Abstract
Background
Cilengitide is a selective integrin inhibitor that is well tolerated and has demonstrated biological activity in patients with recurrent malignant glioma. The primary objectives of this randomized phase II trial were to determine safety and efficacy of cilengitide when combined with radiation and temozolomide for newly diagnosed glioblastoma (GBM) and to select a dose for comparative clinical testing.
Methods
A total of 112 patients were accrued. Eighteen patients received standard RT+TMZ with cilengitide in a safety run-in phase followed by a randomized phase II with ninety-four patients assigned to either 500 or 2000 mg dose groups. The trial was designed to estimate overall survival benefit when compared with the NABTT internal historical control or the published EORTC 26981 data.
Results
Cilengitide at all doses studied was well tolerated with radiation and temozolomide. The median survival was 19.7 months for all patients, 17.4 months for those receiving the 500 mg dose, 20.8 months for those receiving the 2000mg dose, 30 months for patients with methylated MGMT promoters and 17.4 months for unmethylated patients. For patients ages 70 and younger, the median survival and survival at 24 months was superior to that observed in the EORTC trial (20.7 months vs 14.6 months and 41% vs 27% (p=0.008) respectively).
Conclusions
Cilengitide is well tolerated when combined with standard chemoradiation and may improve survival for patients newly diagnosed with GBM regardless of MGMT status. From an efficacy and safety standpoint, future trials of this agent in this population should utilize the 2000 mg dose.
Keywords: glioblastoma, integrin, cilengitide, clinical trial, angiogenesis
INTRODUCTION
Cilengitide (EMD121974, cyclo-L-Arg-Gly-L-Asp-D-Phe-N (Me) L-Val, Merck KGaA, Darmstadt, Germany) is a cyclic RGD containing peptide that binds to αvβ3 and αvβ5 with nanomolar affinity (1). In cell-based assays, cilengitide inhibited both αvβ3 and αvβ5 mediated function with IC50 values in the low micromolar range and inhibited angiogenesis in in vitro model systems (2, 3). Preclinical animal studies of glioma in both mice and rat models demonstrated tumor control and survival advantages (4-7).
The experience of cilengitide in patients with glioma has also demonstrated biological activity as measured by responses, progression-free survival, and overall survival. The initial phase I study in recurrent malignant glioma by Nabors et al failed to define a maximum tolerated dose (MTD) but suggested higher doses to be more active by measuring improvements in tumor blood flow with perfusion MRI (8). A randomized phase II trial in recurrent disease evaluated low and high dosees of the drug and likewise suggest greater biological activity at the higher dose (2000 mg twice a week) (9). In newly diagnosed patients with GBM, cilengitide at 500 mg twice a week was combined with standard therapy, temozolomide and radiation (10). When compared to historical controls, the authors noted improvements in both progression free survival and overall survival that appeared to be enhanced in the MGMT methylated patient population.
The rationale for the study of integrin antagonists in the setting of primary brain malignancies is thus provided. The present study was undertaken to determine the toxicities of cilengitide in patients with newly diagnosed GBM and determine the overall survival for patients treated with different doses of the agent.
PATIENTS AND METHODS
Eligibility Criteria
This study was sponsored by the Cancer Therapy Evaluation Program at the National Cancer Institute and conducted by the New Approaches to Brain Tumor Therapy CNS Consortium (www.nabtt.org for participating institutions). The protocol was reviewed and approved by the Institutional Review Board at each participating institution and all patients signed informed consent.
Patients eligible for enrollment met the following criteria: ≥18 years of age, newly diagnosed and histologically proven glioblastoma, maintained on a stable dose of corticosteriods for ≥5 days, recovered from previous surgery, Karnofsky performance status of ≥60%, adequate hematologic, renal, and hepatic function, agree to practice acceptable birth control method, a mini mental status exam score of ≥15, and capable of providing informed consent.
