Abstract
Abnormal T-cell signaling and activation is a characteristic feature in systemic lupus erythematosus (SLE). Lupus T cells are shifted towards an over-activated state, important signaling pathways are rewired, and signaling molecules are replaced. Disturbances in metabolic and organelle homeostasis, importantly within the mitochondrial, endosomal, and autophagosomal compartments, underlie the changes in signal transduction. Mitochondrial hyperpolarization, enhanced endosomal recycling, and dysregulated autophagy are hallmarks of pathologic organelle homeostasis in SLE. This review is focused on the metabolic checkpoints of endosomal traffic that control immunological synapse formation and mitophagy and may thus serve as targets for treatment in SLE.
Keywords: Systemic lupus erythematosus, T cell, mitochondria, endocytic recycling, mammalian target of rapamycin, autophagy
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disease of complex etiology where immune system deregulation results in widespread inflammation and tissue damage [1]. Pathogenic factors include influences from the environment [2], genetics [3], and epigenetic modifications including DNA hypomethylation [4]. Genetic factors result in a 20–60% concordance of lupus in monozygotic twins, indicating that genetics alone are not solely responsible for development of autoimmunity in SLE [5]. Early environmental exposures to ultraviolet irradiation, chemicals, and infectious agents can permanently alter plasticity of the developing immune system, where overproduction of pro-inflammatory cytokines, including IL-6, results in impaired tolerance induction [2].
SLE is characterized by autoantibody formation against nucleosome components, resulting in anti-nuclear antibody (ANA) production by auto-reactive B cells [1]. Autoantibody production occurs secondary to activation of dendritic cells by necrotic, but not apoptotic, debris [6]. SLE T cells have an increased propensity to undergo necrosis upon stimulation, providing substrates for autoantibody generation. Circulating auto-antibodies form immune complexes which deposit in vasculature, activate complement, and incite inflammation, resulting in end-organ damage [7].
While SLE T cells have increased necrosis, activation induced cell death (AICD) is reduced, allowing for persistence of autoreactive lymphocytes [8]. The balance between apoptotic and necrotic cell death in SLE T cells is disturbed due to mitochondrial dysfunction, characterized by increased mass and transmembrane potential (↑Δψm) [9]. Mitochondrial accumulation may occur as a result of defective autophagic turnover of mitochondria (mitophagy) [10] and increased nitric oxide (NO)-dependent biogenesis [11, 12]. Increased mitochondrial mass and ↑ Δψm allows for sustained T cell activation [13, 14].
Increased T cell activation is central to SLE pathogenesis, as lupus T cells provide help to autoreactive B cells, produce pro-inflammatory cytokines, and stimulate dendritic cell function [15]. Lupus T cells are more sensitive to T cell antigen receptor (TCR) stimulation, with a decreased threshold of activation required to induce intracytoplasmic calcium (Ca2+) fluxing [16]. Pre-clustering of lipid raft domains, which are required for IS formation, and altered activity of protein tyrosine kinases and phosphatases within these raft domains, contribute to increased sensitivity to T cell receptor (TCR) stimulation [17]. Over-expression of endosomal Rab GTPases in SLE T cells [18] contributes to altered recruitment of TCR-associated components to the IS [19] and is required for activation of the mammalian target of rapamycin (mTOR) [20].
mTOR is a key integrator of nutrient signals and regulator of cellular metabolism, which is over-expressed in SLE T cells [18]. mTOR activation controls differentiation of CD4+ and CD8+ T cells through transcriptional regulation [21]. Lupus T cells exhibit altered lineage specification, characterized by depletion of CD4+CD25+Foxp3+ regulatory T cells (Tregs) [15], and accumulation of uncommitted CD4−CD8− double negative (DN) T cells [22, 23], which could result from increased mTOR activity. The lower frequency of Tregs results in failure to maintain immune tolerance [15]. Correcting this deficit may be clinically beneficial in SLE [24]. DN T cells are the major source of IL-17 production, which promotes inflammation through stimulating cytokine and NO production with secretion of IL-1, IL-6, and TNFα [25, 26]. These inflammatory mediators enhance T cell priming, as well as stimulate dendritic cells and macrophages [25]. Blockade of the IL-23/IL-17 axis could be a therapeutic target in SLE [27], through reversal in commitment or depletion of Th17 cells [28, 29].
Mitochondrial dysfunction, endocytic pathway activation, calcium homeostasis, mTOR activation, and autophagy in T cells can serve as biomarkers for SLE and act as potential targets for therapy. New biologic therapies aimed at regulation of T and B cell activation and metabolism show promise in clinical studies and animal models of SLE.
2. Altered T cell receptor signaling machinery in lupus
T cell receptor (TCR) signal transduction is aberrant in SLE. Early T cell receptor associated molecules, which contribute to formation of the immunological synapse, are altered within SLE T cells. CD3ζ and CD4 co-receptor are degraded by lysosomal degradation via the endosomal GTPase HRES-1/Rab4 [18]. A consequence of CD3ζ depletion in lupus CD4+ T cells is re-wiring of the TCR with replacement of CD3ζ for FcεRIγ [30]. FcεRIγ activates tyrosine kinase Syk replacing CD3ζ-ZAP-70 interactions, with Syk being a more potent kinase than ZAP-70, capable of propagating signaling downstream of the TCR under conditions of reduced TCR ligation [31]. Signaling through FcεRIγ-Syk induces stronger and faster Ca2+ fluxing in response to T cell activation than CD3ζ-ZAP-70, providing SLE T cells with a lower threshold for activation [32]. Inhibition of Syk has shown to be therapeutic in lupus-prone mice [33]. CD3ζ depletion also results in reduced CTLA-4 mediated immune suppression, as CTLA-4 requires phosphorylated CD3ζ for its function [34]. Deficiency of CD3ζ results in systemic autoimmunity in mice [35], similar to CTLA-4 deletion [36, 37], with T cell function normalized upon reconstitution of these molecules [38, 39].
CD44 surface expression is increased on T cells, resulting in defective homing and kidney infiltration [40, 41]. Additionally, SLE T cells have deficient production of the homeostatic cytokine IL-2, required during autocrine T cell activation [42]. IL-2 deprivation might contribute to defective function of CD8+ cytotoxic T cells. These changes shape the pathogenic T cell phenotype in SLE.
3. Metabolic dysfunction in lupus T cells
SLE T cells exhibit mitochondrial dysfunction, characterized by increased mass, ↑Δψm, and reduced production of ATP [43]. Mitochondria are essential organelles within all cells, functioning as an energy source and a reservoir for Ca2+ [44]. Increased mitochondrial mass and ↑ Δψm in SLE T cells leads to increased Ca2+ stores within mitochondria, resulting in enhanced intracytosolic Ca2+ fluxing upon stimulation [45]. Mitochondria integrate signals during apoptosis by regulating the balance between pro- and anti-apoptotic proteins, producing reactive oxygen species, and maintaining ψm [46]. Mitochondria are highly dynamic organelles, with organized movement within the cell [44]. They undergo fusion and fission as a response to various stimuli [47] and damaged mitochondria are consumed as a result of organelle autophagy, which is called mitophagy [48].
Mitochondrial function is crucially important in T cells for energy homeostasis. While developing in the thymus, autoreactive T cells undergo apoptosis during negative selection to prevent autoimmunity. The mitochondrial content of thymocytes is regulated by mitophagy, suggesting that cells with improper mitochondrial clearance can contribute to the defective T cell compartment [49]. IL-15 is an important regulator of mitochondrial biogenesis, required of survival and function of CD8+ memory T cells to respond to stimuli [50]. This is mediated through increased expression of carnitine palmitoyl transferase, which increases fatty acid oxidation, oxidative phosphorylation, and ATP production [51]. The circulating, but not the urinary, CD8+ T cell memory compartment is reduced in SLE suggesting that the mitochondrial homeostasis may be affected [52]. In lupus, there is an increased response to IL-15 [50], which contributes to increased mitochondrial biogenesis. IL-15 expression is up-regulated upon T cell activation [53] and is required for survival and function of cytotoxic cells, including CD8+ T cells and natural killer cells [54]. Up-regulation of IL-15 production during autoimmunity can promote accumulation of mitochondria, but may be a protective measure to maintain NK cell function, as NK deficiency promotes systemic autoimmunity and tumor formation [53]. Further studies are required to elucidate the role of cytokines in mitochondrial biogenesis in SLE.
During antigen-specific T cell activation, and following antigen presentation to T cells, redistribution and polarization of the mitochondria occurs towards the site of the immunological synapse (IS) [44, 55]. In T cells, movement of mitochondria is extremely important during IS formation, regulated by the dynamin related protein-1 (Drp-1) which regulates mitochondrial fission [56, 56, 57]. Morphologically, SLE T cells have megamitochondria [58] which could result from disturbed mitophagy.
Persistent mitochondrial hyperpolarization (MHP) predisposes SLE T cells to undergo necrosis in response to stimulation [59, 60], compared to normal T cells which exhibit only transient MHP following activation by CD3/CD28 [60]. Necrotic, but not apoptotic, T cell debris is responsible for the activation of the innate immune system, especially pDCs. pDCs respond to DNA and RNA remnants of the necrotic cells, produce IFN-α, and infiltrate sites of inflammation [61]. ATP depletion and increased reactive oxygen species generation is also characteristic of lupus T cells and contribute to necrosis [9, 62].
Lupus T cell have a characteristic mitochondrial gene signature. Elevated VDAC (voltage dependent anion channel), SOD2 (superoxide dismutase), and transaldolase expression contribute to increased mitochondrial mass and elevated potential in lupus T cells [18]. The main pathogenic driver of the MHP is NO which is released by monocytes and produced by the activation of inducible nitric oxide synthase (iNOS) [12]. T cells express the endothelial and the neuronal isoforms of nitric oxide synthase [60], which contribute to nitrosative stress in lupus. In patients with SLE and multiple sclerosis (MS), polymorphisms of electron transport chain proteins were observed, which could provide genetic predisposition to mitochondrial dysfunction [63].
