Abstract
Purpose
Radiation-induced gastric bleeding has been poorly understood. In this study, we describe dosimetric predictors for gastric bleeding after fractionated radiotherapy and compare several predictive models.
Materials & Methods
The records of 139 sequential patients treated with 3-dimensional conformal radiotherapy (3D-CRT) for intrahepatic malignancies between January 1999 and April 2002 were reviewed. Median follow-up was 7.4 months. Logistic regression and Lyman normal tissue complication probability (NTCP) models for the occurrence of ≥ grade 3 gastric bleed were fit to the data. The principle of maximum likelihood was used to estimate parameters for all models.
Results
Sixteen of 116 evaluable patients (14%) developed gastric bleeds, at a median time of 4.0 months (mean 6.5 months, range 2.1–28.3 months) following completion of RT. The median and mean of the maximum doses to the stomach were 61 and 63 Gy (range 46 Gy–86 Gy), respectively, after bio-correction to equivalent 2 Gy daily fractions. The Lyman NTCP model with parameters adjusted for cirrhosis was most predictive of gastric bleed (AUROC=0.92). Best fit Lyman NTCP model parameters were n =0.10, and m =0.21, with TD50(normal) =56 Gy and TD50(cirrhosis) = 22 Gy. The low n value is consistent with the importance of maximum dose; a lower TD50 value for the cirrhosis patients points out their greater sensitivity.
Conclusion
This study demonstrates that the Lyman NTCP model has utility for predicting gastric bleeding, and that the presence of cirrhosis greatly increases this risk. These findings should facilitate the design of future clinical trials involving high-dose upper abdominal radiation.
Keywords: NTCP, Gastric Bleed, Hepatic Irradiation, Complications
Introduction
Gastric bleeding is an important dose-limiting toxicity of radiation treatment for upper GI malignancies. Despite description of dose-volume-toxicity relationships for several normal tissues, including the lungs, liver, parotid glands, and pharyngeal constrictors over the past several years, recently summarized by the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) group,(1) there is still a paucity of data on the tolerance of the stomach to radiation. It is still common to rely on the tolerances published by Emami et al in 1991(2), which, although useful, were based on a consensus of opinions, rather than data. In this study, we characterize dose-volume effects for the stomach, specifically addressing clinically significant gastric bleeding.
One of the reasons for poor understanding of gastric bleeding risks is the lack of detailed dosimetric data, especially in the higher dose range, as, traditionally, doses above approximately 50–54 Gy are rarely used in the abdomen. Over the past 20 years, we have treated several hundred patients with dose-escalated radiotherapy for intrahepatic malignancies. A small number of patients with tumors in the left lobe of the liver developed gastric bleeding after completion of RT. All of these patients underwent full 3D planning and had a median survival of in excess of a year. Thus, this group of patients represents a unique data set for the quantification of the relationships between dose, volume, and risk of gastric bleeding. In addition to dose-volume parameters, we also assessed the effect of clinical parameters on the risk of bleeding.
Materials & Methods
Patients and toxicity
The records of 139 sequential patients who received three-dimensional conformal RT (3D-CRT) to partial liver volumes between January 1999 and April 2002 in the Department of Radiation Oncology at the University of Michigan were reviewed as part of an IRB-approved retrospective study. 116 patients treated for unresectable intrahepatic cancer (hepatocellular carcinoma, cholangiocarcinoma, or colorectal carcinoma metastatic to the liver) and adequate available dosimetric information, were included in this analysis. A gastric bleed event was defined as a gastric hemorrhage that required transfusion, or radiologic, endoscopic, or elective operative intervention (Grade ≥3 per CTCAE v4.0).
Radiotherapy
The median dose of radiation prescribed to the intrahepatic tumor was 54.0 Gy (range 12.0–90.5 Gy). Most patients were treated once daily with 5 fractions per week, 1.8–4.67 Gy per fraction. 55 patients were treated on a prospective clinical trial,(3) twice daily, 11 fractions per week, at 1.5–1.65 Gy per fraction, with a minimal inter-fraction interval of 4–6 hours. These patients received concurrent continuous infusion hepatic artery floxuridine (FUdR) during the first 4 weeks of RT, with a 2-week break in RT after 2 weeks to minimize the risk of complications related to the hepatic artery catheter used for chemotherapy delivery.