Treatment Plan
This study was designed as an open-label, randomized phase II study to evaluate the safety and efficacy of cilengitide in terms of overall survival. In addition, it was designed to pick one of two cilengitide doses in combination with standard RT+TMZ to be used in future clinical trials in this patient population. The phase II study was preceded by a safety run-in combining cilengitide with standard chemoradiation for patients newly diagnosed with glioblastoma. A total of 3 predefined doses (500, 1000, and 2000 mg twice a week) were selected. The study drug was infused intravenously over a one hour period on a twice a week schedule with a minimum of 72 hours between infusions for 4 weeks. This four week interval was considered a treatment cycle. The starting dose and schedule were selected based on previous studies of cilengitide in malignant glioma (8, 9). Toxicity was evaluated according to the National Cancer Institute Common Toxicity Criteria version 3. Temozolomide was administered daily at 75 mg/M2 concurrent with radiation therapy followed by a maintenance phase of 150-200 mg/M2 for 5 consecutive days out of every 28 day time interval. Radiation therapy was conventional treatment as utilized by the Radiation Therapy Oncology Group (RTOG). This consisted of treatment to the tumor plus a generous margin for a total dose of 6000 cGy in 30 fractions. During the randomized phase II portion, patients were randomized into one of two treatment groups (500 mg or 2000 mg) using a stratified and randomly permuted blocks method. The stratification was based on common prognostic factors of the disease to include age (≤50 or >50 years), KPS (≤80 or >80), and tumor status (measurable or unmeasurable disease). Patients were treated with adjuvant temozolomide for six months and with cilengitide until there was disease progression, unacceptable toxicity, or voluntary withdrawal.
Study Requirements
Tumor tissue from the patient’s surgery at diagnosis was collected for MGMT methylation status determination. Patients underwent evaluation of measurable disease by cranial magnetic resonance (MR) imaging four weeks after the complete of cilengitide plus chemoradiation and then after eight weeks thereafter. Laboratory studies including a chemistry profile and liver function enzymes were determined at baseline and prior to each odd numbered maintenance cycle. Complete blood counts were determined at baseline, weekly during radiation therapy, and monthly during maintenance. A urinalysis, EKG, and chest XRAY were completed at baseline.
MGMT Promoter Methylation Testing
Patient samples were tested for MGMT promoter methylation status using previously described procedures (11) by MDxHealth (Liege, Belgium). Briefly, forty microns of a formalin-fixed, paraffin embedded (FFPE) tumor sample were deparaffinated, digested and the genomic DNA isolated by the standard phenol-chloroform method. Up to 1,500 ng of gDNA was then treated by sodium bisulfite resulting in the deamination of non-methylated cytosine bases to uracil bases. Quantitative PCR for the methylated version of the MGMT gene and for the beta-Actin gene was then performed on the sample. The beta-Actin gene was used to establish sample quality and also used to normalize the test output. A threshold of 1,250 copies of actin (ACTB) was necessary in order to properly interpret the sample. The normalized ratio of mMGMT copy number to ACTB copy number was then calculated (mMGMT/ACTB × 1000). Samples with mMGMT:ACTB ratio of 2 or greater were considered methylated below this, the samples were considered unmethylated.
Statistical Considerations
The trial was designed to accrue 94 patients and analysis required 63 deaths. This would provide 94% power to detect a 30% or larger reduction in hazard of death (the hazard rate) compared to the NABTT internal historical control at an alpha level of 0.1. Comparison with the EORTC data was also proposed. Survival time was calculated from histological diagnosis until death from any cause, and surviving patients were censored at the time of last follow-up. The trial also was designed to further evaluate the safety and to identify either the 500 mg or the 2000mg dose as being the best for future clinical trials in this setting. This trial design was chosen as a consequence of our previous NABTT phase I study of this agent which suggested that the toxicities at the two dose levels woul be similar and that activity was seen across the range of doses tested (8) The overall hazard rate of death was defined as number of death per person-year of follow-up. A decision rule was included in the trial desgn specifying that a ≥30% reduction in the hazard rate compared to the NABTT internal historical control (which did not include temozolomide treated patients) would provide strong evidence to proceed to a formal comparative trial.
Patient characteristics and toxicities were summarized using appropriate descriptive statistics. Chi-Square statistic was used for proportion comparison. Student-T test was used for comparison of continuous data.
The method of Kaplan and Meier (12) was used to estimate overall survival. Confidence intervals were calculated using standard methods. Cox proportional-hazards regression model was used to estimate the hazard ratio for death attributable to the treatment and to prognostic factors. To pick a more efficacious dose in term of overall survival advantage, the two doses was compared using a non-parametric Log-Rank test.
