Abstract
Background
Renal injury as a result of ischemia/reperfusion (I/R) is a major clinical problem with a high mortality rate and a lack of therapeutic treatment. During I/R, cellular homeostasis is disrupted due to energy depletion, leading to cell death. Fatty acid β-oxidation is the major metabolic pathway for generating ATP in the kidneys, which is governed by carnitine palmitoyltransferase 1 (CPT1). C75 is a synthetic compound that up-regulates CPT1 activity. Thus, we hypothesized that C75 treatment could increase energy production and alleviate the renal I/R injury.
Methods
Male adult rats were subjected to renal I/R by bilateral renal pedicle clamping with microvascular clips for 60 min, followed by administration of 8% DMSO (vehicle) or C75 (3 mg/kg BW) with 5 animals per group. Blood and renal tissues were collected 24 h after reperfusion and subjected to various measurements and histological examination.
Results
C75 treatment restored the loss of CPT1 activity and intracellular ATP levels in the kidneys after I/R. Administration of C75 significantly lowered serum creatinine, BUN, AST, and LDH levels elevated by I/R. C75 treatment preserved morphological features of the kidneys with a significant improvement in the damage score. In addition, C75 treatment inhibited the increase of TNF-α levels in serum and kidneys, and lowered MPO activity in the kidneys after I/R.
Conclusions
Stimulation of CPT1 activity by C75 recovered ATP depletion, improved renal function, attenuated tissue injury, and inhibited proinflammatory cytokine production and neutrophil infiltration after renal I/R injury. Therefore, enhancing the metabolism pathways for energy production may provide a novel modality to treat renal I/R injury.
Keywords: renal ischemia and reperfusion injury, carnitine palmitoyltransferase 1, C75, ATP, inflammation
INTRODUCTION
Acute renal failure (ARF) which remains a major clinical problem, that usually results from ischemia/reperfusion (I/R) injury. Renal I/R injury commonly occurs in situations such as trauma, aortic bypass surgery, hemorrhagic shock, and renal transplantation [1]. Especially in renal transplantation, I/R injury contributes significantly to delayed graft function, which occurs in 40–60% of cadaveric kidney recipients [2]. A recent multinational/multicenter study shows that major surgeries and septic shock are the most contributing factors for the development of ARF [3]. Moreover, ARF has been reported to be associated with long hospital stay and high mortality rate [4]. Unfortunately, effective therapeutic modalities are still lacking for treating I/R injury and its care currently is mainly supportive. Thus, development of therapeutic interventions to alleviate renal I/R injury and improve clinical outcome is highly needed.
The pathophysiology of renal I/R is complex [5, 6]. During I/R process, occurrence of several events, including adenosine triphosphate (ATP) depletion, intracellular calcium accumulation, phospholipase activation, membrane lipid alterations, cytoskeletal dysfunction, generation of proinflammatory cytokines along with neutrophil infiltration, and production of reactive oxygen/nitrogen species, leads to cell death by apoptosis and necrosis occasioning renal dysfunction [7, 8]. In order to repair I/R-induced damages and return to homeostasis, numerous energy-consuming processes in renal cells will be activated for the recovery [9]. Therefore, stimulation of energy production provides a rational strategy to facilitate the rescue of renal cellular structure and function after I/R.
Fatty acid β-oxidation is an important energy source for kidney tubule cells [10]. Carnitine palmitoyltransferase-1 (CPT1) is the rate-limiting enzyme for fatty acid β-oxidation [11]. CPT1 is located at the outer membrane of the mitochondria and esterifies carnitine to long-chain fatty acyl-CoAs, permitting their entry into the mitochondria for fatty acid oxidation and energy production [12]. CPT1 activity can be allosterically inhibited by the binding of malonyl-CoA, the product of acetyl CoA carboxylase (ACC) [13–15]. In addition to be regulated by the endogenous natural compound, CPT1 activity can be stimulated by C75, a synthetic compound of α-methylene-γ-butyrolactones [16]. C75 increases CPT1 activity even in the presence of inhibitory concentrations of malonyl-CoA [12]. Recently, C75 has been explored for treating obesity by provoking dramatic and sustained weight loss. One of the proposed mechanisms in weight loss after C75 administration is the resilient stimulation of CPT1 activity and increased fatty acid oxidation in peripheral tissues [12].
In this study, we tested the hypothesis that enhancement of energy production by stimulating CPT1 activity in kidney cells could improve renal function and ameliorate the tissue damage induced by I/R. We utilized a rat model where we inquired the effect of C75 treatment on CPT1 activity and ATP generation in the kidneys after I/R. Moreover, we analyzed the impact of the drug over renal cell structure and function along with the proinflammatory responses led by cytokine production and leukocyte tissue infiltration in the kidneys after I/R.
MATERIALS AND METHODS
Experimental Animals
Male Sprague-Dawley rats (250 ~ 300g) were purchased from Charles River Laboratories (Wilmington, MA). The rats were housed in a temperature controlled room and on a 12-h light/dark cycle. The rats were fed a standard Purina rat chow diet and allowed water ad libitum. Animal experimentation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources). This project was approved by the Institutional Animal Care and Use Committee at the Feinstein Institute for Medical Research.
Animal Model of Renal I/R Injury
Prior to surgery, rats were fasted overnight, but water was given ad libitum. Rats were anesthetized with isoflurane inhalation. A midline laparotomy incision was performed to expose the abdomen. Intestines were covered with warm, moist gauze and retracted to the right to expose the left renal pedicle. An atraumatic microvascular clamp was placed around the left renal vascular pedicle (artery and vein). Cessation of blood flow to the kidney was judged by the blanching of the kidney. Same procedure was conducted to the right kidney. The total clamp time was 60 min based on the previous study in our laboratory [17]. Restoration of blood flow into the kidneys was judged visually. The incision was closed in layers and the animals were returned to their cages with food and water for recovery. At 24 h after reperfusion, the animals were euthanized and blood and tissue samples were harvested for further analyses.
Experimental Groups
Vehicle and C75 were administered via a femoral venous catheter in 1 ml solution over a period of 30 min, immediately followed by the removal of the microvascular clamps. Group 1, sham-operated animals underwent a midline laparotomy incision and the kidneys were isolated, but neither clamping nor infusion was performed. Group 2, renal I/R rats were treated with saline containing 8% DMSO as vehicle. Group 3, renal I/R rats treated with C75 (Sigma-Aldrich, St. Louis, MO) at 3 mg/kg BW. The dose of C75 was chosen based on other studies showing effectiveness in rats without generating toxicity [12, 18]. Each group contained 5 animals.
