Abstract
Reduced defense against infection is commonly observed as a consequence of reproductive activity, but little is known about how post-mating immunosuppression occurs. In this work, we use Drosophila melanogaster as a model to test the role of seminal fluid components and egg production in suppressing post-mating immune defense. We also evaluate whether systemic immune system activity is altered during infection in mated females. We find that post-mating reduction in female defense depends critically on male transfer of sperm and seminal fluid proteins, including the accessory gland protein known as “sex peptide.” However, the effect of these male factors is dependent on the presence of the female germline. We find that mated females have lower antimicrobial peptide gene expression than virgin females in response to systemic infection, and that this lower expression correlates with higher systemic bacterial loads. We conclude that, upon receipt of sperm and seminal fluid proteins, females experience a germline-dependent physiological shift that directly or indirectly reduces their overall ability to defend against infection, at least in part through alteration of humoral immune system activity.
Keywords: Immune defense, Drosophila melanogaster, sex peptide, Accessory gland proteins, antimicrobial peptides
1. Introduction
Evidence that immune defense is involved in trade-offs with multiple life-history traits is abundantly apparent in a diversity of organisms ranging from invertebrates such as snails and insects to birds and mammals (reviewed in Schmid-Hempel, 2003; Sheldon and Verhulst, 1996). Defense against systemic infection by many bacterial pathogens (measured as resistance to infection or survival after infection) is reduced by mating in female D. melanogaster (Fedorka et al., 2007; Short and Lazzaro, 2010). In the present study, we investigate the immunological and reproductive bases for this post-mating depression in immune defense.
The insect immune system consists of multiple components, including the cellular immune response, the humoral immune response, and melanization. The cellular response functions mainly in the encapsulation or phagocytosis of parasites and pathogens, respectively (reviewed in Lemaitre and Hoffmann, 2007). The humoral immune response is activated upon detection of bacteria and fungi in the hemocoel. It includes production of antimicrobial peptides by the fat body and is stimulated when pattern recognition receptors recognize microbial cell wall compounds and trigger signaling through the Toll and IMD pathways (reviewed in Wang and Ligoxygakis, 2006). Melanization occurs in response to wounding, parasitization or infection and is regulated by the enzyme phenoloxidase (reviewed in Cerenius and Söderhäll, 2004).
These immune system components have been shown to be important for overall defense against infection in insects, which is defined as the ability to tolerate or eliminate infection (Ayres and Schneider, 2008). For this reason, quantitative immune system activity is often measured as a proxy for overall immune defense, under the implicit assumption that increased immune system activity correlates with heightened resistance to infection. This may or may not be the case (Fedorka et al., 2007), and this uncertainty can complicate the interpretation of immunity studies, an issue that has specifically been raised in the context of interactions between mating and immune defense (Lawniczak and Barnes et al., 2007). Regardless, much of the evidence for trade-offs between immune defense and reproductive success comes from studies demonstrating that mating and/or reproduction reduces proximal measures of systemic immune system activity or capability. In damselflies, the ability to encapsulate a foreign object inserted into the hemocoel decreases with increasing oviposition in females (Siva-Jothy et al., 1998), and sperm storage is negatively correlated with encapsulation ability in leaf-cutting ant queens (Baer and Armitage et al., 2006). In the beetle Tenebrio molitor, mating results in a decrease in phenoloxidase activity in both males and females (Rolff and Siva-Jothy, 2002). Mating has mixed effects on the immune system of the cricket Allonemobius socius, reducing hemocyte number, encapsulation ability and lytic activity in both males and females, but increasing phenoloxidase activity in females (Fedorka et al., 2004).
While measurements of immune system activity certainly are informative, increases in immune activity do not always correlate with improved tolerance of infection or with heightened ability to eliminate pathogens (Adamo, 2004; Lawniczak and Barnes et al., 2007; Viney et al., 2005). For this reason, it is informative to also assess the efficacy of immune defense, which we measure in this study as the ability to fight and/or survive systemic infection. In D. melanogaster, multiple studies have investigated how mating affects both immune system activity and organism-level defense against infection. Females have been shown to demonstrate a short-term increase in the expression of at least one and often many antimicrobial peptide (AMP) genes after mating, at least in the reproductive tract and possibly in other tissues (Fedorka et al., 2007; Innocenti and Morrow, 2009; Kapelnikov et al., 2008; Lawniczak and Begun, 2004; Mack et al., 2006; McGraw et al., 2004; Peng et al., 2005b; Wigby et al., 2008). These data would seem to predict higher immunocompetence after mating. In fact, however, female D. melanogaster suffer reduced ability to defend against infection by pathogenic bacteria after mating (Fedorka et al., 2007; Short and Lazzaro, 2010), although the ability to eliminate non-pathogenic bacteria injected into the body cavity is not compromised (McKean and Nunney, 2005; Wigby et al., 2008). As of now, no mechanism has been demonstrated for the observed reductions in defense against infection after mating. Notably, all previous studies documenting the increase in AMP expression after mating have been performed using uninfected females. Whether mating affects AMP expression in flies suffering from pathogenic infection remains an important but untested question.
During copulation, males transfer sperm and seminal fluid proteins in their ejaculates. Seminal fluid proteins, especially those made in the male accessory glands (accessory gland proteins, or Acps), have dramatic effects on female behavior and physiology. For example, Acp36DE causes conformational changes of the uterus (Avila and Wolfner, 2009) and is required for proper sperm storage after mating (Neubaum and Wolfner, 1999). Acp26Aa (ovulin) stimulates ovulation in mated females for approximately one day post-mating (Heifetz et al., 2000; Herndon and Wolfner, 1995). The Acp known as sex peptide (SP, also called Acp70A) has many effects on mated females, including reducing their receptivity to subsequent mating (Chapman et al., 2003; Chen et al., 1988; Liu and Kubli, 2003), promoting proper release of sperm from female storage organs (Avila et al., 2010), increasing intake of food (Carvalho et al., 2006) and decreasing siesta sleep (Isaac et al., 2010). SP has also been shown to be at least in part responsible for increased AMP gene expression in females after mating (Domanitskaya et al., 2007; Peng et al., 2005b). Interestingly, however, SP induces increases in juvenile hormone III-bisepoxide production in corpora allata incubated in vitro (Moshitzky et al., 1996), and juvenile hormone (JH) has been shown to suppress immune system activity (Flatt et al., 2008; Rolff and Siva-Jothy, 2002). Furthermore, seminal fluid, particularly SP, stimulates long-term increases in egg production (Chen et al., 1988; Soller et al., 1997), and egg production has been shown to trade-off physiologically (Fellowes et al., 1999) and evolutionarily (McKean et al., 2008) with immune defense. It is therefore possible that, despite inducing short-term modest increases in AMP expression, SP and other ejaculate components might cause overall reductions in systemic defense against infection. To begin to elucidate the mechanism by which females suffer reduced defense against infection after mating, we tested the effect of mating on expression of immune genes during infection and used genetic manipulations to identify critical steps in copulation and reproduction that depress immune defense.