All p-values are reported as two-sided and all analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Between April 2005 and December 2007, 112 patients were enrolled into the study. Their characteristics are described in Table 1. All patients had prior surgery and were histologically diagnosed with glioblastoma. The patient characteristics and prognostic factors were well balanced between treatment groups and comparable to historical controls. At the time of data cut-off (May 30, 2010), 6 patients were continuing to receive study drug infusions.
Table 1.
Patient Characteristics at Baseline
| NABTT 0306 | RT+TMZ+EMD All doses combined | RT+TMZ +EMD 500mg | RT+TMZ +EMD 2000mg | NABTT Historical RT+Chemo |
|---|---|---|---|---|
| Characteristics | N=112 | N=46 | N=48 | N=235 |
| Age – year | ||||
| Median | 55.5 | 56.3 | 54.9 | 55 |
| Range | 22--88 | 35—88 | 22--81 | 21--82 |
| Sex – no .(%) | ||||
| Male | 66 (59) | 28 (61) | 29 (60) | 151 (64) |
| Female | 46 (41) | 18 (39) | 19 (40) | 84 (36) |
| Karnofsky Performance Status no. (%) | ||||
| 100 | 27 (24) | 8 (17) | 15 (31) | 33 (18) |
| 90 | 51 (46) | 27 (59) | 18 (38) | 93 (50) |
| 80 | 15 (13) | 3 (7) | 7 (15) | 31 (17) |
| 70 | 15 (13) | 6 (13) | 6 (13) | 21 (11) |
| 60 | 4 (4) | 2 (4) | 2 (4) | 7 (4) |
| Surgical Procedure no. (%) | ||||
| Biopsy | 25 (22) | 10 (22) | 9 (19) | 29 (16) |
| Craniotomy | 86 (77) | 35 (76) | 39 (81) | 156 (84) |
| Other | 1 (1) | 1 (2) | ||
| Corticosteroid Therapy - no. (%) | ||||
| Yes | 82 (74) | 33 (72) | 36 (77) | 157 (67) |
| No | 28 (24) | 13 (28) | 11 (23) | 24 (10) |
| Data missing | 2 (2) | 54 (23) | ||
| Histological Diagnosis - no. (%) | * | |||
| Glioblastoma | 112 (100) | 46 (100) | 48 (100) | 181 (98) |
| Other | 3 (2) | |||
| MGMT no. (%) | ||||
| Methylated | 21 (19) | 11 (24) | 7 (15) | |
| Unmethylated | 48 (43) | 21 (46) | 18 (37) | |
| Unknown | 43 (38) | 14 (30) | 23 (48) |
Toxicity
Patient infusion with cilengitide did not produce any acute toxicities. During the safety run-in portion of the study, there were no DLTs at any dose level which were sufficient to define a MTD. During the randomized phase, hematologic and non-hematologic grade 3 or 4 adverse events were relatively evenly distributed with 48 occuring in the 500 mg cohort and 35 in the 2000 mg cohort. Hematological toxicities comprised 58% of the adverse events in patients receiving the 500 mg dose and 34% in the 2000 mg dose. These adverse events were uniformly attributed to the radiation and temozolomide. The non-hematological toxicities were also fairly evenly spread across the dose groups and exhibited no consistent concerning toxicity profiles. The range of adverse events seen is presented in Table 2.
Table 2.
