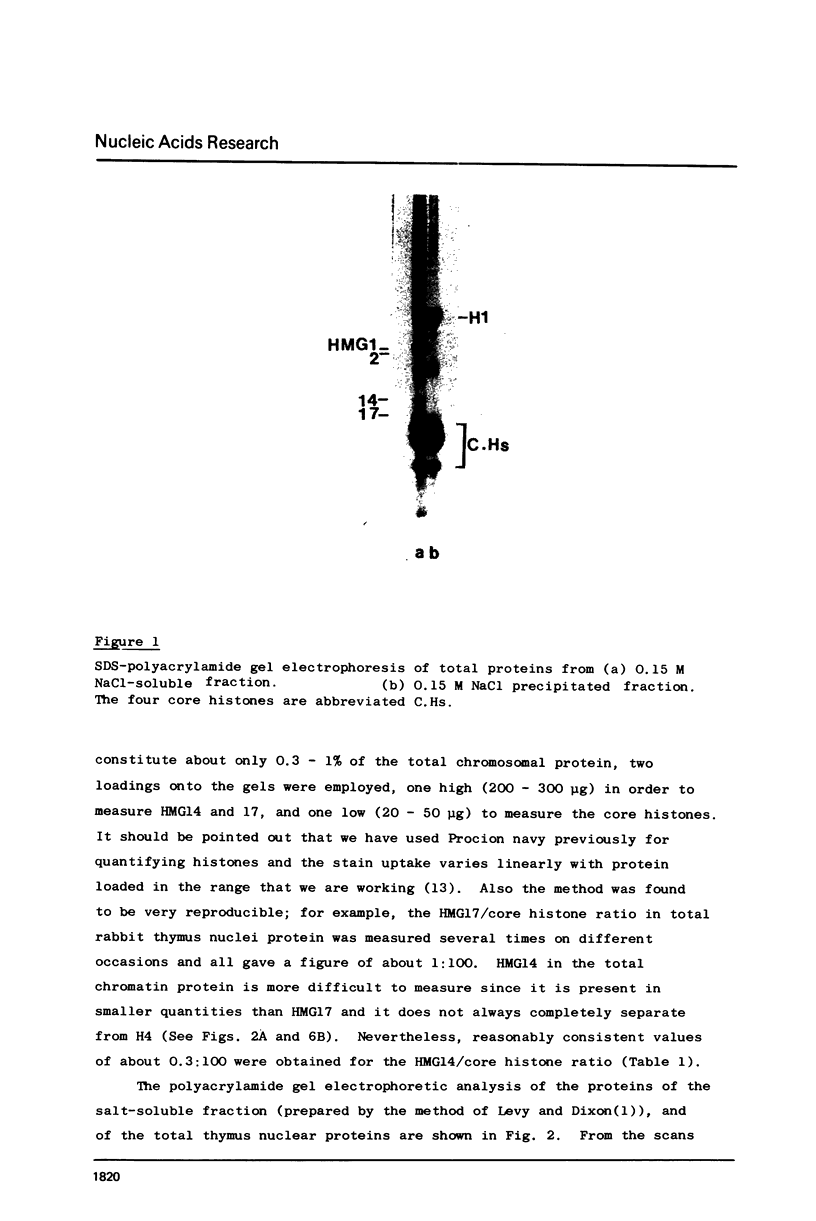
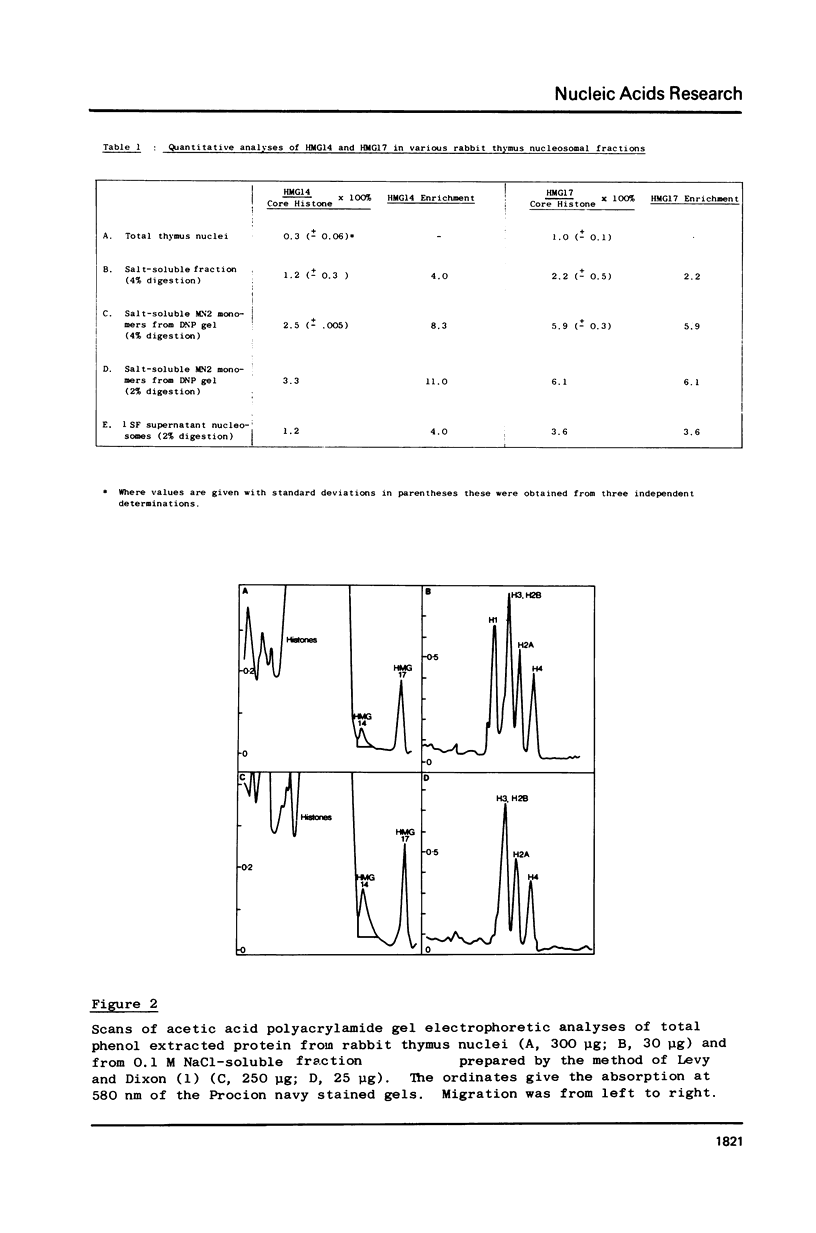
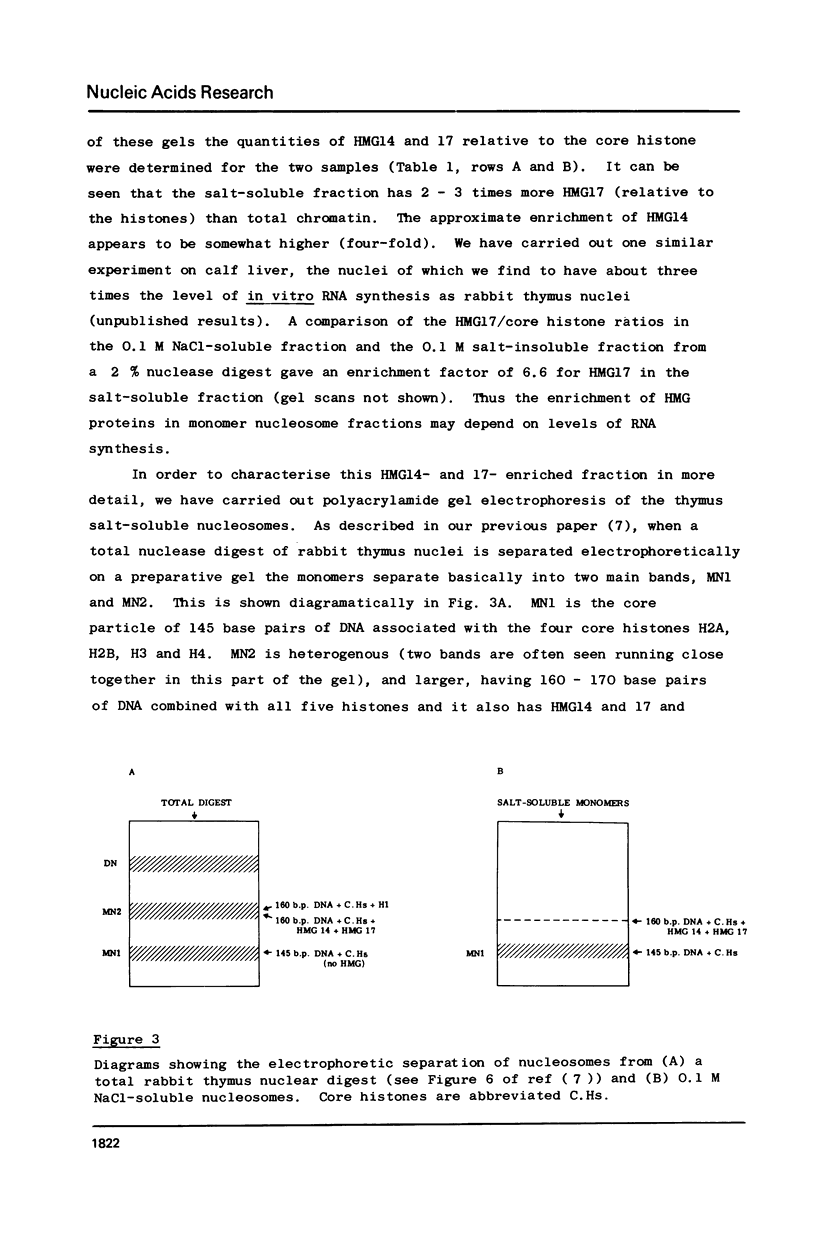
Abstract
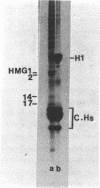
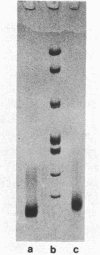
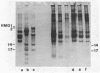
Two methods have recently been described for the isolation of monomer nucleosomes enriched in transcribed sequences which depend on their solubility in 0.1 M NaCl (Levy, W.B. and Dixon (1978), Nucleic Acid Res., 5, 4155-4163) or solutions containing divalent metal ions (Bloom, K.S. and Anderson, J.N. (1978), Cell, 15, 141-150). Using these procedures the proteins associated with such nucleosomes from rabbit thymus, calf liver and hen oviduct nuclei were isolated and analysed. Increased amounts of proteins HMG14 AND HMG17 and small amounts of HMG1 and HMG2 were found associated with the four core histones H2A, H2B, H3 and H4 in these nucleosomes. HMG14 and HMG17 were found to be enriched 2 - 7 fold, suggesting an involvement of these two proteins with transcribed sequences. 0.1 M NaCl-soluble monomer nucleosomes prepared by the method of Levy and Dixon were analysed by polyacrylamide gel electrophoresis and found to be composed of principally two types of particle: 1. Core particles of 145 base pairs of DNA associated with the four core histones only. 2. Nucleosomes with 160 base pairs of DNA associated with the four core histones, increased amounts of HMG14 and 17, and no H1. Small amounts of HMG1 and HMG2 are also detected. These results suggest that HMG14 and HMG17 might be interacting with the 15 base pair linker DNA. A model is presented for the structure of transcriptionally active chromatin.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie B. D., Kneale G. G., Crane-Robinson C., Bradbury E. M., Goodwin G. H., Walker J. M., Johns E. W. Studies on the conformational properties of the high-mobility-group chromosomal protein HMG 17 and its interaction with DNA. Eur J Biochem. 1978 Mar;84(1):173–177. doi: 10.1111/j.1432-1033.1978.tb12154.x. [DOI] [PubMed] [Google Scholar]
