Abstract
In the model organism Caenorhabditis elegans, a class of small molecule signals called ascarosides regulate development, mating and social behaviors. Ascaroside production has been studied in the predominant sex, the hermaphrodite, but not in males, which account for less than 1% of wild-type worms grown under typical laboratory conditions. Using HPLC-MS-based targeted metabolomics, we show that males also produce ascarosides and that their ascaroside profile differs markedly from that of hermaphrodites. Whereas hermaphrodite ascaroside profiles are dominated by ascr#3, containing an α,β-unsaturated fatty acid, males predominantly produce the corresponding dihydro-derivative ascr#10. This small structural modification profoundly affects signaling properties: hermaphrodites are retained by attomole-amounts of male-produced ascr#10, whereas hermaphrodite-produced ascr#3 repels hermaphrodites and attracts males. Male production of ascr#10 is population density-dependent, indicating sensory regulation of ascaroside biosynthesis. Analysis of gene expression data supports a model in which sex-specific regulation of peroxisomal β-oxidation produces functionally different ascaroside profiles.
C. elegans is rapidly being developed as a model organism for the study of endogenous small molecule signals that regulate diverse aspects of animal life history, including development, lifespan, and social behaviors.(1–3) Recently, we used targeted comparative metabolomics to investigate the biosynthesis of the ascarosides in C. elegans, a family of small molecule signals based on the dideoxy sugar ascarylose and additional building blocks from lipid and amino acid metabolism (Figure 1).(3) Ascarosides were originally identified as the main components of the dauer pheromone(4, 5) and have since been shown to mediate several additional aspects of worm behavior, including mating, aggregation, avoidance, and olfactory learning.(6–9)
Figure 1.
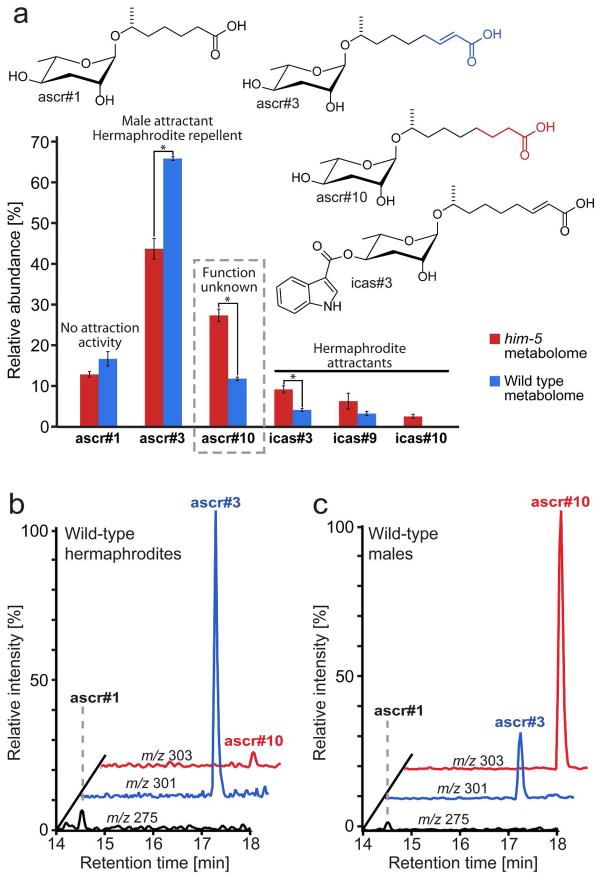
Identification of male-specific ascarosides in C. elegans. a) Relative abundances of ascarosides ascr#3, ascr#10, and several indole ascarosides markedly differs between wild-type (N2) and him-5 metabolomes, whereas abundances of many other ascarosides, for example the dauer pheromone component ascr#1, remains unchanged. Relative abundances were calculated in % of the total amount of the six ascarosides in this Figure (*P < 0.05 unpaired t-test with Welch’s correction; error bars: SD). b) Ion chromatograms for ascr#1, ascr#3, and ascr#10 from negative-ion ESI–LC/MS analysis of exo-metabolome samples obtained from 200 hermaphrodite worms c) Ion chromatogram for LC/MS analysis of exo-metabolome samples derived from 200 male worms.