2. Methods
2.1. Fly stocks and maintenance
Wild type flies are Canton S (CS) in all cases. “Spermless” males and “eggless” females are tud1 bw sp/CS and are generated from a cross between tud1 bw sp females and CS males. tud1 is a recessive maternal effect mutation, and offspring of tud1 mothers fail to form a germline (Boswell and Mahowald, 1985). Sons of tudor females do transfer accessory gland proteins during mating (Kalb et al., 1993). Egg-producing control females, which serve as a genotype control for eggless females, are also tud1 bw sp/CS. However, they are generated from a cross between tud1 bw sp/CyO females and CS males, and therefore produce eggs normally. “Spermless/Acpless” (DTA-E) males have ablated accessory glands due to expression of diphtheria toxin subunit A in their accessory gland main cells (Kalb et al., 1993). They fail to produce sperm and main cell accessory gland proteins (Kalb et al., 1993). Sex peptide null males are SP0/Δ130 and were generated from a cross between SP0/TM3, Sb ry and Δ130/TM3, Sb ser (Liu and Kubli, 2003). Sex peptide null flies were donated by Eric Kubli.
All flies were reared on standard Cornell media (8.3% glucose, 8.3% Brewer’s yeast, and 1% agar, plus 0.04% phosphoric acid and 0.4% propionic acid added to inhibit microbial growth in the food). Flies were kept at 24°C on a 12 hour light-dark cycle.
2.2. Mating setup
Male and female virgins were collected within 8 hours of eclosion, separated by sex, and aged in groups of ~25 with ad libitum access to food. All flies were three days post-eclosion at the time of mating. The day before each experiment, females were anaesthetized on CO2, put into individual glass mating vials, randomly allocated to a mating treatment and allowed to recover overnight. Females that were to remain virgins were anaesthetized and also put into individual vials. The following day, single, unanaesthetized males were aspirated into vials containing females within three hours of incubator “dawn.” Mating pairs that copulated for less than 15 minutes were discarded before infection in order increase our confidence that the male had adequate time to transfer the full complement and amount of ejaculate (where appropriate) and to ensure that females mated to mutant males mated for similar lengths of time as females mated to wild type males. More than 95% of all copulations lasted for longer than 15 minutes, so the number discarded from our experiment represents a small fraction of the total number of copulating pairs. After mating, males were removed, and females that ceased mating within roughly 10 minutes of each other were combined into vials of ~10 flies per vial. Virgin females were combined in similarly sized groups to control for possible housing effects.
2.3. Bacterial infection
Mated females were infected 2–3 hours after mating unless otherwise noted. In all cases, control virgin females were infected in parallel with their mated counterparts. Females were anaesthetized on CO2 and pierced in the thorax with a 0.15mm anodized steel needle (FST) dipped in a dilute overnight culture of Providencia rettgeri. The strain of P. retteri used in this experiment is a natural bacterial pathogen of D. melanogaster, and resistance to it has been shown to be reduced by mating (Short and Lazzaro, 2010). P. rettgeri is a moderate bacterial pathogen, causing ~40% mortality over 3–7 days in virgin D. melanogaster infected under our procedures. Overnight cultures were started from a single bacterial colony, grown overnight at 37ºC to saturation in liquid Luria Broth (LB), then diluted in additional LB to A600=1.0 (±0.05).
2.4. Bacterial load assay
Bacterial load was assayed 24 hours after infection in all cases with the exception of the data presented in the gene expression experiment, when we assayed bacterial load at multiple time points after infection. To determine bacterial load, females were pooled in groups of 3 and homogenized in 500μl LB with a sterile pestle. Homogenates were diluted as described below with additional LB, and 50μl of the homogenate was plated onto LB agar using a WASP 2 spiral plater (Microbiology International, Bethesda, MD, USA). For measurements taken 24 hours after infection, the homogenate was diluted 1:1000 prior to plating. For measurements taken 12 hours after infection, the homogenate was diluted 1:100 prior to plating. Plates were grown overnight at 37ºC, and resulting colonies were counted using a ProtoCOL plate counting system (Microbiology International) to estimate the number of colony forming units in each pool of three flies at the time of homogenization. Plates were routinely checked for contamination by visual inspection of colony morphology. Additionally, we periodically amplified 16S rDNA from a subset of colonies using the primers fd1 and rp2 (Weisburg et al., 1991), and amplified the same sequence in colonies grown from a pure freezer stock of P. rettgeri. We performed a restriction digestion on the amplifications from both the experimental colonies and the pure stock using MspI, ran each digest product on a 1% agarose gel, and compared banding patterns. In all cases, the banding pattern of the experimental colonies was an exact match to that of the positive control colonies from the freezer stock. Control sets of females were wounded with a sterile needle, and the plates from these flies yielded zero colonies.
2.5. Survival assay
Immediately after infection, females were placed into vials in groups of 10 with ad libitum access to food. Females were observed shortly after this to confirm that they had recovered from infection, and those that did not recover were not included in the experiment. Survival was recorded daily for five days, with surviving females from all treatments transferred to new media approximately every other day. Subsets of females from each treatment were pierced with a sterile needle to verify that survival differences between treatments were a consequence of infection and not injury. In all cases, females pierced with a sterile needle demonstrated negligible mortality (0% for most treatments and < 5% for all treatments)
2.6. Measurement of immune system activity
At 0, 4, 12 and 24 hours after infection, mated CS females and control virgins were sorted into pools of 10, snap frozen in TRIZOL reagent (Invitrogen) and stored at −80ºC. Total RNA was isolated using the TRIZOL manufacturer’s recommended protocol, dissolved in RNase-free water, and stored at −80ºC. We then treated approximately 500ng of total nucleic acid from each sample with DNase (Promega) in order to eliminate any residual DNA contamination and manufactured cDNA using M-MLV reverse transcriptase (Promega). Quantitative real-time PCR was performed using the ABI Prism 7000 Sequence Detection System (Applied Biosystems). Expression levels of all AMP genes are reported relative to expression of RpL32 (also known as rp49), and results were verified using Actin 5C as an additional reference control gene. To quantify expression of Attacin A, Attacin B, Metchnikowin, RpL32 and Actin 5C, we used Power SYBR green PCR master mix (Applied Biosystems). To quantify expression of Defensin, Drosomycin and Diptericin A, we used gene-specific Taqman fluorescent probes (Applied Biosystems). Primers and primer/probe sequences are available upon request.
2.7. Statistical analysis
In all experiments where bacterial load was measured, the data were natural log transformed. We then fit a mixed model ANOVA using SAS (SAS Institute) to determine the effect of mating treatment and, where appropriate, time after mating, female genotype, and the interactions between mating treatment and these additional factors. The residual errors of the ANOVA were adequately normally distributed. To assess the effect of mating at different time points, we sorted by time point and performed contrasts between mating statuses within each subset of data. Replicate experiment was included in each ANOVA as a random effect. In cases with more than two mating statuses, we performed a Tukey’s test to conduct pairwise comparisons between treatments and to correct for multiple comparisons.