Nonhematological Grade 3 or 4 Adverse Events (n= number of pts)
| Toxicity Grade 3-4 | 500mg | 1000mg | 2000mg | Total |
|---|---|---|---|---|
| No. of patients | n=52 | n=6 | n=54 | n=112 |
| ALT | 2 | 2 | ||
| Anorexia | 1 | 1 | ||
| Blood | 1 | 1 | ||
| Dehydration | 1 | 1 | ||
| Fatigue | 3 | 1 | 4 | |
| Head/headache | 2 | 2 | ||
| Heartburn | 0 | |||
| Hyponatremia | 1 | 1 | ||
| Hypophosphatemia | 1 | 1 | ||
| Muscle Weakness | 1 | 1 | ||
| Musculoskeletal | 1 | 1 | ||
| Nausea | 1 | 1 | ||
| Rash | 1 | 1 | ||
| SGPT | 1 | 1 | 2 | |
| Somnolence | 1 | 1 | ||
| Thrombosis | 3 | 3 | ||
| Vomiting | 1 | 1 | 2 |
Response Assessment
Overall survival
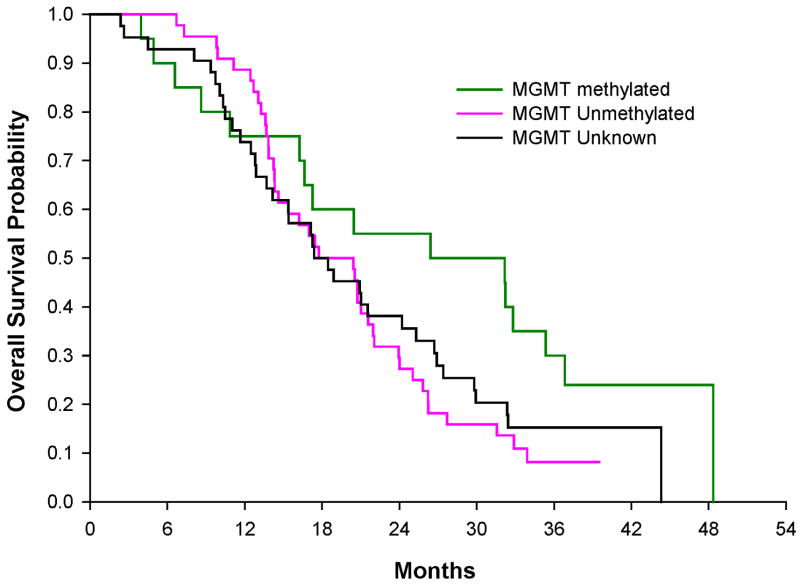
The estimated median survival time for all 112 patients enrolled on this study was 19.7 months (95% Confidence Interval (CI): 16.6 -21.9.months). The overall hazard rate of death was 0.494 (95% CI: 0.4 -0.6) per person-year of follow-up (Figure 1) and the estimated 2 year overall survival (OS) rate was 38% (95% CI: 29-48%). No patients were lost to follow-up.
Figure 1.
Kaplan-Meier survival curve. Kaplan-Meier survival curve for overall survival and 95% CI (shared area) for patients age ≤ 70 treated with RT+TMZ+EMD, the same age range as in the EORTC phase III trial. The black circle with 95% CI represents the mOS of 14.6 months for the EORTC phase III trial. The dash line represents the NABTT internal historical control prior TMZ.
In comparison to the NABTT historical control goup, which did not include patients treated with temozolomide, the unadjusted hazard ratio of death is 0.41 (95%CI: 0.32-0.53; p=0.0001) which constitutes a 59% reduction in the hazard of death. After adjusting for known prognostic factors (age (p=0.0003), KPS (p=0.004), and surgical procedure (p=0.003)), the adjusted hazard ratio of death is 0.39 (95%CI: 0.3-0.5, p<0.0001). In this trial, there were 101 patients age ≤70. Their median survival was 20.7 months (95%CI: 17.2-24.0) which is higher that the similarly aged patients in the EORTC study where the same radiation and temozolomide were used and the median survival was 14.6 months (95%CI: 13.2-16.8). The 24-month survival rate with cilengitide was 41% (95%CI: 31-51) as compared to the EORTC results of 27% (p=0.008, 95%CI: 21-32%).