Ascaroside functions are highly sex- and structure-dependent. For example, whereas ascr#3 attracts male C. elegans but repels hermaphrodites,(6, 10, 11) the related icas#3 acts a general aggregation signal, attracting both hermaphrodites and males.(7) Previous metabolomic analyses have de facto profiled ascaroside production of hermaphrodites due to their much greater abundance in wild-type cultures grown under typical laboratory conditions.(7, 12) Because hermaphrodites and male C. elegans markedly differ in their biological responses to ascaroside-derived signaling molecules,(6, 13) we asked whether male C. elegans biosynthesize ascarosides and whether the male ascaroside profile differs from that of hermaphrodites.
C. elegans is androdioecious and laboratory cultures of wild-type worms are predominantly composed of selfing hermaphrodites.(14) Male C. elegans in the wild-type strain arise due to spontaneous non-disjunction in 0.1% of the progeny.(15, 16) The incidence of males increases in response to stress, and it is possible that in the wild males are more abundant.(17) We began with investigating the metabolome of him-5 worms, a mutant strain that produces a much larger percentage of males (about 30%) than wild-type worms.(15) Targeted mass-spectrometric analyses of the him-5 exo-metabolome, the entirety of small molecules found in worm-conditioned media, revealed the production of the same set of ascarosides found in wild-type controls; however, the relative amounts of some of the most abundant ascarosides in him-5 and wild-type cultures differed significantly (Figure 1a). Specifically, relative amounts of the ascaroside ascr#10, which has not previously been studied in detail, and the known hermaphrodite aggregation factor, icas#3,(7) were higher in him-5 exo-metabolome samples than in wild-type. Notably, ascr#3, a male attractant,(6) was relatively more abundant in the wild-type than in the him-5 exo-metabolome.
To determine whether increased production of these ascarosides was due to the larger percentage of males in him-5 cultures, we developed a protocol for the analysis of the exo-metabolome of small numbers of worms based on selective-ion monitoring (SIM) of ascaroside-associated ions. The identity of peaks whose specific retention times and molecular ions suggested ascarosides was confirmed further by additionally scanning in HPLC-MS/MS mode for precursor ions of m/z 73, a fragment ion characteristic for ascarosides.(3) Using this strategy, attomolar amounts of ascarosides could be detected. We were able to detect ascr#1, ascr#3, and ascr#10 as the major secreted ascarosides in samples derived from as few as 100 wild-type hermaphrodite worms staged as L4 through young adults (Figure 1b). Next we analyzed samples of similarly staged 200 him-5 males, and subsequently wild-type males, which we obtained by increasing male frequency by crossing males and hermaphrodites. These male-only samples revealed the same three ascarosides, ascr#1, ascr#3, and ascr#10, we found in hermaphrodites, but in markedly different proportions (Figures 1b,c). Whereas hermaphrodites excrete the unsaturated ascr#3 as the major component, the corresponding dihydro-derivative, ascr#10, represents the most abundant ascaroside in male samples. Relative production of the saturated ascr#1 did not differ dramatically in males and hermaphrodites. Higher secretion of ascr#10, relative to ascr#3, by males was observed consistently using different growth media (S-media and water) and incubation durations; however, we were unable to detect and reliably quantify the abundance of other ascarosides on this small scale. To check whether increased production of ascr#10 is a general characteristic of male C. elegans and not a specific characteristic of the laboratory wild-type strain N2 Bristol, we also investigated the ascaroside profile of a second C. elegans wild-type strain, Hawaii CB4846.(18) We found that, as in N2 Bristol, ascr#3 is the most abundant ascaroside excreted by hermaphrodites, whereas males excrete predominantly ascr#10 (Figure S1).