- Bakayev V. V., Bakayeva T. G., Schmatchenko V. V., Georgiev G. P. Non-histone proteins in mononucleosomes and subnucleosomes. Eur J Biochem. 1978 Nov 2;91(1):291–301. doi: 10.1111/j.1432-1033.1978.tb20965.x. [DOI] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Carpenter B. G., Baldwin J. P., Bradbury E. M., Ibel K. Organisation of subunits in chromatin. Nucleic Acids Res. 1976 Jul;3(7):1739–1746. doi: 10.1093/nar/3.7.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H., Johns E. W. Are the high mobility group non-histone chromosomal proteins associated with 'active' chromatin? Biochim Biophys Acta. 1978 Jun 22;519(1):279–284. doi: 10.1016/0005-2787(78)90081-3. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Nicolas R. H., Johns E. W. A quantitative analysis of histone H1 in rabbit thymus nuclei. Biochem J. 1977 Nov 1;167(2):485–488. doi: 10.1042/bj1670485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H., Woodhead L., Johns E. W. The presence of high mobility group non-histone chromatin proteins in isolated nucleosomes. FEBS Lett. 1977 Jan 15;73(1):85–88. [PubMed] [Google Scholar]
- Hartman P. G., Chapman G. E., Moss T., Bradbury E. M. Studies on the role and mode of operation of the very-lysine-rich histone H1 in eukaryote chromatin. The three structural regions of the histone H1 molecule. Eur J Biochem. 1977 Jul 1;77(1):45–51. doi: 10.1111/j.1432-1033.1977.tb11639.x. [DOI] [PubMed] [Google Scholar]