Determination of CPT1 Activity
CPT1 activity was analyzed as described [19]. Briefly, 200 mg of the frozen kidney tissue was minced and suspended in homogenization buffer (0.25 M sucrose, 1 mM EDTA, 0.1% ethanol, and protease inhibitors) at a ratio of 1:5 (w/v). The suspension was then homogenized on ice and centrifuged at 300g for 10 min at 4°C. The precleared supernatant was transferred to a new tube and centrifuged at 12,000g for a further 5 min at 4°C.
CPT1 activity was assayed in these supernatants spectrophotometrically by following the release of CoA-SH from palmitoyl-CoA (Sigma-Aldrich) using the general thiol reagent DTNB (Sigma-Aldrich); 175 µl Tris-HCl–DTNB buffer (116 mM Tris-HCl pH 8.0, 2.5 mM EDTA, 2 mM DTNB, and 0.2% Triton X-100). 10 µl homogenization buffer and 10 µl cleared supernatant was added to a 96-well plate. After 5 min preincubation at 30°C, 10 µl palmitoyl-CoA (1 mM dissolved in distilled water) was added. The reaction was then started by adding 2 µl L-carnitine solution (1.2 mM dissolved in 1 M Tris-HCl pH 8.0), immediately followed by photometric measurement at 412 nm for 15 min. Activity was defined as nmol CoA-SH released/min/mg protein or U/mg protein. Protein concentration was determined by DC protein assay (Bio-Rad, Hercules, CA).
Determination of Renal ATP Levels
Twenty-four hours after reperfusion, the right and left kidneys were cut by sagittal sections. Each half of the kidney was immediately frozen in liquid nitrogen. Later, the frozen right and left kidneys were pulverized together with mortar immersed liquid nitrogen. Kidney tissue (25 mg) was homogenized in 100 µl assay buffer and centrifuged to remove insoluble materials at 13,000g for 10 min. The supernatant was deproteinized by perchloric acid precipitation followed by KOH neutralization before subjecting to ATP assay kit from BioVision (Mountain View, CA).
Determination of Serum Levels of Renal Function and Injury Markers
Blood samples were centrifuged at 2,000g for 15 min to collect serum, and then stored at −80°C for measuring the levels of creatinine, blood urea nitrogen (BUN) and aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) by using assay kits from Pointe Scientific (Canton, MI).
Histopathological Analysis
The right kidney was harvested, cut by sagittal section into two portions, and fixed by formalin. Tissue blocks were cut in 4-µm sections, mounted on glass, followed by hematoxylineosin (H&E) staining for light microscopy analysis. Morphological changes were analyzed by an experienced renal pathologist (blinded to the experimental groups). The extent of right renal damage was graded with a modified schema of Kelly et al [20]. The percentage of morphologic alterations (dilatation of Bowman’s space, flattened tubular epithelium, interstitial inflammation, loss of tubular brush borders tissue necrosis and casts) in the outer medulla and corticomedullary junction were estimated and scored as follows: 0, none; 1+, < 10%; 2+, 10–25%; 3+, 26–75%; and 4+, > 75%.
Determination of Renal Water Content
The difference in water content in the kidneys were determined by the difference in the weight of the kidneys after 72 h of desiccation in 70°C from the initial weight, divided by the initial weight and the results are expressed as percentage.
Determination of Serum and Renal Tissue Levels of TNF-α
The concentration of TNF-α in the serum was measured by using a commercially enzyme-linked immunosorbent assay (ELISA) kit from BD Biosciences (San Diego, CA). The TNF-α expression levels in the kidney tissues was determined by real time RT-PCR. Total RNA was extracted by the Trizol reagent (Invitrogen, Carlsbad, CA) and reverse-transcribed into cDNA by using murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA). A PCR reaction was carried out in a 25 µl of final volume containing 0.08 µmol of each forward and reverse primer, cDNA, and 12.5 µl SYBR Green PCR Master Mix (Applied Biosystems). Amplification was conducted in an Applied Biosystems 7300 real-time PCR machine under the thermal profile of 50°C for 2 min, 95°C for 10 min and followed by 45 cycles of 95°C for 15 sec and 60°C for 1 min. The level of rat β-actin mRNA was used for normalization. Relative expression of mRNA was expressed as fold change in comparison to control group.
Determination of Myeloperoxidase (MPO) Activity
Tissues were homogenized in KPO4 buffer containing 0.5% hexa-decyltrimethyl-ammonium bromide (60°C for 2 hours). After centrifuging, the supernatant was diluted in reaction solution, and ΔOD (rate of change in optical density between 1 and 3 min) was measured at 460 nm to calculate MPO activity.
Statistical Analysis
All data are expressed as means ± SEM and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls (SNK) method for multiple group analyses. Differences in value were considered significant if P < 0.05.