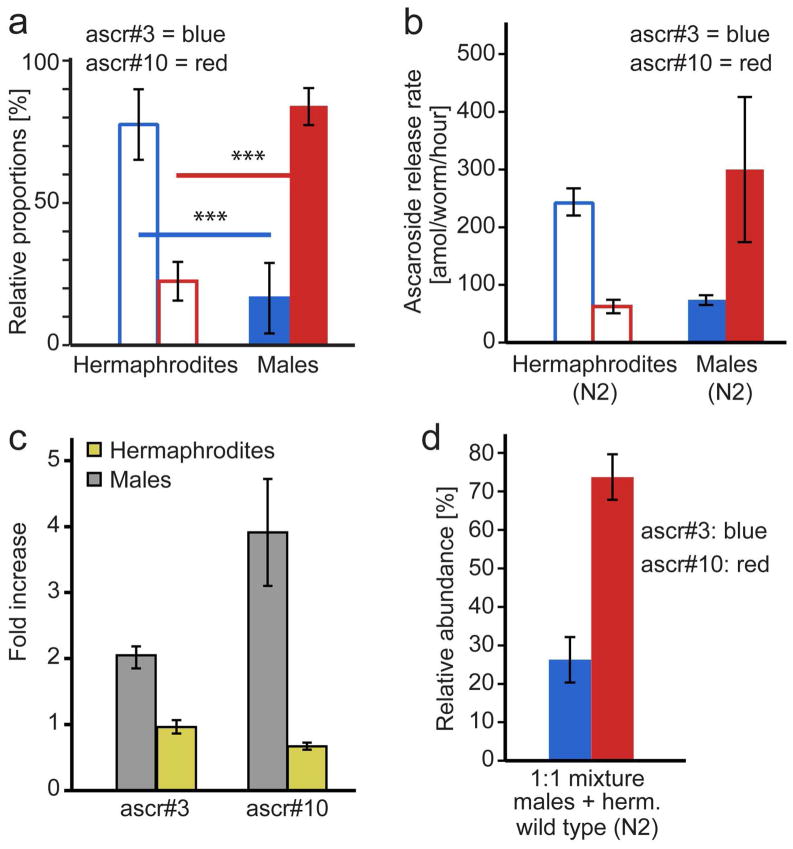
Quantitative LC-MS analyses confirmed that males produce and release much larger amounts of ascr#10 than hermaphrodites (Figure 2a,b, S2). However, whereas relative ascaroside abundances were highly reproducible for both males and hermaphrodites (Figure 2a), we found that ascaroside excretion rates of males, but not hermaphrodites, were highly variable (Figure 2b). We then asked whether ascaroside release rates are dependent on worm population density. Strikingly, we found that a doubling of the density of wild-type males led to an almost 4-fold increase in the excretion rate of ascr#10, as well as a smaller increase in ascr#3 excretion rate (Figure 2c). In contrast, ascaroside production by hermaphrodites did not appear to be density dependent, and the presence of hermaphrodites did not increase male production of ascr#10 and ascr#3 (Figures 2c and S2c). Furthermore, male ascaroside production dominates in mixtures of wild-type males and hermaphrodites (Figure 2d). These results show that C. elegans males produce a sex-specific blend of ascarosides with ascr#10 as the major component and that ascaroside release in wild-type males is promoted by the presence of other worms.
Figure 2.
Males produce large amounts of ascr#10 (error bars: SD). a) Relative abundances of ascr#3 and ascr#10 in wild-type (N2) hermaphrodite and male exo-metabolomes. (***P < 0.0001 unpaired t-test with Welch’s correction). b) Ascaroside release rates of wild-type males and hermaphrodites. c) Fold increase of ascr#3 and ascr#10 production per worm in response to doubling of worm density from 100 to 200 wild-type hermaphrodites or males per well. Ascaroside production of wild-type hermaphrodites does not change significantly, whereas ascr#3 and ascr#10 production of wild-type males increases 2-fold and 4-fold, respectively. d) The ascaroside profile of 1:1 mixtures of wild-type (N2) males and hermaphrodites is dominated by the primarily male-produced ascr#10.