- Levy-Wilson B., Dixon G. H. Limited action of micrococcal nuclease on trout testis nuclei generates two mononucleosome subsets enriched in transcribed DNA sequences. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1682–1686. doi: 10.1073/pnas.76.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. W., Connor W., Dixon G. H. A subset of trout testis nucleosomes enriched in transcribed DNA sequences contains high mobility group proteins as major structural components. J Biol Chem. 1979 Feb 10;254(3):609–620. [PubMed] [Google Scholar]
- Levy B., Dixon G. H. Partial purification of transcriptionally active nucleosomes from trout testis cells. Nucleic Acids Res. 1978 Nov;5(11):4155–4163. doi: 10.1093/nar/5.11.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew C. G., Goodwin G. H., Johns E. W. Studies on the association of the high mobility group non-histone chromatin proteins with isolated nucleosomes. Nucleic Acids Res. 1979 Jan;6(1):167–179. doi: 10.1093/nar/6.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Carlson R. D., Wright E. B., Olins D. E. Chromatin nu bodies: isolation, subfractionation and physical characterization. Nucleic Acids Res. 1976 Dec;3(12):3271–3291. doi: 10.1093/nar/3.12.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Rill R., Van Holde K. E. Properties of nuclease-resistant fragments of calf thymus chromatin. J Biol Chem. 1973 Feb 10;248(3):1080–1083. [PubMed] [Google Scholar]
- Simpson R. T. Structure of the chromatosome, a chromatin particle containing 160 base pairs of DNA and all the histones. Biochemistry. 1978 Dec 12;17(25):5524–5531. doi: 10.1021/bi00618a030. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Felsenfeld G. Pancreatic DNAase cleavage sites in nuclei. Cell. 1977 Mar;10(3):537–547. doi: 10.1016/0092-8674(77)90040-x. [DOI] [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. Overall pathway of mononucleosome production. J Biol Chem. 1979 Apr 25;254(8):3074–3083. [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. Two-dimensional electrophoretic analysis of polynucleosomes. J Biol Chem. 1977 Jul 10;252(13):4729–4738. [PubMed] [Google Scholar]
