did not differ in response time or failure to respond rate, indicating a high level of participation and accuracy in both groups.
3.2. Resting CBF in schizophrenia and health controls
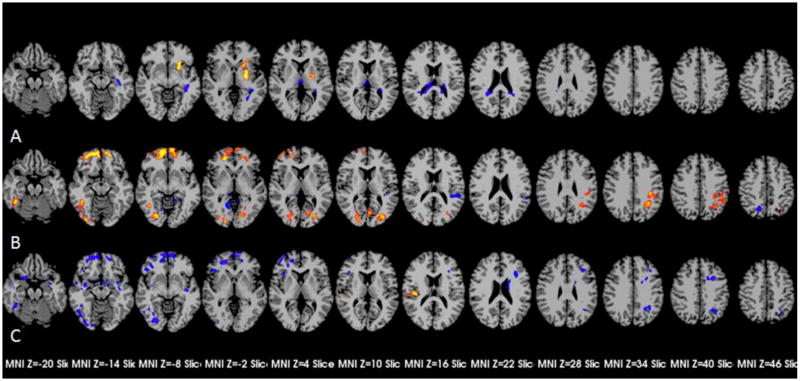
Voxel-wise comparisons indicated that patients had lower resting CBF than controls in bilateral thalami, and higher resting CBF in bilateral base ganglia, with differences in thalami and left basal ganglia surviving cluster criterion (Figure 1-A).
Figure 1.
Group different resting CBF and the effects on task-related BOLD signal increase. A showed the areas with differences of resting CBF between patients and controls with p-corrected ≤ 0.05 (p ≤ 0.04, cluster ≥ 117); B showed the areas with statistical effects of resting CBF on task-related BOLD signal increase with p-corrected ≤ 0.05 (p ≤ 0.01, cluster ≥ 38); C showed the areas with interactions of resting CBF and diagnosis with p-corrected ≤ 0.05 (p ≤ 0.01, cluster ≥ 38).
3.3. Relationship between resting CBF and task-related BOLD signal increases
Figure 1-B shows areas in which task-related BOLD signal increases were associated with regional resting CBF (i.e., significant parameter values for resting CBF). Areas with a positive association between resting CBF and increase in BOLD signal included bilateral prefrontal cortexes, some bilateral visual processing areas, right fusiform gyrus, and left superior marginal area, and those with a negative relationship included some bilateral visual processing areas and an area in left parietal cortex. Areas with interactions of resting CBF and task-related signal increases (i.e., significant parameter values for the interaction between diagnosis and resting CBF) were detected in both hemispheres (Figure 1-C, Table 2). Except the area located in right parietal cortex with positive resting CBF effects on the task-related BOLD signal responses, other areas showed negative effects. Differences between patients and controls in the relationship between resting CBF and delta BOLD were of two types: 0<patients<controls or 0>patients>controls. In all cases the control group showed more consistent or robust associations between resting CBF and delta BOLD than did the patient group.
Table 2.
The areas with interactions of resting CBF and task-related BOLD signal increase
| Region | Centroid MNI
|
Volume (mm3) | Style of Interaction* | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Right inferior frontal gyrus | 42 | 30 | −4 | 1802 | 0<pts<ctr |
| Left middle frontal gyrus | −34 | 26 | 26 | 1480 | 0<pts<ctr |
| Bilateral frontal poles | 12 | 54 | −6 | 7849 | 0<pts<ctr |
| Left occipital superior/middle gyrus | −31 | −57 | 36 | 1659 | 0<pts<ctr |
| Right amygdala and adjacent areas | 33 | 3 | −18 | 1915 | 0<pts<ctr |
| Left amygdala and adjacent areas | −29 | −3 | −16 | 1741 | 0<pts<ctr |
| Right fusiform gyrus | 37 | −70 | −12 | 3756 | 0<pts<ctr |
| Right parietal gyrus | 45 | −23 | 16 | 1048 | 0>pts>ctr |
pts and ctr indicate the resting CBF effects in patients and controls, p-corrected ≤ 0.05 (p ≤ 0.01, cluster ≥ 38) for pts ≠ ctr.