Ascr#3 has previously been shown to attract males at low concentrations and repel hermaphrodites at higher concentrations,(6) whereas the biological roles of ascr#10 have not been investigated in detail. Given that ascr#10 production is strongly upregulated in males, we asked whether ascr#10 plays a specific role in hermaphrodite-male interactions. Using synthetic samples we tested ascr#10 on both sexes in a behavioral assay that measures holding time (the time the worms remain in the scoring region) in response to compound exposure (Figure 3a). Ascr#10 did not affect male behavior at any of the wide range of sample amounts tested, but elicited a very strong response in hermaphrodites at sample amounts as low as 1 attomole, exceeding the potency of previously identified hermaphrodite attractants such as icas#3 by more than 100-fold in this assay (Figure 3b).(7) Next we investigated the effect of ascr#10 on hermaphrodite chemotaxis and aggregation, which revealed significant activity at concentrations of 1 nM (Figure 3c,d, S3). These results show that, compared to icas#3, ascr#10 is much more potent in the spot retention assay but less active in the chemotaxis and aggregation assays.(7) Therefore it appears that ascr#10 serves a specific function as a holding signal, which is also supported by the very long retention times observed with ascr#10 in the spot retention assay (Figure 3b). Given that only 1 attomole of ascr#10 is required to elicit hermaphrodite holding behavior, the amounts of ascr#10 excreted by a single male (about 300 attomole per worm per hour) appear sufficient to induce hermaphrodite retention. We propose that ascr#10, likely in combination with other, less abundant components, is an important part of the male sex pheromone blend in C. elegans.(19)
Figure 3.
Sex-specific behavioral responses to male-produced ascr#10 and regulation of ascaroside biosynthesis. a) Spot retention assay used to measure the effect of ascr#10 on male and hermaphrodite behavior.(6) The red ‘X’ denotes the initial position of the assayed worms. b) Results from spot retention assays reveal that hermaphrodites, but not males, are attracted to ascr#10 sources (see Figure S3 for data for wild-type males). *P < 0.01, **P < 0.001, ***P < 0.0001, unpaired t-test followed by Welch’s correction; error bars: SD. c) Schematic representation of quadrant bioassay used to measure chemotaxis to ascr#10. A red “X” marks the spot where worms are placed at the beginning of the assay. d) Chemotaxis of males and hermaphrodites to ascr#10 as measured by the quadrant assay. **P < 0.01 one-factor ANOVA followed by Dunnett’s post-test; error bars: SD. e) Sex-specific regulation of ascaroside biosynthesis. Shown gene expression ratios are based on DNA microarray experiments of L4-staged worms.(16) The expression ratios highlight differential expression of four peroxisomal enzymes involved in biosynthesis of the ascaroside sidechain in hermaphrodites and males. Relatively decreased acox-1 expression in males is consistent with the observed increase of saturated ascr#10 in the male exo-metabolome.
Next we asked whether sex-specific differences in the regulation of ascaroside biosynthesis enzymes could account for the observed differences between male and hermaphrodite ascaroside profiles. The fatty-acid derived side chains in the ascarosides are derived from peroxisomal β-oxidation of longer-chained precursors (Figure 3e).(3) We found that male worms carrying a mutation in the peroxisomal thiolase daf-22 do not produce ascarosides, confirming that peroxisomal β-oxidation is required for ascaroside biosynthesis in males (Figure S4). Next we analyzed previously published global DNA microarray data for males and hermaphrodites at the L4 stage, corresponding to the developmental stage we used for our metabolomic analyses. We found significant sex-specific differences in the expression levels of all four enzymes known to participate in peroxisomal β-oxidation in L4 worms (Figure 3e).(20) Relative to the other sex, expression of the acyl-CoA oxidase ACOX-1, which introduces the double bond in the biosynthesis of ascr#3 from ascr#10, is upregulated in hermaphrodites, whereas the next two enzymes in the pathway, MAOC-1 and DHS-28, are upregulated in males. Higher expression levels of ACOX-1 in hermaphrodites are consistent with increased abundance of the α,β-unsaturated ascr#3 in this sex. In addition, relatively higher expression of the downstream enzymes MAOC-1 and DHS-28 may result in further depletion of ascr#3 in males. In conjunction with the results from our metabolomic analyses, these findings indicate sex specific regulation of peroxisomal β-oxidation in C. elegans.
