Abstract
Objective
To elucidate the relationship between the two hallmark proteins of Alzheimer's disease (AD), amyloid-β (Aβ) and tau, and clinical decline over time among cognitively normal older individuals.
Design
A longitudinal cohort of clinically and cognitively normal older individuals assessed with baseline lumbar puncture and longitudinal clinical assessments.
Setting
Research centers across the United States and Canada.
Patients
We examined one hundred seven participants with a Clinical Dementia Rating (CDR) of 0 at baseline examination.
Main Outcome Measures
Using linear mixed effects models, we investigated the relationship between CSF p-tau181p, CSF Aβ1-42 and clinical decline as assessed using longitudinal change in global CDR, CDR-Sum of Boxes (CDR-SB), and the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog).
Results
We found a significant relationship between decreased CSF Aβ1-42 and longitudinal change in global CDR, CDR-SB, and ADAS-cog in individuals with elevated CSF p-tau181p. In the absence of CSF p-tau181p, the effect of CSF Aβ1-42 on longitudinal clinical decline was not significantly different from zero.
Conclusions
In cognitively normal older individuals, Aβ-associated clinical decline over a mean of three years may occur only in the presence of ongoing, “downstream” neurodegeneration.
INTRODUCTION
The identification of clinically normal older individuals destined to develop Alzheimer's disease (AD) is of increasing clinical importance as therapeutic interventions for the prevention of dementia are developed. Evidence from both genetic at-risk cohorts and clinically normal older individuals suggests that the pathobiological process of AD begins years before the diagnosis of clinical dementia.1 Based on prior experimental evidence indicating that amyloid-β (Aβ) deposition triggers the neurodegenerative process underlying AD 2, a number of recent human studies have primarily focused on the relationship between Aβ, neurodegeneration, and cognitive decline to identify clinically normal elderly individuals considered to be in the ‘preclinical’ stage of dementia. 3 However, amyloid plaques correlate poorly with memory decline 4 and immunotherapy-induced plaque removal may not prevent progressive neurodegeneration 5 suggesting that other entities may be required for AD-related degeneration.
Recent studies using transgenic mouse models show that the presence of tau is required for Aβ to induce neuronal and synaptic damage. 6 Reductions in tau protect against Aβ-induced neuronal dysfunction 7 while the presence of tau potentiates Aβ-associated synapotoxicity.8 Recent evidence from our laboratory indicates that in older humans at risk for dementia, Aβ-associated volume loss occurs only in the presence of phospho-tau (p-tau). 9 Building upon this work, in this study, using CSF levels of decreased Aβ1-42 and increased p-tau181p, in vivo biomarkers of amyloid-β 10 and p-tau associated neurofibrillary pathology 11, we investigated whether Aβ-associated clinical decline in cognitively normal older individuals occurs only in the presence of p-tau.
SUBJECTS AND METHODS
We evaluated healthy older controls (HC, n = 107) from the Alzheimer's Disease Neuroimaging Initiative (ADNI). The ADNI is a large multi-site collaborative effort launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and non-profit organizations as a public–private partnership aimed at testing whether serial MRI, PET, other biological markers and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. ADNI is the result of many co-investigators from a broad range of academic institutions and private corporations, with subjects recruited from over 50 sites across the US and Canada. For more information, please see http://www.adni-info.org.
Each participant was formally evaluated using eligibility criteria that are described in detail elsewhere (http://www.adniinfo.org/index.php?option=com_content&task=view&id=9&Itemid=43). The institutional review boards of all participating institutions approved the procedures for this study. Written informed consent was obtained from all participants or surrogates. Experienced clinicians conducted independent semi-structured interviews with the participant and a knowledgeable collateral source that included a health history, neurological examination, and a comprehensive neuropsychological battery.
We evaluated participants who were clinically diagnosed at baseline as cognitively and clinically normal. We examined clinical decline (537 longitudinal assessments) using the global Clinical Dementia Rating (CDR) scale, CDR-Sum of Boxes (CDR-SB) 12 and the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog). 13 We restricted participants to those with baseline CSF data and baseline and follow-up clinical data.
Methods for CSF acquisition and biomarker measurement using the ADNI cohort have been reported previously. 14 In brief, CSF was collected and stored at -80°C at the University of Pennsylvania ADNI Biomarker Core Laboratory. Amyloid-β from peptides 1-42, tau phosphorylated at threonine 181, and total tau was measured using the multiplex xMAP Luminex platform (Luminex Corp, Austin TX) with Innogenetics (INNOBIA AlzBio3, Ghent, Belgium) immunoassay kit–based reagents.