In all experiments measuring survival, we assessed the effect of mating status using Cox regression analysis in SAS (SAS Institute). Event data (where an “event” = death) were recorded for flies from each mating status, and flies that were still alive at the end of the observation period were treated as censored data. Mating treatment and replicate were included as factors in all regression analyses, and in experiments with more than two mating treatments, comparisons between mating treatments of interest were performed using contrast statements within the regression analysis. A Bonferroni correction was applied in these situations to correct for multiple testing.
In the analysis of gene expression data, technical replicates for all measurements were averaged and the following model was fitted to the average critical threshold values for all measured AMP genes: Yijkl = μ + RpL32 expression + timei +mating statusj + genek + experimental replicatel + mating statusj*genek + mating statusj*timei + genek*timei +mating statusj*genek*timei, where time (i = 1,4), mating status (j = 1,2) and gene (k = 1,6) are fixed effects and experimental replicate (l = 1,2) is random. Because a significant mating status*time interaction was observed, the data were then sorted by time post-infection and the following model was applied to data from each time point: Yijk = μ + RpL32 expression +mating statusi + genej + experimental replicatek + mating statusi*genej, where mating status (i = 1,2) and gene (j = 1,6) are fixed effects and experimental replicate (k = 1,2) is random. Least squares means for the mating status*gene interaction were obtained from these analyses and, for each time point, we subtracted the mated LS means from the virgin LS means for each gene. We then plotted this difference along a zero axis, where a bar above the zero axis represents a higher level of gene expression at that time point in mated females relative to virgin controls and a bar below the zero axis represents a lower level of expression in mated females relative to virgins. These differences and the standard errors of the differences were determined using the lsmestimate command in SAS (SAS Institute).
3. Results
3.1. The effect of mating on female immune defense lasts for at least twenty-four hours after mating
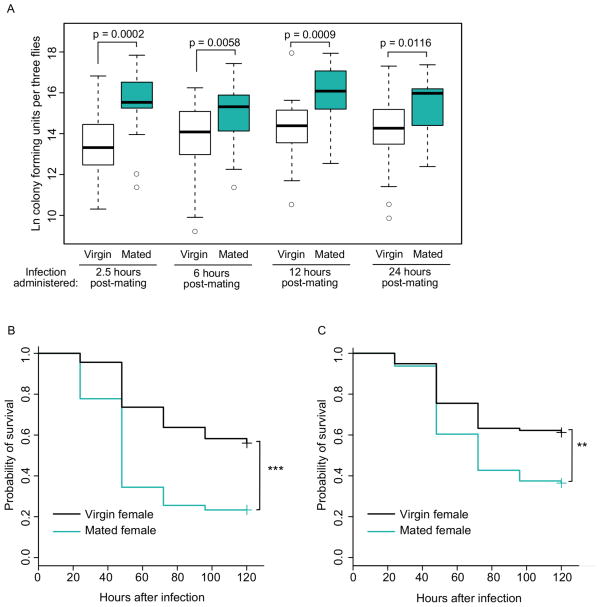
Mated D. melanogaster females suffer a reduction in immune defense against bacterial infection that begins as early as 2.5 hours after mating and may persist for several additional hours beyond this time point (Fedorka et al., 2007; Short and Lazzaro, 2010). To test for persistence of mating-induced immunodepression in our experimental context, we assayed bacterial load in D. melanogaster females (wild type strain Canton S, or CS) infected at 2.5, 6, 12 and 24 hours after mating cessation. We also assayed for differences in survivorship of infection in females infected at 2.5 and 26.5 hours after mating cessation. In both the bacterial load and survival experiments, virgin and mated females were infected in parallel with the bacterium Providencia rettgeri by septic pinprick to the thorax. Systemic bacterial load was recorded 24 hours after infection, and mortality was scored immediately following infection and every day thereafter for a total of five days. For each of the four post-mating infection time points, we found that mated females had significantly higher bacterial loads than virgin control females (Figure 1A; 2.5 hours post-mating, p = 0.0002; 6 hours post-mating, p = 0.0058; 12 hours post-mating, p = 0.0009; 24 hours post-mating, p = 0.0116). We also found that mated females had significantly lower survival than virgin controls over time when infected at both 2.5 (Figure 1B, p < 0.0001) and 26.5 hours after mating (Figure 1C, p = 0.0028). Thus, the effect of mating on defense against systemic bacterial infection is persistent with no sign of decline for at least 24 hours post-mating.
Figure 1. The effect of mating on immune defense lasts for at least 24 hours after mating.
(A) Bacterial load levels of mated Canton S females were higher than those of virgin controls when infected at 2.5 hours (p = 0.0002), 6 hours (p = 0.0058), 12 hours (p = 0.0009) and 24 hours (p = 0.0116) after mated flies finished copulating. All flies were infected with P. rettgeri and virgin controls were aged, housed and infected in parallel with mated females for each time point. Each data point consists of a pool of three females, and the number of pools for each treatment are as follows: for 2.5 hours, nmated = 22 and nvirgin = 22, for 6 hours, nmated = 24 and nvirgin = 26, for 12 hours, nmated = 21 and nvirgin = 20, and for 24 hours, nmated = 21 and nvirgin = 22. Data were collected over three replicate experiments. Flies given a sterile wound always yielded zero colonies (data not shown). (B, C) Survival over time of mated Canton S females was significantly lower than that of virgin controls when infected at 2.5 hours (B, p < 0.0001) and 26.5 hours (C, p = 0.0028) after mated females finished copulating. All flies were infected with P. rettgeri and kept in groups of ~10 flies. Sample sizes are as follows: for 2.5 hours, nmated = 90 and nvirgin = 91, for 26.5 hours, nmated = 96 and nvirgin = 98. Mortality was recorded each day for five days after infection, and data were collected over two replicate experiments. Survival curves were estimated using the Kaplan-Meier method. Control flies given a sterile wound had negligible mortality over the course of the experiment (less than 1%) regardless of mating treatment (data not shown). ** p <0.01, *** p < 0.001.
3.2. Mated females demonstrate higher bacterial loads but lower AMP expression early in bacterial infection
Production of antimicrobial peptides (AMPs) is one important component of the immune response in insects (reviewed in Lemaitre and Hoffmann, 2007). Multiple studies have reported short-term increases in expression of at least one and often multiple AMP genes after mating, where expression level changes have been measured either in the female reproductive tract (Mack et al., 2006; Kapelnikov et al., 2008) or in undissected whole flies (Fedorka et al., 2007; Innocenti and Morrow, 2009; Lawniczak and Begun, 2004; McGraw et al., 2004; Peng et al., 2005b; Wigby et al., 2008). Only two of these studies (Fedorka et al., 2007; Wigby et al., 2008), however, measured organism-level defense against infection in parallel with changes in AMP expression. Fedorka et al. (2007) reported a significant reduction in defense against pathogenic infection after mating, and Wigby et al. (2008) saw no effect of mating on the female’s ability to clear non-pathogenic E. coli from the hemocoel. In neither of these studies does a mating-induced increase in AMP gene expression result in increased immune defense against systemic infection. We posited that, over the course of an infection, mating might actually compromise systemic immune system activity relative to virgin females, thus explaining the observed reduction in overall defense.