RESULTS
Effect of C75 on CPT1 Activity and ATP Levels in Renal after I/R
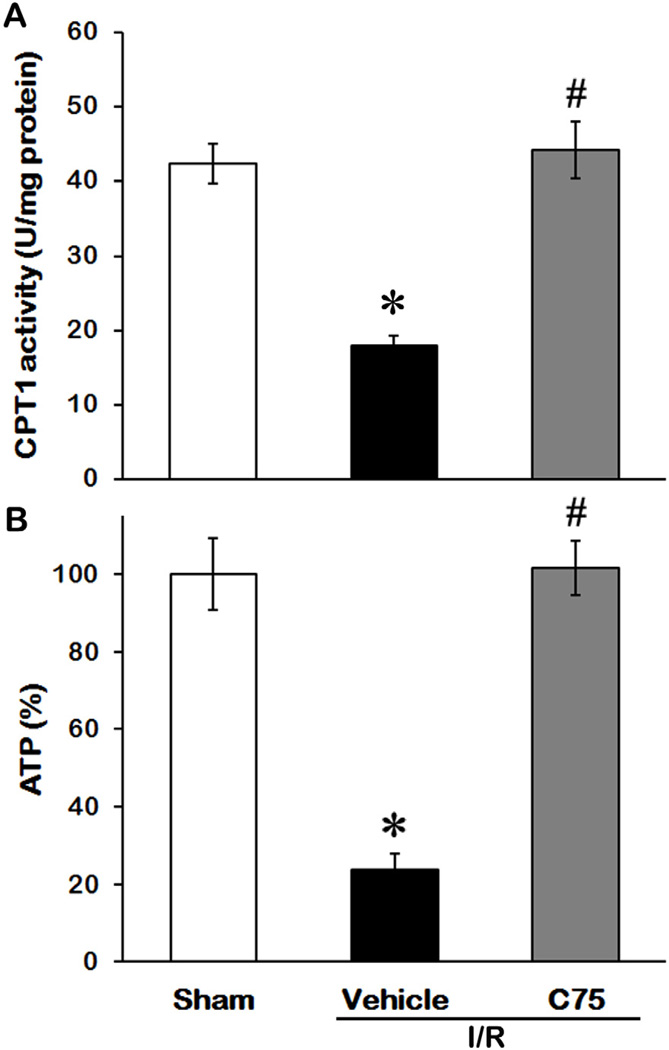
To determine the effectiveness of C75 on regulating energy metabolism, we first measured CPT1 activity in kidney tissues among the sham, vehicle and C75-treated groups (5 rats/group). As shown in Fig. 1A, CPT1 activity in kidney tissues after I/R decreased by 48% in comparison to the sham control 24 h after reperfusion (P < 0.05), while administration of C75 restored CPT1 activity to the levels comparable to the sham control. Correspondingly, I/R process resulted in a 76% decrease of ATP levels in kidney tissues in comparison to the sham group (P < 0.05); however, C75 treatment prevented the reduction of ATP levels after I/R (Fig. 1B). These results indicate the association between activation of CPT1 activity by C75 and energy production in the kidneys after I/R.
Fig. 1.
Effect of C75 on CPT1 activity and ATP levels in the kidneys after renal I/R. Kidney tissues were harvested 24 h after reperfusion to determine CPT1 activity (A) and ATP levels (B). Data are presented as means ± SEM (n = 5 per group) and compared by one-way ANOVA and SNK method. *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
C75 Improves Renal Function and Reduces Injury after I/R
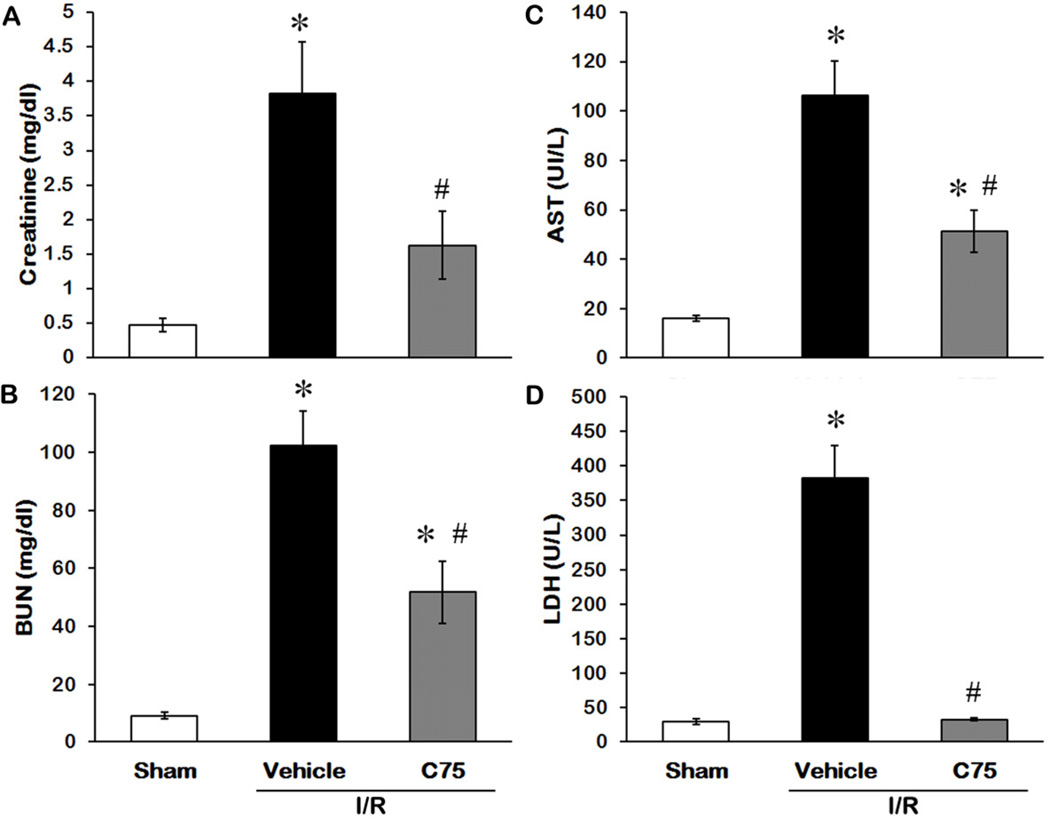
The levels of creatinine and BUN in serum are well-known indicators to monitor the renal function [21]. Creatinine levels increased from 0.48 ± 0.10 to 3.82 ± 0.76 mg/dl after I/R and reduced to 1.63 ± 0.49 mg/dl with C75 treatment (Fig. 2A). BUN levels increased from 9.17 ± 1.10 to 102.34 ± 10.41 mg/dl after I/R and reduced to 50.61 ± 7.58 mg/dl with C75 treatment (Fig. 2B). Serum concentrations of AST and LDH are directly proportional to severity of the tissue damage and are commonly used as markers for cellular injury [21–24]. While AST and LDH levels increased 6.7- and 13.1-fold respectively after I/R compared to the sham group (Figs. 2C and D), C75 treatment significantly reduced AST and LDH activities by 52% and 91% respectively, compared to the vehicle group (Figs. 2C and D).
Fig. 2.
