Abstract
Telomeres are essential for the integrity of chromosomes and for cellular replication. Attrition of telomeres occurs during DNA replication due to the inability of conventional DNA polymerase to replicate chromosomal termini and the insufficient compensation for telomere loss by telomerase, an enzyme that synthesizes telomeric DNA. A number of genetic defects have been described in humans and in animal models which cause accelerated telomere attrition, in turn leading to severe phenotypes of hematopoietic and other proliferating cells. Telomere length, most frequently measured as an average value in heterogeneous peripheral blood leukocyte populations in humans, has been associated with a wide range of health conditions and diseases of immune and non-immune cells. Here, we review recent studies of telomere length dynamics with particular relevance to immune function.
Introduction
The termini of linear chromosomes are called telomeres, consisting of an array of DNA repeats [(TTAGGG)n in mammals] and a number of telomere binding proteins [1;2]. The incomplete replication of chromosomal ends during cell division results in loss of a small fraction of telomere DNA and makes telomere length a marker of the biological age of cells, tissues/organs, and probably of humans [3]. Telomeric DNA is synthesized by telomerase which maintains telomere function via compensating the loss of telomeric DNA resulting from cell-division. Telomerase consists of two core components: telomerase reverse transcriptase (TERT) and telomerase RNA template (TERC). Telomerase activity is tightly regulated as most mature cells express very little telomerase, with the exception of germline cells, stem cells, and lymphocytes.
Telomere length attrition has been well documented during continued in vitro replication of cultured human cells, including fibroblasts and lymphocytes [4;5]; and in leukocytes and fibroblasts with progressive age [6–8] both in normal individuals and at an accelerated rate in the presence of some genetic abnormalities, disease states, or environmental stressors [9–11]. A large body of research has established that intact telomere function is essential for cell proliferation and that impaired telomere maintenance has a severe detrimental impact for cells such as stem cells which rely on proliferation for their function.
Blood leukocytes are composed of multiple cell types that derive from two hematopoietic lineages. Myeloid derived cells include granulocytes (neutrophils, basophils and eosinophils) and monocytes/macrophages, whereas lymphoid derived cells include T cells, B cells, and NK cells. The proportions of these different cell types in blood undergo changes with age, including increased monocytes, decreased lymphocytes, a decrease in naïve lymphocytes, and an increase in memory lymphocytes [12]. Because the mature myeloid lineage cells are non-dividing cells, whereas mature lymphocytes undergo extensive cell division in mediating their function, telomere length measured for leukocytes reflect sums or averages over cells with potentially very different replicative histories. Furthermore, telomeres are on average longer in naïve than in memory T cells [4;7], and are longer in CD28+ than in more differentiated CD28− CD8+ T cells [13]. Therefore, measuring telomeres in defined cell populations whenever possible will facilitate a more informative interpretation of results.
Defects in telomeres cause severe problems in hematopoietic and other systems
The in vivo role of telomeres in cellular replication is best demonstrated from the studies of genetic disorders that impair various components of telomerase and telomeres [9;10]. The first genetic disorder reported to be associated with short telomeres is Dyskeritosis congenita (DC), a rare syndrome with abnormalities of the skin, nails, mucous membranes, and bone marrow [14]. A number of different genetic mutations in components of either telomerase ribonucleoprotein complex (DKC1, TERT, TERC, NOP10, and NHP2) or telomere binding protein-associated protein (TIN2) have been subsequently found in DC families [9;14]. In addition, genetic mutations of telomerase components, with consequent short telomeres, have also been found in association with idiopathic fibrosis of lung [15], fibrosis/cirrhosis of liver [16;17], and hereditary cancer risk [18]. Together, these mutations show defects in the maintenance of telomere maintenance (short telomeres), accompanied by replicative dysfunction in stem cells [19], the hematopoietic system (bone marrow failure) [20], lung (idiopathic pulmonary fibrosis) and liver (cirrhosis), and predisposition of cancer.
A striking feature of these genetic defects and diseases is the phenomenon of genetic anticipation. This is the appearance of progressively shorter telomeres in DC patients and earlier onset of disease manifestations from generation to generation within the same family. It is telomere length, in the presence of an identical genetic mutation, that appears to explain an earlier onset age and severity of the disease in later generations. Thus, genetic and clinical evidence indicates that short telomeres cause genomic instability and lead to cellular dysfunction in those highly proliferative tissues and organs [9;10].