To test post-infection immune performance in mated females relative to virgins, we measured the transcript levels of several AMP genes at different time points after infection in both mated females and virgin controls. Note that for this experiment, and for all subsequent experiments, females were infected at 2.5 hours post mating. Levels of AMP gene expression increased dramatically over the course of infection, but the level of immune system induction varied significantly between mated and virgin females in a time-specific manner (Table 1), with mated females showing lower AMP transcript levels at 4 and 12 hours post-infection (Figure 2, 4 hours p = 0.001, 12 hours p < 0.0001) but higher transcript levels after 24 hours (Figure 2, p < 0.0001). This pattern was consistent across six AMP genes measured (Table 1, Figure 2). Interestingly, we did not observe the induction of AMP gene expression reported by others in response to mating itself (Figure 2, p = 0.1019) (Lawniczak and Begun, 2004; McGraw et al., 2004). Systemic bacterial load did not differ significantly between mated and virgin females at four hours after infection (Figure 2, p = 0.9540), but mated females have significantly higher systemic bacterial loads at 12 hours post-infection (Figure 2, p = 0.0003) and 24 hours post-infection (Figure 1, p = 0.0002). We infer that the lower early AMP gene expression in mated females relative to virgins may contribute to the increased pathogen proliferation observed in mated females, and that the expression of immune system genes in mated females becomes higher than that in virgins at 24 hours post-infection due to greater sustained stimulation of the immune system by the correspondingly more severe infection (Figure 2).
Table 1.
The effect of mating status on transcript levels of six AMP genes at four time points post-infection.
| Factor | Effect type | d.f. | F value | P-value |
|---|---|---|---|---|
| RpL32 expression (CT value) | < 0.0001 | |||
| Time | Fixed | 3 | 3747.3 | < 0.0001 |
| Mating status | Fixed | 1 | 3.64 | 0.0573 |
| Gene | Fixed | 5 | 1055.4 | < 0.0001 |
| Experimental rep | Random | 0.4995 | ||
| Mating status * gene | Fixed | 5 | 1.41 | 0.2219 |
| Mating status * time | Fixed | 3 | 15.13 | < 0.0001 |
| Gene * time | Fixed | 15 | 99.32 | < 0.0001 |
| Mating status * gene * time | Fixed | 15 | 0.80 | 0.6756 |
Figure 2. Antimicrobial peptide gene expression in mated females relative to virgins during the course of infection.
Mated and virgin Canton S females were infected 2.5 hours after mating with a 1.0 A600 culture of P. rettgeri. Bacterial load was measured at 4, 12 and 24 hours after infection in independent experiments. Bacterial loads in mated females did not significantly differ from virgin females at 4 hours post infection (p = 0.9540) but did at 12 hours (p = 0.0003) and 24 hours post infection (p = 0.0002, data taken from Figure 1A). Each data point is a pool of three females, and the number of data points per treatment are as follows: at 4 hours, nmated = 23 and nvirgin = 24, at 12 hours, nmated = 23 and nvirgin = 27, and at 24 hours, nmated = 22 and nvirgin = 22. Flies given a sterile wound always yielded zero colonies (data not shown). AMP gene expression was assayed in a subsequent experiment at 0, 4, 12 and 24 hours after infection. Gene expression increased significantly over the course of infection in both virgin and mated females, but because the effect of mating status varied by time point, data were sorted by time and least squares means for each mating status/AMP gene combination were found by a mixed-model ANOVA. P-values reported on gene expression graphs are from these models and indicate the effect of mating status on overall AMP gene expression. Data are presented as the Log2 fold difference in mated female LS means relative to virgin control LS means for each gene, where a bar above or below the virgin level of expression represents a higher or lower level of expression due to mating, respectively. Because the differences are Log2, an increase of “1” corresponds to 2x the virgin level of gene expression at that time point, and a decrease of “1” corresponds to half the virgin level. Sample sizes are as follows: 0 hours, nmated = 6, nvirgin = 6, 4 hours, nmated = 8, nvirgin = 8, 12 hours, nmated = 8, nvirgin = 8, 24 hours, nmated = 8, nvirgin = 8, where each sample consists of a pool of 9–10 females collected over two replicate experiments.
3.3 Post-mating suppression in female immune defense depends on transfer of both sperm and sex peptide
3.3.1. The role of sperm and Acps in female immune defense
Male-derived seminal fluid has many dramatic effects on female physiology (reviewed in Avila et al., 2011), so we hypothesized that seminal fluid signals might elicit changes in female immune defense. In order to determine whether sperm or accessory gland proteins elicit post-mating reductions in female defense, we compared bacterial load and survival after P. rettgeri infection in CS females from four different mating treatments: (1) virgin females and females mated to (2) wild type males, (3) males who do not produce sperm, or (4) males who produce neither sperm nor accessory gland proteins (Acps). Spermless males have the genotype tud1 bw sp/CS and are sons of tudor homozygous mutant mothers and CS fathers. Because tudor is a maternal effect mutation, these males lack pole cells and fail to form a germline (Boswell and Mahowald, 1985). Spermless/Acpless males have accessory glands whose main cells have been ablated by cell-specific expression of diptheria toxin subunit A (Kalb et al., 1993). These males also fail to produce sperm. By comparing the response of females mated to wild type males and females mated to spermless males, we can determine the specific importance of sperm. By comparing the response of females mated to spermless males to those mated to spermless/Acpless males, we can determine the additional effect of accessory gland proteins (Kalb et al., 1993).
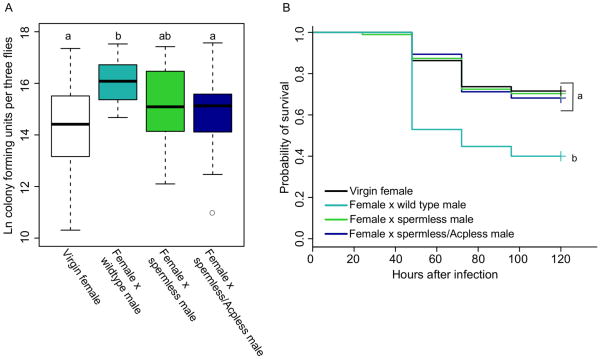
Females that were mated to wild type males sustained significantly higher bacterial loads than did virgin females by 24 hours post-infection (p < 0.0001), but females mated to spermless/Acpless males sustained systemic bacterial loads equivalent to those of virgin females (Figure 3A, p =0.442). Females mated to spermless males had bacterial loads that were intermediate between those of females mated to wild type males and those of virgin females (Figure 3A; virgin vs. female × spermless male, p = 0.0781, female × wild type male vs. female × spermless male, p = 0.0831).
Figure 3. The effect of sperm and accessory gland protein transfer on post-mating female immune defense.