Effect of C75 on renal function and injury after I/R. Serum samples were collected 24 h after reperfusion for measuring creatinine (A), BUN (B), AST (C), and LDH (D). Data are presented as means ± SEM (n = 5 per group) and compared by one-way ANOVA and SNK method. *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
Effect of C75 on Renal Morphology after I/R
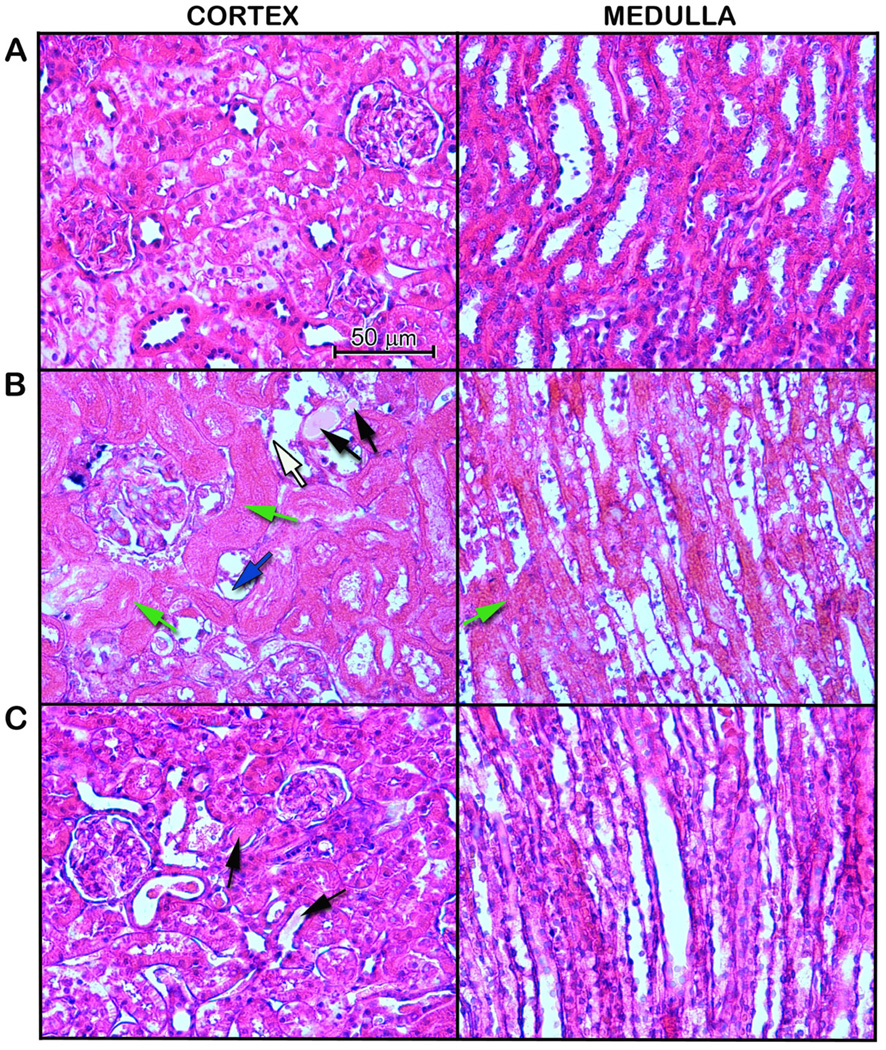
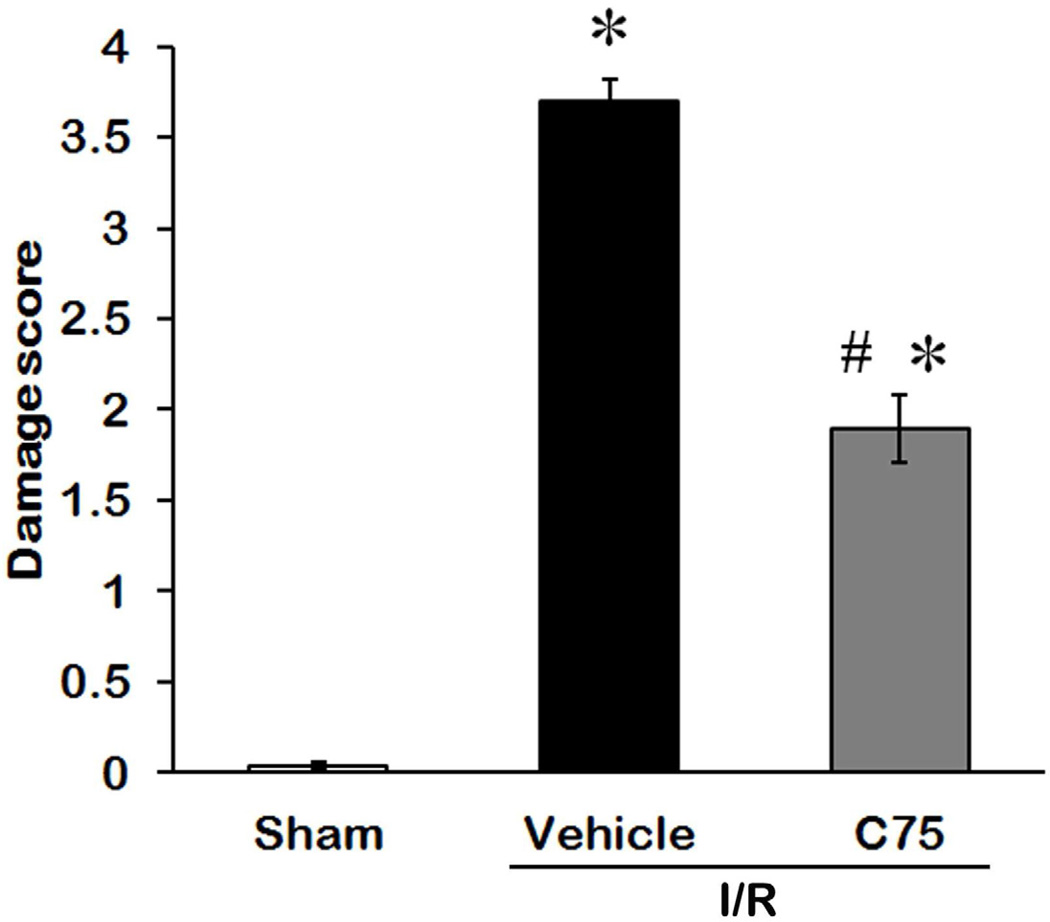
To evaluate the efficacy of the treatments on alleviating the renal tissue damage after I/R, we examined the morphological features of the kidneys by histological staining 24 h after reperfusion (Fig. 3). The right renal cortex and medulla in the vehicle group (Fig. 3B) were severely damaged, showing large areas of tubular dilation, necrosis, hemorrhage, formation of proteinaceous casts, and loss of tubular brush borders in comparison to the sham group (Fig. 3A). C75 treatment exhibited some degrees of improvement in renal morphology (Fig. 3C). In renal I/R injury, the cells located in the outer medulla are more serve damage than cells located at other areas [25]. Thus, the damage levels in the outer medulla and corticomedullary junction were examined by histology and graded in a semiquantitative manner. The damage score of each group is indicated in Fig. 4. The vehicle-treated rats obtained 3.7 ± 0.1, closely to the maximum score. C75 treatment had a significant reduction in damage score of 1.9 ± 0.1, compared to the vehicle group.