- Villeponteaux B., Lasky L., Harary I. Lysine-rich histones and the selective digestion of the globin gene in avian red blood cells. Biochemistry. 1978 Dec 12;17(25):5532–5536. doi: 10.1021/bi00618a031. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Goodwin G. H., Johns E. W. The primary structure of the nucleosome-associated chromosomal protein HMG 14. FEBS Lett. 1979 Apr 15;100(2):394–398. doi: 10.1016/0014-5793(79)80378-6. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Hastings J. R., Johns E. W. The primary structure of a non-histone chromosomal protein. Eur J Biochem. 1977 Jun 15;76(2):461–468. doi: 10.1111/j.1432-1033.1977.tb11616.x. [DOI] [PubMed] [Google Scholar]
- Watson D. C., Peters E. H., Dixon G. H. The purification, characterization and partial sequence determination of a trout testis non-histone protein, HMG-T. Eur J Biochem. 1977 Mar 15;74(1):53–60. doi: 10.1111/j.1432-1033.1977.tb11365.x. [DOI] [PubMed] [Google Scholar]
- Watson D. C., Wong N. C., Dixon G. H. The complete amino-acid sequence of a trout-testis non-histone protein, H6, localized in a subset of nucleosomes and its similarity to calf-thymus non-histone proteins HMG-14 and HMG-17. Eur J Biochem. 1979 Mar 15;95(1):193–202. doi: 10.1111/j.1432-1033.1979.tb12953.x. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener S., Urivetzky M., Lendvai S., Shafer S., Meilman E. The indole method for determination of DNA: conditions for maximal sensitivity. Anal Biochem. 1976 Apr;71(2):579–582. doi: 10.1016/s0003-2697(76)80027-9. [DOI] [PubMed] [Google Scholar]