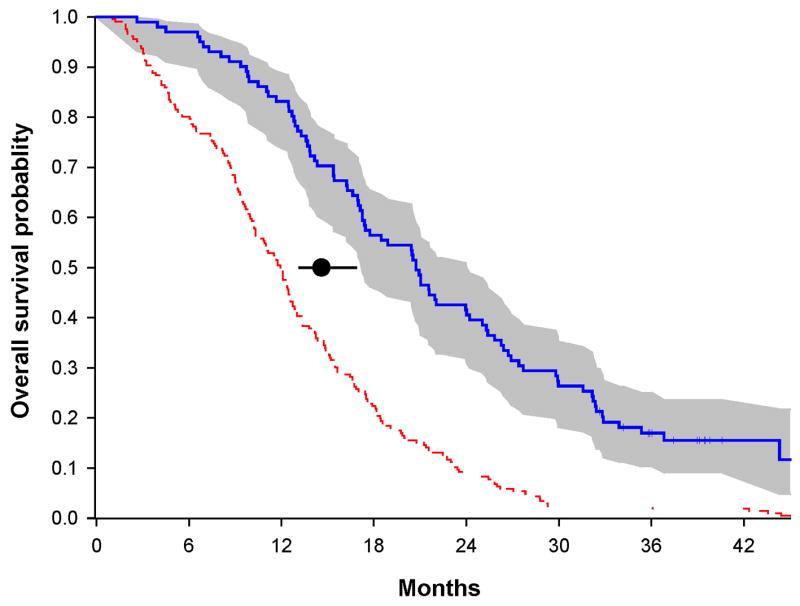
In the randomized portion of the trial for dose selection, ninety-four patients were assigned into either the 500 mg (46 pts) or the 2000 mg (48 pts) dose groups. An estimated median OS for the 500 mg group was 17.4 months (95% CI: 13.3-21.6 months) compared to a median OS of 20.8 months in the 2000 mg dose group (Figure 2). The unadjusted hazard ratio of death was 0.83 (95%CI: 0.5-1.3; p=0.4), a 17% reduction in the hazard of death for patient treated in the 2000 mg dose group compared to patients in the 500 mg dose group. After adjusting for stratification factors and well-known prognostic factors such as age, KPS, surgical procedure, and MGMT status at baseline, the adjusted hazard ratio of death was 0.8 (95%CI: 0.5-1.28), a 20% reduction in hazard of death for patient treated in the 2000 mg dose group compared to patients in the 500 mg dose group. The two years survival rates among those randomized patients are 28% (95%CI: 16-44%) and 44% (95%CI: 30-59%) for the 500 mg dose and the 2000 mg dose group, respectively.
Figure 2.
Kaplan-Meier overall survival curves for patients treated in the randomized phase II
Progression-free survival (PFS)
PFS was not a primary objective of this trial. However, disease progression was recorded in patient follow-up form as an off treatment criteria. The PFS was calculated from the date of diagnosis to the date of disease progression. A total of 103 (92%) out 112 patients had progressive disease or had died by the data cut-off date. The median progression-free survival was 9.97 months (95% CI: 8.3-11.1 months) for all adult patients and 10.6 months (95%CI: 8.5-13.2 months) for patients aged from 18 to 70. Among the 94 randomized patients, the PFS were similar between the two doses. The median PFS was 9.5 months (95%CI: 6.9-12.2 months) and 9.3 month (95% CI: 7.7-11.1months) for the 500mg group and the 2000mg group respectively. After adjusting for age, KPS, surgical procedure, and MGMT status at baseline, the adjusted hazard ratio of PFS is 1.07 (95%CI: 0.67-1.68) for patients in the 2000 mg group versus the 500 mg group.
MGMT status
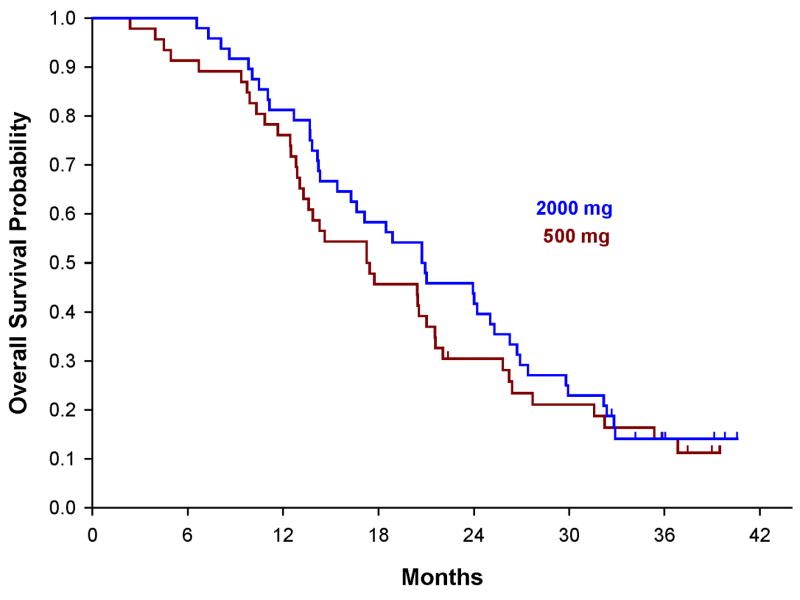
A total of 88 patients (79%, 88/112) had tumor tissue collected from baseline. Following neuropathological review, 79 were felt to have adequate tissue for baseline MGMT assessment. Twenty-one patients (19%) out of 112 had MGMT methylation at baseline, 48 (43%) unmethylated, and 43 (38%) methylation status unknown, hence, a MGMT methylation frequency of evaluable cases of 30% (21/69). The methylation status unknown includes those tested but judged invalid due to insufficient number of beta-actin gene copies or tumor tissue was not collected or available. The overall survival stratified by MGMT status at the baseline alone for all 112 patients is shown in Figure 3. The estimated median OS of patients who were methylated, unmethylated and unknown 30 months (95% CI: 16.6-35.4 months), 19.1 months (95% CI: 14.6-21.9 months), and 17.4 months (95% CI: 13.7-24.2 months), respectively.