Short telomeres of leukocytes associated with aging and immune dysfunctions
Cellular proliferation is a key component of an effective adaptive immune response. Therefore, the role of telomeres in leukocytes, particularly in lymphocytes, is of great interest [21]. A number of intriguing observations have indicated that a shortening of telomeres occurs in cells of the human immune system as a function of in vivo lineage differentiation and with aging, as well as during in vitro culture. The loss of telomeres has been observed during naïve T cell differentiation to memory T cells [4;7], during CD28+ to CD28− CD8 T cell differentiation [13], in long-term cultured T cells [4;5], and in chronic viral infections [22]. In the cultured CD8 T cells, significantly shortened telomeres appear to be to cause senescence as the ectopic expression of telomerase is capable of rescue telomere shortening and prolong their proliferation [23]. Furthermore, cross-sectional analyses show that telomere shortening occurs in leukocytes or PBMC and in isolated granulocytes and lymphocytes (CD4, CD8 T cells and B cells) with age [6–8]. Collectively, these findings show that telomere attrition occurs in blood leukocytes of both myeloid and lymphoid lineages, and that telomere length associates with leukocyte differentiation and age. However, the degree to which telomere attrition contributes to the overall decline of immune function with age remains to be determined.
Short telomeres of leukocytes or PBMC have also been described in association with a number of immune-related diseases [24]. Patients with some common autoimmune syndromes including rheumatoid arthritis [25], and diabetes mellitus (type 1 and type 2) [26;27] display significantly shortened telomeres in leukocytes or PBMC compared to age-matched healthy controls. In addition, patients with viral infections including HIV, EBV, and CMV undergo extensive proliferative response of T cells in response to these infections, and it has been observed that viral reactive T cells have short telomeres associated with replicative impairment [22;28]. A common pathological basis of these immune dysfunctions is the extensive proliferation of lymphocytes, leading to the exhaustion of telomeres and replicative potential.
Finally, short telomeres of leukocytes or PBMC are also found in patients with malignancies and as a risk factor for some type of tumors, whether or not the malignancies are of immune cell lineages [29] or other cell types [30]. In malignancies of immune cell origin such as leukemia, it is currently unclear whether short telomeres contribute causally to leukemia pathogenesis or are the consequence of extensive proliferation of tumor cells, or both. Even less is known about the cause of short telomeres of leukocytes or PBMC in patients with tumors of non-immune cells. Whether the burden of tumor bearing affects immune cell function, and more specifically how telomere maintenance is affected requires further study. Nevertheless, these findings suggest that telomere shortening may be a cause and/or a consequence of immune cell dysfunction.
Some known factors influencing telomere lengths in blood leukocytes
An increasing number of publications in recent years show that various physiological and/or psychological factors have an impact on overall health as well as an effect on telomere length of peripheral blood leukocytes [31–36]. Early studies showed an association of sustained stress, such as that experienced by the mothers of sick children or the caregivers of Alzheimer's diseases, with short telomere length in leukocytes or PBMC compared to healthy controls [31;32]. Individuals with major depression over a long period of time have also been reported to have shorter telomeres in leukocytes than healthy controls [33]. More recently, short telomeres have also been found in the offspring of mothers who had experienced a severe stressor in the index pregnancy, suggesting that prenatal stress exposure is linked to subsequent shorter telomere length in offspring [34]. Other studies have shown that a healthy lifestyle such as exercise, healthy weight, lower meat and higher fruit/vegetable consumption is linked to longer telomeres of leukocytes [35] whereas obesity is associated with shorter telomeres [36]. It has further been suggested that a sustained healthy lifestyle may even attenuate the effect of stressors on telomere length [37].