3.4. Task-related signal increases unadjusted and adjusted by resting CBF
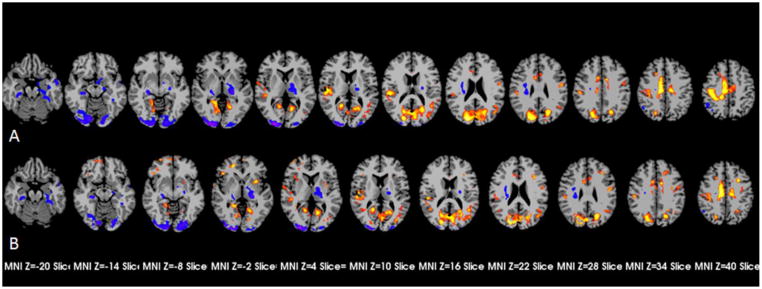
Voxel-wise t tests comparing task-related group signal increases without adjustments (i.e., M0), showed multiple areas of significant differences (Figure 2-A). Patients failed to show task-related signal decreases evident in controls in peri- posterior branch of right lateral sulcus cortex, left pericentral cortex, bilateral dorsal cingulate gyri, right pericentral cortex, posterior part of left middle temporal gyrus extending to angular gyrus and bilateral inferior parts of lingual gyrus and cuneus. Patients showed greater signal decreases than controls in left parahippacampal gyrus and adjacent areas and left middle temporal gyrus. Controls showed greater signal increases than patients in left thalamus, right parahippacampal gyrus and adjacent areas, and the lingual gyri bilaterally extending to the fusiform gyri (Table 3).
Figure 2.
The patients-controls differences of task-related BOLD signal increases. A showed the areas with group differences before adjustments with p-corrected ≤ 0.05 (p ≤ 0.01, cluster ≥ 38); B showed the areas with p-corrected ≤ 0.05 (p ≤ 0.01, cluster ≥ 38) after determining and applying the best model incorporating resting CBF for each voxel.
Table 3.
The areas with group different face-related BOLD signal increases without adjustment
| Region | Centroid MNI | Volume (mm3) | T-score | T-score | T-score | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| x | y | z | Contrast(Patients-Controls)* | Patients | Controls | ||
| Cortex peri- right lateral sulcus, posterior branch | 49 | −26 | 14 | 7253 | 3.20 ± 0.50 | −0.33 ± 2.35 | −4.40 ± 2.14 |
| Left pericentral cortex | −47 | −7 | 31 | 1715 | 3.03 ± 0.27 | 0.69 ± 2.96 | −3.22 ± 2.52 |
| Bilateral dorsal cingulate gyrus | 3 | −10 | 40 | 12538 | 3.42 ± 0.66 | 2.29 ± 2.06 | −2.70 ± 1.97 |
| Right pericentral cortex | 37 | −29 | 48 | 6620 | 3.61 ± 0.67 | 0.02 ± 2.40 | −4.48 ± 1.97 |
| Posterior part of left middle temporal gyrus extending to angular gyrus | −42 | −66 | 16 | 4722 | 2.91 ± 0.23 | −1.25 ± 1.73 | −5.82 ± 1.96 |
| Bilateral inferior part of lingual gyrus and cuneus | 1 | −69 | 17 | 27273 | 3.38 ± 0.63 | 1.16 ± 1.97 | −3.51 ± 1.68 |
| Left thalamus | −19 | −16 | 3 | 4344 | −3.17 ± 0.42 | −2.00 ± 0.96 | 2.53 ± 1.31 |
| Right parahippacampal gyrus and adjacent areas | 27 | −26 | −11 | 2073 | −3.29 ± 0.49 | −2.63 ± 1.64 | 1.89 ± 1.76 |
| Right lingual gyrus extending to fusiform gyrus | 26 | −88 | −6 | 9801 | −3.72 ± 0.83 | 5.21 ± 2.80 | 9.18 ± 2.26 |
| Left lingual gyrus extending to fusiform gyrus | −21 | −91 | −6 | 6086 | −3.22 ± 0.49 | 7.48 ± 3.28 | 9.15 ± 2.21 |
| Left parahippacampal gyrus and adjacent areas | −29 | −24 | −18 | 1870 | −2.94 ± 0.24 | −4.00 ± 0.67 | −0.90 ± 0.88 |
| Left middle temporal gyrus | −49 | −2 | −21 | 1204 | −3.19 ± 0.40 | −4.85 ± 0.92 | −1.13 ± 0.81 |
p-corrected ≤ 0.05 (p ≤ 0.01, cluster ≥ 38)