In summary, we have demonstrated that ascaroside biosynthesis and functions are sex-specific. Male C. elegans produce a sex-specific blend of ascarosides, the major component of which, ascr#10, strongly retains and attracts hermaphrodites, whereas C. elegans hermaphrodites most abundantly produce the corresponding dehydro-derivative ascr#3, which serves as a male attractant but repels hermaphrodites. A single double bond differentiates ascr#10 and ascr#3, yet dramatically changes the signaling properties of these two molecules. The differences between male and hermaphrodite expression levels of the four genes involved in peroxisomal side chain biosynthesis as well as the results from our worm-body ascaroside analyses suggest that the observed sex-specific differences in ascaroside profiles are not simply the result of relative differences in ascaroside secretion rate, but rather result from sex-specific control of ascaroside biosynthesis. We further show that male C. elegans respond to the presence of other worms with increased ascaroside production, a type of response not seen in hermaphrodites. Whether sensing of the male-specific ascarosides plays a role in male population density signaling remains to be determined. Given the large quantities of ascr#10 produced by males and its potent activity, it is likely that ascr#10 constitutes an important component of the male sex pheromone blend in C. elegans. Based on our bioassay results it appears that the amounts of ascr#10 excreted by a single male are sufficient to affect hermaphrodite behavior. It is likely that other, less abundant ascarosides contribute to hermaphrodite retention and attraction, for example, males may also produce some of the indole ascarosides we found to be upregulated in the male-rich him-5 liquid cultures (Figure 1a). Notably, hermaphrodite worms also release ascr#10, although in smaller quantities, which could contribute to indole ascaroside-mediated aggregation or counteract dispersal behavior driven by ascr#3.(7)
Our study shows that minute changes in ascaroside structures can dramatically affect their signaling properties. HPLC-MS-based metabolic profiling of small numbers of worms, as demonstrated here, provides the basis for more detailed exploration of the biological functions and underlying sensory mechanisms of this diverse library of small molecule signals.
Methods
Preparation of metabolite extracts
Large liquid culture exo-metabolome samples of wild-type and him-5 mutant worms were prepared as described previously.(3) Briefly, wild-type or him-5 mutant worms were grown for two generations on 6 cm NGM plates seeded with E. coli OP50 bacteria. Worms from three NGM plates were washed into 100 mL of S-media into a 500 mL Erlenmeyer flask and grown at 22 °C and 220 pm. Concentrated bacteria from 1 L cultures, grown overnight, were added on day 1 and day 3. On day 5, the liquid culture was split into two 500-mL Erlenmeyer flasks and S-media was added to maintain a volume of 100 ml per flask. Additional concentrated bacteria derived from 1 L OP50 culture was added as food upon splitting. The cultures were harvested on day 7 by centrifugation at 4750 rpm. The supernatant was lyophilized and the residue extracted with 95% ethanol (300 mL) at room temperature for 12 h. The resulting suspension was filtered and evaporated in vacuo at room temperature.
For preparation of small-scale exo-metabolome samples, wild-type (N2), him-5, and daf-22 worms were grown for at least two generations on NGM plates seeded with OP50. Male production in wild-type (N2) and daf-22 worms was induced by placing L4 hermaphrodite worms at 30 °C with ethanol for 6 h (WormAtlas, http://www.wormatlas.org/). Wild type and daf-22 mutant worms were synchronized by timed egg lay from mated parents. him-5 worms were synchronized by timed egg lay.
For exo-metabolome collection, 100 or 200 L4 male or hermaphrodite worms were picked with an aluminum wire pick and placed into 250 μL of water or S-media in a 96 well plate (BD Biosciences) and incubated for 17 hours at 22 °C and 220 pm. The solution was filtered over cotton to remove worms and evaporated in vacuo at room temperature. The extract was taken up in 100 μL methanol for subsequent HPLC-MS analysis. For mixed-gender experiments a mixture of 50 male and 50 hermaphrodite L4 worms (all wild-type) was incubated in 250 μL of water in a 96 well plate for 17 h at 22 °C and 220 pm. Samples were extracted and prepared for analysis as described above.