These epidemiological analyses of mostly case-control studies reveal a correlative link between telomere length of leukocytes and various health conditions. Considering that telomere length is highly polymorphic in the population, a large sample size and preferably a longitudinal follow-up will be needed to further test these findings. More importantly, it will be critical to understanding the underlying mechanism of these correlative findings. Do these physiological and/or psychological factors affect the length of telomeres by actual shortening/lengthening of telomeres in leukocytes, or do they alter telomere length in heterogeneous leukocyte populations by altering the relative proportions of different cell types known to differ in telomere length? A parallel analysis of telomere length and leukocyte composition, or more rigorously still, a direct analysis of telomere length in leukocyte subsets with longitudinal follow-up may provide some answers. Only limited studies to date have carried out any longitudinal analysis of leukocyte subsets [8], and still more refined subpopulation analysis will be necessary to better define the potentially complex effects of telomere length change. It has also been suggested that the length of individual telomeres in a cell, rather than the overall average of telomere length, may be important to function [38]. It remains to be determined whether this is in fact relevant to normal and pathological human conditions. As noted in the summary of available methodologies, measurement of this parameter remains highly challenging in human population studies.
Comprehensive assessment of immune function including telomere length
Whether telomere length of leukocytes can be a useful parameter for predicting health and diseases is a subject of current debate [39;40]. Here we focus on whether telomere length of leukocyte or PBMC is a useful measure for assessing the competence of immune system function. From the study of genetic syndromes of telomere/telomerase dysfunction that were described above, there is little question that telomere length can influence bone marrow function. However, it remains to be determined whether there are measurable effects of telomere length on immune function, either with normal aging, during normal immune responses, or in defined pathologic states.
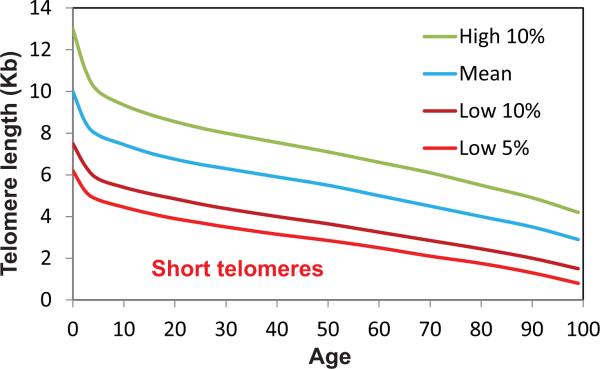
It has been shown that patients with various genetic defects in telomere maintenance have leukocyte/PBMC telomere length in the lower 10% of the age-matched normal population [41]. Therefore, telomere length equal to the lower 10% or 5% in a population of a given age could be used as an operational criterion of short telomeres (Figure 1). Considering the variation of telomere length in populations, applying this criterion requires establishing a standard telomere length range of leukocytes and/or PBMC in the normal population and its change with age. As telomere length of leukocytes and PBMC is dynamic [42;43], repeated measurements of telomere length over time (months to years) is necessary to accurately determine the rate of telomere length change, a parameter that has critical value in assessing the telomere status in addition to the absolute length.
Figure 1.
Distribution and attrition of telomere length in human leukocytes with age. Telomere length is highly polymorphic in the general population. The distributions of telomere length with age are presented as percentages (modified based on [41]).
Considering the heterogeneous nature of cell lineages, types, and proliferative history, and the heterogeneity of telomere length on individual chromosomes within a cell, the measurement of average telomere length of heterogeneous cell populations alone is likely to be insufficient for assessing immune competence. As the adaptive immune response is antigen-specific, an average telomere length does not always predict the telomere length of those antigen-reactive lymphocytes which represent a small percentage of total lymphocytes. The most specific assessment of telomere function in a specific response thus must be conducted by analysis of antigen-specific populations. A more comprehensive assessment of immune function in human must also include other measures, such as detailed analysis of composition of different cell type such as monocytes and lymphocytes, and the relative percentages of naïve, effector, and memory cells, and levels of cytokine/chemokines [44]. Integrating these measures will reveal new insight into the human immune system and its change with age (Table 1). This in turn will help to further assess how telomere length may contribute in predicting immune competency.
Table 1.