When group difference was considered after determining and applying the best model incorporating resting CBF data for each voxel (i.e., Model 0, 1, or 2, Figure 2-B), four areas were identified in which patients had greater task-related increases in BOLD than controls: right inferior frontal cortex, left insula extending to putamen, left middle frontal cortex and bilateral frontal poles (Fig 2-c, Table 4). Three of the four newly detected areas of significantly greater signal increase in patients showed numerically greater but statistically non-significant greater signal in patients in unadjusted Model 0. In the area in bilateral frontal poles, patients had non-significantly lower signal increases than controls in the unadjusted maps but significantly greater activity than controls when resting CBF was considered. In other areas, introduction of resting CBF led to changes in shape and size of group differences. (Figure 2-B, Table 5).
Table 4.
The newly areas with group different face-related BOLD signal increases with adjustment
| Rengion | Centroid MNI | Volume (mm3) | T-score* | Signal increases | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| x | y | z | Adjusted | Unadjusted | Patients | Controls | ||
| Right inferior frontal gyrus | 42 | 29 | −5 | 1292 | 3.20 ± 0.44 | 1.12 ± 0.64 | 0.00 ± 0.02 | −0.03 ± 0.02 |
| Left insula extending to putamen | −31 | 8 | −1 | 1068 | 3.13 ± 0.35 | 1.29 ± 0.45 | −0.01 ± 0.03 | −0.04 ± 0.02 |
| Left middle frontal gyrus | −29 | 27 | 28 | 1753 | 3.15 ± 0.40 | 1.21 ± 0.76 | 0.02 ± 0.03 | −0.02 ± 0.04 |
| Bilateral frontal poles | 17 | 58 | −7 | 1737 | 3.09 ± 0.39 | −0.48 ± 1.13 | −0.11 ± 0.10 | −0.07 ± 0.19 |
p-corrected ≤ 0.05 (p ≤ 0.01, cluster ≥ 8)
Table 5.
The overlay areas with group different task-related BOLD signal increases with and without adjustment
| Region | Centroid MNI (x,y,z) | Volume (mm3) | Contrasts* | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | ||||||
| Cortex peri- right lateral sulcus, posterior branch | 49 | −26 | 14 | 50 | −24 | 14 | 7253 | 6525 | pts>ctr |
| Left pericentral cortex | −47 | −7 | 31 | −51 | −6 | 28 | 1715 | 1172 | pts>ctr |
| Bilateral dorsal cingulate gyrus | 3 | −10 | 40 | 4 | −10 | 41 | 12538 | 12344 | pts>ctr |
| Right pericentral cortex | 37 | −29 | 48 | 36 | −29 | 48 | 6620 | 7461 | pts>ctr |
| Posterior part of left middle temporal gyrus extending to angular gyrus | −42 | −66 | 16 | −44 | −63 | 17 | 4722 | 3401 | pts>ctr |
| Bilateral inferior part of lingual gyrus and cuneus | 1 | −69 | 17 | 2 | −69 | 17 | 27273 | 29810 | pts>ctr |
| Left thalamus | −19 | −16 | 3 | −19 | −17 | 2 | 4344 | 4845 | pts<ctr |
| Right parahippacampal gyrus and adjacent areas | 27 | −26 | −11 | 27 | −26 | −11 | 2073 | 2040 | pts<ctr |
| Right lingual gyrus extending to fusiform gyrus | 26 | −88 | −6 | 26 | −88 | −6 | 9801 | 11290 | pts<ctr |
| Left lingual gyrus extending to fusiform gyrus | −21 | −91 | −6 | −19 | −91 | −5 | 6086 | 6012 | pts<ctr |
| Left parahippacampal gyrus and adjacent areas | −29 | −24 | −18 | −21 | −11 | −17 | 1870 | 2422 | pts<ctr |
| Left middle temporal gyrus | −49 | −2 | −21 | −50 | −1 | −23 | 1204 | 1248 | pts<ctr |
p-corrected ≤ 0.05 (p ≤ 0.01, cluster ≥ 38)
4. Discussions
Patients showed significantly abnormal resting CBF in the left basal ganglion and bilateral thalami. The relationship between resting CBF and task-related BOLD signal increases was significant in multiple brain areas and was also different in patients than controls in multiple areas. Considering resting CBF when comparing task-related BOLD signal changes in patients and controls led to identification of additional areas of significant group differences.