Figure 3.
Kaplan-Meier plots of the overall survival curves stratified by patient’s MGMT status at the baseline
DISCUSSION
In this report, we extend our previous studies with the integrin antagonist, cilengitide to the newly diagnosed glioblastoma population and augment the safety and toxicity description of the compound with standard radiation and chemotherapy. Overall cilengitide was well tolerated without new or unexpected toxicities in this patient population when combined with radiation and temozolomide. The majority of the adverse events noted in the trial are consistent with the expected toxicities of standard chemoradiation. Specific events that may be induced or attributed by an agent with anti-angiogenic or anti-invasive properties such as hemorrhage were not noted. These findings are consistent with other studies of cilengitide for newly diagnosed GBM (10).
The primary endpoint of this study was to evaluate overall survival in patients newly diagnosed with GBM treated with cilengitide in conjunction with standard chemoradiation and to select a dose for comparative clinical trial testing. The trial achieved a 61% reduction in the hazard rate of death compared to NABTT historical control, which exceeded the protocol decision rule of a 30% or greater reduction in the failure rate. As the NABTT historical database did not include the use of temozolomide administered with and following radiation, the analysis planned before the 2005 publication of the EORTC results is of marginal importance in the current therapeutic environment. However, the observed median OS for all 112 patients enrolled in the trial was 19.7 months which compare very favorably with the 16.1 months reported from a recent study of low dose cilengitide (500 mg) in the same setting(10) and with the 14.6 months noted in the EORTC phase III trial (13) which included a better prognostic group of only patients age 70 or less. In addition, the celingitide treated patients had an improved two year survival for similarly aged patients (41% vs 27%).
Secondary objectives were to examine toxicity and survival in patients treated with low (500 mg) and high doses (2000 mg) of cilengitide and to choose the dose that should be studied in future phase III trials. The toxicities were similar. However, overall survival demonstrates a median OS of 20.8 months for the 2000 mg group and 17.4 months for the 500 mg group (adjusted hazard ratio of death is 0.8) and 2-year survival rates, of 44% in the 2000 mg group and 28% in the 500 mg group. Although these differences are not statistically different using a Cox Regression model (data not shown), this trial was not intended or powered to conduct such a comparison as our goal was to simply select the best dose to use in future studies. This data strongly suggest that Cilengitide dosed at 2000 mg twice a week with standard radiation and chemotherapy is the superior choice for phase III trials in patients with newly diagnosed GBM.
The determination of MGMT status in GBM has been shown to predict survival (14). We were able to determine MGMT status in 62% of the patients on study with 21 (30%) methylated of 69 with valid test results. In figure 3, Kaplan-Meier survival curves illustrate improved survival for MGMT methylated patients relative to unmethylated and unknowns. This observation is in keeping with other studies. It was not possible to make a reasonable analysis of the effect MGMT status had on survival based on the dose of cilengitide due to the small number. It does appear cilengitide impacted survival for the unmethylated as well as the methylated patients. We observed a median OS of 30 months for methylated and 19.1 months for unmethylated patients. This would be an improvement for both genotypes compared to EORTC 26981 where methylated patients treated with TMZ+RT had a median OS of 21.7 months and unmethylated 12.7.
The use of an integrin antagonist in the treatment of malignant glioma is emerging as a strong complement to radiation and temozolomide in patients with newly diagnosed GBM. The ability to exploit the novel integrin mediated signaling cascade brings to bear a new class of compounds for this disease. The randomized nature of the current study provides strong support for the use of 2000 mg dose and lays a foundation for the current comparative trials ongoing with cilengitide (CENTRIC). In addition, the different mechanism of action and excellent tolerability may permit cilengitide to also be combined with anti-VEGF agents for newly diagnosed GBM (15).
Table 3.