Suggested panel of tests for evaluating the status of human immune function
| Test | Method |
|---|---|
|
| |
| Blood cell counts of all types of leukocytes | CBC |
| Telomere length of leukocytes or PBMC | qPCR or Flow-FISH |
| Leukocyte composition including lymphocyte subsets | Multi-color flow cytometry |
| Serum cytokine profile | Multiplex assay |
Potential applications of telomere length measurement in leukocytes
What is the usefulness of knowing one's telomere length of leukocytes? First, identifying individuals, children in particular, with short telomeres may reveal genetic defects of telomere maintenance and allow early diagnosis prior to development of the typical phenotype. Second, individuals with short telomeres but without any known genetic defects may need additional follow-up measurements and observation to determine if they develop any symptoms due to currently unknown mutations or not. Third, it may be feasible to use pharmacological agents to enhance telomere length and reduce telomere attrition for those adults who have short telomeres. Recent in vitro studies show that telomerase activator (TAT2) modestly retards telomere shortening and increases proliferative potential of CD8 T cells [45] and that inhibition of mitogen-activated protein kinase (p38) enhances telomerase activity and survival after TCR activation [46], suggesting that enhancing telomerase activity in immune cells by a pharmacological approach could restore or improve immune function that is limited by the telomeres in genetic disorders as well as in aging.
Vaccination and immunization has been perhaps the most cost-effective intervention in modern medicine. Regardless of what type or kind of vaccine, often some individuals do not respond well to the vaccine which could be due to different possible reasons. Dysfunctional telomeres may be a contributing factor. As observed in various chronic viral infections, repeated stimulation causes extended proliferation and short telomeres of antigen-specific T cells, which eventually leads to exhaustion [22;28;47]. Since a successful immune response to a vaccine requires substantial division of naïve lymphocytes, individuals with short telomeres may be susceptible to exhaustion of these vaccine reactive lymphocytes, limiting their ability to respond to an eventual real encounter with a pathogen [47]. This is of particular relevance to the vaccination of the elderly. Older adults (over 75 years old) have diminishing numbers of naïve lymphocytes, the primary target cells for an effective vaccination, and have limited residual replicative capacity due to generally short telomeres of their lymphocytes. It has been observed that the efficacy of seasonal influenza vaccine shows an inverse correlation with age in this elderly population (the efficacy is measured by the frequency of antibody conversion in sera: 50% from 60–70 years old, 31% from 70–80, and 11% after 80 years old) [48]. Although it remains to be directly determined whether short telomere length of lymphocytes in the elderly contributes to their decline of vaccine response, these observations suggest that a comprehensive assessment of immune function including the measurement of telomere length might be informative prior to giving a vaccine to the elderly. For those elderly with immune function limited by lymphocyte exhaustion, which can be the result of telomere shortening, active immunization may be of limited efficacy, and passive immunization strategies may be more effective. Therefore, developing the protective antibodies against influenza and other viruses with broad specificity will be a useful alternative for those elderly [49].
Concluding remarks
A plethora of data has been generated in characterizing the role of telomeres in cell proliferation, in identifying factors influencing telomere length regulation, and in demonstrating an association of telomere length with various health and abnormal conditions. Studies of genetic abnormalities associated with telomere impairment demonstrate a critical role of telomeres in proliferation of stem cells. It is conceivable that shortened telomeres in lymphocytes will have a detrimental impact on their ability to combat pathogens which requires their robust proliferation. Studies of chronic viral infection show that exhausted viral reactive T cells have very short telomeres while other T cells have normal telomere length [22;28], suggesting that telomere shortening may limit T cell function in antigen-dependent and antigen-specific manner. Thus, lymphocytes with short telomeres will have reduced ability to mount a strong and sustained immune response.
Like many biological parameters, telomere length in peripheral blood is an average value of different chromosomes within a cell and of a heterogeneous population of cells. It is therefore not a precise reading with high specificity and resolution. Nevertheless, such measurements may reflect a general status, which will be useful as an initial screening test. Longitudinal monitoring of telomere length will be of particular importance in providing a dynamic picture of telomere length change over time, which is equally important in predicting the immune cell function as the absolute telomere length. Longitudinal measurement of telomere length in combination with other immune parameters may offer valuable information in analyzing of the status of immune function across the age range, in children, adult, and elderly.
Highlights
Telomeres are essential for the integrity of chromosomes and for cellular replication.
Attrition of telomeres in leukocytes occurs during DNA replication and observed with aging.
A number of genetic defects cause accelerated telomere attrition and severe phenotypes of hematopoietic and other proliferating cells.
Telomere length, an average value in heterogeneous peripheral blood leukocyt epopulations, has been associated with a wide range of health conditions and diseases of immune and non-immune cells.
Telomere length in leukocytes in combination with other measurements can be used for evaluation of immune function, which will have clinical implications.