Fig. 3.
Effect of C75 on renal morphology after I/R. Histological changes of the right kidneys in the sham, vehicle, and C75-treated rats were examined. Kidneys were harvested 24 h after reperfusion, processed and stained with hematoxylin and eosin. Representative photomicrographs from renal cortex and renal medulla are shown. Compared with the sham group (essentially normal histology), examination of kidneys obtained from the vehicle group demonstrated a significant degree of renal injury with proteinaceous casts (black arrow), loss of tubular brush borders (blue arrow), flattened epithelium (white arrow), and necrosis of renal tubular cells (green arrow) The integrity of renal morphology in the C75-treated rats was lay between the sham and vehicle groups. Original magnification 400×. (A) Sham; (B) Vehicle; and (C) C75.
Fig. 4.
Histological score of renal I/R injury and C75 treatment. The extent damage of right renal in the outer medulla and corticomedullary junction was graded with a modified schema as described in Materials and Methods. The score represents the percentage of morphologic alterations as follows: 0, none; 1+, < 10%; 2+, 10–25%; 3+, 26–75%; and 4+, > 75%. Data are presented as means ± SEM (n = 5 per group) and compared by one-way ANOVA and SNK method. *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
C75 Reduces Renal Edema after I/R
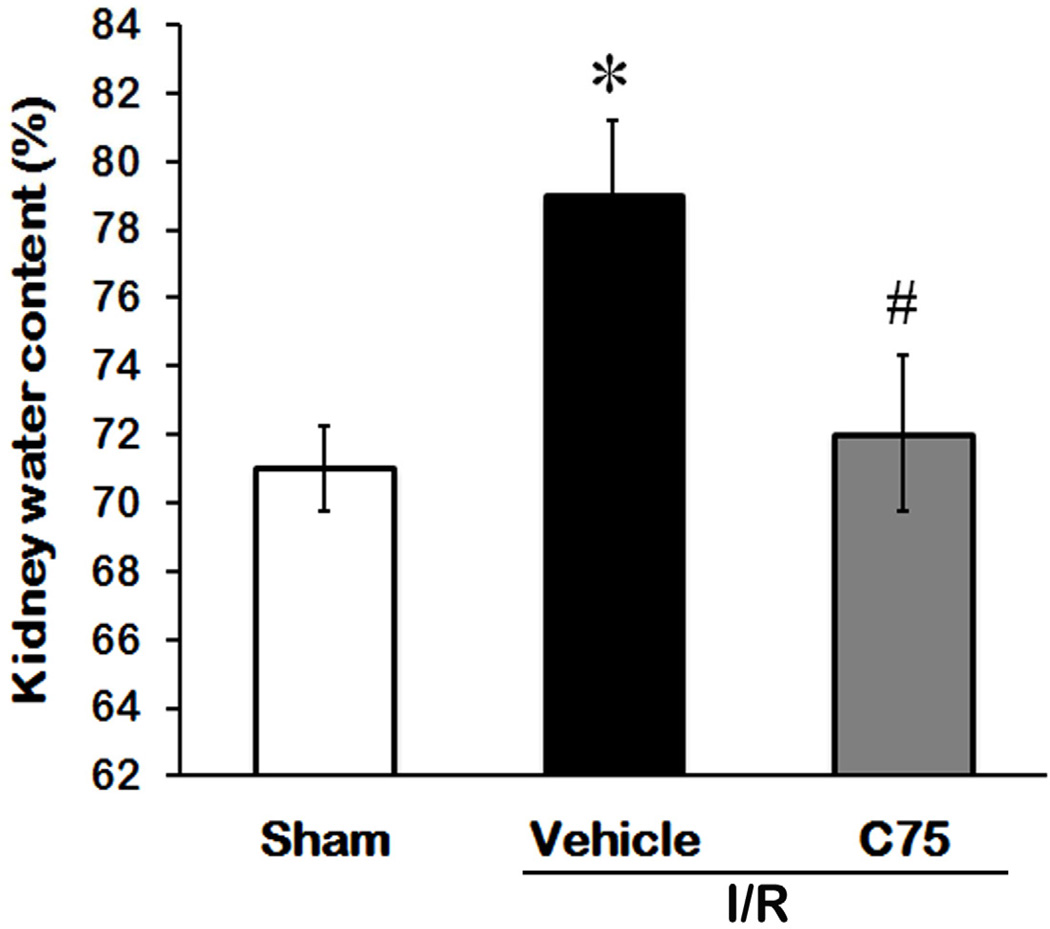
Development of edema is another indicator of tissue injury. We measured the water content of kidney tissues to quantify the degree of edema among the experimental groups. As shown in Fig. 5, the water content of kidney tissues in the vehicle group was 7.96% higher than that in the sham group (P < 0.05), while it was only 0.96% increased in the C75-treated group in comparison to the sham group.
Fig. 5.