Comparison of baseline and overall survival for all subjects, those age 18-70 only, in RT+TMZ+EMD to EORTC phase III cohort
| Trial | RT+TMZ+EMD | EORTC Phase III |
|---|---|---|
| Age 18-70 (n=101) | Age 18-70 (n=287) | |
| No. of patients (%) | No. of patients (%) | |
| Baseline: | ||
| Age Median (range), yr | 55 (23-70) | 56 (19-70) |
| KPS, 90-100 | 72 (71) | 249 (86) |
| Surgery, Debulking | 77 (76) | 239 (83) |
| Corticosteroids, yes | 75 (76) | 193 (67) |
| Histology, GBM | 101 (100) | 221 (92) |
| Outcome: | ||
| Median overall survival (95% CI) | 20.7 (17-24) | 14.6 (13-17) |
| % overall survival at 24 months (95% CI)* | 41 (31 -51) | 27 (21-32) |
p< 0.05
Acknowledgments
Supported by National Institutes of Health [Grant #: CA-62475]
Footnotes
Financial Disclosures: All Authors reports no financial disclosures except T. Mikkelsen reports research funding support from Merck KGaA; and T. Batchelor reports EMD-Serono consultant in 2009.
References
- 1.Goodman SL, Holzemann G, Sulyok GA, Kessler H. Nanomolar small molecule inhibitors for alphav(beta)6, alphav(beta)5, and alphav(beta)3 integrins. J Med Chem. 2002 Feb 28;45(5):1045–51. doi: 10.1021/jm0102598. [DOI] [PubMed] [Google Scholar]
- 2.Germer M, Kanse SM, Kirkegaard T, Kjoller L, Felding-Habermann B, Goodman S, et al. Kinetic analysis of integrin-dependent cell adhesion on vitronectin--the inhibitory potential of plasminogen activator inhibitor-1 and RGD peptides. Eur J Biochem. 1998 May 1;253(3):669–74. doi: 10.1046/j.1432-1327.1998.2530669.x. [DOI] [PubMed] [Google Scholar]
- 3.Nisato RE, Tille JC, Jonczyk A, Goodman SL, Pepper MS. alphav beta 3 and alphav beta 5 integrin antagonists inhibit angiogenesis in vitro. Angiogenesis. 2003;6(2):105–19. doi: 10.1023/B:AGEN.0000011801.98187.f2. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee S, Matsumura A, Schradermeier J, Gillespie GY. Human malignant glioma therapy using anti-alpha(v)beta3 integrin agents. J Neurooncol. 2000;46(2):135–44. doi: 10.1023/a:1006444300504. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald TJ, Taga T, Shimada H, Tabrizi P, Zlokovic BV, Cheresh DA, et al. Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery. 2001 Jan;48(1):151–7. doi: 10.1097/00006123-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 6.Yamada S, Bu XY, Khankaldyyan V, Gonzales-Gomez I, McComb JG, Laug WE. Effect of the angiogenesis inhibitor Cilengitide (EMD 121974) on glioblastoma growth in nude mice. Neurosurgery. 2006;59(6):1304–12. doi: 10.1227/01.NEU.0000245622.70344.BE. discussion 12. [DOI] [PubMed] [Google Scholar]
- 7.Mikkelsen T, Brodie C, Finniss S, Berens ME, Rennert JL, Nelson K, et al. Radiation sensitization of glioblastoma by cilengitide has unanticipated schedule-dependency. Int J Cancer. 2009 Jun 1;124(11):2719–27. doi: 10.1002/ijc.24240. [DOI] [PubMed] [Google Scholar]
- 8.Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007 May 1;25(13):1651–7. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008 Dec 1;26(34):5610–7. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 10.Stupp R, Hegi ME, Neyns B, Goldbrunner R, Schlegel U, Clement PM, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010 Jun 1;28(16):2712–8. doi: 10.1200/JCO.2009.26.6650. [DOI] [PubMed] [Google Scholar]
- 11.Vlassenbroeck I, Califice S, Diserens AC, Migliavacca E, Straub J, Di Stefano I, et al. Validation of real-time methylation-specific PCR to determine O6-methylguanine-DNA methyltransferase gene promoter methylation in glioma. J Mol Diagn. 2008 Jul;10(4):332–7. doi: 10.2353/jmoldx.2008.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 13.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005 Mar 10;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 14.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005 Mar 10;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 15.Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011 Jan 10;29(2):142–8. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]