Acknowledgement
I thank Richard Hodes for comments and suggestions for this review. This research was supported by the Intramural Research Programs of the National Institute on Aging and National Cancer Institute, National Institutes of Health (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended readings
- 1.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat.Med. 2006;12:1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 2.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greider CW. Telomeres and senescence: the history, the experiment, the future. Curr Biol. 1998;8:R178–R181. doi: 10.1016/s0960-9822(98)70105-8. [DOI] [PubMed] [Google Scholar]
- 4.Weng NP, Levine BL, June CH, Hodes RJ. Human naive and memory T lymphocytes differ in telomeric length and replicative potential. Proc Natl Acad Sci U S A. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford RL, Kronenberg M, Cohen D, Schachter F. Decline in CD28+ T cells in centenarians and in long-term T cell cultures: a possible cause for both in vivo and in vitro immunosenescence. Exp.Gerontol. 1994;29:601–609. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 6.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am.J.Hum.Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 7.Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J.Exp.Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Son NH, Murray S, Yanovski J, Hodes RJ, Weng N. Lineage-specific telomere shortening and unaltered capacity for telomerase expression in human T and B lymphocytes with Age. J.Immunol. 2000;165:1191–1196. doi: 10.4049/jimmunol.165.3.1191. [DOI] [PubMed] [Google Scholar]
- 9.Armanios M. Syndromes of telomere shortening. Annu.Rev.Genomics Hum.Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savage SA, Bertuch AA. The genetics and clinical manifestations of telomere biology disorders. Genet.Med. 2010;12:753–764. doi: 10.1097/GIM.0b013e3181f415b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calado RT, Young NS. Telomere diseases. N.Engl.J.Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, Franceschi C, Passeri M. The immune system in extreme longevity. Exp.Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28−CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J.Immunol. 1996;156:3587–3590. [PubMed] [Google Scholar]
- 14.Kirwan M, Dokal I. Dyskeratosis congenita, stem cells and telomeres. Biochim.Biophys.Acta. 2009;1792:371–379. doi: 10.1016/j.bbadis.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz de LA, Cronkhite JT, Katzenstein AL, Godwin JD, Raghu G, Glazer CS, Rosenblatt RL, Girod CE, Garrity ER, Xing C, Garcia CK. Telomere lengths, pulmonary fibrosis and telomerase (TERT) mutations. PLoS.One. 2010;5:e10680. doi: 10.1371/journal.pone.0010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calado RT, Brudno J, Mehta P, Kovacs JJ, Wu C, Zago MA, Chanock SJ, Boyer TD, Young NS. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology. 2011;53:1600–1607. doi: 10.1002/hep.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartmann D, Srivastava U, Thaler M, Kleinhans KN, N'kontchou G, Scheffold A, Bauer K, Kratzer RF, Kloos N, Katz SF, Song Z, Begus-Nahrmann Y, Kleger A, von FG, Strnad P, Lechel A, Gunes C, Potthoff A, Deterding K, Wedemeyer H, Ju Z, Song G, Xiao F, Gillen S, Schrezenmeier H, Mertens T, Ziol M, Friess H, Jarek M, Manns MP, Beaugrand M, Rudolph KL. Telomerase gene mutations are associated with cirrhosis formation. Hepatology. 2011;53:1608–1617. doi: 10.1002/hep.24217. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Delgado B, Yanowsky K, Inglada-Perez L, Domingo S, Urioste M, Osorio A, Benitez J. Genetic anticipation is associated with telomere shortening in hereditary breast cancer. PLoS.Genet. 2011;7:e1002182. doi: 10.1371/journal.pgen.1002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batista LF, Pech MF, Zhong FL, Nguyen HN, Xie KT, Zaug AJ, Crary SM, Choi J, Sebastiano V, Cherry A, Giri N, Wernig M, Alter BP, Cech TR, Savage SA, Reijo Pera RA, Artandi SE. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature. 2011;474:399–402. doi: 10.1038/nature10084. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study shows that the extended culture of dyskeratosis congenita (DKC1)-mutant iPSCs leads to progressive telomere shortening and eventual loss of self-renewal ability and suggests that undifferentiated iPSCs accurately recapitulate features of a human stem cell disease.