For density experiments, synchronized wild-type (N2) L4 lavae were used. 100 males, 100 hermaphrodites, 200 males, 200 hermaphrodites, or 100 males and 100 hermaphrodites (all L4 stage) were placed into 250 μL of water in a 96 well plate and for 17 h at 22 °C and 220 pm. Samples were extracted and prepared for analysis as described above.
Small-scale worm body extracts were prepared similarly to worm exo-metabolome samples except for the following modifications. 200 synchronized L4 male or hermaphrodite worms were picked onto a seeded NGM plate. M9 buffer was used to wash the worms into a 10 mL falcon tube, and the worms were washed twice with M9 buffer. A suspension of the worms in a small amount of M9 buffer (~50 μL) was lyophilized and the residue was extracted with 95% ethanol (2 ml). After filtration, the extract was evaporated to dryness and re-dissolved in 100 μL of methanol for subsequent HPLC-MS analysis. For all worm exo-metabolome analyses at least 2 independent replicates were performed. Figure 2a summarizes data from 11 replicates.
Mass spectrometric analysis
HPLC-MS analysis was performed using an Agilent 1100 Series HPLC system equipped with an Agilent Eclipse XDB-C18 column (9.4 × 250 mm, 5 μm particle diameter) connected to a Quattro II spectrometer (Micromass/Waters) using a 10:1 split. Samples were analyzed using a water (0.1% acetic acid) – acetonitrile gradient with a flow rate of 3.6 mL min−1. Acetonitrile content was held at 5% for the first 5 minutes then increased to 100% over 40 minutes. Samples were analyzed by HPLC-ESI-MS in negative and positive ion modes. Single Ion Monitoring (SIM) in negative-ion ionization mode was used to detect the molecular ions ([M–H]−) of ascr#1, ascr#3, and ascr#10 at m/z 247.2, 301.2 and 303.2, respectively. Ascaroside identification was confirmed by MS/MS analysis and comparison with published retention times,(3) and injection of synthetic standards.
Quantification of ascarosides
Relative ascaroside content was quantified by integration of LC-MS signals from corresponding ion traces. Absolute quantification of ascarosides was achieved by injection of solutions of known concentration of synthetic ascr#1, ascr#3, and ascr#10. Ascaroside release was calculated in attomoles of ascarosides per hour per worm, and ascaroside content in worm-body extracts was calculated in attomoles of ascarosides per worm.
Spot retention assays
Spot retention assays were performed as described previously.(6, 10) Briefly, for both hermaphrodites and males, we harvested 50–60 L4-stage worms daily and stored them segregated by sex at 20 °C overnight to be used as young adults the following day. Aliquots of ascr#10 assay solutions, dissolved in 10% ethanol, were stored at 20 °C in 20 μL tubes. 10% ethanol in water was used as control.
Quadrant chemotaxis assays
Chemotaxis was assessed on 10-cm four-quadrant Petri plates.(7) Each quadrant was separated from adjacent ones by plastic spacers (Figure 3c). Pairs of opposite quadrants were filled with NGM agar with or without ascr#10. Worms were placed in the center of the plate and scored after 30 min. A chemotaxis index was calculated as (number of animals on ascaroside quadrants minus number of animals on control quadrants)/(total number of animals).
Aggregation assays
Aggregation assays were conducted as described previously.(7, 21)
Supplementary Material
Acknowledgments
We thank S. Lee (Cornell University) for helpful discussions and J. Liu (Cornell University) for providing strain CB4846. This work was supported in part by the National Institutes of Health (GM088290 to FCS, GM008500 to YI, and GM085285 to FCS and PWS), and the Howard Hughes Medical Institute, with which PWS is an Investigator, as well as the Vassar College Mary Landon Sague Fellowship (to YI).