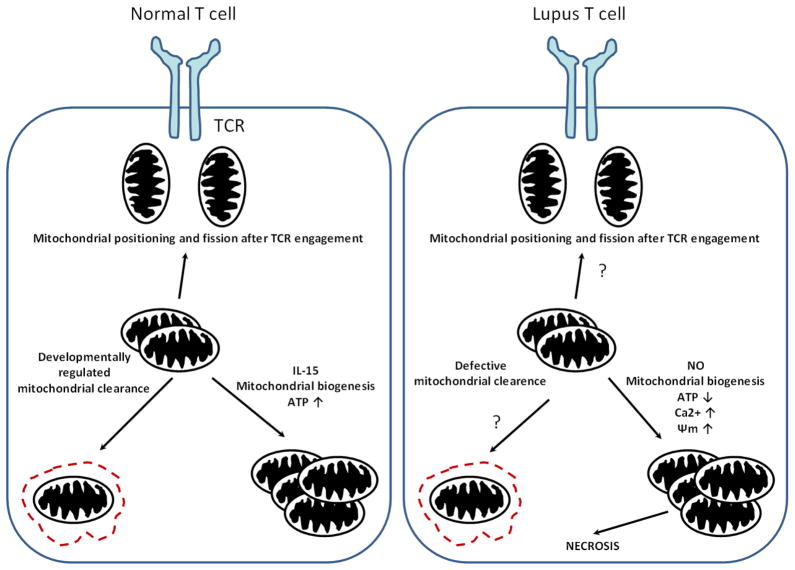
Mitochondrial membrane potential is regulated by pyridine nucleotides and reduced glutathione (GSH) levels in lupus T cells. GSH is elevated in whole blood of lupus patients but is depleted within T cells, suggesting that production is not impaired, but GSH deficiency could result from oxidation [64]. Global mitochondrial dysfunction affecting both innate (neutrophils and monocytes) and adaptive immune components (T and B cells), has been identified in patients with anti-phospholipid syndrome, a common comorbidity in SLE patients, providing evidence that mitochondrial dysfunction is not restricted to T cells. Anti-phospholipid antibodies could elicit membrane changes on monocytes via Fc-receptor signaling, which induces alterations in mitochondrial homeostasis and promotes mitochondrial fission [65]. The main differences in mitochondrial homeostasis between a normal and the lupus T cell are depicted schematically in Figure 1.
Fig. 1.
Mitochondrial homeostasis in a normal and a lupus T cells. During the formation of the IS, mitochondria are shuttled and redistributed towards the TCR signaling complex. Mitochondrial content is regulated during T cell differentiation allowing damaged mitochondria to be eliminated by autophagy. During T cell memory formation, mitochondrial biogenesis and ATP content are increased via the effect of IL-15.
However, in lupus T cell, higher mitochondrial mass and elevated potential is observed, which could be due to higher NO production in these cells. Mitochondria are larger in size and have elevated Ca2+, which alters mitochondrial movement and contributes to defective IS architecture. Removal of mitochondria could be disturbed during T cell differentiation. Contrary to the normal T cell, SLE T cells produce less ATP. The result of mitochondrial dysfunction can be necrotic cell death upon stimulation.
3.1. Activation of the mammalian target of rapamycin (mTOR) in SLE
mTOR is a serine/threonine protein kinase conserved from fission yeast to humans that integrates environmental and metabolic signals to modulate the innate and adaptive immune responses [21]. Two multi-protein complexes comprise mTOR, complex 1 (mTORC1) and complex 2 (mTORC2). mTORC1 activity is acutely sensitive to suppression by the antibiotic rapamycin through binding to cellular receptor, FK506 binding protein of 12 kDa, while mTORC2 activity is reduced with higher concentrations and prolonged exposure to rapamycin through preventing assembly of mTORC2 components [66].
Inhibition of mTOR by rapamycin results in impaired maturation of dendritic cells [67], reduced cellular proliferation [68], and hyporesponsiveness of CD4+ T cells [69]. These immunosuppressive effects have led to its efficacy and approval by the Food and Drug Administration (FDA) for anti-rejection treatment post-renal transplantation. mTOR activation has been implicated in the pathogenesis of multiple cancers and autoimmune diseases, and its inhibition was found to be therapeutic in cancer [70], epilepsy [71, 72], SLE [45], tuberous sclerosis [73], and autoimmune lymphoproliferative syndrome [74].
mTORC1 is activated in lymphocytes from human lupus patients and in murine lupus models, measured by increased phosphorylation of downstream targets which regulate protein translation, p70 S6 kinase and 4E-BP1 [45, 75]. mTORC1 activity is implicated in disease pathogenesis, as inhibition of mTOR by rapamycin prevents the onset [76, 77]and treats established disease in lupus-prone mice [78] and reduces disease activity in patients with SLE [45]. Rapamycin treatment prevents antinuclear antibody production [75, 76, 79, 80] and suppresses production of pro-inflammatory cytokines produced by CD4+ T cells, including interferon-γ and IL-17A [79, 81], which have been shown to play a key role in lupus pathogenesis [29, 82–85].
One mechanism through which rapamycin exerts therapeutic efficacy in lupus is through shifting lymphocyte cellular metabolism away from glycolysis. Increased mTORC1 activity results in an increased dependence of glycolytic activity and lipid biosynthesis, with reduced oxidative phosphorylation and lipid oxidation [86]. Increased dependence on glycolytic activity supports TH1, TH2, and TH17 metabolism, with these CD4+ T cells expressing high levels of Glut1 receptor [87]. Enhanced mTORC1 activity and reduced lipid oxidation suppresses activation of cAMP-associated protein kinase (AMPK) [88] required for regulatory T cell metabolism, shifting the balance to support effector T over regulatory T cell function [87].
mTORC1 activity directs metabolism in T lymphocytes through control of mitochondrial function. Changes in the mitochondrial transmembrane potential are sensed by mTOR through formation of a complex containing voltage-dependent anion channel and Bcl-xL, components of the outer mitochondrial membrane [89]. mTORC1 activation is associated with increased mitochondrial transmembrane potential, oxygen consumption, and ATP synthetic capacity, as treatment with rapamycin reduces these parameters in vitro [90]. Rapamycin may reduce mitochondrial oxidative capacity through transcriptional regulation [91]. SLE T cells have increased mTOR activity [18] and mitochondrial transmembrane potential [9], however, mTOR activation is only one factor regulating mitochondrial function, as treatment with rapamycin in vivo in SLE patients has not been found to reduce mitochondrial mass or potential in T cells [45].
Inhibition of mTOR activity by rapamycin promotes T cell anergy [69] and induces peripheral tolerance. mTOR exerts a dominant role in determining antigen responsiveness, reducing effector and promoting Treg lineage commitment in naïve CD4+ T cells [92]. In the absence of mTOR, CD4+ T cells are unable to undergo TH1, TH2, or TH17 lineage commitment in response to polarizing cytokines. mTORC1 promotes TH1 and TH17 differentiation through activation of lineage specific transcription factors T-bet and RORγT, respectively [93]. mTORC2 activity promotes TH2 specification via activation of TH2 transcription factor GATA-3 [93]. In the absence of mTOR, T cells will undergo Treg specification. Treg commitment occurs through release of inhibition of Foxo1 and Foxo3a transcription factors and increased Smad3 activation, which promotes peripheral generation of Tregs in the presence of TGFβ [92]. mTOR enhances Treg generation, rapamycin promotes tolerance through selective expansion of CD3+CD4+CD25+Foxp3+ T regs, which retain suppressive activity in vitro and in vivo [94].
Oxidative stress stimulates mTORC1 activity, with increased interaction of Raptor with mTOR and subsequent phosphorylation of S6 kinase in the presence of thiol oxidants [95]. Increased mTOR activity in response to oxidative stress is promoted through inhibition of the tuberous sclerosis 1/2 complex, an upstream negative regulator of mTORC1 [96]. Amelioration of oxidative stress has been shown to reduce mTOR activity in vitro [18] and in vivo [64] and is therapeutic in human and murine SLE [64, 97, 98]. In a placebo-controlled clinical trial testing the efficacy of antioxidant N-acetyl-cysteine (NAC) in SLE, NAC was found to reduce mTOR activity in T cells and promote expansion of regulatory T cells (Tregs) in vivo [64]. This correlated with reduced disease activity by SLEDAI and BILAG indices, reduced fatigue assessment scores, and reduced titers of antinuclear autoantibodies (ANA) [64]. The exact mechanism of mTOR inhibition by NAC remains to be established.
An additional point of regulation for mTOR activation is through altering its intracellular distribution via the endocytic pathway. Early endosomal Rab GTPases, Rab4A and Rab5A, are over-expressed in SLE T cells [18] and regulate recycling and endocytosis of receptors from the plasma membrane, respectively. mTOR colocalizes with HRES-1/Rab4 and Rab5 on early endosomes and with Rab7 of the late endosome [18][20]. Target of rapamycin components have been identified on isolated endosomes, suggesting endocytic trafficking controls localization of mTOR within the cell [99]. mTORC1 function was found to require intact early to late endosomal conversion, as expression of a GTP-locked constitutively active form of Rab5 or knockdown of a Rab7 guanine nucleotide exchange factor, resulted in the inability to activate mTORC1 in response to amino acids [100]. This is due to preventing association of mTOR with Rag GTPases when GDP/GTP cycling is impaired on hybrid early/late endosomes [101]. mTOR is recruited to Rab7+ late endosomes by the Ragulator complex, which associates RagB and RagD, small GTPases which bind raptor, and the amino acid transporter PAT1 to recruit mTOR to localize to endosomal/lysosomal compartments during nutrient activation by amino acids [102–104].
Activity of endosomal Rab GTPases was found to be indispensible for mTOR activation [100]. To sustain activation, mTOR participates in a positive feedback loop with the early endosome. mTOR over-expression in SLE T cells results in increased expression of HRES-1/Rab4, modified by rapamycin treatment [18].
3.2. Enhanced endosome traffic mediates increased T cell activation through reorganization of the immunological synapse
In addition to enhancement of mTOR activity, activation of the endocytic pathway in SLE T cells increases recycling of the T cell antigen receptor (TCR) and CD4 co-receptor in T cells [18], which enhances T cell activation. Increased endocytic activity results in enrichment of TCRs and other components to lipid raft microdomains that make up the T cell-antigen presenting cell interface at the immunological synapse by polarized exocytosis [105]. This process is mediated in part by Rab GTPases and the guanine nucleotide activating proteins that regulate their activity [19]. Recycling of TCRs occurs constitutively [106], allowing T cells to maintain responsiveness upon serial activation by peptide-MHC complexes when an immunological synapse forms [105]. Conversely, chronic stimulation should result in down-regulation of TCR surface expression through blockade of endocytic recycling, while maintaining the same TCR internalization rate [107]. This is a protective measure to limit T cell exhaustion and resulting antigen-induced cell death, which is impaired in SLE T cells, allowing for persistence of autoreactive T cells [8].