All patients were treated using 3D-CRT. Target and normal liver volumes were contoured on axial CT cuts, and the targets were expanded to account for occult disease and setup uncertainty. Most patients were treated utilizing active breathing control to eliminate respiratory-related target and normal tissue motion. For those who could not cooperate with this system, additional margin was added to account for respiratory motion. Treatment planning was performed using an in-house planning system, UMPLAN. Tumor dose was individualized, based on a nominal 10–15% risk of radiation-induced liver disease, as described previously.(4, 5)
Normal stomach DVHs were obtained for all 116 patients. The stomach volume was defined as the volume between the inner wall and outer wall contours. Using UMPLAN, the physical doses from the three-dimensional dose distributions of the treatment plans were converted to normalized iso-effective doses at 2.0 Gy per fraction using the linear quadratic model (α/β = 2.5 Gy) before computation of the DVHs.
Dose Variables
Mean dose (DMEAN) was calculated by summing the dose values for all voxels within the organ and dividing that by the total number of voxels. Maximum dose (DMAX) was defined as the minimum dose value in the highest 1cc of the organ wall dose volume histogram. The generalized equivalent uniform dose (gEUD) was determined based on the estimated Lyman NTCP model parameters, as described in the next section.
Models for Toxicity Risk Evaluation
Lyman NTCP Models
Maximum likelihood estimates (MLEs) of the Lyman normal tissue complication probability (NTCP) model parameters (TD50, m, and n) were computed as described previously in Dawson et al.(6) In brief, the Lyman NTCP model assumes, for a population of patients, a normal distribution of complications as a function of generalized equivalent uniform dose (gEUD). This distribution is characterized by a mean TD50 and a standard deviation m·TD50. Summation of that distribution with increasing gEUD produces a sigmoidal cumulative NTCP function for the population. Each patient’s gEUD was computed from their normalized iso-effective dose DVH via the generalized mean,(7)
where vi and di are the fractional volume and dose value of the ith dose bin of the direct DVH, and n is the Lyman NTCP model power law parameter. MLEs were computed for a model with a single TD50 parameter for all patients and for a model with separate TD50 parameters for cirrhotic and non-cirrhotic patients. 95% profile likelihood confidence intervals were determined for each parameter. The goodness of fit of the models was compared by means of a likelihood ratio test.
Logistic Regression Models
SAS v9.2 (SAS Institute, Cary, NC) was used for all logistic regression modeling. The parameters of logistic regression models for the presence or absence of gastric bleed as a function of mean dose, maximum dose and cirrhosis were fit by the method of maximum likelihood. 95% profile likelihood confidence intervals were determined for each parameter. Wald-type confidence intervals for the area under the observed ROC curve (AUROC) were calculated using standard errors calculated by the Mann-Whitney method. In exploratory analyses, stepwise logistic regression was used to determine if other demographic or clinical variables mediated the relationship between radiation dose (maximum dose, mean dose or Lyman NTCP) and gastric bleed.
Results
Patient Characteristics
The median follow-up for the 116 assessable patients was 7.4 months (range=0.2–61.5 months). Of these, a total of 16 patients (14%) developed gastric bleeds: 4/8 (50%) patients with cirrhosis and 12/108 (11%) patients without cirrhosis(Table 1). 11 patients had clinical risk factors for GI bleeding, including 2 patients with a pre-existing history of GI bleeds, 9 patients with portal hypertension, 2 with portal hypertensive gastropathy (PHG), 4 with gastric antral vascular ectasia (GAVE), and 5 with gastric varices. Of these patients, 6 with portal hypertension, 1 with PHG, 3 with GAVE, and 4 with varices had a gastric bleed after radiotherapy (Table 1).
Table 1.
