Abstract
Objective
In pediatric patients fluid overload (FO) at continuous renal replacement (CRRT) initiation is associated with increased mortality. The aim of this study was to characterize the association between fluid overload at CRRT initiation, fluid removal during CRRT, the kinetics of fluid removal and mortality in a large pediatric population receiving CRRT while on extracorporeal membrane oxygenation (ECMO).
Design
Retrospective chart review.
Setting
Tertiary children’s hospital
Patients
ECMO patients requiring CRRT from July 2006 to September 2010
Interventions
None
Measurements and Main Results
Overall ICU survival was 34% for 53 patients that were initiated on CRRT while on ECMO during the study period. Median FO at CRRT initiation was significantly lower in survivors compared to non-survivors (24.5 vs. 38%, p=0.006). Median FO at CRRT discontinuation was significantly lower in survivors compared to non-survivors (7.1 vs. 17.5%, p=0.035). After adjusting for percent FO at CRRT initiation, age and severity of illness, the change in FO at CRRT discontinuation was not significantly associated with mortality (p=0.212). Models investigating the rates of fluid removal in different periods, age, severity of illness and fluid overload at CRRT initiation found that fluid overload at CRRT initiation was the most consistent predictor of survival.
Conclusions
Our data demonstrates an association between FO at CRRT initiation and mortality in pediatric patients receiving ECMO. The degree of FO at CRRT discontinuation is also associated with mortality, but appears to reflect the effect of FO at initiation. Furthermore, correction of FO to ≤ 10% was not associated with improved survival. These results suggest that intervening prior to the development of significant FO may be more clinically effective than attempting fluid removal after significant fluid overload has developed. Our findings suggest a role for earlier initiation of CRRT in this population, and warrant further clinical studies.
Keywords: Acute kidney injury, pediatric intensive care, fluid overload, continuous renal replacement therapy, extracorporeal membrane oxygenation
Introduction
Extracorporeal membrane oxygenation (ECMO) is a life-saving therapy for pediatric patients with severe cardiac and respiratory failure. For patients on ECMO, the development of acute kidney injury (AKI) and fluid overload (FO) are associated with increased mortality (1–7). As such, continuous renal replacement therapy (CRRT) has become an important tool in managing severe AKI in patients undergoing ECMO (2, 4, 8, 9).
FO is a clinically important target for intervention with CRRT in patients requiring ECMO (3, 5). FO at the initiation of CRRT is independently associated with increased mortality in a variety of clinical scenarios (10–13), although the ECMO population has not been independently examined. FO is a key component of the cardiorenal syndrome, which makes it of particular importance for patients on ECMO with cardiac dysfunction (14). CRRT provides flexibility and control in the management of fluid balance, and CRRT enhances the ability to achieve dry weight and negative fluid balance during ECMO (15, 16).
The impact of different CRRT fluid removal strategies on outcomes in AKI patients has not been extensively studied. Michael et al studied patients following stem-cell transplants who developed AKI and showed that the ability to maintain or achieve dry weight was associated with improved survival (17). Bouchard et al found in adults that greater duration of FO while on RRT was associated with higher mortality (18). To date, there have been no prospective randomized clinical trials comparing different CRRT fluid management strategies. The question remains as to whether CRRT can truly correct pre-existing FO and whether such correction improves outcomes.
The aim of this study was to characterize the association between FO and outcomes in pediatric ECMO patients receiving CRRT. In particular, we sought to determine the impact of FO on survival at both CRRT initiation and discontinuation. We also examined the kinetics of CRRT-mediated fluid removal as a potential predictor of outcomes. We hypothesized that the ability to remove fluid and restore fluid balance with CRRT would be associated with improved survival.
Methods
Study Population
We conducted a retrospective review of all pediatric patients undergoing concurrent CRRT and ECMO between July 2006 and October 2010 at the University of Michigan. For patients with multiple CRRT episodes separated by more than 24 hours while on ECMO, the first episode was included. Patients on CRRT prior to ECMO were excluded. The institutional investigational review board at the University of Michigan approved this study.
Prior to September 2009, ECMO was provided by a servo-regulated roller-pump system with a silicone lung as described by Swaniker et al (5). Subsequently, ECMO was performed using a centrifugal pump (Centrimag, Levitronix LLC, Waltham, Mass.). Prior to 2007 we used only Medtronic Silicone membrane oxygenators, from 2007 through 2009 we used Quadrox oxygenators on all children over 10kg because of lack of experience with Quadrox oxygenators on the smaller children. We converted to Quadrox oxygenators for all patients in November of 2009. The method of bypass performed was at the discretion of the treating physician. Typically, patients requiring ECMO for cardiac failure were placed on venoarterial bypass and those requiring respiratory support were placed on venovenous bypass.