Abnormalities in resting CBF measured with radioactive tracers have been reported repeately in patients with schizophrenia since 1974(Davidson and Heinrichs, 2003; Hill et al., 2004; Ingvar and Franzen, 1974). Recently, Horn et al. used ASL techniques similar to ours and also found a generalized but non-significant decrease of global CBF in schizophrenia (Horn et al., 2009). We but not Horn et al found statistically significant reductions in the thalami bilaterally and increases in left basal ganglion. This difference between studies is probably due to greater sensitivity of the 3.0-Tesla scanner used in the present study (Golay and Petersen, 2006). In another study using continuous ASL (CASL), the decreases of resting CBF in patients were significant in still more areas (Scheef et al., 2010). CASL which uses continuous radio frequency may yield higher signal-to-noise ratio than the PASL we used. However, it is important to note that the specific techniques of PASL used in the present study have been shown to produce findings closely matching PET findings in the same participants (Qiu et al., 2010). Another difference is that chronic schizophrenia patients with long-time antipsychotic medication were enrolled in the present study, while unmedicated patients earlier in the course of illness were studied by Scheef et al. Some studies have reported that antipsychotic drugs modify regional CBF, however, it is still difficult to draw specific conclusions because of complexity of the effect itself and associated methodological challenges(Vita and De Peri, 2007).
The particular areas of significantly abnormal resting CBF in the present study are of interest. Lower resting CBF is observed in thalami, which constitute a major subcortical switchboard crucial for processing among sensory organs, subcortical areas and cortexes. Structural and functional abnormalities of thalamus in schizophrenia have been found in many studies (Alelu-Paz and Gimenez-Amaya, 2008; Byne et al., 2009; Cronenwett and Csernansky, 2010; Pinault, 2011; Tomelleri et al., 2009), and dysfunctions of processing networks that include the thalami have also been previously reported in schizophrenia, including thalamocorticostriatal circuit dysfunction associated with cognitive dysmetria (Andreasen et al., 1999; Pinault, 2011). Reversely, damage to the thalamus or thalamus-related networks could induce schizophrenia-like symptoms in otherwise healthy individuals (Crick, 1984; Cronenwett and Csernansky, 2010; Ferrarelli and Tononi, 2011). Patients had higher resting CBF of basal ganglia than controls. Basal ganglia are critical for regulating motor activity and for goal-oriented behavior and action selection, and are also involved in cognitive and emotional functions (Chakravarthy et al., 2010). Neural imaging studies have confirmed abnormal basal ganglia activations of schizophrenia in cognitive, emotional and reward-related processing (Li et al., 2010; Perez-Costas et al., 2010). The abnormal increase of basal ganglia resting CBF in patients may be the result of medications, as previous studies provide strong evidence suggesting this can be a direct effect of anti-psychotic medications (Corson et al., 2002; Lahti et al., 2003; Miller et al., 2001).