- 20.Gadalla SM, Cawthon R, Giri N, Alter BP, Savage SA. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging (Albany.NY) 2010;2:867–874. doi: 10.18632/aging.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weng NP. Telomere and adaptive immunity. Mech.Ageing Dev. 2008;129:60–66. doi: 10.1016/j.mad.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van BD, Nanlohy NM, Otto S, Plunkett FJ, Fletcher JM, Akbar AN. Progressive telomere shortening of Epstein-Barr virus-specific memory T cells during HIV infection: contributor to exhaustion? J.Infect.Dis. 2008;198:1353–1357. doi: 10.1086/592170. [DOI] [PubMed] [Google Scholar]; * This study demonstrates that chronic EBV loads during HIV infection causes exhaustion of EBV-specific T cells with enhanced telomere shortening and suggests that chronic exposure to high antigen levels may lead to the progressive shortening of telomeres of antigen-specific T cells.
- 23.Menzel O, Migliaccio M, Goldstein DR, Dahoun S, Delorenzi M, Rufer N. Mechanisms regulating the proliferative potential of human CD8+ T lymphocytes overexpressing telomerase. J Immunol. 2006;177:3657–3668. doi: 10.4049/jimmunol.177.6.3657. [DOI] [PubMed] [Google Scholar]
- 24.Andrews NP, Fujii H, Goronzy JJ, Weyand CM. Telomeres and immunological diseases of aging. Gerontology. 2010;56:390–403. doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koetz K, Bryl E, Spickschen K, O'Fallon WM, Goronzy JJ, Weyand CM. T cell homeostasis in patients with rheumatoid arthritis. Proc.Natl.Acad.Sci.U.S.A. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fyhrquist F, Tiitu A, Saijonmaa O, Forsblom C, Groop PH. Telomere length and progression of diabetic nephropathy in patients with type 1 diabetes. J.Intern.Med. 2010;267:278–286. doi: 10.1111/j.1365-2796.2009.02139.x. [DOI] [PubMed] [Google Scholar]
- 27.Testa R, Olivieri F, Sirolla C, Spazzafumo L, Rippo MR, Marra M, Bonfigli AR, Ceriello A, Antonicelli R, Franceschi C, Castellucci C, Testa I, Procopio AD. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabet.Med. 2011;28:1388–1394. doi: 10.1111/j.1464-5491.2011.03370.x. [DOI] [PubMed] [Google Scholar]
- 28.van de Berg PJ, Griffiths SJ, Yong SL, Macaulay R, Bemelman FJ, Jackson S, Henson SM, ten Berge IJ, Akbar AN, Van Lier RA. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J.Immunol. 2010;184:3417–3423. doi: 10.4049/jimmunol.0903442. [DOI] [PubMed] [Google Scholar]; ** This study shows that telomere shortening is more rapid in CMV-seropositive individuals and correlates with the amount of differentiated T cells in both CD4 and CD8 T cells.
- 29.Lan Q, Cawthon R, Shen M, Weinstein SJ, Virtamo J, Lim U, Hosgood HD, III, Albanes D, Rothman N. A prospective study of telomere length measured by monochrome multiplex quantitative PCR and risk of non-Hodgkin lymphoma. Clin.Cancer Res. 2009;15:7429–7433. doi: 10.1158/1078-0432.CCR-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mirabello L, Garcia-Closas M, Cawthon R, Lissowska J, Brinton LA, Peplonska B, Sherman ME, Savage SA. Leukocyte telomere length in a population-based case-control study of ovarian cancer: a pilot study. Cancer Causes Control. 2010;21:77–82. doi: 10.1007/s10552-009-9436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc.Natl.Acad.Sci.U.S.A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damjanovic AK, Yang Y, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Zou Y, Beversdorf DQ, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study shows that the caregivers of Alzheimer's diseases have significantly shorter telomere lengths in PBMC, T cells and monocytes than controls and that the telomere attrition in caregivers is not due to an increase of shorter telomere possessing T cell subsets in PBMC. In addition, this study demonstrates that basal telomerase activity in PBMC and T cells is significantly higher in caregivers than in controls, pointing to an unsuccessful attempt of cells to compensate the excessive loss of telomeres in caregivers.