Patients received CVVH by having a dialysis filter placed in parallel to the ECMO circuit by connecting to the ECMO circuit pre-oxygenator, until December 2008. After December 2008 we performed CVVHDF using the Prismaflex system (Gambro, Lund, Sweden) similarly in parallel. For patients weighing < 25 kg, CRRT was performed using an AN-69 M60 filter (Hospal, France). Otherwise, polysulfone HF 400 (Renalflo II, MN) or Optiflux (Fresenius, Germany) filters were used in line based on body surface area. Prior to 2008 heparin was used for anti-coagulation in patients requiring CRRT in line with ECMO. Anticoagulation was performed using a standardized regional citrate protocol after December 2008 to standardize the institutional Prismafelx CRRT protocols (19).
Data Collection
Data collection included demographic data, laboratory data, and characteristics of the ICU course (Table 1). Pediatric Risk of Mortality III (PRISM III) scores at ICU admission for all patients and Risk Adjusted Congenital Heart Surgery-1 (RACHS-1) scores for cardiac surgical patients were calculated (20, 21). The indication for CRRT and ECMO was determined from the pediatric nephrology and critical care notes. The timing of CRRT initiation was at the treating physician’s discretion.
Table 1.
Baseline patient characteristics overall and by survival status
| Variable | Overall | ICU Survival | p- value | |
|---|---|---|---|---|
| Yes (N = 18) | No (N = 35) | |||
| Age (months) | 0 (0, 10) | 10.5 (0, 112) | 0 (0, 1) | < 0.001 |
| Sex: Female | 21 (39.6) | 5 (27.8) | 16 (45.7) | 0.206 |
| ICU Admission Weight | 3.6 (3.2, 8.0) | 10.5 (3.5, 63.7) | 3.4 (3.0, 4.3) | 0.002 |
| Hospital days prior to CRRT | 6 (3, 11) | 4.5 (3, 6) | 9 (4, 13) | 0.035 |
| ICU days prior to CRRT | 5 (3, 10) | 4.5 (3, 6) | 6 (3, 11) | 0.299 |
| Hours on ECMO | 244 (151, 347) | 281 (186, 369) | 233 (138, 345) | 0.320 |
| Hours ECMO prior to CRRT | 43 (21, 92) | 48.5 (29, 74) | 40 (19, 94) | 0.614 |
| Days on CRRT | 7 (4, 12) | 8 (6, 16) | 7 (4, 12) | 0.385 |
| > 2 vasoactive medications at initiation | 34 (64.2) | 5 (27.8) | 29 (82.9) | < 0.001 |
| Number of vasoactive agents at initiation | 3 (2, 4) | 1.5 (1, 3) | 4 (3, 4) | < 0.001 |
| Diuretic Exposure | 48 (90.6) | 16 (88.9) | 32 (91.4) | 0.765 |
| Diuretic Infusion | 35 (66) | 12 (66.7) | 23 (65.7) | 0.945 |
| Therapeutic Plasma Exchange | 6 (11.3) | 4 (22.2) | 2 (5.7) | 0.072 |
| PRISM III Score at ICU admission | 15 (9,20) | 16 (5, 19) | 13 (9, 22) | 0.660 |
ECMO: extracorporeal membrane oxygenation; CRRT: continuous renal replacement therapy
Continuous variables are expressed as median (interquartile range). Categorical variables are expressed as count (%).
FO was determined using daily patient weights obtained during CRRT with the ICU admission weight as baseline. The standard of care at our institution is to weigh ECMO patients daily, in a standardized manner similar to patients not on ECMO with care taken to monitor the cannulas during the procedure. The following previously reported formula was used to calculate percent FO at CRRT initiation (13):
Similarly, FO at CRRT discontinuation was calculated as follows:
The change in FO while on CRRT was determined as follows:
Change in Fluid Overload = Initiation FO − Discontinuation FO
FO was also examined as a categorical variable using a cut-off of 10% (<=10% versus >10%) and 20% (<=20% versus >20%) based on published literature (10, 22).
The primary outcome was ICU mortality.
Statistical Methods
Due to skewness of data, continuous variables are presented as median (interquartile range). Univariate comparisons were made using the Mann-Whitney U-test and chi-square or Fisher’s exact tests, as appropriate. Pearson correlation coefficients were computed to assess the linear relationship between initiation and discontinuation FO. Logistic regression models were used to assess the relationship between FO at each time point, the rate of fluid removal in various intervals and ICU mortality while adjusting for age and PRISM score. Separate models were used to evaluate the impact of FO as a continuous variable and as a categorical variable. Multi-collinearity was ruled out in all multivariate models using estimates of the variance inflation factor for each predictor. When examining fluid removal kinetics, the degree of FO on each day of CRRT was calculated, and a rate of change was determined by calculating the slope of a linear regression model for each patient. Rates were determined for the following periods: the first 3 days on CRRT, the first 7 days on CRRT, and the entire course of CRRT. Analyses were performed in SAS 9.2 (SAS Institute, Cary, NC) and R 2.10.1 (23), and statistical significance was set at p ≤ 0.05.