Effect of C75 on renal water content after I/R. Kidney tissues were harvested 24 h after reperfusion for determining the percentage of the amount of water retained in the kidney tissues. Data are presented as means ± SEM (n = 5 per group) and compared by one-way ANOVA and SNK method. *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
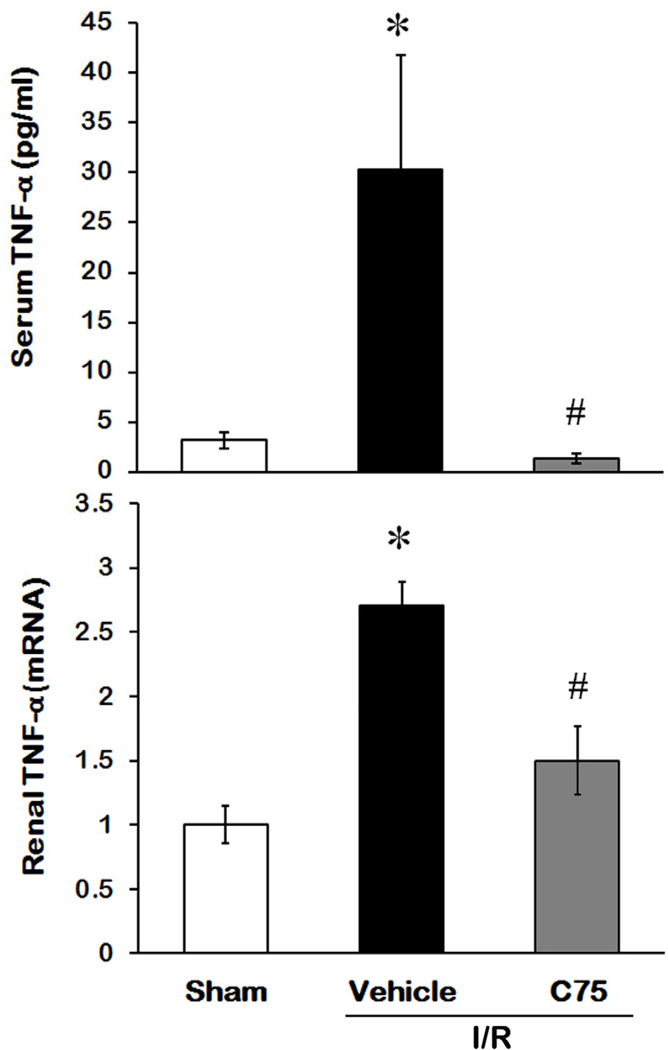
Effect of C75 on TNF-α Production after I/R
Inflammation is one of the major factors in contribution to the renal damage after I/R [6]. To monitor the effect of the treatment on systemic inflammation after I/R, we measured the levels of proinflammatory cytokine TNF-α in serum 24 h after reperfusion. Serum TNF-α levels in the vehicle group were 9.5-fold higher than the sham group (P < 0.05), while there was no significant difference between C75-treated and sham groups (Fig 6A). We also assessed the TNF-α expression in kidney tissues by real time RT-PCR. The TNF-α mRNA levels in the vehicle group were reduced by C75 treatment from 2.71- to 1.52-fold, compared to the sham group (Fig. 6B).
Fig. 6.
Effect of C75 on TNF-α production after I/R. (A) Serum samples were collected 24 h after reperfusion for measuring TNF-α by ELISA. (B) Kidney tissues were harvested 24h after reperfusion for total RNA isolation. The levels of TNF-α mRNA were determined by real-time RT-PCR. Results are normalized by β-actin as an internal control and are expressed as fold induction in comparison to the sham group. Data are presented as means ± SEM (n = 5 per group) and compared by one-way ANOVA and SNK method. *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
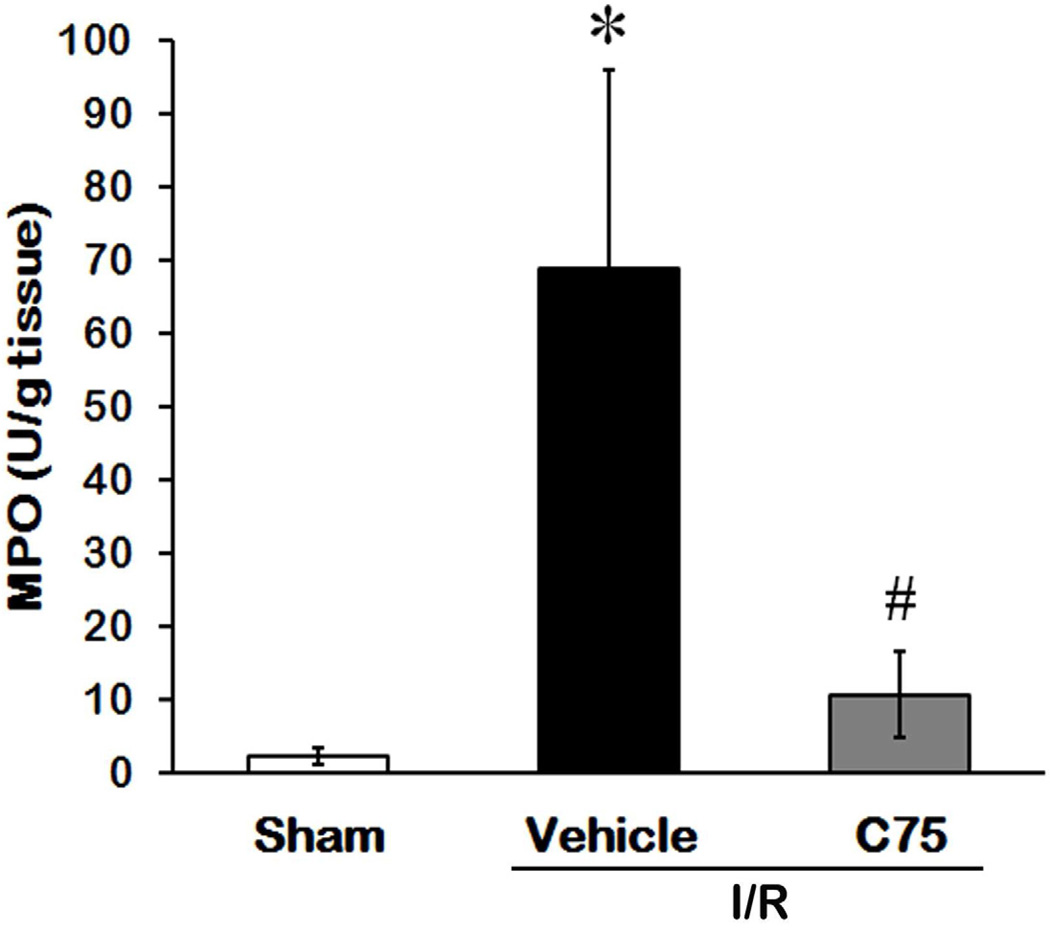
C75 Attenuates MPO Activity in Renal after I/R
Excessive neutrophils infiltrated into the inflamed sites can cause tissue damage [26]. We measured MPO activity to compare the amount of neutrophil infiltration to the kidney tissues in all three groups. The MPO activity in the vehicle group was reduced by C75 treatment from 29.5- to 4.5-fold in comparison to the sham group (Fig. 7).
Fig. 7.