Resting CBF showed region-specific associations with task-related BOLD response. Although the mechanism of correlation of BOLD and resting CBF in these areas is not clear, changed base tone of local neurovasular units(Arima et al., 2006) may be important. In neurovascular units, astrocytes bridge neural activities and vascular responses. Higher concentration of vasoactive neurotransmitter (e.g. glutamate) and more receptors in local astrocytes (Filosa and Blanco, 2007), or lower concentration of inhibitory neurotransmitter(e.g. GABA) and fewer receptors in local astrocytes (Cauli et al., 2004; Kocharyan et al., 2008) could alter resting regional CBF. The balance between activitating and inhibiting vasoactive neurotransmitter receptors may also alter sensitivity of neurovasular units to behavior responses. Additionally, non-specific agents in the local microenviroment such as nitric oxide synthase andarachidonic acid derivatives, which can alter vascular tone(Akgoren et al., 1994; Straub and Nelson, 2007), may be the possible causes as well.
Interactions of resting CBF and task-related BOLD response were different in patients and controls, primarily in prefrontal and occipitotemporal regions(Figure 1-B). In these areas, positive and negative relationships between resting CBF amd BOLD response were weaker (smaller absolute value of parameters) across patients than across controls. Abnormal BOLD responses have previously been identified in these regions in patients with schizophrenia (Gur et al., 2002; Habel et al., 2010; Li et al., 2012; Quintana et al., 2011; Seiferth et al., 2009; Singer et al., 2004). This abnormality in the relationship between resting CBF and BOLD responsivity appears to be independent of differences in resting CBF itself; the abnormal relationships were not in the areas of greatest differernce between groups in resting CBF (i.e., thalami and left basal ganglion). Disruption of the normal relationship between resting and dynamic aspects of regional blood flow is most likely another reflection of pathological changes in neurovascular units including abnormalities in glutamate, GABA, and dopamine (Benes and Berretta, 2001; Kantrowitz and Javitt, 2012; Schmidt and Mirnics, 2012; Shen et al., 2012) and anatomic injuries of vessels per se(Uranova et al., 2010), which may affect both baseline and dynamic vascular responsivity.
When the patient-control comparison of BOLD response was adjusted for effects of individual differences in resting CBF and for differences between the patient and control groups in the relationship between resting CBF and change in BOLD, four additional areas of difference became evident: left insula extending to putamen, right inferior frontal cortext, left middle frontal cortex and bilateral frontal poles. The four areas have previously been reported to be associated with emotions, social inference or social interactions (Izuma et al., 2008; Li et al., 2012; Lieberman, 2007; Singer et al., 2004; Sprengelmeyer et al., 1998; Surguladze et al., 2011), and abnormalities of similar areas have been reported in emotion- or social-related studies of schizophrenia (Bogerts, 1997; Chakravarthy et al., 2010; Gur et al., 2002; Li et al., 2012; Pinault, 2011; Quintana et al., 2011; Surguladze et al., 2011). The previous findings of patient abnormalities in the same areas where significant group differences emerged in our study after including resting CBF differences in the model suggest that including measurement of resting CBF increases sensitivity of fMRI.
The interactions of resting CBF and BOLD response provide new information about schizophrenia pathology, however, the mechanism of the relationship is not clear, and other methods are needed to more fully characterize the pathololgies of arterioles, venules and capillaries and regulation of vascular response to neural activaion. This relationship may be altered in more regions than we detected because we could detect it only in areas with enough variations across subjects in resting CBF and BOLD response, and because our scanning did note extend fully to the top of the brain.
In summary, ASL imaging to assess resting CBF can be done along with fMRI to increase sensitivity of the fMRI BOLD imaging and identify additional aspects of brain pathology. In addition to the well established abnormalities in task-related brain activation and in resting CBF, the relationship between resting CBF and task-related changes in blood flow may be altered. These pathologies are widely distributed throughout the brain but are more prominent in some areas than others.
Supplementary Material
Acknowledgments
Role of the funding source
This study was supported by National Institute of Health grants to Dr. Wexler (R01MH084079 and KO2 MH01296).