Results
Patient Characteristics
A total of 203 patients underwent ECMO during the study period and 57 (28%) of these patients received concurrent CRRT. Four patients received CRRT prior to ECMO and were excluded. Forty-six (87%) patients received venoarterial ECMO and 7 (13%) patients received venovenous ECMO. One patient received multiple runs of CRRT. This study included 33 neonates (1 month of age or younger) and median patient age was 0 (0,10) months. Baseline characteristics of the study population are summarized in Table 1.
The indications for the initiation of CRRT were fluid overload (48 patients), electrolyte abnormalities (3 patients) and multiple indications (2 patients). The indication for ECMO was respiratory failure (19 patients, 36%), cardiac arrest (8 patients, 15%), low cardiac output syndrome (12 patients, 23%) and failure to separate from bypass following surgery (14 patients, 26%). The most common underlying diagnosis was surgical heart disease (N=28, 53%, Table 2).
Table 2.
Primary underlying disease necessitating extracorporeal membrane oxygenation
| Primary Disease | N=53 | |
|---|---|---|
| Number | % of Total Patients |
|
| Primary Myocardial Failure | 8 | 15.1 |
| Post-Operative Myocardial Failure | 28 | 52.8 |
| Primary Renal Disease | 1 | 1.9 |
| Sepsis | 7 | 13.2 |
| Congenital Diaphragmatic Hernia | 6 | 11.3 |
| Other | 3 | 5.7 |
Outcome Data
Survival for patients requiring ECMO during the study period was 58%. For ECMO patients requiring CRRT, survival to ICU discharge was 34%. Survival was lower in the 36 patients that received CRRT with the filter placed in parallel with the ECMO circuit (prior to December, 2008) compared to the 17 who received CRRT with the Prismaflex machine (25% vs 52.9%, p = 0.045). Neonates (≤ 1 month) had a significantly lower rate of survival to ICU discharge (15.1% vs 65%, p < 0.001). Additional variables that were significantly different between survivors and non-survivors included age, ICU admission weight, and number of vasoactive medications at CRRT initiation (Table 1). The median initiation FO was significantly lower in survivors compared to non-survivors (24.5% vs. 38.0%, p=0.006, Table 3). Similarly, the median discontinuation FO was significantly lower in survivors compared to non-survivors (7.1% vs. 17.5%, p=0.035).
Table 3.
Fluid overload among ECMO patients requiring CRRT, overall and by survival status.
| Variable | Overall | Survival | p- value | |
|---|---|---|---|---|
| N = 53 | Yes (N = 18) |
No (N = 35) |
||
| % FO at CRRT Initiation | 30 (21, 49) | 24.5 (7, 29) | 38 (26, 51) | 0.006 |
| % FO at CRRT Discontinuation | 13.8 (2.6, 28.9) | 7.1 (−3, 18.4) | 17.5 (6.7, 40.6) | 0.035 |
| Patients with Surgical Heart Disease | ||||
| N = 28 | Yes (N = 3) |
No (N = 25) |
||
| % FO at CRRT Initiation | 37.5 (25, 49.5) | 14 (2, 27) | 38 (30, 50) | 0.039 |
| % FO at CRRT Discontinuation | 14.3 (1.1, 29.3) | 3 (−11.3, 18.3) | 14.8 (4.3, 29.8) | 0.248 |
| Patients without Surgical Heart Disease | ||||
| N = 25 | Yes (N = 15) |
No (N = 10) |
||
| % FO at CRRT Initiation | 26 (16, 41) | 25 (7, 31) | 35 (21, 60) | 0.162 |
| % FO at CRRT Discontinuation | 13.3 (2.6, 24.6) | 9.7 (−3, 20.9) | 29.2 (11.1, 44.2) | 0.034 |
Variables are expressed as median (interquartile range).
Univariate analysis demonstrated a significant association between initiation FO and increased mortality (OR 1.04, 95% CI 1.01–1.08, Table 4), and between discontinuation FO and increased mortality (OR 1.04, 95% CI 1.00–1.07, Table 4). In other words, for each 1% increase in FO at CRRT initiation or discontinuation, the odds of mortality increased by 4%. Multivariate analysis correcting for patient age and PRISM III score at ICU admission demonstrated a borderline significant association between initiation FO and mortality (OR 1.05, 95% CI 1.00–1.10, Table 4). In a similar model, discontinuation FO was associated with increased mortality (OR 1.06, 95% CI 1.00–1.12). Because we observed a high correlation (Pearson correlation 0.62) between degree of FO at CRRT initiation and discontinuation, we were unable to directly assess their individual effects in the same model. In order to assess the association between fluid removal and mortality, we examined the change in degree of FO during CRRT while adjusting for FO at CRRT initiation. This model revealed that change in percent FO was not significantly associated with mortality (OR 0.96, 95% CI 0.89–1.03, Table 4).