- 33.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, Reus VI, Rosser R, Burke HM, Kupferman E, Compagnone M, Nelson JC, Blackburn EH. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS.One. 2011;6:e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wust S, Wadhwa PD. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc.Natl.Acad.Sci.U.S.A. 2011;108:E513–E518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study shows that prenatal stress exposure is a significant predictor of subsequent adult leukocyte telomere length in the offspring. The effect is substantially unchanged after adjusting for potential confounders and is more pronounced in women, which provides the first evidence in humans of an association between prenatal stress exposure and subsequent shorter telomere length.
- 35.Diaz VA, Mainous AG, III, Everett CJ, Schoepf UJ, Codd V, Samani NJ. Effect of healthy lifestyle behaviors on the association between leukocyte telomere length and coronary artery calcium. Am.J.Cardiol. 2010;106:659–663. doi: 10.1016/j.amjcard.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Buxton JL, Walters RG, Visvikis-Siest S, Meyre D, Froguel P, Blakemore AI. Childhood obesity is associated with shorter leukocyte telomere length. J.Clin.Endocrinol.Metab. 2011;96:1500–1505. doi: 10.1210/jc.2010-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study shows that the obese children have a shorter leukocyte telomere length than do nonobese children and telomere length is inversely associated with age, weight, and height, which highlights a potentially deleterious impact of early onset obesity on future health.
- 37.Puterman E, Lin J, Blackburn E, O'Donovan A, Adler N, Epel E. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS.One. 2010;5:e10837. doi: 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study shows that among non-exercisers a one unit increase in the Perceived Stress Scale is related to a 15-fold increase in the odds of having short telomeres, whereas in exercisers, perceived stress appears to be unrelated to telomere length. Vigorous physical activity appears to protect those experiencing high stress by buffering its relationship with telomere length.
- 38.Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am.J.Hum.Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leslie M. Cell biology. Are telomere tests ready for prime time? Science. 2011;332:414–415. doi: 10.1126/science.332.6028.414. [DOI] [PubMed] [Google Scholar]
- 40.Eisenstein M. Telomeres: All's well that ends well. Nature. 2011;478:S13–S15. doi: 10.1038/478S13a. [DOI] [PubMed] [Google Scholar]
- 41.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 42.Nordfjall K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS.Genet. 2009;5:e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study shows that telomere length in leukocytes is oscillating in short time span but levels out at the longer term (e.g. 10 years follow up) and suggests that individual blood cell telomere length is a dynamic feature.
- 43.Ehrlenbach S, Willeit P, Kiechl S, Willeit J, Reindl M, Schanda K, Kronenberg F, Brandstatter A. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int.J.Epidemiol. 2009;38:1725–1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- 44.Maecker HT, McCoy JP, Nussenblatt R. Standardizing immunophenotyping for the Human Immunology Project. Nat.Rev.Immunol. 2012;12:191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fauce SR, Jamieson BD, Chin AC, Mitsuyasu RT, Parish ST, Ng HL, Kitchen CM, Yang OO, Harley CB, Effros RB. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J.Immunol. 2008;181:7400–7406. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study provides evidence that a telomerase activator (TAT2) is able to modestly retard telomere shortening, increases proliferative potential, and, importantly, enhances cytokine/chemokine production and antiviral activity CD8 T cellss from HIV-infected human donors.
- 46.Di Mitri D, Azevedo RI, Henson SM, Libri V, Riddell NE, Macaulay R, Kipling D, Soares MV, Battistini L, Akbar AN. Reversible senescence in human CD4+CD45RA+ J.Immunol. 2011;187:2093–2100. doi: 10.4049/jimmunol.1100978. [DOI] [PubMed] [Google Scholar]; ** This study identifies activation of the p38 MAPK pathway is directly involved in certain senescence characteristics of highly differentiated CD4 T cells and showes that inhibition of p38 signaling in CD4 EMRA T cells enhances telomerase activity and survival after TCR activation.
- 47.Wherry EJ. T cell exhaustion. Nat.Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 48.Bouree P. Immunity and immunization in elderly. Pathol.Biol.(Paris) 2003;51:581–585. doi: 10.1016/j.patbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, Silacci C, Fernandez-Rodriguez BM, Agatic G, Bianchi S, Giacchetto-Sasselli I, Calder L, Sallusto F, Collins P, Haire LF, Temperton N, Langedijk JP, Skehel JJ, Lanzavecchia A. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]