The authors wish to thank Dr. Silvia Corbera for her help in subject enrollment and Karen Martin, Cheryl Lea McMurray and Hickey Terry in MRI data collection. The authors also would like to thank Cheryl Lacadie for giving instruction to Bioimagesuite.
Footnotes
Conflict of interest
None of the authors have financial relationships with commercial interests related to the work reported.
Financial Disclosures
None of the authors have financial relationships with commercial interests related to the work reported.
Contributors
Jiacheng Liu, MD & PhD
Dr. Liu was the main person to work on the whole project including data collection, analyzing the data and summarizing the findings.
Maolin Qiu, PhD
Dr. Qiu set up the MRI sequences and the parameter. He also provided technique support for data analysis.
R. Todd Constable, PhD
Dr. Constable worked as technique consultant and reviewed the manuscript.
Bruce E. Wexler, MD
Dr. Wexler was PI and pivotal in the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akgoren N, Fabricius M, Lauritzen M. Importance of nitric oxide for local increases of blood flow in rat cerebellar cortex during electrical stimulation. Proc Natl Acad Sci U S A. 1994;91(13):5903–5907. doi: 10.1073/pnas.91.13.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alelu-Paz R, Gimenez-Amaya JM. The mediodorsal thalamic nucleus and schizophrenia. J Psychiatry Neurosci. 2008;33(6):489–498. [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46(7):908–920. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima H, Tzourio C, Butcher K, Anderson C, Bousser MG, Lees KR, Reid JL, Omae T, Woodward M, MacMahon S, Chalmers J. Prior events predict cerebrovascular and coronary outcomes in the PROGRESS trial. Stroke. 2006;37(6):1497–1502. doi: 10.1161/01.STR.0000221212.36860.c9. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Berman KF, Zec RF, Weinberger DR. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. II. Role of neuroleptic treatment, attention, and mental effort. Arch Gen Psychiatry. 1986;43(2):126–135. doi: 10.1001/archpsyc.1986.01800020032005. [DOI] [PubMed] [Google Scholar]
- Bogerts B. The temporolimbic system theory of positive schizophrenic symptoms. Schizophr Bull. 1997;23(3):423–435. doi: 10.1093/schbul/23.3.423. [DOI] [PubMed] [Google Scholar]
- Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathol. 2009;117(4):347–368. doi: 10.1007/s00401-008-0404-0. [DOI] [PubMed] [Google Scholar]
- Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci. 2004;24(41):8940–8949. doi: 10.1523/JNEUROSCI.3065-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy VS, Joseph D, Bapi RS. What do the basal ganglia do? A modeling perspective. Biol Cybern. 2010;103(3):237–253. doi: 10.1007/s00422-010-0401-y. [DOI] [PubMed] [Google Scholar]
- Corson PW, O’Leary DS, Miller DD, Andreasen NC. The effects of neuroleptic medications on basal ganglia blood flow in schizophreniform disorders: a comparison between the neuroleptic-naive and medicated states. Biol Psychiatry. 2002;52(9):855–862. doi: 10.1016/s0006-3223(02)01421-x. [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81(14):4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronenwett WJ, Csernansky J. Thalamic pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:509–528. doi: 10.1007/7854_2010_55. [DOI] [PubMed] [Google Scholar]
- Davidson LL, Heinrichs RW. Quantification of frontal and temporal lobe brain-imaging findings in schizophrenia: a meta-analysis. Psychiatry Res. 2003;122(2):69–87. doi: 10.1016/s0925-4927(02)00118-x. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37(2):306–315. doi: 10.1093/schbul/sbq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Blanco VM. Neurovascular coupling in the mammalian brain. Experimental physiology. 2007;92(4):641–646. doi: 10.1113/expphysiol.2006.036368. [DOI] [PubMed] [Google Scholar]