Using recently proposed CSF cutoffs 14, we classified all participants based on high (>23 pg/ml, “positive”) and low (<23 pg/ml, “negative”) p-tau181p levels and based on low (<192 pg/ml, “positive”) and high (>192 pg/ml, “negative”) Aβ1-42 levels (Table 1). We investigated the relationship between CSF Aβ1-42 status and clinical decline using a linear mixed effects model, controlling for the effects of age and sex. Specifically, Δc = β0 × Δt + β1 + β1CSF_Aβ1-42_status × Δt + covariates × Δt + ε Here, Δc = global CDR, CDR-SB or ADAS-cog score and Δt = change in time from baseline clinical assessment (years).
Table 1.
Demographic, clinical, and imaging data for all healthy older controls (HC) in this study. MMSE = Mini-mental status exam, CDR-SB = CDR-Sum of Box Scores, ADAS-cog = Alzheimer's Disease Assessment Scale-cognitive subscale, APC = Annualized Percent Change, SE = Standard error of the mean
| Aβ -/P-tau - (n = 46) | Aβ -/P-tau + (n = 19) | Aβ +/P-tau - (n = 20) | Aβ +/P-tau + (n = 21) | |
|---|---|---|---|---|
| Age, Mean (SE) | 74.3 (0.6) | 78.0 (1.4) | 74.9 (1.1) | 78.2 (1.0) |
| Female, % | 24 | 29 | 31 | 38 |
| Education Years, Mean (SE) | 15.5 (0.4) | 15.5 (0.4) | 14.8 (0.8) | 16.7 (0.6) |
| MMSE, Mean (SE) | 29.1 (0.1) | 28.8 (0.3) | 29.1 (0.2) | 29.3 (0.2) |
| Years Follow-up, Mean (SE) | 3.3 (0.1) | 3.3 (0.2) | 3.0 (0.2) | 2.9 (0.2) |
| ADAS- cog APC, Mean (SE) | -0.13 (0.7) | 0.43 (0.6) | 0.61 (0.7) | 1.6 (0.9) |
| CDR-SB APC, Mean (SE) | 0.03 (0.01) | 0.006 (0.01) | -0.03 (0.1) | 0.2 (0.1) |
RESULTS
We first evaluated whether there was a relationship between CSF Aβ1-42 status and longitudinal clinical decline. Consistent with prior studies 15-17, across all participants, we found that positive CSF Aβ1-42 status significantly correlated with change in global CDR (β1 = 0.03, Standard Error (SE) = 0.01, p = 0.04), CDR-SB (β1 = 0.09, SE = 0.05, p = 0.048), and ADAS-cog (β1 = 0.59, SE = 0.23, p = 0.0098). To ensure that our results were not due to a categorical treatment of variables, we examined CSF Aβ1-42 as a continuous variable and found significant associations between decreased CSF Aβ1-42 levels and change in global CDR (β-coefficient = -0.0002, SE = 0.0001, p = 0.030), CDR-SB (β-coefficient = -0.0009, SE = 0.0004, p = 0.041), and ADAS-cog (β-coefficient = -0.005, SE = 0.002, p = 0.016).
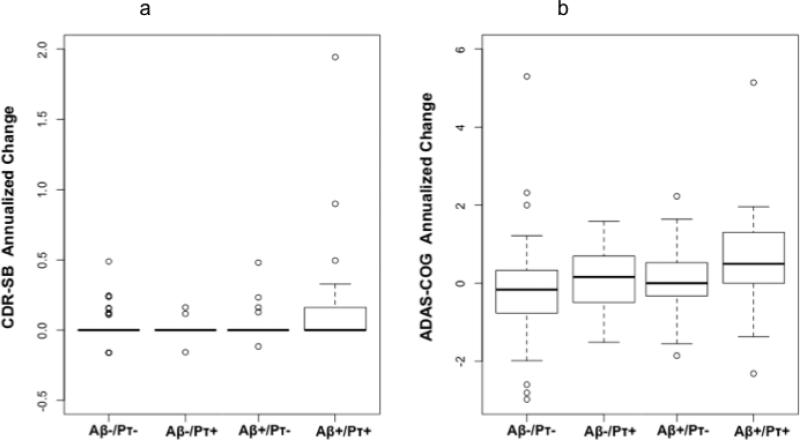
We next investigated whether the presence of CSF p-tau181p influenced the relationship between CSF Aβ1-42 and longitudinal clinical decline. We found that positive CSF Aβ1-42 status was associated with change in global CDR only among CSF p-tau181p positive individuals (β1 = 0.06, SE = 0.02, p = 0.01). There was no association between CSF Aβ1-42 status and change in global CDR among CSF p-tau181p negative individuals (β1 = -0.02, SE = 0.02, p = 0.35). Similarly, we found that positive CSF Aβ1-42 status was associated with change in CDR-SB scores only among CSF p-tau181p positive individuals (β1 = 0.24, SE = 0.11, p = 0.036) (Figure 1a). There was no association between CSF Aβ1-42 status and change in CDR-SB scores among CSF p-tau181p negative individuals (β1 = -0.003, SE = 0.04, p = 0.94). Consistent with these results, we found that positive CSF Aβ1-42 status was associated with change in ADAS-cog scores only among CSF p-tau181p positive individuals (β1 = 0.94, SE = 0.32, p = 0.0043) (Figure 1b). There was no association between CSF Aβ1-42 status and change in CDR-SB scores among CSF ptau181p negative individuals (β1 = 0.41, SE = 0.34, p = 0.23).