Recycling of CD3 and CD4 surface receptors are increased on SLE T lymphocytes, associated with increased expression of HRES-1/Rab4, an early endosomal small GTPase over-expressed in SLE T cells [18]. HRES-1/Rab4 forms direct interactions with CD4 and CD3ζ, and its over-expression results in targeting of CD4 and CD3ζ to lysosomes for degradation [18, 108]. The E3 ubiquitin ligase Cbl down-regulates the TCR upon sustained engagement through targeting CD3ζ [109], which could be mediated by HRES-1/Rab4 through formation of a Rab4-CD2 adaptor protein-Cbl complex [110]. HRES-1/Rab4 could also direct CD3ζ for degradation by microautophagy, a catabolic process that delivers cytosolic cargo into multivesicular bodies formed from endosomes, which transfers cargo to lysosomes by fusion [111].
Activation of early endocytic recycling could also enhance lymphocyte activation in SLE through regulation of antigen processing in B cells. Peptide-MHC class II complexes are loaded into endocytic compartments [112] and early endosomal recycling is required for antigen processing within B cells and presentation to CD4+ T lymphocytes [113]. Rab4 is required for efficient presentation of antigens that are internalized by the B cell receptor (BCR) and is important in processing of receptor-bound antigens [114, 115]. Rab4 transcription is promoted by the MHC class II transactivator, which could allow B cells to enhance their ability to present antigen through coordinately increasing Rab4-dependent recycling. Increased antigen processing by Rab4 could contribute to SLE pathogenesis through promoting generation of autoantigens through molecular mimicry of endocytosed material, and increased MHC class II processing would enhance presentation of these autoantigens, leading to stimulation of autoreactive CD4+ T cells [116].
Antigen presentation can be promoted by Rab GTPases through increased autophagy [117]. Several Rab GTPases that localize to membrane sources (endoplasmic reticulum, mitochondria) or endosomes (early and late) have been implicated in formation and maturation of autophagosomes [118]. Increased phagocytosis from early endosomes results in a need for increased biogenesis of degradative compartments to eliminate pathogens and foreign substances that are internalized by the cell [119]. Production of pro-inflammatory cytokines, including interferon-γ and IL-6, are up-regulated in SLE [120, 121], which can contribute to increased biogenesis and maturation of phagocytic compartments, through promoting Rab5 transcription [122–124].
3.3. Autophagy is up-regulated within lupus T cells
Autophagy is a well conversed cellular regulatory mechanism in which proteins (microautophagy) or organelles (e.g. mitochondria, previously mentioned as mitophagy) are sequestered and degraded by the autophagosome and autolysosome [125]. The key event of autophagic flux is the assembly of the autophagosome in which several proteins of the Atg family are implicated. Besides Atg proteins, the most notable component is the microtubule associated protein LC3, which is used as marker for identifying autophagosomes [126].
Autophagy regulates the biology of both the innate and adaptive immune systems [127]. Induction of autophagy is essential for the proliferation, homeostatic maintainence, and survival of T lymphocytes. Deficiency of autophagic proteins in knockout mice results in reduced intrathymic development of T cells (Atg6, [128]), impairment of T cell survival and proliferation (Atg5, [129]), defective activation-induced effector cytokine production upon activation (Atg7, [130]), and exaggerated T cell apoptosis upon stimulation (Atg6, [131]). Defective autophagy prevents turnover of damaged endoplasmic reticulum (ER), resulting in its accumulation within effector T cells of knockout mice (Atg3, [132], Atg 7 [133]). Impaired ER homeostasis due to deficient autophagy results in reduced recruitment of stromal interaction molecule-1 (STIM-1) towards Orai1 [133], preventing store-operated Ca2+ release activated Ca2+ current (CRAC) from the ER, required to sustain T cell activation [134]. Up-regulation of autophagy during T cell activation spares mitochondria [130], which elongate and concentrate towards the immunological synapse to meet the energetic requirements of stimulation [57, 135].
In accordance with these observations, increased autophagy promotes autoimmunity through enhanced survival and reduced apoptosis of autoreactive lymphocytes. Phosphoinositide-3 kinase Vps34, plays an important role in autophagosome formation by producing phosphoinositide 3-phosphate [PI(3)P], which regulates Rab5-directed vesicle traffic. Vps34 expression promotes increased mitochondrial mass and enhanced production of reactive oxygen intermediates within T cells [136]. Lupus T cells exhibit reduced activation-induced cell death [8], increased intracytoplasmic Ca2+ fluxing upon stimulation [137], and over-production of effector cytokines [138], all of which could be related to increased autophagy.
Both murine and human lupus T cells have elevated numbers of autophagosomes. Despite increased autophagosome formation, mitochondrial mass remains increased in SLE T cells [58]. HRES-1/Rab4 over-expression, which occurs in SLE T cells, was found to increase microautophagy (with increased lysosomal degradation of proteins CD3ζ, CD4), while inhibiting macroautophagy of mitochondria through degradation of Drp1, a mitochondrial fission initiator required early during mitophagy [139]. Drp1 is depleted in SLE T cells [139], resulting in defective clearance and promoting the formation of megamitochondria.
Increased autophagy can lead to the survival of auto-reactive T cells in lupus, a defect which precedes disease onset [140]. Up-regulation of autophagy in SLE T cells could be mediated by circulating auto-antibodies, as complement-inactivated autoimmune sera from patients with diabetes mellitus has been shown to stimulate autophagy in vitro [141]. Polymorphic alleles of autophagy-related genes in SLE patients may also be responsible. In genome-wide association studies in SLE, single nucleotide polymorphisms within the locus of autophagy-related gene ATG5 were identified, resulted in increased transcription, and were linked to lupus susceptibility [142]. Although functional consequences of ATG5 over-expression have not been investigated in SLE, it is thought to be pathogenic through promoting survival and expansion of autoreactive T cells, identified in studies of ATG5 up-regulation during acute demyelination in MS [143].
Modulation of autophagy may improve outcomes in SLE and several medications currently used in SLE management affect the autophagic machinery. Anti-malarial drugs, including chloroquine and hydroxychloroquine, act through raising the pH of endosomes and lysosomes, resulting in an accumulation of ineffective autophagosomes [144]. The P140 phosphopeptide, an inhibitor of Hsc70, prevents formation of autolysosomes [145, 146], and shows promise in a recent phase II clinical trial [147]. Up-regulation of autophagy occurs in SLE lymphocytes despite increased mTORC1 activation, and agents that reduce mTOR activity are effective in SLE although they act as autophagy inducers, including corticosteroids, proteasome inhibitors, and rapamycin. Glucocorticoids reduce mTOR activity and induce autophagy by inhibiting Ca2+ signaling, resulting in up-regulation of AMPK, which inhibits mTORC1 activation [148]. Proteasome inhibitors, including Bortezomib, inhibit mTORC1 through blockade of the ubiquitin-proteasome system [149].
4. New therapies target B and T cell signal transduction in SLE
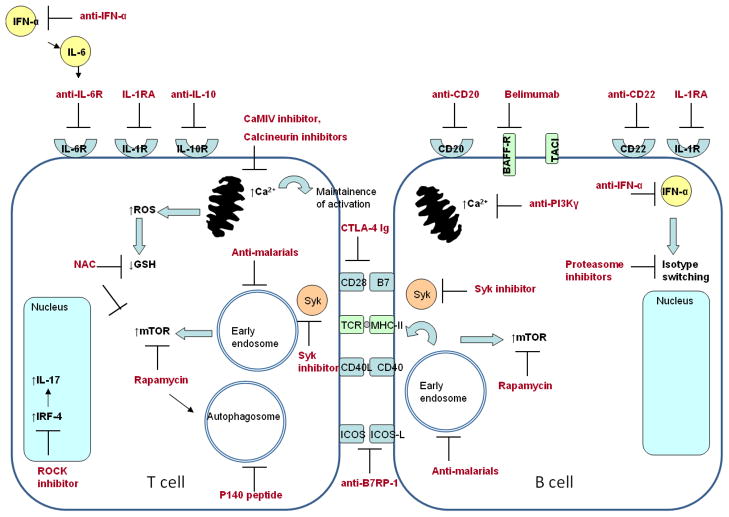
Currently, available therapeutics in SLE are limited and are strong immunosuppressants. Serious side effects, such as infections and impaired wound healing, limit long-term use and efficacy of immunosuppressive regimens. Only four therapies have been approved by the Food and Drug Administration (FDA) for management of SLE [150]. These include 1) glucocorticoids [150] 2) aspirin [150]3) anti-malarial drugs [151]and 4) belimumab [152], an inhibitor of B cell activation. There is an unmet need for safer medications for SLE treatment that can be used during disease flares and for long-term management. There are increasing number of biological targets currently under investigation for SLE, focusing on blockade of major pathways involved in activation and survival of auto-reactive B and T lymphocytes due to metabolic and autophagic disturbances. Some of these targets have been studied in murine models of SLE, but have yet to be investigated in clinical trials. A schematic diagram of these targets is shown in figure 2.
Fig. 2.
Biological targets under investigation for treatment of SLE. Depletion of autoreactive B cells is achieved by treatment with monoclonal antibodies against CD20 and CD22. CD22 also depletes plasma cells, which can also be targeted through blockade of isotype-switching by proteasome inhibition or anti-IFNα antibodies. Activation of lupus B cells can be inhibited by targeting survival (BAFF targeted by Belimumab, APRIL targeted by TACI-Ig) and co-stimulatory signals. Lupus T cell activation is targeted by blockade of cytokine action (IL-6, IL-1, and IL-10), cytokine production (IL-17), and co-stimulation (CD28-B7 interaction by CTLA-4 Ig, ICOS-ICOS ligand interaction by anti-B7RP-1 antibodies).