Effect of C75 on MPO activity in renal after I/R. Kidney tissues were harvested 24 h after reperfusion for measuring MPO activity. Data are presented as means ± SEM (n = 5 per group) and compared by one-way ANOVA and SNK method. *P < 0.05 vs. sham; #P < 0.05 vs. vehicle.
DISCUSSION
In this study, we demonstrated that administration of C75 in early reperfusion effectively restored the loss of CPT1 activity and ATP levels from I/R in the kidneys, which led to improvement of the renal function and attenuation of organ injury after I/R. This observation corresponds to our previous study shown that stimulation of CPT1 activity by combination of carnitine and 5-Aminoimidazole-4-carboxyamide ribonucleoside (AICAR) associates with an increase of renal ATP levels and the improvement of the overall outcome after severe I/R injury [27]. Although both treatments can stimulate CPT1 activity, C75 acts by direct binding to CPT1, while AICAR attenuates malonyl-CoA levels by inhibiting ACC activity. Using acceleration of ATP recovery as strategy to reduce I/R-induced renal injury has also been shown in another recent study by administration of SS-31, a mitochondria-targeted tetra-peptide, for protecting mitochondrial structure and preventing deterioration of mitochondrial function [28].
We observed a dramatic reduction of renal ATP levels, down to 24% of sham group, 24 h after ischemia. This result is consistent to a previous report shown that renal ATP levels fall to below 10% of preischemic values within 5 to 10 minutes of ischemia and remain low for the duration of ischemia [29–31]. It also has been reported that after I/R, a persistent perfusion deficit exists even at 24 h after reperfusion, and the outer medullary partial pressure of oxygen is restored to only 10% of its normal levels, rendering this region susceptible to injury at both the tubular and vascular levels [32, 33]. In addition, oxidative phosphorylation, a major process for ATP synthesis, is inhibited during hypoxia and deteriorates upon reoxygenation [34, 35].
In I/R injury, fair amount of renal cells die by necrosis. Those necrotic tissues can initiate an inflammatory cascade, which causes further tissue damage [36, 37]. Thus, inhibition of inflammatory responses after I/R injury is very crucial to protect the kidneys from secondary damage and facilitate its recovery. We demonstrated that C75 administration suppressed the I/R-induced elevation of TNF-α levels in serum as well as its expression in the kidney tissues. This result indicates that inhibition of proinflammatory cytokine production is one of the mechanisms by which C75 reduces renal tissue injury.
The release of proinflammatory cytokines (e.g., TNF-α) from damaged renal epithelial cells and macrophages can increase the expression of adhesion molecules to facilitate leukocyte adhesion and infiltration to the inflamed sites [38]. Activated neutrophils release proteolytic enzymes such as elastase and MPO, and reactive oxygen species including hydrogen peroxide and superoxide. Excessive production of these agents engages in disruption of endothelial barrier functions and promotes extravascular host tissue damage during uncontrolled inflammation [39, 40]. As expected, MPO activity, a marker of leukocyte infiltration into the renal parenchyma, is highly induced in the vehicle group. When administration of C75, the MPO activity is significantly reduced, indicating that neutrophil infiltration into the kidney tissues is blocked by C75. Overall, C75 can modulate proinflammatory responses in renal induced by I/R injury. Whether C75 directly acts on immune cells or mainly through preventing cell death for triggering a proinflammatory cascade needs further investigation.
The majority of research studies perusing a cure for renal I/R injury, had found encouraging results in the settings of preventive strategies, i.e., the agents are applied prior to ischemia when renal cells are still intact [41]. However, these modalities are beyond from practical use since most of the time the common clinical scenario resembles a patient with already established kidney injury and deterioration of renal function. In this study we showed that the beneficial effect of C75 on renal I/R injury was administered after the ischemia stage. Thus, C75 is a potential candidate to develop as a post-injury therapeutic agent to treat patients suffering from renal I/R injury, although the treatment window of C75 needs to be further identified. Recently, several new biomarkers, including neutrophil gelatinase-associated lipocalin (NGAL), liver-type fatty acid binding protein (L-FABP) or kidney injury molecule-1 (KIM-1) that increase prior to the serum creatinine elevation show very promising in clinical use [41, 42]. In conjunction with these new markers, early detection of the deteriorated kidney tissues will definitely enhance the effectiveness of C75 treatment at the early injury stage. So far, C75 is still under the preclinical stage for developing it as an antitumor agent by utilizing its capability of inhibiting fatty acid synthase activity [43]. However, clinical development of C75 has been hampered by anorexia and weight loss caused by drug treatment [44]. Whether these side effects can be tolerated in a short-term administration for treating renal I/R injury needs to be further studied. The toxicity and solubility of C75 are additional considerations for moving it to a phase I clinical trial.
In summary, infusion of C75 into ischemic-damaged kidneys at the early reperfusion stage improves renal function, lowers proinflammatory cytokine levels, reduces neutrophil infiltration and attenuates tissue damage in an animal model. The beneficial activity of C75 is associated with the stimulation of CPT1 activity and recovery of ATP depletion after I/R. Thus, stimulating metabolism pathways for energy production provides an attractive therapeutic strategy to treat patients with delayed graft function following kidney transplantation as well as acute renal failure after major surgery, trauma or hypotensive shock.