Patient, Tumor, and Treatment Characteristics
| No Bleed (n=100) | Bleed (n=16) | All Patients | |
|---|---|---|---|
| Male | 61.0% | 62.5% | 61.2% |
| Caucasian | 88.0% | 93.8% | 88.8% |
| Cirrhosis | 4.0% | 25.0%* | 6.9% |
| Previous History of GI Bleeding | 8.0% | 12.5% | 8.6% |
| Prior NSAID use | 7.0% | 6.2% | 6.9% |
| Other Risk Factors for GI Bleeding | 6.0% | 31.2%* | 9.5% |
| GI Prophylaxis | 34.0% | 62.5%* | 37.9% |
| Histology | |||
| Hepatocellular Carcinoma | 31.0% | 43.8% | 32.8% |
| Cholangiocarcinoma | 36.0% | 43.8% | 37.1% |
| Metastatic | 30.0% | 12.5% | 27.6% |
| Other | 3.0% | 0.0% | 2.6% |
| Concurrent Chemotherapy | |||
| FUDR | 46.0% | 56.3% | 47.4% |
| Xeloda/5FU-LV | 2.0% | 12.5% | 3.5% |
| Maximum Stomach Dose (Gy) | 40.0±20.5† | 62.5±11.0* | 43.1±21.0 |
| Mean Stomach Dose (Gy) | 13.1±9.0 | 21.3±16.9 | 14.3±11.4 |
Indicates significant difference between patients with and without gastric complications (p<0.05) by either Fisher’s Exact Test or Wilcoxon Test.
Mean±Standard Deviation.
Regarding the timing of gastric bleeds, 14 of 16 occurred within 1 year of completion of radiotherapy, with median and mean times of 4.0 and 6.5 months (range: 2.1–28.3), respectively. Two bleeds occurred late, at 20.2 and 28.3 months (Figure 2). 6 of 16 patients with gastric bleeds required transfusions (median number of units 4, range 2–6). No patients died from gastric bleeding. Median and mean stomach maximum doses for patients with gastric bleeds were 61 and 63 Gy (range 46 Gy–86 Gy, std dev 11 Gy, Table 1), respectively, after bio-correction to equivalent 2 Gy daily fractions. For patients without gastric bleeds, median and mean stomach maximum doses were 43 and 40 Gy (range 0.3–88 Gy, std dev 21 Gy), respectively.
Figure 2.
Time to gastric bleed
For patients without cirrhosis, bleeds occurred after a mean maximum stomach dose of 66±10 Gy (range 53–86 Gy). Patients with cirrhosis experienced bleeds at a mean maximum stomach dose of 52±6 Gy (range 46–60). Of 8 total patients with cirrhosis, all 4 patients with stomach maximum dose ≥46 Gy developed a gastric bleed, while all 4 with a maximum dose ≤25 Gy did not.
Models Predicting Gastric Bleeding
Mean stomach dose, NTCP, and maximum stomach dose were calculated for all patients and plotted (Figure 1). Maximum likelihood estimates (MLEs) of Lyman-NTCP parameters and logistic regression model parameters for DMAX and DMEAN were calculated. MLEs of the Lyman-NTCP model parameters are TD50= 59 Gy, m = 0.30 and n = 0.09 (Lyman-NTCP Model 1 in Table 2). Writing π as the probability of gastric bleeding, the logistic regression MLE for maximum stomach dose is logit(π)=log(π/(1-π))=−5.79+0.075×DMAX, and the MLE for the logistic regression on mean stomach dose is logit(π)=−2.72+0.053×DMEAN (DMAX Logistic Regression Model 1 and DMEAN Logistic Regression Model 1 in Table 2). Based on the AUROC metric, the Lyman-NTCP model 1 and DMAX logistic regression model 1were similar in predicting gastric bleeding (Figure 3 and Table 2), while the DMEAN logistic regression had significantly (p<0.05) worse goodness of fit (although all three models were better than the intercept-only model, with p<0.02 for the weakest, DMEAN, model). The similar performance of logistic regression on DMAX and Lyman-NTCP models 1 is not surprising, given their high correlation (Spearman r=0.97) and the small estimated value of n in the latter model.
Figure 1.
Mean stomach dose (A), Lyman NTCP (B) and maximum stomach dose (C) of patients who did not (left) and did (right) experience gastric bleed. Both the raw data and boxplots are presented. The bottom and whiskers of the boxplots represent the 10th and 90th percentiles, respectively, the box edges are the first and third quartiles, and the center line is the median.