Canton S females were assigned to one of four mating treatments: virgin (V), mated to CS males (MCS), mated to spermless males (MSL) or mated to spermless/Acpless males (MSL/AcpL). In both experiments, females from all mating treatments were infected with a 1.0 A600 culture of P. rettgeri. (A) Bacterial load was assayed in females from all mating treatments and Tukey’s test was used to make the following treatment comparisons: V vs. MCS, p < 0.0001; V vs. MSL, p = 0.07; V vs. MSL/AcpL, p =0.442; MCS vs. MSL, p = 0.08; MCS vs. MSL/AcpL, p = 0.0084; MSL vs. MSL/AcpL, p = 0.885. Each data point is a pool of three flies, and the number of data points collected for each treatment are as follows: nvirgin = 26, n×CSmale = 29, n×spermless = 26, n×spermless/Acpless=25. Samples were collected over three replicate experiments, and flies given a sterile wound always yielded zero colonies (data not shown). (B) Survival was assayed for females from all mating treatments, and Cox regression analysis was used to determine the effect of mating treatment on survival. Independent contrasts were performed within the regression analysis, and the Bonferroni corrected p-value for the six pairwise comparisons is 0.0083. V vs. MCS, p < 0.0001; V vs. MSL, p = 0.8705; V vs. MSL/AcpL, p =0.727; MCS vs. MSL, p = 0.0001; MCS vs. MSL/AcpL, p = 0.0011; MSL vs. MSL/AcpL, p = 0.8417. Sample sizes: nvirgin = 95, n×CSmale = 85, n×spermless = 95, n×spermless/Acpless=66. Each data point represents a single fly and samples were collected over two replicate experiments. Flies given a sterile wound had 0% mortality in all treatments.
Females mated to spermless/Acpless males also survived their infections significantly better than females mated to wild type males (p = 0.0011), and equivalently to virgin females (Figure 3B, p = 0.7270). Interestingly, females mated to males lacking sperm were significantly more likely to survive infection than females mated to wild type males (p = 0.0001), also surviving equivalently to virgin females (Figure 3B, p = 0.8705). Thus, failure to transfer sperm alone was sufficient to eliminate the effect of mating on survival. The probability of survival after infection for females mated to spermless/Acpless males was not significantly different from that of females mated to spermless males (Figure 3B, p = 0.8417).
3.3.2. The role of sex peptide in female immune defense
Previous studies have shown an important role for the Acp known as sex peptide (SP) on female physiology and behavior (e.g. Avila et al., 2010; Carvalho et al., 2006; Chapman et al., 2003; Chen et al., 1988; Isaac et al., 2010; Liu and Kubli, 2003; Moshitzky et al., 1996; Soller et al., 1997). The effects of SP persist for days in the female, but only if sperm are also successfully transferred and stored (Manning, 1962). SP is tethered to sperm in the female reproductive tract, and it is thought that this allows SP to persist in the female and to be slowly released over time (Peng et al., 2005a). We hypothesized that SP may indeed play a role in female immune defense, but that its effect may be dependent on sperm transfer. We therefore contrasted systemic bacterial load and survival after infection of females from four different mating treatments: (1) virgin females and females mated to (2) wild type males, (3) spermless males or (4) males null for sex peptide (Liu and Kubli, 2003). SP null males (SP0/Δ130) carry a null mutation of the sex peptide gene uncovered by deficiency Δ130 and fail to produce functional sex peptide, but are normal in their other seminal fluid components, sperm production, and mating biology.
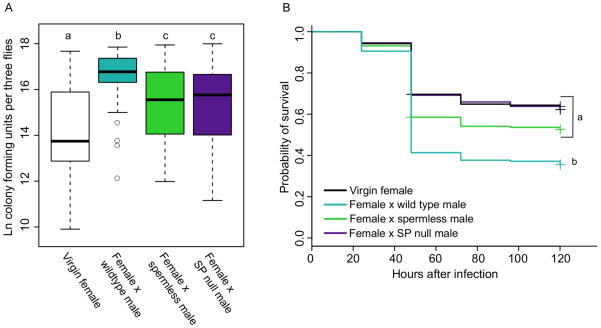
As in our previous experiments, females mated to wild type males had significantly higher bacterial loads than virgin females at 24 hours post-mating (Figure 4A, p < 0.0001). Again, females that failed to receive sperm during copulation demonstrated intermediate bacterial loads, significantly lower than those of females mated to wild type males (p = 0.0225) but still significantly higher than those of virgin females (Figure 4A, p = 0.0027). Females mated to sex peptide null males showed this same pattern, exhibiting bacterial loads significantly lower than those of females mated to wild type males (p = 0.0136) but significantly higher than virgins (Figure 4A, p = 0.0097). Bacterial loads of females mated to spermless males or to SP null males were equivalent (Figure 4A, p = 0.9933). These data are consistent with the hypothesis that proper delivery of sex peptide is crucial for immune defense to be reduced after mating, but also indicate that other components of the seminal fluid must contribute to mating-induced changes in bacterial load since elimination of sex peptide is not sufficient to return females to virgin defense levels.
Figure 4. The effect of sperm and sex peptide transfer on post-mating female immune defense.
CS females were assigned to one of four mating treatments: virgin (V), mated to CS males (MCS), mated to spermless males (MSL) or mated to sex peptide null males (MSP). In both experiments, females from all mating treatments were infected with P. rettgeri. (A) Bacterial load was assayed in females from all mating treatments 24 hours after infection and Tukey’s test was used to make the following comparisons: V vs. MCS, p < 0.0001; V vs. MSL, p = 0.0027; V vs. MSP, p =0.0097; MCS vs. MSL, p = 0.0225; MCS vs. MSP, p = 0.0136; MSL vs. MSP males, p = 0.993. Each data point is a pool of three flies, and the number of data points for each treatment are as follows: nvirgin = 45, n×CSmale = 40, n×spermless = 42, n×SPnullmale=37. Samples were collected over five replicate experiments, and flies given a sterile wound always yielded zero colonies (data not shown). (B) Survival was assayed for all mating treatments, and Cox regression analysis was used to determine the effect of mating treatment on survival. Independent contrasts were performed within the regression analysis, and the Bonferroni corrected p-value for the six pairwise comparisons is 0.0083. V vs. MCS, p < 0.0001; V vs. MSL, p = 0.0844, V vs. MSP, p = 0.86; MCS vs. MSL, p = 0.007; MCS vs. MSP, p < 0.0001; MSL vs. MSP, p = 0.13. Sample sizes: nvirgin = 191, nxCSmale = 191, nxspermless = 205, nxSPnullmale=175. Each data point represents a single fly and samples were collected over five replicate experiments. Flies given a sterile wound had 0% mortality.