ACKNOWLEDGMENTS
The authors thank Dr. Asha Jacob for critically reviewing this manuscript. This study was supported by the National Institutes of Health grant, R01HL076179 (to PW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 2.Cecka JM, Cho YW, Terasaki PI. Analyses of the UNOS Scientific Renal Transplant Registry at three years--early events affecting transplant success. Transplantation. 1992;53:59. doi: 10.1097/00007890-199201000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Liangos O, Wald R, O'Bell JW, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 5.Lameire NH, Vanholder R. Pathophysiology of ischaemic acute renal failure. Best Pract Res Clin Anaesthesiol. 2004;18:21. doi: 10.1016/j.bpa.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 7.Weight SC, Bell PR, Nicholson ML. Renal ischaemia--reperfusion injury. Br J Surg. 1996;83:162. [PubMed] [Google Scholar]
- 8.Paller MS. The cell biology of reperfusion injury in the kidney. J Investig Med. 1994;42:632. [PubMed] [Google Scholar]
- 9.Szabo C, Dawson VL. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci. 1998;19:287. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- 10.Razanoelina M, Freund N, Bismuth J, Geloso JP, Delaval E. Effect of lipid diet on mitochondrial palmitoyl-l-carnitine oxidation in kidney at postnatal development. J Dev Physiol. 1991;16:283. [PubMed] [Google Scholar]
- 11.Bonnefont JP, Djouadi F, Prip-Buus C, et al. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med. 2004;25:495. doi: 10.1016/j.mam.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Thupari JN, Landree LE, Ronnett GV, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci U S A. 2002;99:9498. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGarry JD, Takabayashi Y, Foster DW. The role of malonyl-coA in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978;253:8294. [PubMed] [Google Scholar]
- 14.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 15.Zammit VA. The malonyl-CoA-long-chain acyl-CoA axis in the maintenance of mammalian cell function. Biochem J. 1999;343(Pt 3):505. [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhajda FP, Pizer ES, Li JN, et al. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci U S A. 2000;97:3450. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah KG, Rajan D, Jacob A, et al. Attenuation of renal ischemia and reperfusion injury by human adrenomedullin and its binding protein. J Surg Res. 2010;163:110. doi: 10.1016/j.jss.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clegg DJ, Wortman MD, Benoit SC, McOsker CC, Seeley RJ. Comparison of central and peripheral administration of C75 on food intake, body weight, and conditioned taste aversion. Diabetes. 2002;51:3196. doi: 10.2337/diabetes.51.11.3196. [DOI] [PubMed] [Google Scholar]
- 19.Bieber LL, Abraham T, Helmrath T. A rapid spectrophotometric assay for carnitine palmitoyltransferase. Anal Biochem. 1972;50:509. doi: 10.1016/0003-2697(72)90061-9. [DOI] [PubMed] [Google Scholar]
- 20.Kelly KJ, Williams WW, Jr, Colvin RB, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterjee PK, Patel NS, Sivarajah A, et al. GW274150, a potent and highly selective inhibitor of iNOS, reduces experimental renal ischemia/reperfusion injury. Kidney Int. 2003;63:853. doi: 10.1046/j.1523-1755.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- 22.Feilleux-Duche S, Garlatti M, Aggerbeck M, et al. Cell-specific regulation of cytosolic aspartate aminotransferase by glucocorticoids in the rat kidney. Am J Physiol. 1993;265:C1298. doi: 10.1152/ajpcell.1993.265.5.C1298. [DOI] [PubMed] [Google Scholar]
- 23.Hauet T, Mothes D, Goujon J, et al. Trimetazidine reverses deleterious effects of ischemia-reperfusion in the isolated perfused pig kidney model. Nephron. 1998;80:296. doi: 10.1159/000045190. [DOI] [PubMed] [Google Scholar]
- 24.Heyman SN, Rosen S, Epstein FH, Spokes K, Brezis ML. Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney Int. 1994;45:981. doi: 10.1038/ki.1994.132. [DOI] [PubMed] [Google Scholar]
- 25.Gobe G, Willgoss D, Hogg N, Schoch E, Endre Z. Cell survival or death in renal tubular epithelium after ischemia-reperfusion injury. Kidney Int. 1999;56:1299. doi: 10.1046/j.1523-1755.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- 26.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Idrovo JP, Yang WL, Matsuda A, et al. POST-TREATMENT With the Combination of 5-Aminoimidazole-4-Carboxyamide Ribonucleoside and Carnitine Improves Renal Function After Ischemia/Reperfusion Injury. Shock. 2012;37:39. doi: 10.1097/SHK.0b013e31823185d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szeto HH, Liu S, Soong Y, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel NJ, Avison MJ, Reilly HF, Alger JR, Shulman RG. Enhanced recovery of renal ATP with postischemic infusion of ATP-MgCl2 determined by 31P-NMR. Am J Physiol. 1983;245:F530. doi: 10.1152/ajprenal.1983.245.4.F530. [DOI] [PubMed] [Google Scholar]
- 30.Gaudio KM, Stromski M, Thulin G, et al. Postischemic hemodynamics and recovery of renal adenosine triphosphate. Am J Physiol. 1986;251:F603. doi: 10.1152/ajprenal.1986.251.4.F603. [DOI] [PubMed] [Google Scholar]
- 31.Arnold PE, Van Putten VJ, Lumlertgul D, Burke TJ, Schrier RW. Adenine nucleotide metabolism and mitochondrial Ca2+ transport following renal ischemia. Am J Physiol. 1986;250:F357. doi: 10.1152/ajprenal.1986.250.2.F357. [DOI] [PubMed] [Google Scholar]
- 32.Bonventre JV. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto K, Wilson DR, Baumal R. Outer medullary circulatory defect in ischemic acute renal failure. Am J Physiol. 1984;116:253. [PMC free article] [PubMed] [Google Scholar]
- 34.Weinberg JM, Venkatachalam MA, Roeser NF, et al. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Renal Physiol. 2000;279:F927. doi: 10.1152/ajprenal.2000.279.5.F927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veitch K, Hombroeckx A, Caucheteux D, Pouleur H, Hue L. Global ischaemia induces a biphasic response of the mitochondrial respiratory chain. Anoxic pre-perfusion protects against ischaemic damage. Biochem J. 1992;281(Pt 3):709. doi: 10.1042/bj2810709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daemen MA, de Vries B, Buurman WA. Apoptosis and inflammation in renal reperfusion injury. Transplantation. 2002;73:1693. doi: 10.1097/00007890-200206150-00001. [DOI] [PubMed] [Google Scholar]
- 37.Heinzelmann M, Mercer-Jones MA, Passmore JC. Neutrophils and renal failure. Am J Kidney Dis. 1999;34:384. doi: 10.1016/s0272-6386(99)70375-6. [DOI] [PubMed] [Google Scholar]
- 38.Donnahoo KK, Shames BD, Harken AH, Meldrum DR. Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162:196. doi: 10.1097/00005392-199907000-00068. [DOI] [PubMed] [Google Scholar]
- 39.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 40.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7:1. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 41.Kunzendorf U, Haase M, Rolver L, Haase-Fielitz A. Novel aspects of pharmacological therapies for acute renal failure. Drugs. 2010;70:1099. doi: 10.2165/11535890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 43.Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 44.Orita H, Coulter J, Lemmon C, et al. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin Cancer Res. 2007;13:7139. doi: 10.1158/1078-0432.CCR-07-1186. [DOI] [PubMed] [Google Scholar]