Activation of SLE T and B cells results in a rise of cellular Ca2+, which results from mitochondrial dysfunction, mTOR activation, and endocytic pathway activation. Intracellular Ca2+ can be modulated by treatment with calcium calmodulin kinase inhibitors, calcineurin inhibitors, and anti-PI3Kγ. Early endosome and mTOR activation in SLE T and B cells are inhibited with anti-malarials and rapamycin. Autophagy in SLE lymphocytes can be reduced by treatment with P140 peptide and anti-malarial drugs.
Of these targets, antimalarial drugs and Belimumab have been FDA-approved for SLE disease management. The other targets mentioned are under intensive investigation in pre-clinical and clinical studies.
B cell depletion therapies targeting CD20 and CD22 have been utilized to reduce the source of auto-antibody generation and reduce activation of auto-reactive T cells [153–156]. These have achieved limited success due to survival of long-lived plasma cells. However, proteasome inhibitors, including Bortezomib, result in plasma cell depletion and have been shown to be effective in preventing onset of disease in mice [157]. Generation of immune complexes (ICs) by ANA produced by plasma cells results in activation of TLRs and complement activation, which have been effectively blocked by toll-like receptor antagonists, monoclonal antibodies, and complement inhibitors in lupus-prone mice [158–160].
Targets to inhibit B cell activation with reduced B cell depletion are also under investigation, including Atacicept, a fusion protein that targets TACI, which interacts with BAFF during B cell activation in a phase II/III clinical trial [161]. Medications aimed at tolerizing auto-reactive B or T cells have achieved limited success [162, 163], but additional tolerogenic therapies are under investigation. Additionally, blockade of T cell-B cell co-stimulation with CTLA-4 Ig, has been shown to have modest effects in reducing flares in SLE [164]. Co-stimulatory inhibition by blockade of B7RP-1 is therapeutic in lupus-prone mice and is under investigation in a phase I clinical trial [165]. B7RP-1 inhibition prevents activation of CD4+ T cells, including follicular helper T cells which provide B cell help in splenic germinal center reactions [165].
Blocking co-stimulation prevents T and B cell activation. Exaggerated Ca2+ responses result from enhanced T and B cell activation and promote survival and proliferation of autoreactive lymphocytes. Enhanced intracytoplasmic Ca2+ fluxing is a target for treatment in SLE, which can be mitigated by mTOR blockade by rapamycin [45], or inhibition of calcium-activated calmodulin kinase [166], PI3Kγ [167], or calcineurin [168].
Blockade of pro-inflammatory cytokines produced by B cells, T cells, or macrophages by monoclonal antibodies has shown promise. Toclizumab, which targets IL-6R, reduces disease activity, ANA, and IL-17 production through reduction in IL-21, which prevents differentiation of pathogenic Th17 cells from naïve CD4+ T cells and DN T cells [169]. IL-17 and IL-21 are over-produced in SLE due to activation of IRF4, and result in increased generation of pathogenic Th17 cells in lupus-prone mice, reversed by Rho kinase 2 inhibitor, Fasudil [170]. Inhibition of IL-10 reduces Th2 differentiation and reduces disease activity [171]. Effects of IL-1 over-production by activated macrophages can be blocked by Anakinra, an IL-1 receptor antagonist [172]. Neutralization of IFN-α produced by pDCs, has shown promise in murine lupus models and a phase I trial [173]. Production of pro-inflammatory cytokines can also be effectively reduced by treatment with rapamycin [45]. Modulation of oxidative stress through use of high-potency anti-oxidant N-acetylcysteine (NAC), has been found to be effective in reducing disease activity in SLE [64]. Descriptions of these therapeutic targets are included in Table 1.
Table 1.
Current and prospective therapies for SLE
| Molecular target | Treatment | References |
|---|---|---|
| FDA approved therapies: | ||
| Homeostatic survival of T cells, B cells, & macrophages | Glucocorticoids Prednisone, Triamcinolone hexacetonide | 148, 150 |
| Non-steroidal anti- inflammatory drugs | Aspirin | 150 |
| Endosome function, activation of toll-like receptors, antigen processing / presentation | Chloroquine; hydroxychloroquine | 151 |
| BLγS/BAFF (B cell cytokine) | Belimumab | 152 |
| B cell targeted therapies: | ||
| B cell depletion | Rituximab (anti-CD20) Epratuzumab (anti-CD22) Atacicept (TACI-Ig fusion protein) |
153. 154 155, 156 161 |
| Proteasome (↓ plasma cells) | Bortezomib (MRL/lpr; NZB/WF1 mice) | 157 |
| Syk | Fostamatinib (MRL/lpr; BAX/BAK mice) | 33 |
| T cell targeted therapies: | ||
| Glutathione depletion | N-acetylcysteine | 64 |
| Follicular helper T cells | Anti-B7RP-1 Ab (NZB/WF1 mice) | 165 |
| Rho kinase (ROCK) Inhibits IRF4 phosphorylation | Fasudil, ROCK2 inhibitor (NZB/WF1 mice) | 170 |
| Blockade of B cell – T cell co-stimulation: | ||
| T cell-B cell costimulation | Abatacept (CTLA-4 Ig) | 164 |
| Tolerogenic therapies | Edratide (hCDR1 peptide, T cell tolerogen) Abetimus (B cell tolerogen) |
162 163 |
| PI3Kγ | AS605240 (MRL/lpr mice) | 167 |
| Regulation of intracellular Ca2+: | ||
| Calcineurin | Dipyridamole (MRL/lpr mice) | 168 |
| Calcium-activated calmodulin kinase | KN-93, CaMKIV inhibitor (MRL/lpr mice) | 166 |
| Cytokine blockade: | ||
| Monoclonal antibodies | Tocilizumab (anti-IL-6R) Anti-IFNα IgG1κ neutralizing antibody Anti-IL-10 monoclonal antibody (B-N10) Anakinra (IL-1 receptor antagonist) |
169 173 171 172 |
| mTOR | Rapamycin | 45 |
5. Discussion
Numerous disturbances in T cell signaling occur within lupus lymphocytes. These changes are characterized by the altered metabolic flux, mitochondrial homeostasis, early endosome activation, and autophagic activity of T cells. Activation of the mTOR pathway that affects lineage specification, MHP which increases ROS production and predisposes activated cells to necrosis, over-expression of Rab GTPases which enhances recycling and degradation of TCR signaling components, and increased autophagy within T cells contributes to these abnormalities. These can function as potential biomarkers and therapeutic targets to reverse aberrant T cell activation and reduce clinical severity in SLE.
Highlights.
Organelles, such as endosomes and mitochondria, regulate T cell activation
Endosomes recycle surface receptors that transmit signals from the T-cell receptor
Endosome control traffic of proteins and organelles for disposal via autophagy
Mitochondria control T-cell activation and death
Organelle dysfunction represents target for treatment in SLE
Abbreviations
- ↑Δψm
increased mitochondrial transmembrane potential
- AICD
activation-induced cell death
- AMPK
cAMP-activated protein kinase
- ANA
antinuclear autoantibodies
- ATG
autophagy-related gene
- BCR
B cell receptor
- BILAG
British Isles lupus assessment group
- Ca2+
calcium
- CRAC
calcium release activated calcium current
- CTLA-4
cytotoxic T lymphocyte antigen-4
- DN T cell
CD4−CD8− double negative T cell
- Drp1
dynamin-related protein 1
- ECLAM
European consensus lupus activity measurement index
- ER
endoplasmic reticulum
- FDA
Food and Drug Adminstration
- GN
glomerulonephritis
- GSH
reduced glutathione
- IC
immune complex
- IFN
interferon
- IL-
interleukin-
- iNOS
inducible nitric oxide synthase
- IS
immunological synapse
- MHP
mitochondrial hyperpolarization
- MRL/lpr mouse
murine research laboratory lymphoproliferative mouse
- MS
multiple sclerosis
- NAC
N-acetylcysteine
- NK
natural killer
- NO
nitric oxide
- NZB/WF1
New Zealand Black × New Zealand White F1 progeny, a mouse model of SLE
- mTOR
mammalian target of rapamycin
- pDC
plasmacytoid dendritic cell
- Rab
Ras-associated in brain
- SLE
systemic lupus erythematosus
- SLEDAI
systemic lupus erythematosus disease activity score
- STAT
signal transducer and activator of transcription
- SOCS
suppressor of cytokine signaling
- Syk
spleen tyrosine kinase
- TCR
T cell antigen receptor
- TH
T helper, CD4+ T cell
- TLR
toll-like receptor
- Treg
regulatory T cell, CD3+CD4+CD25+Foxp3+ cell
- ZAP-70
CD3 zeta associated protein of 70 kDa
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsokos GC. Mechanisms of disease: Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Edwards CJ, Cooper C. Early environmental exposure and the development of lupus. Lupus. 2006;15:814–819. doi: 10.1177/0961203306069347. [DOI] [PubMed] [Google Scholar]
- 3.Moser KL, Kelly JA, Lessard CJ, Harley JB. Recent insights into the genetic basis of systemic lupus erythematosus. Genes Immun. 2009;10:373–379. doi: 10.1038/gene.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Lu Q. DNA methylation in T cells from idiopathic lupus and drug-induced lupus patients. Autoimmunity Reviews. 2008;7:376–383. doi: 10.1016/j.autrev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Deapen D, Escalante A, Weinrib L, Horwitz D, Bachman B, Roy-Burman P, Walker A, Mack TM. A revised estimate of twin concordance in systemic lupus erythematosus. Arthritis Rheum. 1992;35:311–318. doi: 10.1002/art.1780350310. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, Chan K, Trendell-Smith NJ, Wu A, Tian L, Lam AC, Chan AK, Lo C, Chik S, Ko K, To CKW, Kam S, Li X, Yang C, Leung SY, Ng M, Stott DI, MacPherson GG, Huang F. Systemic autoimmune disease induced by dendritic cells that have captured necrotic but not apoptotic cells in susceptible mouse strains. Eur J Immunol. 2005;35:3364–3375. doi: 10.1002/eji.200535192. [DOI] [PubMed] [Google Scholar]
- 7.Lövgren T, Eloranta M, Båve U, Alm GV, Rönnblom L. Induction of interferon-α production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 8.Kovacs B, Vassilopoulos D, Vogelgesang SA, Tsokos GC. Defective CD3-mediated cell death in activated T cells from patients with systemic lupus erythematosus: Role of decreased intracellular TNF- Clin Immunol Immunopathol. 1996;81:293–302. doi: 10.1006/clin.1996.0192. [DOI] [PubMed] [Google Scholar]