Figure 1.
Box and whisker plots for all participants illustrating change in (a) CDR-SB and (b) ADAS-cog scores, measured as annualized percent change (APC), based on CSF Aβ1-42 (Aβ) and CSF p-tau181p (pτ) status. For each plot, thick black lines show the median value. Regions above and below the black line show the upper and lower quartiles, respectively. The dashed lines extend to the minimum and maximum values with outliers shown as open circles. As illustrated, the Aβ +/pτ + individuals demonstrated the largest change in CDR-SB and ADAS-cog scores.
Consistent with the results obtained from categorizing subjects on the basis of cutoff values, we found that decreased CSF Aβ1-42 levels significantly associated with change in global CDR only among CSF p-tau181p positive individuals (β-coefficient = - 0.005, SE = 0.0002, p = 0.02). Similarly, decreased CSF Aβ1-42 levels significantly associated with change in ADAS-cog scores (β-coefficient = -0.007, SE = 0.002, p = 0.0064) and showed a trend towards significant association with change in CDR-SB scores (β-coefficient = -0.002, SE = 0.001, p = 0.06) only among CSF p-tau181p positive individuals. Neither CSF p-tau181p status nor CSF p-tau181p level significantly associated with clinical decline, irrespective of CSF Aβ1-42 status.
Finally, we examined whether the presence of a nonspecific form of tau, total tau (t-tau), affected the relationship between CSF Aβ1-42 and longitudinal clinical decline. We classified all participants based on high (“positive”, n = 22) and low (“negative”, n = 85) t-tau levels using a CSF cutoff value of 93 pg/ml. 14 We found that positive CSF Aβ1-42 status did not associate with change in global CDR or CDR-SB either among CSF t-tau positive or negative individuals. Positive CSF Aβ1-42 status significantly associated with change in ADAS-cog scores among CSF t-tau positive individuals (β-coefficient = 1.43, SE = 0.49, p = 0.005) and showed a trend towards significance among CSF t-tau negative individuals (β-coefficient = 0.48, SE = 0.27, p = 0.067).
DISCUSSION
Here, we show that in clinically normal older individuals Aβ-associated longitudinal clinical decline occurs only in the presence of elevated p-tau. In the absence of p-tau, the effect of Aβ on longitudinal clinical decline is not significantly different from zero.
These findings provide important insights into the preclinical stage of Alzheimer's disease. Consistent with prior studies 18-19, 22-23, our results indicate that in clinically normal older individuals Aβ deposition by itself is not associated with clinical decline; the presence of p-tau represents a critical link between Aβ deposition and accelerated clinical decline. Furthermore, our findings point to p-tau as an important marker of AD-associated degeneration. Elevations in CSF total tau are seen in a number of neurologic disorders characterized by neuronal and axonal death whereas increased CSF p-tau correlates with increased neurofibrillary pathology and can distinguish AD from other neurodegenerative disorders 20 suggesting that p-tau may represent a more specific marker of the Alzheimer's pathologic process than total tau. When considered together with recent work from our laboratory 9, these data suggest that the combination of p-tau and Aβ likely reflects underlying pathobiology of the preclinical stage of AD.
Recent experiments using transgenic mice illustrates that the presence of tau potentiates Aβ-associated neurodegeneration. Postsynaptic Aβ toxicity is tau dependent 6,8 and tau reduction prevents premature mortality and memory deficits in APP23 mice. 7-8 Our human data are consistent with these experimental findings.
This study has limitations. One concern is that CSF biomarkers provide an indirect assessment of amyloid and neurofibrillary pathology and may not fully reflect the biological processes underlying Alzheimer's disease. Another concern is that though our findings indicate that CSF Aβ1-42, in combination with CSF p-tau181p, may better predict clinical decline than CSF Aβ1-42, in combination with CSF t-tau, prior studies have shown that CSF p-tau and t-tau, when combined with CSF Aβ1-42, are equally predictive of decline.18-19, 22-23 This difference maybe related to slight differences in CSF measurement assays (ELISA vs. Luminex), the nature of the participant population or other factors. A third limitation is that we primarily focused on CSF biomarkers of the two pathologic hallmarks of AD. Additional markers, such as CSF levels of YKL-40 22 or Visinin-like protein-1 (VILIP-1) 23, may also interact with Aβ to predict clinical decline in cognitively normal elders. Finally, the individuals examined here may represent a group of highly selected, generally healthy older adults who are motivated to participate in research studies. As such, these findings need to be further validated on an independent, community-based cohort of older individuals that would be more representative of the general older population.