Table 4.
Logistic regression models of fluid overload at CRRT initiation, discontinuation and change in fluid overload as predictors of mortality.
| Univariate Analysis | |||
|---|---|---|---|
| Variable # | Odds Ratio | 95% CI | p- value |
| Fluid Overload at CRRT Initiation | 1.04 | 1.01, 1.08 | 0.018 |
| Fluid Overload at CRRT Discontinuation | 1.04 | 1.00, 1.07 | 0.046 |
| Multivariate Analysis Correcting for Age and PRISM III at ICU Admission# | |||
| Variable # | Odds Ratio | 95% CI | p- value |
| Fluid Overload at CRRT Initiation | 1.05 | 1.00, 1.10 | 0.063 |
| Fluid Overload at CRRT Discontinuation | 1.06 | 1.00, 1.12 | 0.047 |
| Multivariate Analysis Evaluating Age, PRISM III Score at ICU Admission, and Fluid Overload at CRRT Initiation | |||
| Variable | Odds Ratio | 95% CI | p- value |
| PRISM III score | 1.19 | 1.00, 1.40 | 0.044 |
| Age in Months | 0.95 | 0.89, 1.01 | 0.077 |
| Fluid Overload at CRRT Initiation | 1.08 | 1.01, 1.16 | 0.033 |
| Change in % Fluid Overload | 0.96 | 0.89, 1.03 | 0.212 |
| Multivariate Analysis Correcting for Age, PRISM III at ICU Admission | |||
| Variable | Odds Ratio | 95% CI | p- value |
| CRRT Initiation <10% FO* | 0.02 | 0.00, 0.77 | 0.035 |
| CRRT Initiation >10% FO and CRRT Discontinuation <10% FO* |
1.22 | 0.13, 11.1 | 0.860 |
| Variable | Odds Ratio | 95% CI | p- value |
| CRRT Initiation <20% FO** | 0.21 | 0.02,2.6 | 0.221 |
| CRRT Initiation >20% FO and CRRT Discontinuation <20% FO** |
0.53 | 0.13,3.87 | 0.687 |
Interpretation of Odds Ratios is odds of ICU mortality per 1% change in Fluid Overload at the specified timepoint.
Reference group for analysis was those patients with >10% FO at CRRT Initiation and >10% FO at CRRT Discontinuation
Reference group for analysis was those patients with >20% FO at CRRT Initiation and >20% FO at CRRT Discontinuation
A subgroup analysis was performed on the patients with underlying surgical cardiac disease (n=28). The survival to ICU discharge was significantly different in patients with underlying surgical cardiac disease (11% vs 60%, p<0.001). There were no differences between survivors and non-survivors in age, sex, timing of ECMO initiation, or duration of ECMO (data not shown). Median RACHS score was 4 (3, 6) and was not predictive of mortality (p=0.608). The degree of FO at CRRT initiation was significantly higher in non-survivors compared to survivors (38.0% vs. 14.0%, p=0.039). FO at CRRT discontinuation was not significantly different between survivors and non-survivors (3% vs 14.8%, p=0.248).