- 9.Gergely P, Jr, Grossman C, Niland B, Puskas F, Neupane H, Allam F, Banki K, Phillips PE, Perl A. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borniquel S, Valle I, Cadenas S, Lamas S, Monsalve M. Nitric oxide regulates mitochondrial oxidative stress protection via the transcriptional coactivator PGC-1alpha. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2006;20:1889–1891. doi: 10.1096/fj.05-5189fje. [DOI] [PubMed] [Google Scholar]
- 12.Nagy G, Barcza M, Gonchoroff N, Phillips PE, Perl A. Nitric oxide-dependent mitochondrial biogenesis generates Ca2+ signaling profile of lupus T cells. Journal of Immunology. 2004;173:3676–3683. doi: 10.4049/jimmunol.173.6.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quintana A, Schwarz EC, Schwindling C, Lipp P, Kaestner L, Hoth M. Sustained activity of calcium release-activated calcium channels requires translocation of mitochondria to the plasma membrane. J Biol Chem. 2006;281:40302–40309. doi: 10.1074/jbc.M607896200. [DOI] [PubMed] [Google Scholar]
- 14.Quintana A, Schwindling C, Wenning AS, Becherer U, Rettig J, Schwarz EC, Hoth M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc Natl Acad Sci U S A. 2007;104:14418–14423. doi: 10.1073/pnas.0703126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crispín JC, Kyttaris VC, Juang Y, Tsokos GC. How signaling and gene transcription aberrations dictate the systemic lupus erythematosus T cell phenotype. Trends Immunol. 2008;29:110–115. doi: 10.1016/j.it.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Perl A, Fernandez DR, Telarico T, Doherty E, Francis L, Phillips PE. T-cell and B-cell signaling biomarkers and treatment targets in lupus. Curr Opin Rheumatol. 2009;21:454–464. doi: 10.1097/BOR.0b013e32832e977c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA, Isenberg DA. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 2004;113:1176–1187. doi: 10.1172/JCI20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernandez DR, Telarico T, Bonilla E, Li Q, Banerjee S, Middleton FA, Phillips PE, Crow MK, Oess S, Muller-Esterl W, Perl A. Activation of mammalian target of rapamycin controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. Journal of immunology (Baltimore, Md: 1950) 2009;182:2063–2073. doi: 10.4049/jimmunol.0803600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patino-Lopez G, Dong X, Ben-Aissa K, Bernot KM, Itoh T, Fukuda M, Kruhlak MJ, Samelson LE, Shaw S. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem. 2008;283:18323–18330. doi: 10.1074/jbc.M800056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridges D, Fisher K, Zolov SN, Xiong T, Inoki K, Weisman LS, Saltiel AR. Rab5 Proteins Regulate Activation and Localization of Target of Rapamycin Complex 1. Journal of Biological Chemistry. 2012;287:20913–20921. doi: 10.1074/jbc.M111.334060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annual Review of Immunology. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crispín JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang Y, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. Journal of Immunology. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crispín JC, Tsokos GC. Human TCR-αβ+ CD4- CD8- T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. Journal of Immunology. 2009;183:4675–4681. doi: 10.4049/jimmunol.0901533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scalapino KJ, Tang Q, Bluestone JA, Bonyhadi ML, Daikh DI. Suppression of Disease in New Zealand Black/New Zealand White Lupus-Prone Mice by Adoptive Transfer of Ex Vivo Expanded Regulatory T Cells. The Journal of Immunology. 2006;177:1451–1459. doi: 10.4049/jimmunol.177.3.1451. [DOI] [PubMed] [Google Scholar]
- 25.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radhakrishnan S, Cabrera R, Schenk EL, Nava-Parada P, Bell MP, Van Keulen VP, Marler RJ, Felts SJ, Pease LR. Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. Journal of Immunology. 2008;181:3137–3147. doi: 10.4049/jimmunol.181.5.3137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of Immune Induction of Collagen-Induced Arthritis in IL-17-Deficient Mice. Journal of Immunology. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 28.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th17 axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. Journal of Immunology. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enyedy EJ, Nambiar MP, Liossis S-C, Dennis G, Kammer GM, Tsokos GC. Fcε receptor type I γ chain replaces the deficient T cell receptor ζ chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:1114–1121. doi: 10.1002/1529-0131(200105)44:5<1114::AID-ANR192>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan S, Farber DL, Tsokos GC. T cell rewiring in differentiation and disease. Journal of Immunology. 2003;171:3325–3331. doi: 10.4049/jimmunol.171.7.3325. [DOI] [PubMed] [Google Scholar]
- 32.Tsokos GC, Nambiar MP, Tenbrock K, Juang Y. Rewiring the T-cell: Signaling defects and novel prospects for the treatment of SLE. Trends Immunol. 2003;24:259–263. doi: 10.1016/s1471-4906(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 33.Deng G, Liu L, Bahjat FR, Pine PR, Tsokos GC. Suppression of skin and kidney disease by inhibition of spleen tyrosine kinase in lupus-prone mice. Arthritis Rheum. 2010;62:2086–2092. doi: 10.1002/art.27452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueda H, Howson JMM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KMD, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RCJ, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithiyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlien DE, R⊘nningen KS, Guja C, Ionescu-Tirgovişte C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SCL. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 35.Holst J, Wang H, Eder KD, Workman CJ, Boyd KL, Baquet Z, Singh H, Forbes K, Chruscinski A, Smeyne R, van Oers NSC, Utz PJ, Vignali DAA. Scalable signaling mediated by T cell antigen receptor-CD3 ITAMs ensures effective negative selection and prevents autoimmunity. Nat Immunol. 2008;9:658–666. doi: 10.1038/ni.1611. [DOI] [PubMed] [Google Scholar]
- 36.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 37.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi S, Kataoka H, Hara S, Yokosuka T, Takase K, Yamasaki S, Kobayashi W, Saito Y, Saito T. In vivo overexpression of CTLA-4 suppresses lymphoproliferative diseases and thymic negative selection. Eur J Immunol. 2005;35:399–407. doi: 10.1002/eji.200324746. [DOI] [PubMed] [Google Scholar]
- 39.Nambiar MP, Fisher CU, Warke VG, Krishnan S, Mitchell JP, Delaney N, Tsokos GC. Reconstitution of deficient T cell receptor ζ chain restores T cell signaling and augments T cell receptor/CD3-induced interleukin-2 production in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1948–1955. doi: 10.1002/art.11072. [DOI] [PubMed] [Google Scholar]
- 40.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annual Review of Immunology. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crispín JC, Keenan BT, Finnell MD, Bermas BL, Schur P, Massarotti E, Karlson EW, Fitzgerald LM, Ergin S, Kyttaris VC, Tsokos GC, Costenbader KH. Expression of CD44 variant isoforms CD44v3 and CD44v6 is increased on T cells from patients with systemic lupus erythematosus and is correlated with disease activity. Arthritis Rheum. 2010;62:1431–1437. doi: 10.1002/art.27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crispín JC, Liossis S-C, Kis-Toth K, Lieberman LA, Kyttaris VC, Juang Y, Tsokos GC. Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol Med. 2010;16:47–57. doi: 10.1016/j.molmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perl A, Gergely P, Jr, Banki K. Mitochondrial dysfunction in T cells of patients with systemic lupus erythematosus. Int Rev Immunol. 2004;23:293–313. doi: 10.1080/08830180490452576. [DOI] [PubMed] [Google Scholar]
- 44.Quintana A, Hoth M. Mitochondrial dynamics and their impact on T cell function. Cell Calcium. 2012 doi: 10.1016/j.ceca.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hüttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr. 2008;40:445–456. doi: 10.1007/s10863-008-9169-3. [DOI] [PubMed] [Google Scholar]
- 47.Palmer CS, Osellame LD, Stojanovski D, Ryan MT. The regulation of mitochondrial morphology: Intricate mechanisms and dynamic machinery. Cell Signal. 2011;23:1534–1545. doi: 10.1016/j.cellsig.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 48.Wang K, Klionsky DJ. Mitochondria removal by autophagy. Autophagy. 2011;7:297–300. doi: 10.4161/auto.7.3.14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pua HH, Guo J, Komatsu M, He Y. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. Journal of Immunology. 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- 50.Baranda L, de la Fuente H, Layseca-Espinosa E, Portales-Pérez D, Niño-Moreno P, Valencia-Pacheco G, Abud-Mendoza C, Alcocer-Varela J, González-Amaro R. IL-15 and IL-15R in leucocytes from patients with systemic lupus erythematosus. Rheumatology. 2005;44:1507–1513. doi: 10.1093/rheumatology/kei083. [DOI] [PubMed] [Google Scholar]
- 51.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolff S, Abdulahad WH, Van Dijk MCRF, Limburg PC, Kallenberg CGM, Bijl M. Urinary T cells in active lupus nephritis show an effector memory phenotype. Ann Rheum Dis. 2010;69:2034–2041. doi: 10.1136/ard.2009.124636. [DOI] [PubMed] [Google Scholar]
- 53.Ivanova AV, Ivanov SV, Pascal V, Lumsden JM, Ward JM, Morris N, Tessarolo L, Anderson SK, Lerman MI. Autoimmunity, spontaneous tumouringenesis, and IL-15 insufficiency in mice with a targeted disruption of the tumour suppressor gene Fus1. J Pathol. 2007;211:591–601. doi: 10.1002/path.2146. [DOI] [PubMed] [Google Scholar]