- Golay X, Petersen ET. Arterial spin labeling: benefits and pitfalls of high magnetic field. Neuroimaging Clin N Am. 2006;16(2):259–268. doi: 10.1016/j.nic.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Gur RE, McGrath C, Chan RM, Schroeder L, Turner T, Turetsky BI, Kohler C, Alsop D, Maldjian J, Ragland JD, Gur RC. An fMRI study of facial emotion processing in patients with schizophrenia. Am J Psychiatry. 2002;159(12):1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- Habel U, Chechko N, Pauly K, Koch K, Backes V, Seiferth N, Shah NJ, Stocker T, Schneider F, Kellermann T. Neural correlates of emotion recognition in schizophrenia. Schizophr Res. 2010;122(1–3):113–123. doi: 10.1016/j.schres.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Hill K, Mann L, Laws KR, Stephenson CM, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004;110(4):243–256. doi: 10.1111/j.1600-0447.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Horn H, Federspiel A, Wirth M, Muller TJ, Wiest R, Wang JJ, Strik W. Structural and metabolic changes in language areas linked to formal thought disorder. Br J Psychiatry. 2009;194(2):130–138. doi: 10.1192/bjp.bp.107.045633. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Shinba T, Sato C, Doi N. Resting hypofrontality in schizophrenia: A study using near-infrared time-resolved spectroscopy. Schizophr Res. 2006;84(2–3):411–420. doi: 10.1016/j.schres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Ingvar DH, Franzen G. Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta Psychiatr Scand. 1974;50(4):425–462. doi: 10.1111/j.1600-0447.1974.tb09707.x. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58(2):284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Kanahara N, Shimizu E, Sekine Y, Uchida Y, Shibuya T, Yamanaka H, Hashimoto T, Asaka T, Sasaki T, Miyatake R, Ohkami T, Fukami G, Fujisaki M, Watanabe H, Shirayama Y, Hayashi H, Hashimoto K, Asano M, Iyo M. Does hypofrontality expand to global brain area in progression of schizophrenia?: a cross-sectional study between first-episode and chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):410–415. doi: 10.1016/j.pnpbp.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Kantrowitz J, Javitt DC. Glutamatergic transmission in schizophrenia: from basic research to clinical practice. Curr Opin Psychiatry. 2012;25(2):96–102. doi: 10.1097/YCO.0b013e32835035b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocharyan A, Fernandes P, Tong XK, Vaucher E, Hamel E. Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J Cereb Blood Flow Metab. 2008;28(2):221–231. doi: 10.1038/sj.jcbfm.9600558. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol Psychiatry. 2003;53(7):601–608. doi: 10.1016/s0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- Li H, Chan RC, McAlonan GM, Gong QY. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophr Bull. 2010;36(5):1029–1039. doi: 10.1093/schbul/sbn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Chan RC, Gong QY, Liu Y, Liu SM, Shum D, Ma ZL. Facial emotion processing in patients with schizophrenia and their non-psychotic siblings: A functional magnetic resonance imaging study. Schizophr Res. 2012;134(2–3):143–150. doi: 10.1016/j.schres.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Miller DD, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD. Comparison of the effects of risperidone and haloperidol on regional cerebral blood flow in schizophrenia. Biol Psychiatry. 2001;49(8):704–715. doi: 10.1016/s0006-3223(00)01001-5. [DOI] [PubMed] [Google Scholar]
- Perez-Costas E, Melendez-Ferro M, Roberts RC. Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J Neurochem. 2010;113(2):287–302. doi: 10.1111/j.1471-4159.2010.06604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault D. Dysfunctional thalamus-related networks in schizophrenia. Schizophr Bull. 2011;37(2):238–243. doi: 10.1093/schbul/sbq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M, Paul Maguire R, Arora J, Planeta-Wilson B, Weinzimmer D, Wang J, Wang Y, Kim H, Rajeevan N, Huang Y, Carson RE, Constable RT. Arterial transit time effects in pulsed arterial spin labeling CBF mapping: insight from a PET and MR study in normal human subjects. Magn Reson Med. 2010;63(2):374–384. doi: 10.1002/mrm.