Failure to transfer and/or store sex peptide is sufficient to entirely eliminate the effect of mating on female survival of infection. Females mated to SP null males were significantly more likely to survive infection than females mated to wild type males (p < 0.0001), and their survival did not significantly differ from that of virgin females (Figure 4B, p = 0.8681). Females mated to spermless males showed this same pattern, and the survivorships of females mated to either spermless or SP null males were equivalent (Figure 4B, p = 0.1311)
3.4. Females that fail to produce eggs demonstrate no effect of mating on immune defense
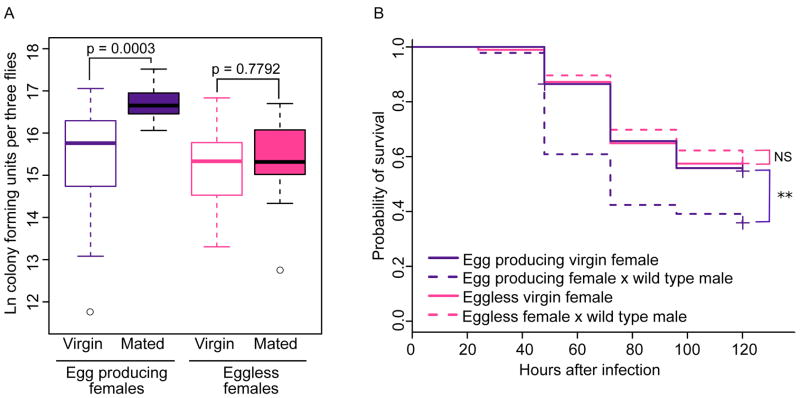
We hypothesized that after mating, females may experience a shift in physiological and molecular signaling toward a state that maximizes vitellogenesis and egg production, and that any such shift might occur at the cost of immunocompetence. If this hypothesis is correct, we would predict that mated females that lack a germline and therefore fail to produce eggs would not demonstrate an immunological cost of mating. To test this hypothesis, we generated genetically identical females that did or did not produce eggs. “Eggless” females (tud1 bw sp/CS) were produced from a cross between tudor mutant females (tud1 bw sp) and CS males. The maternal effect of tudor results in daughters that fail to develop a germline and therefore cannot produce eggs. “Egg producing” control females of the genotype tud1 bw sp/CS were generated from a cross between tud1 bw sp/CyO mothers and CS fathers. We then measured bacterial load and survival in mated and virgin egg-producing and eggless females. We found that egg producing females sustained significantly higher P. rettgeri loads due to mating (Figure 5A, p = 0.0003), but that the bacterial loads of mated eggless females were equivalent to those of virgin eggless females (Figure 5A, p = 0.7792). Similarly, mating resulted in significantly decreased survival of infection in egg producing females (p = 0.0022), but not in eggless females (p = 0.7718; Figure 5B). These results demonstrate the requirement of a female germ line in order for female immune defense to be affected by male seminal signals.
Figure 5. Females that fail to produce eggs do not demonstrate decreased immune defense after mating.
Eggless females are tudor1 bw sp/CS (generated by tudor1 bw sp/tudor1 bw sp females x CS males) and have no germline. Egg producing females are tudor1 bw sp/CS (generated by tudor1 bw sp/CyO females x CS males) and have wild type egg production. All females were infected with P. rettgeri 2–3 hours after mated females completed copulation. (A) Egg producing females demonstrated a significant effect of mating on bacterial load (p = 0.0003), while eggless females did not (p = 0.7792). We assayed bacterial load in females 24 hours after infection. Sample sizes: nvirgin, egg producing = 15, nmated, egg producing = 16, nvirgin, eggless = 14, nmated, eggless = 15. Each data point consists of three pooled females and samples were collected over two replicate experiments, and flies given a sterile wound always yielded zero colonies (data not shown). (B) Egg producing mated females demonstrated significantly lower survival after infection compared to egg producing virgin females (p = 0.0022, Bonferroni corrected cutoff=0.025). Survival of eggless mated females was not significantly different from that of eggless virgin females (p = 0.7718). Sample sizes: nvirgin, egg producing = 96, nmated, egg producing = 92, nvirgin, eggless = 94, nmated, eggless = 106. Each data point represents a single fly and samples were collected over two replicate experiments. Females given a sterile wound had < 5% mortality regardless of treatment. ** p < 0.01.
4. Discussion
While evidence of evolutionary and physiological trade-offs between immune defense and life history traits is abundant, comparatively little is known about how trade-offs occur on a physiological or genetic level. In this work, we demonstrate that post-mating reductions in immune defense persist in wild type females for at least 24 hours after mating, and that mated females are compromised in their ability to induce expression of AMP genes after infection. Further, we demonstrate that seminal fluid elicits reduced overall defense against infection in the mated female, and that sperm and sex peptide play a crucial role in this effect. Finally, we demonstrate that females must have an intact germline in order for mating to drive any difference in overall immune defense. Taken together, these data indicate that reduction in systemic immune defense is a cost of reproduction in females, and that this cost is dependent on transfer of sperm and proteins in the seminal fluid. The immunological cost of mating could be direct, resulting for instance from genetic pleiotropy between egg production and immune signaling, or indirect, resulting from altered resource allocation after mating. Direct and indirect costs are not mutually exclusive, and disentangling them will require substantial additional experimentation.
The fact that a mating-induced reduction in female immune defense is not observed when the female lacks a germline or when the male fails to transfer sperm and seminal fluid proteins reveals that the effect of mating on immune defense is not simply due to wounding or general exertion associated with courting and the act of copulation. We previously reported that females vary genetically for the magnitude of post-mating immune depression they experience (Short and Lazzaro, 2010). Interestingly, however, males were genetically invariant for the degree of post-mating depression they elicit in their mates, despite the presently demonstrated dependence of the effect on male seminal fluid components (Short and Lazzaro, 2010). Considering this in the context of the data we present here, we suggest that mating results in a sustained shift in the female’s physiology as she transitions from virgin homeostasis to active production of mature eggs, representing a genetically variable physiological trade-off between mating and immunity in females. We speculate that this could in part be mediated, for example, by a pleiotropic signaling molecule such as juvenile hormone (Flatt et al., 2005). Notably, the production of juvenile hormone III-bisepoxide is stimulated in vitro by sex peptide (Moshitzky et al., 1996). Juvenile hormone plays an important role in controlling egg production (Soller et al., 1999) and reduces AMP gene expression in cell culture (Flatt et al., 2008), suggesting the hypothesis that juvenile hormone signaling might simultaneously contribute to the mating-induced reductions in overall defense and AMP gene expression we report in this work. We note that genetically variable physiological trade-offs like the one we describe here are likely to lie at the heart of evolutionary life history trade-offs (Flatt et al., 2005).
We analyzed humoral immune system activity during the course of infection in mated and virgin females and found that mated females exhibit lower AMP gene expression than virgin controls at four and twelve hours post-infection, despite mated females having equal and higher bacterial loads at these respective times. This finding is distinct from previously reported increases in AMP gene expression after mating in uninfected females (Fedorka et al., 2007; Innocenti and Morrow, 2009; Lawniczak and Begun, 2004; McGraw et al., 2004; Peng et al., 2005b; Wigby et al., 2008). These previous studies differed in design, from each other and from ours, perhaps accounting for the differences in effects seen between them. Most notably, females in our study were infected when AMP gene expression was assayed. It is possible that some of the increases in AMP gene expression reported by others may be tissue specific, and two studies have specifically identified changes in AMP gene expression in the reproductive tract after mating (Kapelnikov et al., 2008; Mack et al., 2006). AMP expression in the reproductive tract could be important for fighting local infection after mating. Our present data reveal a diminished capability of mated females to induce AMP genes in response to systemic infection as compared to the induction capability of virgin females. While the overall differences in AMP expression due to mating are statistically significant, the actual fold differences between mating treatments for each gene are small (less than two-fold at each time point), and it is unclear whether they are sufficient to be solely responsible for the higher bacterial load and lower survival of mated females. We consider it possible that suppressed induction of the humoral immune system is only one of multiple mechanisms by which mating reduces female defense against infection.