Footnotes
Supporting Information Available. Figures S1–S4. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Gene Dev. 2008;22:2149–2165. doi: 10.1101/gad.1701508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hulme SE, Whitesides GM. Chemistry and the Worm: Caenorhabditis elegans as a Platform for Integrating Chemical and Biological Research. Angew Chem Int Edit. 2011;50:4774–4807. doi: 10.1002/anie.201005461. [DOI] [PubMed] [Google Scholar]
- 3.von Reuss SH, Bose N, Srinivasan J, Yim JJ, Judkins JC, Sternberg PW, Schroeder FC. Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small molecule signals in C. elegans. J Am Chem Soc. 2012;134:1817–1824. doi: 10.1021/ja210202y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong PY, Jung M, Yim YH, Kim H, Park M, Hong EM, Lee W, Kim YH, Kim K, Paik YK. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 5.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 6.Srinivasan J, Kaplan F, Ajredini R, Zachariah C, Alborn HT, Teal PE, Malik RU, Edison AS, Sternberg PW, Schroeder FC. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454:1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan J, von Reuss SH, Bose N, Zaslaver A, Mahanti P, Ho MC, O’Doherty OG, Edison AS, Sternberg PW, Schroeder FC. A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans. PLoS Biol. 2012;10:e1001237. doi: 10.1371/journal.pbio.1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, Bargmann CI. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458:1171–1175. doi: 10.1038/nature07886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada K, Hirotsu T, Matsuki M, Butcher RA, Tomioka M, Ishihara T, Clardy J, Kunitomo H, Iino Y. Olfactory plasticity is regulated by pheromonal signaling in Caenorhabditis elegans. Science. 2010;329:1647–1650. doi: 10.1126/science.1192020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pungaliya C, Srinivasan J, Fox BW, Malik RU, Ludewig AH, Sternberg PW, Schroeder FC. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2009;106:7708–7713. doi: 10.1073/pnas.0811918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choe A, von Reuss SH, Kogan D, Gasser RB, Platzer EG, Schroeder FC, Sternberg PW. Ascaroside Signaling Is Widely Conserved among Nematodes. Curr Biol. 2012;22:772–780. doi: 10.1016/j.cub.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan F, Srinivasan J, Mahanti P, Ajredini R, Durak O, Nimalendran R, Sternberg PW, Teal PEA, Schroeder FC, Edison AS, Alborn HT. Ascaroside Expression in Caenorhabditis elegans Is Strongly Dependent on Diet and Developmental Stage. PLoS One. 2011;6:e17804. doi: 10.1371/journal.pone.0017804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edison AS. Caenorhabditis elegans pheromones regulate multiple complex behaviors. Curr Opin Neurobiol. 2009;19:378–388. doi: 10.1016/j.conb.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chasnov JR, Chow KL. Why are there males in the hermaphroditic species Caenorhabditis elegans? Genetics. 2002;160:983–994. doi: 10.1093/genetics/160.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodgkin J, Horvitz HR, Brenner S. Nondisjunction Mutants of the Nematode Caenorhabditis elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schedl T, Graham PL, Barton MK, Kimble J. Analysis of the Role of Tra-1 in Germline Sex Determination in the Nematode Caenorhabditis elegans. Genetics. 1989;123:755–769. doi: 10.1093/genetics/123.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodgkin J, Doniach T. Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics. 1997;146:149–164. doi: 10.1093/genetics/146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia LR, LeBoeuf B, Koo P. Diversity in mating behavior of hermaphroditic and male-female Caenorhabditis nematodes. Genetics. 2007;175:1761–1771. doi: 10.1534/genetics.106.068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prahlad V, Pilgrim D, Goodwin EB. Roles for mating and environment in C. elegans sex determination. Science. 2003;302:1046–1049. doi: 10.1126/science.1087946. [DOI] [PubMed] [Google Scholar]
- 20.Jiang M, Ryu J, Kiraly M, Duke K, Reinke V, Kim SK. Genome-wide analysis of developmental and sex-regulated gene expression profiles in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2001;98:218–223. doi: 10.1073/pnas.011520898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.