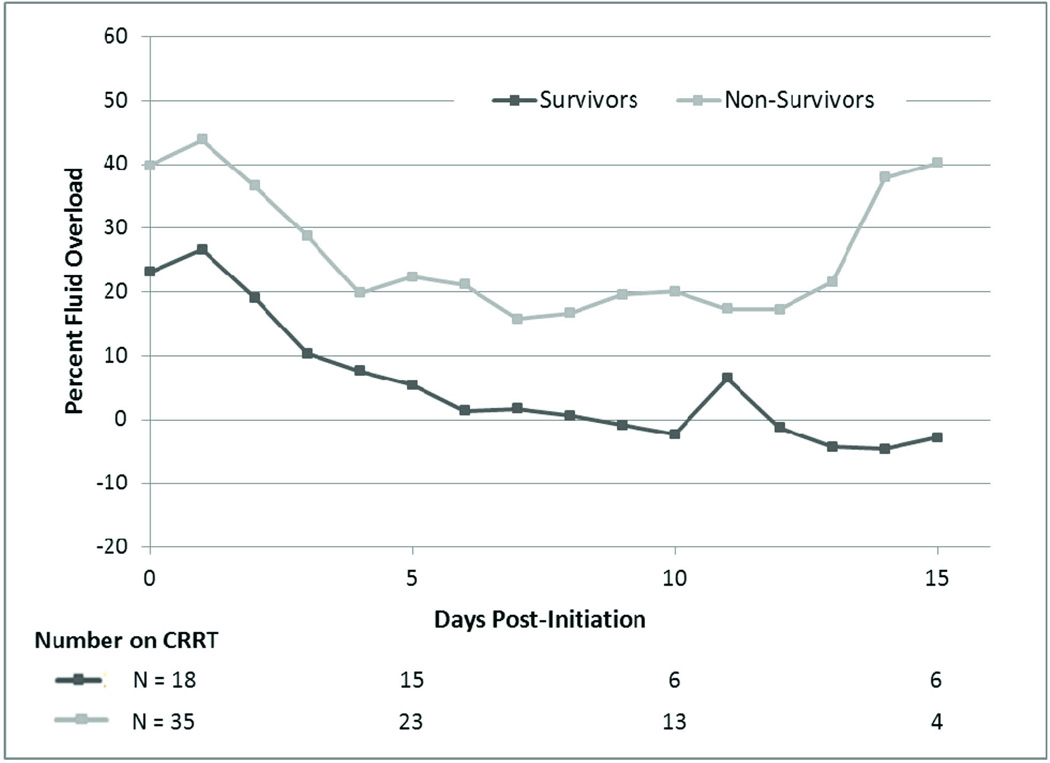
We sought to describe the daily fluid removal while on CRRT by measuring the mean daily FO in survivors and non-survivors from initiation (Figure 1). The average degree of FO remained higher in non-survivors compared to survivors at all timepoints examined. The FO curves of survivors and non-survivors remained parallel, suggesting that fluid removal was similar in both groups. Multivariate analysis accounting for patient age, PRISM III score at ICU admission and rate of fluid removal during 3 different intervals from CRRT initiation demonstrated a consistently significant association between initiation FO and ICU mortality (Table 5). In the same models, a higher rate of fluid removal during each period was not significantly predictive of lower mortality (Table 5). Average fluid removal rates were significantly higher in neonates compared to non-neonates on day 7 and over the entire CRRT course. Fluid removal rates were not significantly different after the change to utilizing the Prsimaflex system for CRRT on ECMO.
Figure 1.
Mean Fluid Overload by Day Post-Initiation of CRRT
Table 5.
Logistic Regression Models for Mortality Incorporating Daily Rate of Change in Fluid Overload
| Rate of Fluid Removal during First Three Days | |||
|---|---|---|---|
| Variable | Odds Ratio | 95% CI | p- value |
| Fluid Overload at Initiation | 1.1 | 1.01, 1.12 | 0.031 |
| Rate of Fluid Removal | 0.79 | 0.62, 1.02 | 0.066 |
| PRISM Score | 1.16 | 0.97, 1.38 | 0.101 |
| Age, Months | 0.89 | 0.76, 1.04 | 0.130 |
| - The median (IQR) rate of fluid removal was 2.34 %/day (0.10, 8.69) | |||
| Rate of Fluid Removal during First Seven Days | |||
| Variable | Odds Ratio | 95% CI | p- value |
| Fluid Overload at Initiation | 1.07 | 1.00, 1.14 | 0.046 |
| Rate of Fluid Removal | 0.74 | 0.53, 1.04 | 0.085 |
| PRISM Score | 1.15 | 0.98, 1.35 | 0.094 |
| Age, Months | 0.87 | 0.71, 1.06 | 0.165 |
| - The median (IQR) rate of fluid removal was 3.31 %/day (1.61, 5.89) | |||
| Rate of Fluid Removal over Entire CRRT Treatment | |||
| Variable | Odds Ratio | 95% CI | p- value |
| Fluid Overload at Initiation | 1.08 | 1.01, 1.15 | 0.034 |
| Rate of Fluid Removal | 0.75 | 0.55, 1.03 | 0.079 |
| PRISM Score | 1.16 | 0.98, 1.37 | 0.092 |
| Age, Months | 0.88 | 0.73, 1.06 | 0.187 |
| - The median(IQR) rate of removal in this period was 1.87 %/day (0.56, 4.62) | |||
Rate of fluid removal is defined as the % change in fluid overload per day during the period evaluated.
Neonate defined as up to 1 month of age.