Our data reveal that female flies are immunocompromised after mating only when they have intact germlines and when they receive sperm and accessory gland proteins from their mates. In particular, we observed the importance of one seminal fluid protein, sex peptide. Importantly, however, our data do not exclude the possibility that additional Acps may play a role, since neither sperm nor sex peptide individually account for the entire effect of mating on systemic bacterial load after infection.
The immune performance of eggless females is unaltered by mating, revealing an important role for the female germline. It is possible that the effect of mating on overall immune defense is due to post-mating changes in molecular or hormonal signaling that fail to occur in daughters of tudor females due to their absence of a germline. Another possibility is that the effect of mating on overall immune defense could be a consequence of producing mature eggs. The process of egg production is energetically demanding requiring females to synthesize large amounts of yolk protein, which is deposited into oocytes at the vitellogenic stages of oogenesis. Upon mating, transcription of yolk protein genes increases dramatically (Soller et al., 1997). The production of vitellogenic oocytes begins to increase in mated females at 6 hours after mating (Heifetz et al., 2001) and reaches very high levels by 14 hours after mating (Soller et al., 1997). This shift toward rapid and continuous egg production is arguably the most obvious and costly effect that mating has on female physiology. Mating may induce changes in physiology or genetic signaling that act to prepare females for this long-term cost by altering utilization of resources to favor reproduction over defense.
In this context, we suggest that male delivery of sperm and SP may reduce female post-mating immune competence by inducing increases in egg production. In support of this hypothesis, we note that, in the first 24 hours after mating, females mated to spermless males have been shown to demonstrate significantly reduced levels of egg laying and fewer vitellogenic oocytes compared to females mated to wild type males (Heifetz et al., 2001). Additionally, in the first day post-mating, females mated to SP null or spermless/Acpless males have virgin-like levels of egg production and/or vitellogenesis (Heifetz et al., 2001; Kalb et al., 1993; Liu and Kubli, 2003). Over the next four days post-mating, females mated to spermless, spermless/Acpless or SP null males have been reported to lay eggs at virgin levels (Kalb et al., 1993; Liu and Kubli, 2003). Of note, our data are consistent with a model where sperm and SP may together contribute to a single effect on female post-mating immunocompetence (Peng et al., 2005a). Sex peptide is known to bind to sperm and be slowly cleaved off over multiple days in the female sperm storage organs (Peng et al., 2005a). If long-term increases in egg production alter female immune defense, it is possible that sperm per se is not eliciting changes in female immune defense, but rather that it acts to facilitate the effect of SP by ensuring its stable storage in the female (Peng et al., 2005a). We note that alteration of immune defense due to long-term maintenance of SP signaling is unlikely to occur as a consequence of any direct effect of SP on JH signaling, as SP cleaved from sperm does not contain the N-terminus, which is crucial to elicit JH production (Fan et al., 2000). Unbound SP may act to alter immune defense by affecting JH levels shortly after mating, while bound SP may have a later effect on defense by prolonging egg production.
Like Fedorka et al. (2007), we observed that females who did not produce late-stage oocytes failed to show reduced immune defense after mating. These results suggest that aspects of female physiology needed to produce mature oocytes - such as high-level production and secretion of yolk proteins, for example - intersect with immune defense ability. Moreover, it is interesting to consider the reasons why we observed no post-mating immune depression in germlineless females, whereas Fedorka et al. (2007) reported that ovoD1mutant Drosophila females responded to mating with reduced immune defense until 9hrs. post-mating. Although the females in both studies were sterile and did not produce mature eggs, the cause of their sterility differs. The tudor-progeny females that we analyzed completely lack a germline and thus never initiate oogenesis (Boswell and Mahowald, 1985). In contrast, ovoD1 females initiate oogenesis, but arrest the process prior to the vitellogenic stages (Busson et al., 1983). Assuming that the difference in findings between the studies does not reflect genetic background differences between fly strains or procedural differences between the labs, they suggest that early post-mating effects on immune defense might be influenced by aspects of pre-vitellogenic signaling. Additional studies examining a range of female reproductive mutants that fail in various stages of egg development will help narrow down the specific aspects of oogenesis that are important for inhibiting immune defense.
5. Conclusions
In summary, we report that reduced overall defense against infection suffered by D. melanogaster females after mating is not a result of the act of copulation, but rather is dependent on sperm and seminal fluid proteins, including sex peptide, transferred from males to females during mating. We also find that the effect is dependent on an intact female germline. We hypothesize that a physiological shift from virgin somatic homeostasis directly or indirectly compromises immune defense, including the ability to induce the humoral immune system. Such physiological trade-offs between mating and immune defense may reveal the mechanisms that underlie life history trade-offs and shape the evolution of both traits involved.
Highlights.
Female Drosophila suffer reduced immune defense for at least one day post-mating.
Mated females demonstrate lower immune gene expression than virgins when infected.
Lower immune gene expression due to mating correlates with higher bacterial loads.
Female post-mating immunosuppression is dependent on transfer of seminal fluid.
Effect of mating on defense depends on proper female germline formation.