From a clinical perspective, these results are consonant with the three stage preclinical AD framework recently proposed by the National Institute on Aging-Alzheimer's Association workgroup 3 and indicate that a biomarker profile consisting of both CSF Aβ1-42 and CSF p-tau181p levels may better identify those older individuals who are at an elevated risk of progressing to eventual AD dementia than either biomarker by itself. Given that Aβ accumulation is ‘necessary but not sufficient’ to express the clinical manifestations of AD dementia 3, early intervention trials should take into account both the CSF p-tau181p and CSF Aβ1-42 status of participants since older individuals with increased CSF p-tau181p and decreased CSF Aβ1-42 levels are likely to have a different rate of clinical progression than individuals with normal CSF p-tau181p and decreased CSF Aβ1-42 levels. These findings also illustrate the need for developing novel therapeutic approaches that specifically target tau. It is feasible that though Aβ initiates the degenerative cascade, elevated levels of tau may represent a second phase of the AD pathologic process where neurodegenerative changes occur largely independent of Aβ. 21 As such, targeting ‘downstream’ events, such as tau phosphorylation and aggregation, in older individuals with both decreased CSF Aβ1-42 and increased CSF p-tau181p levels may be an additionally beneficial treatment strategy.
Acknowledgements
This research was supported by grants from the National Institutes of Health (R01AG031224; K01AG029218; K02 NS067427; T32 EB005970; P01 AG036694; K24 AG035007 and a Young Scholar Award from the Alzheimer's Association San Diego/Imperial Chapter (RSD)). Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co.,Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., and Wyeth, as well as non-profit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation.
Footnotes
Disclosure Statement
Dr. Anders M. Dale is a founder and holds equity in CorTechs Labs, Inc, and also serves on the Scientific Advisory Board. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies.
Dr. Linda K. McEvoy's spouse is CEO of CorTechs Labs, Inc.
References
- 1.Morris JC. Early-stage and preclinical Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19(3):163–5. doi: 10.1097/01.wad.0000184005.22611.cc. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42(3 Pt 1):631–9. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 5.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372(9634):216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 6.Ittner LM, Götz J. Amyloid-β and tau--a toxic pas de deux in Alzheimer's disease. Nat Rev Neurosci. 2011;12(2):65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 7.Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316(5825):750–4. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 8.Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, Wölfing H, Chieng BC, Christie MJ, Napier IA, Eckert A, Staufenbiel M, Hardeman E, Götz J. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142(3):387–97. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Desikan RS, McEvoy LK, Thompson W, Holland D, Roddey JC, Blennow K, Aisen PS, Brewer JB, Hyman BT, Dale AM, Alzheimer's Disease Neuroimaging Initiative Amyloid-β associated volume loss occurs only in the presence of phospho-tau. Ann Neurol. doi: 10.1002/ana.22509. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, LaRossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 11.Buerger K, Ewers M, Pirttilä T, Zinkowski R, Alafuzoff I, Teipel SJ, DeBernardis J, Kerkman D, McCulloch C, Soininen H, Hampel H. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain. 2006;129:3035–41. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 13.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141(11):1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 14.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ, Alzheimer's Disease Neuroimaging Initiative Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, Ackermann U, Cowie TF, Currie J, Chan SG, Jones G, Tochon-Danguy H, O'Keefe G, Masters CL, Rowe CC. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia. 2008;46(6):1688–97. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66(12):1469–75. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66(12):1476–81. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind MA, Zhang J, Peskind ER, Montine TJ. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69(7):631–9. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 19.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64(3):343–9. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 20.Hampel H, Blennow K, Shaw LM, Hoessler YC, Zetterberg H, Trojanowski JQ. Total and phosphorylated tau protein as biological markers of Alzheimer's disease. Exp Gerontol. 2010;45:30–40. doi: 10.1016/j.exger.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol. 2011;68(8):1062–4. doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- 22.Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Mintun MA, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, D'Angelo G, Malone JP, Townsend RR, Morris JC, Fagan AM, Holtzman DM. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biol Psychiatry. 2010;68(10):903–12. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarawneh R, D'Angelo G, Macy E, Xiong C, Carter D, Cairns NJ, Fagan AM, Head D, Mintun MA, Ladenson JH, Lee JM, Morris JC, Holtzman DM. Visinin-like protein-1: diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol. 2011;70(2):274–85. doi: 10.1002/ana.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]