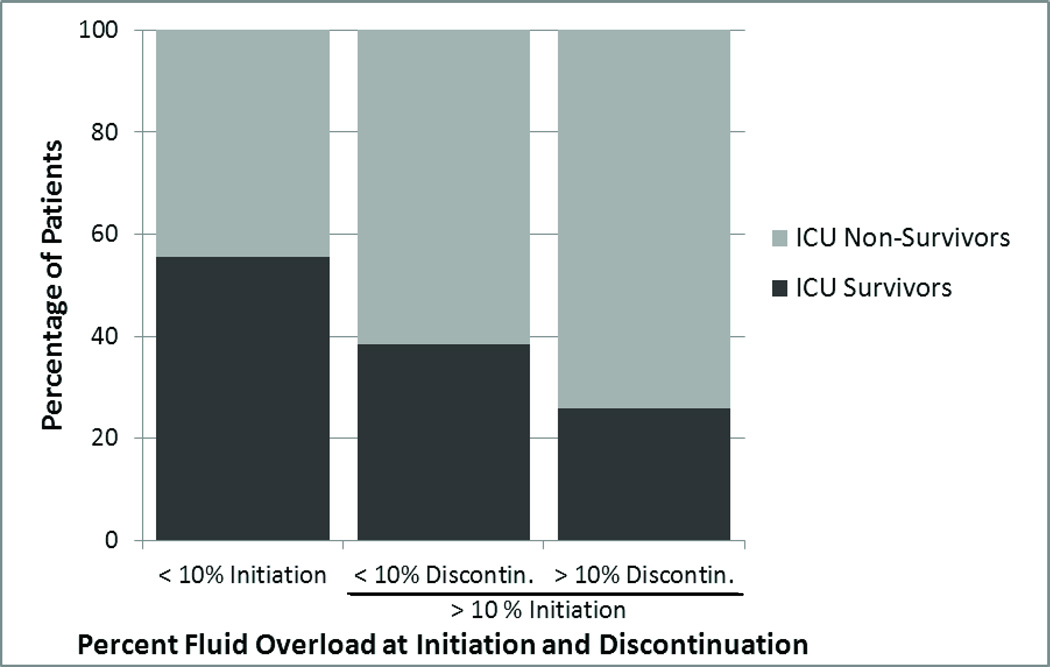
To further examine the impact of fluid removal on outcome, we compared patients that achieved a clinically significant improvement in degree of FO (defined as FO <10% at CRRT discontinuation) versus those that remained >10% FO. Nine patients initiated CRRT at <10% FO and this group had an ICU survival of 55.6%. The survival to ICU discharge of the 44 patients initiating CRRT at >10% FO was 29.5%, and this did not seem to vary by the degree of fluid removal achieved. Compared to patients starting with >10% FO and ending with <10% FO (n=13), patients starting and ending CRRT with >10% FO (n=31) had similar survival rates (38.5% vs. 25.8%, p=0.478, Figure 2). In univariate analysis, the overall ICU survival was not statistically different between the 3 groups (p=0.251). In a multivariate model adjusting for age and PRISM score, starting <10% FO was associated with a lower risk for mortality compared to the reference group of patients starting and ending with >10% FO (OR 0.02, 95% CI 0.00–0.77), while starting with >10% FO and achieving <10% FO did not appear to confer any different mortality risk (OR 1.22, 95% CI 0.13–11.1) (Table 4). A similar analysis was performed using 20% FO as the defining point and did not yield significant results (Table 4).
Figure 2.
Change in the Degree of Fluid Overload from Initiation to Discontinuation
Discussion
In pediatric patients requiring ECMO, AKI is an independent risk factor for mortality (1, 4, 5), and CRRT is an important therapy (2, 24). Previous pediatric studies have found an association between FO at CRRT initiation and subsequent mortality (10–13, 22, 25), and we have confirmed this association in an exclusive ECMO population (13). We demonstrate that CRRT was effective at improving fluid balance in the majority of patients. In addition, we present the first study to investigate the kinetics of fluid removal after CRRT initiation and the impact on mortality. Our data raises important questions about the clinical benefit of fluid removal once significant FO is established.
While the clinical significance of initial FO has been described, few studies have examined the impact of CRRT-mediated fluid removal on outcomes. In a study of 116 patients with multi-organ dysfunction, Goldstein and colleagues noted a significant difference in survival between patients that achieved their dry weight versus those that remained FO while on CRRT (76% vs. 36%, p<0.001) (25). The PICARD study noted increased mortality among adult patients with persistent FO at cessation of dialysis compared to those with resolved FO (35% vs. 56%, p<0.001); similarly, longer duration of FO during dialysis was associated with worse outcomes(18). The implications of FO for patients on ECMO include increased mortality (5) and increased ECMO duration (3). We found that a lower degree of FO at CRRT discontinuation for patients on ECMO was associated with improved survival. However, this finding appeared to result from the high correlation between initiation and discontinuation FO. When directly assessing the impact of fluid removal, the change in percent FO from initiation to discontinuation of CRRT was not a significant predictor of outcome. The degree of FO improved in a parallel fashion in both survivors and non-survivors, demonstrating that fluid removal was equally achievable in both groups. Furthermore, after significant (>10%) FO was established, restoration of fluid balance to <10% FO during CRRT did not appear to significantly improve outcomes compared to patients that remained >10% FO. These data suggest that prevention of significant FO is likely to be more effective at improving outcomes than attempting fluid removal once significant FO is established. The exact definition of “clinically significant” FO remains undefined, but when examining a threshold of 20% FO, we did not observe a significant difference between FO groups with respect to mortality, suggesting that 20% may be too high a threshold level.