Acknowledgments
We would like to thank Punita Juneja, Virginia Howick, Chloe Ota and Mark Jandricic for helpful discussion and Susan Rottschaefer, Madeline Galac, Jacob Crawford and Maria Driscoll for technical assistance as well as thoughtful discussion. We would also like to thank Frank Avila for assistance with the sex peptide null mutant flies and Eric Kubli for providing them. This work was supported by NIH grant R01 AI083932 (BPL) and R01 HD038921 (MFW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamo SA. How should behavioural ecologists interpret measurements of immunity? Animal Behavior. 2004;68:1443–1449. [Google Scholar]
- Avila FW, Ravi Ram K, Bloch Qazi MC, Wolfner MF. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics. 2010;186:595–600. doi: 10.1534/genetics.110.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annual Review of Entomology. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Wolfner MF. Acp36DE is required for uterine conformational changes in mated Drosophila females. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15796–15800. doi: 10.1073/pnas.0904029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres JS, Schneider DS. A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biology. 2008;6:e305. doi: 10.1371/journal.pbio.0060305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B, Armitage SAO, Boomsma JJ. Sperm storage induces an immunity cost in ants. Nature. 2006;441:872–875. doi: 10.1038/nature04698. [DOI] [PubMed] [Google Scholar]
- Boswell RE, Mahowald AP. tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell. 1985;43:97–104. doi: 10.1016/0092-8674(85)90015-7. [DOI] [PubMed] [Google Scholar]
- Busson D, Gans M, Komitopoulou K, Masson M. Genetic analysis of three dominant female-sterile mutations located on the X chromosome of Drosophila melanogaster. Genetics. 1983;105:309–325. doi: 10.1093/genetics/105.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine modulation of feeding behavior by the Sex Peptide of Drosophila. Current Biology. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerenius L, Söderhäll K. The prophenoloxidase-activating system in invertebrates. Immunological Reviews. 2004;198:116–126. doi: 10.1111/j.0105-2896.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Böhlen P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 1988;54:291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Domanitskaya EV, Liu H, Chen S, Kubli E. The hydroxyproline motif of male sex peptide elicits the innate immune response in Drosophila females. Federation of European Biochemical Societies Journal. 2007;274:5659–5668. doi: 10.1111/j.1742-4658.2007.06088.x. [DOI] [PubMed] [Google Scholar]
- Fan Y, Rafaeli a, Moshitzky P, Kubli E, Choffat Y, Applebaum SW. Common functional elements of Drosophila melanogaster seminal peptides involved in reproduction of Drosophila melanogaster and Helicoverpa armigera females. Insect biochemistry and molecular biology. 2000;30:805–812. doi: 10.1016/s0965-1748(00)00052-7. [DOI] [PubMed] [Google Scholar]
- Fedorka KM, Linder JE, Winterhalter W, Promislow D. Post-mating disparity between potential and realized immune response in Drosophila melanogaster. Proceedings of the Royal Society B: Biological Sciences. 2007;274:1211–1217. doi: 10.1098/rspb.2006.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorka KM, Zuk M, Mousseau TA. Immune suppression and the cost of reproduction in the ground cricket, Allonemobius socius. Evolution. 2004;58:2478–2485. doi: 10.1111/j.0014-3820.2004.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Fellowes MDE, Kraaijeveld AR, Godfray HCJ. The relative fitness of Drosophila melanogaster (Diptera, Drosophilidae) that have successfully defended themselves against the parasitoid Asobara tabida (Hymenoptera, Braconidae) Journal of Evolutionary Biology. 1999;12:123–128. [Google Scholar]
- Flatt T, Heyland A, Rus F, Porpiglia E, Sherlock C, Yamamoto R, Garbuzov A, Palli SR, Tatar M, Silverman N. Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. Journal of Experimental Biology. 2008;211:2712–2724. doi: 10.1242/jeb.014878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. BioEssays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Heifetz Y, Lung O, Frongillo EA, Wolfner MF. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Current Biology. 2000;10:99–102. doi: 10.1016/s0960-9822(00)00288-8. [DOI] [PubMed] [Google Scholar]
- Heifetz Y, Tram U, Wolfner MF. Male contributions to egg production: the role of accessory gland products and sperm in Drosophila melanogaster. Proceedings of the Royal Society B: Biological Sciences. 2001;268:175–180. doi: 10.1098/rspb.2000.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon LA, Wolfner MF. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10114–10118. doi: 10.1073/pnas.92.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti P, Morrow EH. Immunogenic males: a genome-wide analysis of reproduction and the cost of mating in Drosophila melanogaster females. Journal of Evolutionary Biology. 2009;22:964–973. doi: 10.1111/j.1420-9101.2009.01708.x. [DOI] [PubMed] [Google Scholar]
- Isaac RE, Li C, Leedale AE, Shirras AD. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proceedings of the Royal Society B: Biological Sciences. 2010;277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb JM, DiBenedetto AJ, Wolfner MF. Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8093–8097. doi: 10.1073/pnas.90.17.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapelnikov A, Zelinger E, Gottlieb Y, Rhrissorrakrai K, Gunsalus KC, Heifetz Y. Mating induces an immune response and developmental switch in the Drosophila oviduct. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13912–13917. doi: 10.1073/pnas.0710997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawniczak MKN, Barnes AI, Linklater JR, Boone JM, Wigby S, Chapman T. Mating and immunity in invertebrates. Trends in Ecology and Evolution. 2007;22:48–55. doi: 10.1016/j.tree.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Lawniczak MKN, Begun DJ. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome. 2004;47:900–910. doi: 10.1139/g04-050. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annual Review of Immunology. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack PD, Kapelnikov A, Heifetz Y, Bender M. Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10358–10363. doi: 10.1073/pnas.0604046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning A. A sperm factor affecting the receptivity of Drosophila melanogaster females. Nature. 1962;194:252–253. [Google Scholar]
- McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Current Biology. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- McKean KA, Nunney L. Bateman’s principle and immunity: phenotypically plastic reproductive strategies predict changes in immunological sex differences. Evolution. 2005;59:1510–1517. [PubMed] [Google Scholar]
- McKean KA, Yourth CP, Lazzaro BP, Clark AG. The evolutionary costs of immunological maintenance and deployment. BMC Evolutionary Biology. 2008;8:76. doi: 10.1186/1471-2148-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshitzky P, Fleischmann I, Chaimov N, Saudan P, Klauser S, Kubli E, Applebaum SW. Sex-peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus allatum. Archives of Insect Biochemistry and Physiology. 1996;32:363–374. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<363::AID-ARCH9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Neubaum DM, Wolfner MF. Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 1999;153:845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Chen S, Büsser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Current Biology. 2005a;15:207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Peng J, Zipperlen P, Kubli E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Current Biology. 2005b;15:1690–1694. doi: 10.1016/j.cub.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Rolff J, Siva-Jothy MT. Copulation corrupts immunity: a mechanism for a cost of mating in insects. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9916–9918. doi: 10.1073/pnas.152271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P. Variation in immune defence as a question of evolutionary ecology. Proceedings of the Royal Society B: Biological Sciences. 2003;270:357–366. doi: 10.1098/rspb.2002.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology and Evolution. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Short SM, Lazzaro BP. Female and male genetic contributions to post-mating immune defence in female Drosophila melanogaster. Proceedings of the Royal Society B: Biological Sciences. 2010;277:3649–3657. doi: 10.1098/rspb.2010.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva-Jothy MT, Tsubaki Y, Hooper RE. Decreased immune response as a proximate cost of copulation and oviposition in a damselfly. Physiological Entomology. 1998;23:274–277. [Google Scholar]
- Soller M, Bownes M, Kubli E. Control of oocyte maturation in sexually mature Drosophila females. Developmental Biology. 1999;208:337–351. doi: 10.1006/dbio.1999.9210. [DOI] [PubMed] [Google Scholar]
- Soller M, Bownes M, Kubli E. Mating and sex peptide stimulate the accumulation of yolk in oocytes of Drosophila melanogaster. European Journal of Biochemistry. 1997;243:732–738. doi: 10.1111/j.1432-1033.1997.00732.x. [DOI] [PubMed] [Google Scholar]
- Viney ME, Riley EM, Buchanan KL. Optimal immune responses: immunocompetence revisited. Trends in Ecology and Evolution. 2005;20:665–669. doi: 10.1016/j.tree.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Wang L, Ligoxygakis P. Pathogen recognition and signalling in the Drosophila innate immune response. Immunobiology. 2006;211:251–261. doi: 10.1016/j.imbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigby S, Domanitskaya EV, Choffat Y, Kubli E, Chapman T. The effect of mating on immunity can be masked by experimental piercing in female Drosophila melanogaster. Journal of Insect Physiology. 2008;54:414–420. doi: 10.1016/j.jinsphys.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]