- 54.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JCL, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwindling C, Quintana A, Krause E, Hoth M. Mitochondria positioning controls local calcium influx in T cells. Journal of Immunology. 2010;184:184–190. doi: 10.4049/jimmunol.0902872. [DOI] [PubMed] [Google Scholar]
- 56.Chang C, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Annals of the New York Academy of Sciences. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baixauli F, Martín-Cófreces NB, Morlino G, Carrasco YR, Calabia-Linares C, Veiga E, Serrador JM, Sánchez-Madrid F. The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J. 2011;30:1238–1250. doi: 10.1038/emboj.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagy G, Koncz A, Fernandez D, Perl A. Nitric oxide, mitochondrial hyperpolarization, and T cell activation. Free Radical Biology and Medicine. 2007;42:1625–1631. doi: 10.1016/j.freeradbiomed.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perl A, Gergely P, Jr, Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: A checkpoint of T-cell life, death and autoimmunity. Trends Immunol. 2004;25:360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagy G, Koncz A, Perl A. T Cell Activation-Induced Mitochondrial Hyperpolarization is Mediated by Ca2+- and Redox-Dependent Production of Nitric Oxide. Journal of Immunology. 2003;171:5188–5197. doi: 10.4049/jimmunol.171.10.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kis-Toth K, Tsokos GC. Dendritic cell function in lupus: Independent contributors or victims of aberrant immune regulation. Autoimmunity. 2010;43:121–130. doi: 10.3109/08916930903214041. [DOI] [PubMed] [Google Scholar]
- 62.Gergely P, Jr, Niland B, Gonchoroff N, Pullmann R, Jr, Phillips PE, Perl A. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. Journal of Immunology. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vyshkina T, Sylvester A, Sadiq S, Bonilla E, Canter JA, Perl A, Kalman B. Association of common mitochondrial DNA variants with multiple sclerosis and systemic lupus erythematosus. Clinical Immunology. 2008;129:31–35. doi: 10.1016/j.clim.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lai Z, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, Miklossy G, Jimah J, Doherty E, Tily H, Francis L, Garcia R, Dawood M, Yu J, Ramos I, Coman I, Faraone SV, Phillips PE, Perl A. N-acetylcysteine reduces disease activity by blocking mTOR in T cells of lupus patients. Arthritis & Rheumatism. 2012 doi: 10.1002/art.34502. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perez-Sanchez C, Ruiz-Limon P, Aguirre MA, Bertolaccini ML, Khamashta MA, Rodriguez-Ariza A, Segui P, Collantes-Estevez E, Barbarroja N, Khraiwesh H, Gonzalez-Reyes JA, Villalba JM, Velasco F, Cuadrado MJ, Lopez-Pedrera C. Mitochondrial dysfunction in antiphospholipid syndrome: implications in the pathogenesis of the disease and effects of coenzyme Q10 treatment. Blood. 2012;119:5859–5870. doi: 10.1182/blood-2011-12-400986. [DOI] [PubMed] [Google Scholar]
- 66.Sarbassov DD, Ali SM, Sengupta S, Sheen J, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged Rapamycin Treatment Inhibits mTORC2 Assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 67.Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, Pulendran B, Hörl WH, Säemann MD, Weichhart T. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. Journal of Immunology. 2010;185:3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 68.Song J, Salek-Ardakani S, So T, Croft M. The kinases aurora B and mTOR regulate the G1-S cell cycle progression of T lymphocytes. Nat Immunol. 2007;8:64–73. doi: 10.1038/ni1413. [DOI] [PubMed] [Google Scholar]
- 69.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. Journal of Immunology. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 70.Rao RD, Buckner JC, Sarkaria JN. Mammalian target of rapamycin (mTOR) inhibitors as anti-cancer agents. Current Cancer Drug Targets. 2004;4:621–635. doi: 10.2174/1568009043332718. [DOI] [PubMed] [Google Scholar]
- 71.Zeng L, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang X, Zhang H, Yang J, Wu J, McMahon J, Lin Y, Cao Z, Gruenthal M, Huang Y. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–199. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sampson JR. Therapeutic targeting of mTOR in tuberous sclerosis. Biochem Soc Trans. 2009;37:259–264. doi: 10.1042/BST0370259. [DOI] [PubMed] [Google Scholar]
- 74.Teachey DT, Greiner R, Seif A, Attiyeh E, Bleesing J, Choi J, Manno C, Rappaport E, Schwabe D, Sheen C, Sullivan KE, Zhuang H, Wechsler DS, Grupp SA. Treatment with sirolimus results in complete responses in patients with autoimmune lymphoproliferative syndrome. Br J Haematol. 2009;145:101–106. doi: 10.1111/j.1365-2141.2009.07595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis Rheum. 1994;37:289–297. doi: 10.1002/art.1780370219. [DOI] [PubMed] [Google Scholar]
- 76.Lui SL, Yung S, Tsang R, Zhang F, Chan KW, Tam S, Chan TM. Rapamycin prevents the development of nephritis in lupus-prone NZB/W F 1 mice. Lupus. 2008;17:305–313. doi: 10.1177/0961203307088289. [DOI] [PubMed] [Google Scholar]
- 77.Ramos-Barrón Á, Piñera-Haces C, Gõmez-Alamillo C, Santiuste-Torcida I, Ruiz JC, Buelta-Carrillo L, Merino R, ÁDe Francisco LM, Arias M. Prevention of murine lupus disease in (NZB X NZW)F 1 mice by sirolimus treatment. Lupus. 2007;16:775–781. doi: 10.1177/0961203307081401. [DOI] [PubMed] [Google Scholar]
- 78.Lui SL, Tsang R, Chan KW, Zhang F, Tam S, Yung S, Chan TM. Rapamycin attenuates the severity of established nephritis in lupus-prone NZB/W F1 mice. Nephrology Dialysis Transplantation. 2008;23:2768–2776. doi: 10.1093/ndt/gfn216. [DOI] [PubMed] [Google Scholar]
- 79.Hou L, He S, Li X, Yang Y, He P, Zhou Y, Zhu F, Yang Y, Li Y, Tang W, Zuo J. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011;63:2445–2455. doi: 10.1002/art.30392. [DOI] [PubMed] [Google Scholar]
- 80.Reddy PS, Legault HM, Sypek JP, Collins MJ, Goad E, Goldman SJ, Liu W, Murray S, Dorner AJ, O’Toole M. Mapping similarities in mTOR pathway perturbations in mouse lupus nephritis models and human lupus nephritis. Arthritis Research and Therapy. 2008;10 doi: 10.1186/ar2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshizaki A, Yanaba K, Yoshizaki A, Iwata Y, Komura K, Ogawa F, Takenaka M, Shimizu K, Asano Y, Hasegawa M, Fujimoto M, Sato S. Treatment with rapamycin prevents fibrosis in tight-skin and bleomycin-induced mouse models of systemic sclerosis. Arthritis Rheum. 2010;62:2476–2487. doi: 10.1002/art.27498. [DOI] [PubMed] [Google Scholar]
- 82.Schwarting A, Tesch G, Kinoshita K, Maron R, Weiner HL, Kelley VR. IL-12 drives IFN-γ-dependent autoimmune kidney disease in MRL-Fas(lpr) mice. Journal of Immunology. 1999;163:6884–6891. [PubMed] [Google Scholar]
- 83.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-γ receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. Journal of Immunology. 1998;161:494–503. [PubMed] [Google Scholar]
- 84.Balomenos D, Rumold R, Theofilopoulos AN. Interferon-γ is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–371. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jie G, Jiang Q, Rui Z, Yifei Y. Expression of interleukin-17 in autoimmune dacryoadenitis in MRL/lpr mice. Curr Eye Res. 2010;35:865–871. doi: 10.3109/02713683.2010.497600. [DOI] [PubMed] [Google Scholar]
- 86.Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4 + T cell subsets. Journal of Immunology. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hahn-Windgassen A, Nogueira V, Chen C, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 89.Ramanathan A, Schreiber SL. Direct control of mitochondrial function by mTOR. Proc Natl Acad Sci U S A. 2009;106:22229–22232. doi: 10.1073/pnas.0912074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schieke SM, Phillips D, McCoy JP, Jr, Aponte AM, Shen R, Balaban RS, Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J Biol Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- 91.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1α transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 92.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR Kinase Differentially Regulates Effector and Regulatory T Cell Lineage Commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–304. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Battaglia M, Stabilini A, Roncarolo M. Rapamycin selectively expands CD4+CD25+FoxP3 + regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 95.Sarbassov DD, Sabatini DM. Redox regulation of the nutrient-sensitive raptor-mTOR pathway and complex. J Biol Chem. 2005;280:39505–39509. doi: 10.1074/jbc.M506096200. [DOI] [PubMed] [Google Scholar]
- 96.Yoshida S, Hong S, Suzuki T, Nada S, Mannan AM, Wang J, Okada M, Guan K, Inoki K. Redox regulates mammalian target of rapamycin complex 1 (mTORC1) activity by modulating the TSC1/TSC2-Rheb GTPase pathway. J Biol Chem. 2011;286:32651–32660. doi: 10.1074/jbc.M111.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suwannaroj S, Lagoo A, Keisler D, McMurray RW. Antioxidants suppress mortality in the female NZB × NZW F1 mouse model of systemic lupus erythematosus (SLE) Lupus. 2001;10:258–265. doi: 10.1191/096120301680416940. [DOI] [PubMed] [Google Scholar]