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M, Ramani R, Swetye M, Rajeevan N, Constable RT. Anesthetic effects on regional CBF, BOLD, and the coupling between task-induced changes in CBF and BOLD: an fMRI study in normal human subjects. Magn Reson Med. 2008;60(4):987–996. doi: 10.1002/mrm.21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana J, Lee J, Marcus M, Kee K, Wong T, Yerevanian A. Brain dysfunctions during facial discrimination in schizophrenia: selective association to affect decoding. Psychiatry Res. 2011;191(1):44–50. doi: 10.1016/j.pscychresns.2010.09.005. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. [Google Scholar]
- Scheef L, Manka C, Daamen M, Kuhn KU, Maier W, Schild HH, Jessen F. Resting-state perfusion in nonmedicated schizophrenic patients: a continuous arterial spin-labeling 3.0-T MR study. Radiology. 2010;256(1):253–260. doi: 10.1148/radiol.10091224. [DOI] [PubMed] [Google Scholar]
- Schmidt MJ, Mirnics K. Modeling Interneuron Dysfunction in Schizophrenia. Dev Neurosci. 2012 doi: 10.1159/000336731. [DOI] [PubMed] [Google Scholar]
- Seiferth NY, Pauly K, Kellermann T, Shah NJ, Ott G, Herpertz-Dahlmann B, Kircher T, Schneider F, Habel U. Neuronal correlates of facial emotion discrimination in early onset schizophrenia. Neuropsychopharmacology. 2009;34(2):477–487. doi: 10.1038/npp.2008.93. [DOI] [PubMed] [Google Scholar]
- Shen LH, Liao MH, Tseng YC. Recent advances in imaging of dopaminergic neurons for evaluation of neuropsychiatric disorders. J Biomed Biotechnol. 2012;2012:1–14. doi: 10.1155/2012/259349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Kiebel SJ, Winston JS, Dolan RJ, Frith CD. Brain responses to the acquired moral status of faces. Neuron. 2004;41(4):653–662. doi: 10.1016/s0896-6273(04)00014-5. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proc Biol Sci. 1998;265(1409):1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub SV, Nelson MT. Astrocytic calcium signaling: the information currency coupling neuronal activity to the cerebral microcirculation. Trends in cardiovascular medicine. 2007;17(6):183–190. doi: 10.1016/j.tcm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, Chu EM, Marshall N, Evans A, Anilkumar AP, Timehin C, McDonald C, Ecker C, Phillips ML, David AS. Emotion processing in schizophrenia: fMRI study of patients treated with risperidone long-acting injections or conventional depot medication. J Psychopharmacol. 2011;25(6):722–733. doi: 10.1177/0269881110363316. [DOI] [PubMed] [Google Scholar]
- Tomelleri L, Jogia J, Perlini C, Bellani M, Ferro A, Rambaldelli G, Tansella M, Frangou S, Brambilla P. Brain structural changes associated with chronicity and antipsychotic treatment in schizophrenia. Eur Neuropsychopharmacol. 2009;19(12):835–840. doi: 10.1016/j.euroneuro.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Zimina IS, Vikhreva OV, Krukov NO, Rachmanova VI, Orlovskaya DD. Ultrastructural damage of capillaries in the neocortex in schizophrenia. World J Biol Psychiatry. 2010;11(3):567–578. doi: 10.3109/15622970903414188. [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L. The effects of antipsychotic treatment on cerebral structure and function in schizophrenia. Int Rev Psychiatry. 2007;19(4):429–436. doi: 10.1080/09540260701486332. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF. Speculation on the meaning of cerebral metabolic hypofrontality in schizophrenia. Schizophr Bull. 1988;14(2):157–168. doi: 10.1093/schbul/14.2.157. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43(2):114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Halwes T. Increasing the power of dichotic methods: the fused rhymed words test. Neuropsychologia. 1983;21(1):59–66. doi: 10.1016/0028-3932(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Okazawa H, Kishibe Y, Sugimoto K, Takahashi M. The effect of acetazolamide on the changes of cerebral blood flow and oxygen metabolism during visual stimulation. Neuroimage. 2003;20(1):543–549. doi: 10.1016/s1053-8119(03)00283-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.