Our study is among the first to examine the kinetics of fluid removal following CRRT initiation. When viewing FO as a pathologic state, a more rapid improvement in the degree of FO may limit damage from this condition or may reflect clinical improvement. We hypothesized that a higher rate of fluid removal would be associated with lower mortality. In contrast, we observed that the rate of FO correction was similar between survivors and non-survivors (Figure 1), and the rate of fluid removal was not a significant predictor of mortality when considered in either early (first 3 days) or later (first 7 days or entire course) periods following CRRT initiation. In all the models we examined, the degree of FO at CRRT initiation remained the most consistent predictor of mortality. These findings suggest that efforts at fluid removal may not be effective once significant FO is established, and are consistent with our other observations that correction of FO did not appear to be associated with improved outcomes. Clinical trials are needed to test whether prevention of FO may result in better outcomes.
The clinical importance of FO prevention is particularly relevant in patients on ECMO when one considers that trials off ECMO are more successful if patients are near their dry weight. At our institution, it is standard of care to remove no greater than 3 mL/kg/hour (6–7% of dry weight/day) of fluid while on CRRT based on the dry weight, a practice consistent with other institutions (4). Patients receiving CRRT in our study were a median 30% FO at the initiation of CRRT. At a fluid removal rate of 3 mL/kg/hour, it would take 4–5 days for these patients to achieve their dry weight, as compared to 1–2 days if the patient was 10% FO at CRRT initiation. Therefore, the degree of FO at CRRT initiation may in part drive the duration of ECMO and contribute to morbidity resulting from prolonged therapy.
We included a large pediatric cardiac patient population in this study, in whom FO may be of particular importance given the complex interplay between renal and cardiac dysfunction in the cardiorenal syndrome (26–29). The finding that initiation FO was not significantly predictive of mortality in non-cardiac patients further highlights the cardiac subgroup as a potentially high-yield target population. Early CRRT following cardiac surgery in adult studies has been shown to potentially improve outcomes (30). Such studies are lacking in the pediatric cardiac patient population, and our study suggests that 10% FO may be a potential target for future prospective interventional studies.
Limitations of this study include that it is a single center retrospective analysis. Although we were able to find statistically significant associations, our small sample size limited the precision of our estimates in sub-populations. In addition, we were not able to completely stratify for severity of illness or multiple organ dysfunction as there is not a defined severity of illness score for patients on ECMO (PRISM III score at ICU admission served as a surrogate). During the study period two methods were used to perform CRRT for patients on ECMO and these patients had significantly different rates of survival, but no differences in fluid removal. This finding likely reflects improvements in institutional practices and not the precision of FO measurements by pumps, but warrants further multi-center investigation. We acknowledge that there is no standardized protocol for the timing of the initiation of CRRT at our institution, which likely contributed to study heterogeneity. Lastly, as an observational study we cannot offer firm clinical recommendations, but we hope to generate interest in prospective clinical trials.
Conclusion
We have demonstrated an association between FO at CRRT initiation and increased mortality not previously reported in an exclusive pediatric ECMO patient population. The degree of FO at CRRT discontinuation is also predictive of survival, but likely reflects the effect of FO at initiation, as correction of FO during CRRT did not appear to improve outcomes. In addition, we observed that the kinetics of fluid removal were actually similar between survivors and non-survivors. Taken together, these results suggest that intervening prior to the development of significant FO may be more clinically effective than attempting fluid removal after significant FO has developed. Our findings suggest a role for earlier initiation of CRRT in this population, and further clinical study is warranted in this area.
Acknowledgements
David T. Selewski, MD is supported by the “Research Training in Pediatric Nephrology” grant (T-32 F023015). Timothy T. Cornell, MD is supported by the Pediatric Critical Care Scientist Development Program (K12HD047349) and an individual Career Development Award (K08HD062142).
Neal B. Blatt, MD was supported by a Child Health Research Career Development Award (National Institutes of Health, K12 HD 028820).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have not disclosed any potential conflicts of interest.