- 98.Tewthanom K, Janwitayanujit S, Totemchockcyakarn K, Panomvana Na Ayudhya D. The effect of high dose of N-acetylcysteine in lupus nephritis: A case report and literature review. J Clin Pharm Ther. 2010;35:483–485. doi: 10.1111/j.1365-2710.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 99.Michelot A, Costanzo M, Sarkeshik A, Boone C, Yates JR, III, Drubin DG. Reconstitution and protein composition analysis of endocytic actin patches. Current Biology. 2010;20:1890–1899. doi: 10.1016/j.cub.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Flinn RJ, Yan Y, Goswami S, Parker PJ, Backer JM. The late endosome is essential for mTORC1 signaling. Mol Biol Cell. 2010;21:833–841. doi: 10.1091/mbc.E09-09-0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li L, Kim E, Yuan H, Inoki K, Goraksha-Hicks P, Schiesher RL, Neufeld TP, Guan K. Regulation of mTORC1 by the Rab and Arf GTPases. J Biol Chem. 2010;285:19705–19709. doi: 10.1074/jbc.C110.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141:290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ögmundsdóttir MH, Heublein S, Kazi S, Reynolds B, Visvalingam SM, Shaw MK, Goberdhan DCI. Proton-assisted Amino acid transporter PAT1 complexes with Rag GTPases and activates TORC1 on late endosomal and lysosomal membranes. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu H, Rhodes M, Wiest DL, Vignali DAA. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 2000;13:665–675. doi: 10.1016/s1074-7613(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 106.Krangel MS. Endocytosis and recycling of the T3-T cell receptor complex. The role of T3 phosphorylation. J Exp Med. 1987;165:1141–1159. doi: 10.1084/jem.165.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tax WJM, Van der Heijden HMW, Capel PJA, Koene RAP. Internalization (but no recycling) of T cell receptor, T3 antigen, and other T cell antigens. Transplant Proc. 1987;19:273–276. [PubMed] [Google Scholar]
- 108.Nagy G, Ward J, Mosser DD, Koncz A, Gergely P, Jr, Stancato C, Qian Y, Fernandez D, Niland B, Grossman CE, Telarico T, Banki K, Perl A. Regulation of CD4 expression via recycling by HRES-1/RAB4 controls susceptibility to HIV infection. J Biol Chem. 2006;281:34574–34591. doi: 10.1074/jbc.M606301200. [DOI] [PubMed] [Google Scholar]
- 109.Naramura M, Jang I, Kole H, Huang F, Haines D, Gu H. C-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3:1192–1199. doi: 10.1038/ni855. [DOI] [PubMed] [Google Scholar]
- 110.Cormont M, Metón I, Mari M, Monzo P, Keslair F, Gaskin C, McGraw TE, Le Marchand-Brustel Y. CD2AP/CMS regulates endosome morphology and traffic to the degradative pathway through its interaction with Rab4 and c-Cbl. Traffic. 2003;4:97–112. doi: 10.1034/j.1600-0854.2003.40205.x. [DOI] [PubMed] [Google Scholar]
- 111.Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, Santambrogio L. Microautophagy of Cytosolic Proteins by Late Endosomes. Developmental Cell. 2011;20:131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Drake JR, Webster P, Gambier JC, Mellman I. Delivery of B cell receptor-internalized antigen to endosomes and class II vesicles. J Exp Med. 1997;186:1299–1306. doi: 10.1084/jem.186.8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wolf PR, Ploegh L. How MHC class II molecules acquire peptide cargo: Biosynthesis and trafficking through the endocytic pathway. Annual Review of Cell and Developmental Biology. 1995;11:267–306. doi: 10.1146/annurev.cb.11.110195.001411. [DOI] [PubMed] [Google Scholar]
- 114.Pochynyuk O, Stockand JD, Staruschenko A. Ion channel regulation by Ras, Rho, and Rab small GTPases. Exp Biol Med. 2007;232:1258–1265. doi: 10.3181/0703-MR-76. [DOI] [PubMed] [Google Scholar]
- 115.Lazzarino DA, Blier P, Mellman I. The monomeric guanosine triphosphatase rab4 controls an essential step on the pathway of receptor-mediated antigen processing in B cells. J Exp Med. 1998;188:1769–1774. doi: 10.1084/jem.188.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lleo A, Invernizzi P, Selmi C, Coppel RL, Alpini G, Podda M, Mackay IR, Gershwin ME. Autophagy: Highlighting a novel player in the autoimmunity scenario. J Autoimmun. 2007;29:61–68. doi: 10.1016/j.jaut.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Crotzer VL, Blum JS. Autophagy and Its Role in MHC-Mediated Antigen Presentation. The Journal of Immunology. 2009;182:3335–3341. doi: 10.4049/jimmunol.0803458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chua CEL, Gan BQ, Tang BL. Involvement of members of the Rab family and related small GTPases in autophagosome formation and maturation. Cellular and Molecular Life Sciences. 2011;68:3349–3358. doi: 10.1007/s00018-011-0748-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pei G, Bronietzki M, Gutierrez MG. Immune regulation of Rab proteins expression and intracellular transport. Journal of Leukocyte Biology. 2012 doi: 10.1189/jlb.0212076. [DOI] [PubMed] [Google Scholar]
- 120.Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive Production of IFN-γ in Patients with Systemic Lupus Erythematosus and Its Contribution to Induction of B Lymphocyte Stimulator/B Cell-Activating Factor/TNF Ligand Superfamily-13B. The Journal of Immunology. 2008;181:2211–2219. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 121.Davas EM, Tsirogianni A, Kappou I, Karamitsos D, Economidou I, Dantis PC. Serum IL-6, TNFα, p55 srTNFα, p75 srTNFα, srIL-2α levels and disease activity in systemic lupus erythematosus. Clin Rheumatol. 1999;18:17–22. doi: 10.1007/s100670050045. [DOI] [PubMed] [Google Scholar]
- 122.Alvarcz-Dominguez C, Stahl PD. Interferon-γ/selectively induces Rab5a synthesis and processing in mononuclear cells. J Biol Chem. 1998;273:33901–33904. doi: 10.1074/jbc.273.51.33901. [DOI] [PubMed] [Google Scholar]
- 123.Wainszelbaum MJ, Proctor BM, Pontow SE, Stahl PD, Barbieri MA. IL4/PGE2 induction of an enlarged early endosomal compartment in mouse macrophages is Rab5-dependent. Exp Cell Res. 2006;312:2238–2251. doi: 10.1016/j.yexcr.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 124.Bhattacharya M, Ojha N, Solanki S, Mukhopadhyay CK, Madan R, Patel N, Krishnamurthy G, Kumar S, Basu SK, Mukhopadhyay A. IL-6 and IL-12 specifically regulate the expression of Rab5 and Rab7 via distinct signaling pathways. EMBO J. 2006;25:2878–2888. doi: 10.1038/sj.emboj.7601170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Florey O, Overholtzer M. Autophagy proteins in macroendocytic engulfment. Trends Cell Biol. 2012 doi: 10.1016/j.tcb.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mizushima N, Yoshimori T, Levine B. Methods in Mammalian Autophagy Research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kuballa P, Nolte WM, Castoreno AB, Xavier RJ. Autophagy and the immune system. Annual Review of Immunology. 2012;30:611–646. doi: 10.1146/annurev-immunol-020711-074948. [DOI] [PubMed] [Google Scholar]
- 128.Arsov I, Adebayo A, Kucerova-Levisohn M, Haye J, MacNeil M, Papavasiliou FN, Yue Z, Ortiz BD. A role for autophagic protein Beclin 1 early in lymphocyte development. Journal of Immunology. 2011;186:2201–2209. doi: 10.4049/jimmunol.1002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He Y. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hubbard VM, Valdor R, Patel B, Singh R, Cuervo AM, Macian F. Macroautophagy regulates energy metabolism during effector T cell activation. Journal of Immunology. 2010;185:7349–7357. doi: 10.4049/jimmunol.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kovacs JR, Li C, Yang Q, Li G, Garcia IG, Ju S, Roodman DG, Windle JJ, Zhang X, Lu B. Autophagy promotes T-cell survival through degradation of proteins of the cell death machinery. Cell Death Differ. 2012;19:144–152. doi: 10.1038/cdd.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jia W, He Y. Temporal regulation of intracellular organelle homeostasis in T lymphocytes by autophagy. Journal of Immunology. 2011;186:5313–5322. doi: 10.4049/jimmunol.1002404. [DOI] [PubMed] [Google Scholar]
- 133.Jia W, Pua HH, Li Q, He Y. Autophagy regulates endoplasmic reticulum homeostasis and calcium mobilization in T lymphocytes. Journal of Immunology. 2011;186:1564–1574. doi: 10.4049/jimmunol.1001822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Sen D, Roos J, Stauderman KA, Cahalan MD. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci U S A. 2008;105:2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gomes LC, Benedetto GD, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Willinger T, Flavell RA. Canonical autophagy dependent on the class III phosphoinositide-3 kinase Vps34 is required for naive T-cell homeostasis. Proc Natl Acad Sci U S A. 2012;109:8670–8675. doi: 10.1073/pnas.1205305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vassilopoulos D, Kovacs B, Tsokos GC. TCR/CD3 complex-mediated signal transduction pathway in T cells and T cell lines from patients with systemic lupus erythematosus. Journal of Immunology. 1995;155:2269–2281. [PubMed] [Google Scholar]
- 138.Jacob N, Stohl W. Cytokine disturbances in systemic lupus erythematosus. Arthritis Research and Therapy. 2011;13 doi: 10.1186/ar3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Telarico T, Doherty E, Clair B, Malorni W, Perl A. Overexpression of Lupus-Susceptibility Gene HRES-1/Rab4 Causes Enhanced Microautophagy and Defective Mitochondrial Macroautophagy (Mitophagy) in Lupus T cells. Arthritis & Rheumatism. 2011;63(10 supplement):S649. [Google Scholar]
- 140.Gros F, Arnold J, Page N, Decossas M, Korganow A, Martin T, Muller S. Macroautophagy is deregulated in murine and human lupus T lymphocytes. Autophagy. 2012;8(7) doi: 10.4161/auto.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Towns R, Kabeya Y, Yoshimori T, Guo C, Shangguan Y, Hong S, Kaplan M, Klionsky DJ, Wiley JW. Sera from patients with type 2 diabetes and neuropathy induce autophagy and colocalization with mitochondria in SY5Y cells. Autophagy. 2005;1:163–170. doi: 10.4161/auto.1.3.2068. [DOI] [PubMed] [Google Scholar]
- 142.Zhou X, Lu X, Lv J, Yang H, Qin L, Zhao M, Su Y, Li Z, Zhang H. Genetic association of PRDM1-ATG5 intergenic region and autophagy with systemic lupus erythematosus in a Chinese population. Ann Rheum Dis. 2011;70:1330–1337. doi: 10.1136/ard.2010.140111. [DOI] [PubMed] [Google Scholar]