Figure 3.
ROC Curves for logistic regression on the mean stomach dose (A), Lyman-NTCP (B) and logistic regression on the maximum stomach dose (C). The black ROC curves do not account for cirrhosis, while the gray ROC curves do account for cirrhosis.
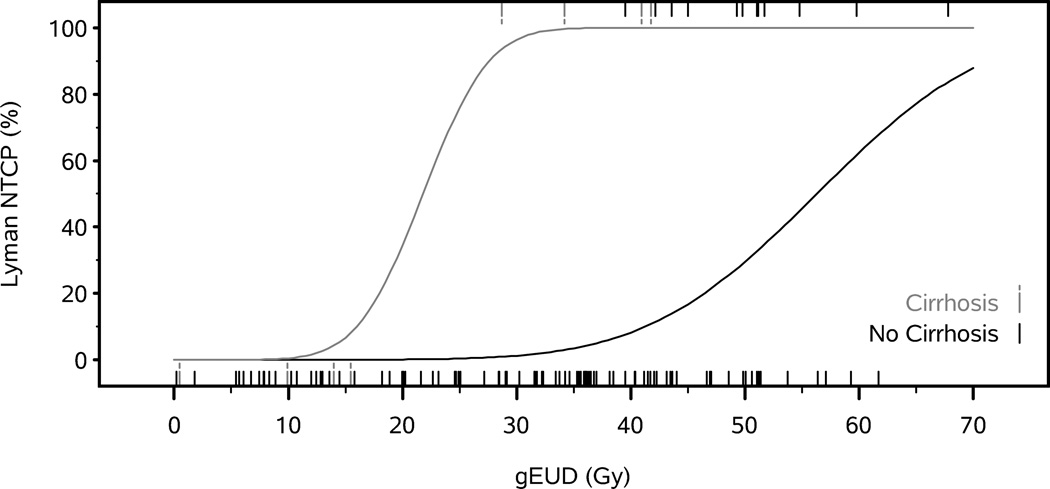
In the exploratory (model 1) stepwise logistic regression analysis, Lyman-NTCP, DMAX, DMEAN, age, sex, histology, use of either intrahepatic or systemic chemotherapy, previous history of ulcers or upper GI bleed, use of predisposing medications (aspirin or NSAIDs) or use of GI prophylactic medications (PPI or H2-blockers) were proposed as predictors of gastric bleed. Only Lyman-NTCP and cirrhosis were statistically significant (p<0.05) predictors; eliminating Lyman-NTCP from the list of candidates induced the selection of DMAX and cirrhosis. Based on these results, we re-fit the Lyman-NTCP model with separate TD50 estimates for non-cirrhotic and cirrhotic patients, and included non-cirrhotic and cirrhotic intercepts (analogous to shifting TD50) in the logistic regressions on DMAX and DMEAN (Lyman-NTCP, DMAX and DMEAN Models 2, Table 2). All three model 2s demonstrated significant improvement in the AUROC and goodness of fit compared to their model 1 counterparts that did not consider cirrhosis. The ROC curves of Lyman-NTCP, DMAX and DMEAN are presented in Figure 3. The Lyman NTCP and logistic regression DMAX models for patients with and without cirrhosis are presented in Figure 4. For patients without cirrhosis, a gEUD of 41 Gy is associated with a 10% NTCP for gastric bleed, while 46 Gy gives a 20% risk, and 56 Gy gives a 50% NTCP. For patients with cirrhosis, there is a steep curve, such that only 16 Gy gEUD is associated with a 10% NTCP for bleed, while 18 Gy gives 20% risk, and 22 Gy gives 50% risk.
Figure 4.
Estimated probability of gastric bleed as a function of maximum stomach dose (from logistic regression, A) and as a function of gEUD (from Lyman NTCP Model, B) for patients with (gray) and without (black) cirrhosis. Tick marks at bottom of graph indicate patients without gastric complications, while tick marks at top of graph indicate patients with gastric complications.
Discussion
In this study, we found that of the several models we evaluated, the Lyman NTCP model with parameters adjusted for cirrhosis was most prognostic for gastric bleeds. The maximum stomach dose was also prognostic, but with a slightly lower AUC. Mean dose to the stomach was not correlated with subsequent gastric bleeds.