Bibliography
- 1.Weber TR, Connors RH, Tracy TF, Jr, et al. Prognostic determinants in extracorporeal membrane oxygenation for respiratory failure in newborns. Ann Thorac Surg. 1990;50(5):720–723. doi: 10.1016/0003-4975(90)90669-w. [DOI] [PubMed] [Google Scholar]
- 2.Heiss KF, Pettit B, Hirschl RB, et al. Renal insufficiency and volume overload in neonatal ECMO managed by continuous ultrafiltration. ASAIO Trans. 1987;33(3):557–560. [PubMed] [Google Scholar]
- 3.Kelly RE, Jr, Phillips JD, Foglia RP, et al. Pulmonary edema and fluid mobilization as determinants of the duration of ECMO support. J Pediatr Surg. 1991;26(9):1016–1022. doi: 10.1016/0022-3468(91)90665-g. [DOI] [PubMed] [Google Scholar]
- 4.Smith AH, Hardison DC, Worden CR, et al. Acute renal failure during extracorporeal support in the pediatric cardiac patient. ASAIO J. 2009;55(4):412–416. doi: 10.1097/MAT.0b013e31819ca3d0. [DOI] [PubMed] [Google Scholar]
- 5.Swaniker F, Kolla S, Moler F, et al. Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg. 2000;35(2):197–202. doi: 10.1016/s0022-3468(00)90009-5. [DOI] [PubMed] [Google Scholar]
- 6.Kolovos NS, Bratton SL, Moler FW, et al. Outcome of pediatric patients treated with extracorporeal life support after cardiac surgery. Ann Thorac Surg. 2003;76(5):1435–1441. doi: 10.1016/s0003-4975(03)00898-1. discussion 1441-1432. [DOI] [PubMed] [Google Scholar]
- 7.Gadepalli SK, Selewski DT, Drongowski RA, et al. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg. 2011;46(4):630–635. doi: 10.1016/j.jpedsurg.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 8.Meyer RJ, Brophy PD, Bunchman TE, et al. Survival and renal function in pediatric patients following extracorporeal life support with hemofiltration. Pediatr Crit Care Med. 2001;2(3):238–242. doi: 10.1097/00130478-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Paden ML, Warshaw BL, Heard ML, et al. Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12(2):153–158. doi: 10.1097/PCC.0b013e3181e2a596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. 2010;55(2):316–325. doi: 10.1053/j.ajkd.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein SL, Currier H, Graf C, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 12.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32(8):1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 13.Selewski DT, Cornell TT, Lombel RM, et al. Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med. 2011;37(7):1166–1173. doi: 10.1007/s00134-011-2231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronco C, Giomarelli P. Current and future role of ultrafiltration in CRS. Heart Fail Rev. 2011;16(6):595–602. doi: 10.1007/s10741-010-9198-y. [DOI] [PubMed] [Google Scholar]
- 15.Hoover NG, Heard M, Reid C, et al. Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med. 2008;34(12):2241–2247. doi: 10.1007/s00134-008-1200-y. [DOI] [PubMed] [Google Scholar]
- 16.Sell LL, Cullen ML, Whittlesey GC, et al. Experience with renal failure during extracorporeal membrane oxygenation: treatment with continuous hemofiltration. J Pediatr Surg. 1987;22(7):600–602. doi: 10.1016/s0022-3468(87)80107-0. [DOI] [PubMed] [Google Scholar]
- 17.Michael M, Kuehnle I, Goldstein SL. Fluid overload and acute renal failure in pediatric stem cell transplant patients. Pediatr Nephrol. 2004;19(1):91–95. doi: 10.1007/s00467-003-1313-z. [DOI] [PubMed] [Google Scholar]
- 18.Bouchard J, Soroko SB, Chertow GM, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 19.Bunchman TE, Maxvold NJ, Barnett J, et al. Pediatric hemofiltration: Normocarb dialysate solution with citrate anticoagulation. Pediatr Nephrol. 2002;17(3):150–154. doi: 10.1007/s00467-001-0791-0. [DOI] [PubMed] [Google Scholar]
- 20.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins KJ, Gauvreau K. Center-specific differences in mortality: preliminary analyses using the Risk Adjustment in Congenital Heart Surgery (RACHS-1) method. J Thorac Cardiovasc Surg. 2002;124(1):97–104. doi: 10.1067/mtc.2002.122311. [DOI] [PubMed] [Google Scholar]
- 22.Hayes LW, Oster RA, Tofil NM, et al. Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care. 2009;24(3):394–400. doi: 10.1016/j.jcrc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Team RDC. R: A Language and Environment for Statistical Computing. Vienna: Austria R Foundation for Statistical Computing; 2011. [Google Scholar]
- 24.Shaheen IS, Harvey B, Watson AR, et al. Continuous venovenous hemofiltration with or without extracorporeal membrane oxygenation in children. Pediatr Crit Care Med. 2007;8(4):362–365. doi: 10.1097/01.PCC.0000269378.76179.A0. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(2):653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 26.Ronco C, Giomarelli P. Current and future role of ultrafiltration in CRS. Heart Fail Rev. 2010 doi: 10.1007/s10741-010-9198-y. [DOI] [PubMed] [Google Scholar]
- 27.Udani SM, Murray PT. The use of renal replacement therapy in acute decompensated heart failure. Semin Dial. 2009;22(2):173–179. doi: 10.1111/j.1525-139X.2008.00542.x. [DOI] [PubMed] [Google Scholar]
- 28.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121(23):2592–2600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 29.Price JF, Goldstein SL. Cardiorenal syndrome in children with heart failure. Curr Heart Fail Rep. 2009;6(3):191–198. doi: 10.1007/s11897-009-0027-3. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Fernandez N, Perez-Valdivieso JR, Bes-Rastrollo M, et al. Timing of renal replacement therapy after cardiac surgery: a retrospective multicenter Spanish cohort study. Blood Purif. 2011;32(2):104–111. doi: 10.1159/000324195. [DOI] [PubMed] [Google Scholar]