- 143.Alirezaei M, Fox HS, Flynn CT, Moore CS, Hebb ALO, Frausto RF, Bhan V, Kiosses WB, Whitton JL, Robertson GS, Crocker SJ. Elevated ATG5 expression in autoimmune demyelination and multiple sclerosis. Autophagy. 2009;5:152–158. doi: 10.4161/auto.5.2.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kužnik A, Benčina M, Švajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. Journal of Immunology. 2011;186:4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 145.Page N, Schall N, Strub J, Quinternet M, Chaloin O, Décossas M, Cung MT, Van Dorsselaer A, Briand J, Muller S. The spliceosomal phosphopeptide P140 controls the lupus disease by interacting with the HSC70 protein and via a mechanism mediated by γδ T cells. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0005273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Page N, Gros F, Schall N, Décossas M, Bagnard D, Briand J, Muller S. HSC70 blockade by the therapeutic peptide P140 affects autophagic processes and endogenous MHCII presentation in murine lupus. Ann Rheum Dis. 2011;70:837–843. doi: 10.1136/ard.2010.139832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Muller S, Monneaux F, Schal N, Rashkov RK, Oparanov BA, Wiesel P, Geiger J, Zimmer R. Spliceosomal peptide P140 for immunotherapy of systemic lupus erythematosus: Results of an early phase II clinical trial. Arthritis Rheum. 2008;58:3873–3883. doi: 10.1002/art.24027. [DOI] [PubMed] [Google Scholar]
- 148.Harr MW, McColl KS, Zhong F, Molitoris JK, Distelhorst CW. Glucocorticoids downregulate Fyn and inhibit IP3-mediated calcium signaling to promote autophagy in T lymphocytes. Autophagy. 2010;6:912–921. doi: 10.4161/auto.6.7.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu WKK, Sakamoto KM, Milani M, Aldana-Masankgay G, Fan D, Wu K, Lee CW, Cho CH, Yu J, Sung JJY. Macroautophagy modulates cellular response to proteasome inhibitors in cancer therapy. Drug Resistance Updates. 2010;13:87–92. doi: 10.1016/j.drup.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 150.Francis L, Perl A. Pharmacotherapy of systemic lupus erythematosus. Expert Opin Pharmacother. 2009;10:1481–1494. doi: 10.1517/14656560902971003. [DOI] [PubMed] [Google Scholar]
- 151.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: A systematic review. Ann Rheum Dis. 2010;69:20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 152.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, Stohl W, Ginzler EM, Hough DR, Zhong ZJ, Freimuth W, Van Vollenhoven RF. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ramos-Casals M, Soto MJ, Cuadrado MJ, Khamashta MA. Rituximab in systemic lupus erythematosus A systematic review of off-label use in 188 cases. Lupus. 2009;18:767–776. doi: 10.1177/0961203309106174. [DOI] [PubMed] [Google Scholar]
- 154.Melander C, Sallée M, Trolliet P, Candon S, Belenfant X, Daugas E, Rémy P, Zarrouk V, Pillebout E, Jacquot C, Boffa J, Karras A, Masse V, Lesavre P, Elie C, Brocheriou I, Knebelmann B, Noël L, Fakhouri F. Rituximab in severe lupus nephritis: Early B-cell depletion affects long-term renal outcome. Clinical Journal of the American Society of Nephrology. 2009;4:579–587. doi: 10.2215/CJN.04030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dörner T, Kaufmann J, Wegener WA, Teoh N, Goldenberg DM, Burmester GR. Initial clinical trial of epratuzumab (humanized anti-CD22 antibody) for immunotherapy of systemic lupus erythematosus. Arthritis Research and Therapy. 2006;8 doi: 10.1186/ar1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Daridon C, Blassfeld D, Reiter K, Mei HE, Giesecke C, Goldenberg DM, Hansen A, Hostmann A, Frölich D, Dörner T. Epratuzumab targeting of CD22 affects adhesion molecule expression and migration of B-cells in systemic lupus erythematosus. Arthritis Research and Therapy. 2010;12 doi: 10.1186/ar3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, Wiethe C, Winkler TH, Kalden JR, Manz RA, Voll RE. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. 2008;14:748–755. doi: 10.1038/nm1763. [DOI] [PubMed] [Google Scholar]
- 158.Wang Y, Hu Q, Madri JA, Rollins SA, Chodera A, Matis LA. Amelioration of lupus-like autoimmune disease in NZB/W F1 mice after treatment with a blocking monoclonal antibody specific for complement component C5. Proc Natl Acad Sci U S A. 1996;93:8563–8568. doi: 10.1073/pnas.93.16.8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bao L, Haas M, Kraus DM, Hack BK, Rakstang JK, Holers VM, Quigg RJ. Administration of a soluble recombinant complement C3 inhibitor protects against renal disease in MRL/lpr mice. Journal of the American Society of Nephrology. 2003;14:670–679. doi: 10.1097/01.asn.0000051597.27127.a1. [DOI] [PubMed] [Google Scholar]
- 160.Barrat F, Meeker T, Chan J, Guiducci C, Coffman R. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol. 2007;37:3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]
- 161.Dall’Era M, Chakravarty E, Wallace D, Genovese M, Weisman M, Kavanaugh A, Kalunian K, Dhar P, Vincent E, Pena-Rossi C, Wofsy D, Alum N, Dubois A, Kinnman N, Picard M, Bortolotti A, O’Grady L, Hill J, Nestorov I, Salmon E. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: Results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. 2007;56:4142–4150. doi: 10.1002/art.23047. [DOI] [PubMed] [Google Scholar]
- 162.Sthoeger ZM, Sharabi A, Molad Y, Asher I, Zinger H, Dayan M, Mozes E. Treatment of lupus patients with a tolerogenic peptide, hCDR1 (Edratide): Immunomodulation of gene expression. J Autoimmun. 2009;33:77–82. doi: 10.1016/j.jaut.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 163.Cardiel MH, Tumlin JA, Furie RA, Wallace DJ, Joh T, Linnik MD, Abeles M, Aelion J, Appel GB, Aranow C, Ballou S, Becker MA, Belmont HM, Boling EP, Bombardieri S, Brodeur J, Buyon J, Condemi JJ, Cronin ME, Cush JJ, DeHoratius R, Desir D, Donohue J, Edwards M, El-Shahawy MA, Emery P, Ensworth S, Espinoza LR, Fondal M, Fortin P, Geppert T, Gilkeson GS, Ginzler E, Gorevic P, Granda J, Grossman J, Hiepe F, Howard P, Hura CE, Jaffer A, Jakes J, Kalden JR, Kammer GM, Kaplan MJ, Kaplan S, Katz R, Kennedy A, Kenney HM, Khamashta M, Kivitz AJ, Kovacs C, Krishnan M, Kurtzman NA, Liebling M, Lourie SH, Loveless J, Manion CV, Manzi S, Martin K, McKay J, Mease PJ, Merrill JT, Moreland LW, Moritz C, Neuwelt CM, Petri M, Pogue BC, Quinet RJ, Ramsey-Goldman R, Roth A, Rothfield N, Scarpa N, Schneider M, Shergy WJ, Sherrer Y, Sibilia J, Sisay M, Smith D, Spinowitz B, Spira M, Stevens MP, Sturfelt G, Surbeck W, Tindall EA, Torres A, Van Vollenhoven R, Vijayan A, Vilardell-Tarres M, Weaver C, Williams GW, Zummer M. Abetimus sodium for renal flare in systemic lupus erythematosus: Results of a randomized, controlled phase III trial. Arthritis Rheum. 2008;58:2470–2480. doi: 10.1002/art.23673. [DOI] [PubMed] [Google Scholar]
- 164.Merrill JT, Burgos-Vargas R, Westhovens R, Chalmers A, D’Cruz D, Wallace DJ, Bae SC, Sigal L, Becker J, Kelly S, Raghupathi K, Li T, Peng Y, Kinaszczuk M, Nash P. The efficacy and safety of abatacept in patients with non?life-threatening manifestations of systemic lupus erythematosus: Results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis & Rheumatism. 2010;62:3077–3087. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- 165.Hu Y, Metz DP, Chung J, Siu G, Zhang M. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. Journal of Immunology. 2009;182:1421–1428. doi: 10.4049/jimmunol.182.3.1421. [DOI] [PubMed] [Google Scholar]
- 166.Ichinose K, Juang Y, Crispín JC, Kis-Toth K, Tsokos GC. Suppression of autoimmunity and organ pathology in lupus-prone mice upon inhibition of calcium/calmodulin-dependent protein kinase type IV. Arthritis & Rheumatism. 2011;63:523–529. doi: 10.1002/art.30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Barber DF, Bartolomé A, Hernandez C, Flores JM, Redondo C, Fernandez-Arias C, Camps M, Rückle T, Schwarz MK, Rodríguez S, Martinez-A C, Balomenos D, Rommel C, Carrera AC. PI3Kγ inhibition blocks glomerulonephritis and extends lifespan in a mouse model of systemic lupus. Nat Med. 2005;11:933–935. doi: 10.1038/nm1291. [DOI] [PubMed] [Google Scholar]
- 168.Kyttaris VC, Zhang Z, Kampagianni O, Tsokos GC. Calcium signaling in systemic lupus erythematosus T cells: A treatment target. Arthritis & Rheumatism. 2011;63:2058–2066. doi: 10.1002/art.30353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Illei GG, Shirota Y, Yarboro CH, Daruwalla J, Tackey E, Takada K, Fleisher T, Balow JE, Lipsky PE. Tocilizumab in systemic lupus erythematosus: Data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 2010;62:542–552. doi: 10.1002/art.27221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stirzaker RA, Biswas PS, Gupta S, Song L, Bhagat G, Pernis AB. Administration of fasudil, a ROCK inhibitor, attenuates disease in lupus-prone NZB/W F1 female mice. Lupus. 2012;21:656–661. doi: 10.1177/0961203312436862. [DOI] [PubMed] [Google Scholar]
- 171.Llorente L, Richaud-Patin Y, García-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, Alcocer-Varela J, Grangeot-Keros L, Alarcón-Segovia D, Wijdenes J, Galanaud P, Emilie D. Clinical and biologic effects of anti?interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis & Rheumatism. 2000;43:1790–1800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 172.Ostendorf B, Iking-Konert C, Kurz K, Jung G, Sander O, Schneider M. Preliminary results of safety and efficacy of the interleukin 1 receptor antagonist anakinra in patients with severe lupus arthritis. Ann Rheum Dis. 2005;64:630–633. doi: 10.1136/ard.2004.025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yao Y, Richman L, Higgs BW, Morehouse CA, de los Reyes M, Brohawn P, Zhang J, White B, Coyle AJ, Kiener PA, Jallal B. Neutralization of interferon-?/??inducible genes and downstream effect in a phase I trial of an anti?interferon-? monoclonal antibody in systemic lupus erythematosus. Arthritis & Rheumatism. 2009;60:1785–1796. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]