To our knowledge, this is the largest and only collection of patients with detailed 3-dimensional dosimetric information, a wide range of radiation doses to the stomach, and close follow-up for gastric bleeding. Our new TD50s, m, and n values for the Lyman model suggest a lower stomach tolerance to radiation for gastric bleeding than previously estimated for ulceration/perforation,(8) especially for patients already prone to gastric bleeding at baseline. The response of the stomach to radiation resembles a serial organ structure for the endpoint of gastric bleeding.
Prior information on radiation-induced gastric bleeding has been sparse, mostly due to historic limitations placed on doses to structures in the upper abdomen. Although we, and a few others, have escalated radiation dose to liver tumors, dose escalation in other upper abdominal site has not been frequently attempted. Ceha, et al, reported a phase II study for unresectable pancreatic cancer, (9) during which 41 patients received 70–72 Gy to the primary tumor, with dose limited to 50 Gy over 5 weeks to the stomach. Two patients had endoscopically confirmed grade 3 gastric bleeding and 1 patient had a fatal GI bleed with blood seen in the stomach without progression of the primary tumor. In a small retrospective review by Haque, et al, which describes 13 patients treated with two courses of radiotherapy, separated by an average of 2 years,(10) one patient developed grade 4 bleeding from the gastrojejunal anastomosis, which had received a cumulative maximum dose of 63 Gy. In our population, the presence of cirrhosis dramatically increased the risk of a gastric bleed. Patients with cirrhosis are at risk for gastric bleeding at baseline, due to a number of factors. Portal hypertensive gastropathy (PHG), found in 20–80% of patients with cirrhosis, and gastric antral vascular ectasia (GAVE), found in 2–3%, have a risk of both chronic and acute gastric hemorrhage, and are often asymptomatic and undiagnosed.(11) Additionally, gastric varices are found in 5–33% of patients with portal hypertension, and have an approximately 25% risk of bleeding over 2 years.(12)
The direct effects of radiation on gastric mucosa may also result in gastric bleeding. The etiology of radiation induced gastric ulcers is most likely due to desquamation and erosion of the damaged mucosa. In patients with portal hypertensive gastropathy and gastric antral vascular ectasia, the gastric mucosa can be prone to injury and likely has an impaired ability to heal after an insult such as radiotherapy.(11) Although our policy has been to recommend GI prophylaxis with a proton pump inhibitor (PPI) for all patients receiving high dose radiation near the stomach, it did not reduce the bleeding risk. Sucralfate, which exerts its effects via coating and protection of the gastric mucosa, rather than through neutralization or inhibition of gastric acid, therefore may potentially be useful prophylaxis, especially for cirrhotics.(13, 14) In addition to sucralfate, one may consider a non-selective beta-blocker, such as propranolol, which is commonly used to prevent bleeding from portal hypertensive gastropathy, gastric antral vascular ectasia, and varices.
Although Lyman-NTCP% based models or maximum stomach dose may be useful for describing the probability of gastric bleeding, there are limitations in this study. The stomach wall position can demonstrate both inter- and intra-fraction variability, which can confound dose calculations (18). However, since the majority of our patients were treated on an empty stomach with active breathing control, which eliminates respiratory motion, stomach wall position was relatively stable in our patients. Any differences in stomach filling would be anticipated to be small along the lesser curvature of the stomach, which was typically near the treated liver, and whose position is more consistent than the greater curvature. Additionally, our data do not include the location of the gastric bleeds relative to the high dose region, as accurate localization is difficult with endoscopy. With only 8 patients with cirrhosis included in this series, there is still uncertainty about the exact risk of bleeding, especially in the 25 Gy – 46 Gy maximum dose range and the 16–28 Gy gEUD range, since no patients received these doses. However, none of the 4 cirrhotic patients who received ≤25 Gy maximum dose and ≤16 Gy gEUD developed a gastric bleed, while all of the 4 patients who received ≥46 Gy maximum dose and 28 gEUD did.
We feel these findings will be important in the design of our next generation of clinical trials. We have found that improvements in radiation planning and delivery can aid in maximizing target radiotherapy dose for optimal local control. Rather than relying on traditional homogeneous dose distributions within targets, in which the maximum dose permitted in an adjacent organ at risk is the prescribed dose in the target, permitting heterogeneity can allow for higher doses in the target while still respecting normal tissue constraints. With IMRT, we have demonstrated that this is feasible, and allows for meaningful dose escalation by an average of 10 Gy, to intrahepatic tumors as measured by EUD.(19) Precise patient positioning for each treatment utilizing volumetric imaging, such as cone beam CT, can also ensure that the stomach does not receive a higher dose than planned, by employing a prioritized positioning strategy emphasizing stomach avoidance, especially if this area is near a steep dose gradient. Thus, the integration of treatment planning, delivery, and the quantitation of the effect of radiation on stomach bleeding presented in this study should improve the safe and effective use of high dose radiotherapy for patients with tumors near the stomach.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors have no conflict of interest in connection with this paper.
References
- 1.Marks LB, Ten Haken RK, Martel MK, editors. Quantitative analyses of normal tissue effects in the clinic. International Journal of Radiation Oncology Biology Physics. 2010;Vol 76:S1–S160. doi: 10.1016/j.ijrobp.2009.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence TS, Ten Haken RK, Kessler ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–788. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 5.McGinn CJ, Ten Haken RK, Ensminger WD, et al. Treatment of intrahepatic cancers with radiation doses based on a normal tissue complication probability model. J Clin Oncol. 1998;16:2246–2252. doi: 10.1200/JCO.1998.16.6.2246. [DOI] [PubMed] [Google Scholar]
- 6.Dawson LA, Normolle D, Balter JM, et al. Analysis of radiation-induced liver disease using the Lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53:810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 7.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burman C, Kutcher GJ, Emami B, et al. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991;21:123–135. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- 9.Ceha HM, van Tienhoven G, Gouma DJ, et al. Feasibility and efficacy of high dose conformal radiotherapy for patients with locally advanced pancreatic carcinoma. Cancer. 2000;89:2222–2229. doi: 10.1002/1097-0142(20001201)89:11<2222::aid-cncr10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 10.Haque W, Crane CH, Krishnan S, et al. Reirradiation to the abdomen for gastrointestinal malignancies. Radiat Oncol. 2009;4:55. doi: 10.1186/1748-717X-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ripoll C, Garcia-Tsao G. The management of portal hypertensive gastropathy and gastric antral vascular ectasia. Dig Liver Dis. 2011;43:345–351. doi: 10.1016/j.dld.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 13.Yokel RA, Dickey KM. Mucosal injury and gamma-irradiation produce persistent gastric ulcers in the rabbit. Evaluation of antiulcer drug binding to experimental ulcer sites. Gastroenterology. 1991;100:1201–1205. [PubMed] [Google Scholar]
- 14.Rees WD. Mechanisms of gastroduodenal protection by sucralfate. Am J Med. 1991;91:58S–63S. doi: 10.1016/0002-9343(91)90452-4. [DOI] [PubMed] [Google Scholar]
- 15.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 16.Antonadou D, Coliarakis N, Synodinou M, et al. Randomized phase III trial of radiation treatment +/− amifostine in patients with advanced-stage lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:915–922. doi: 10.1016/s0360-3016(01)01713-8. [DOI] [PubMed] [Google Scholar]
- 17.Simone NL, Menard C, Soule BP, et al. Intrarectal amifostine during external beam radiation therapy for prostate cancer produces significant improvements in Quality of Life measured by EPIC score. Int J Radiat Oncol Biol Phys. 2008;70:90–95. doi: 10.1016/j.ijrobp.2007.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wysocka B, Kassam Z, Lockwood G, et al. Interfraction and respiratory organ motion during conformal radiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2010;77:53–59. doi: 10.1016/j.ijrobp.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 19.Thomas E, Chapet O, Kessler ML, et al. Benefit of using biologic parameters (EUD and NTCP) in IMRT optimization for treatment of intrahepatic tumors. Int J Radiat Oncol Biol Phys. 2005;62:571–578. doi: 10.1016/j.ijrobp.2005.02.033. [DOI] [PubMed] [Google Scholar]