Abstract
A vast majority of human vaccines rely on neutralizing antibodies for protection. With the exception of vaccines against human papillomavirus, despite a great amount of dedicated effort by the scientific community, development of vaccines against sexually transmitted viruses has generally been unsuccessful. Understanding the immunobiology of the genital tract is key to designing vaccines that prevent spreading of these viruses. Recent studies demonstrate that adaptive immunity in the vaginal mucosa is uniquely regulated compared to other mucosal organs. In particular, development of virus-specific CD4+ and CD8+ T cells is critically important for antiviral defense in vagina. In this review, we provide an overview of our current understanding of a wide spectrum of immune responses in vagina - from innate viral sensing to memory development.
Introduction
The lower reproductive tract represents a unique site for both pathogen entry and dissemination between individuals. However, despite its importance in sexually transmitted diseases, mechanisms of antiviral immunity in the genital mucosa have attracted relatively little attention compared to those in other mucosal surfaces such as gastrointestinal and respiratory tracts. Compared to the monolayer epithelia in the intestine and in the lung, the vaginal tract is covered with stratified epithelia. In addition, the vaginal mucosa differs from other mucosae with respect to mucus composition, microbiota and innate and adaptive immune mechanisms. Here we discuss recent progress in understanding how antiviral immunity is initiated and maintained in the female genital tract.
Constitutive barrier mechanisms in the female genital mucosa
The female genital tract consists of two different types of mucosal surfaces. The upper genital tract (endocervix and endometrium) surfaces represents the type I mucosal surface, which is covered with a monolayer of columnar epithelial cells with tight junctions and secretory IgA. In contrast, the lower genital tract (vagina and ectocervix) represents the type II mucosal surface, lined by stratified squamous epithelia that lack luminal IgA and mucosa-associated lymphoid tissues [1,2]. The boundary between type I and type II mucosa, known as the cervical transformation zone, is most vulnerable to invasion by pathogens (Figure 1) and populated heavily with T cells and antigen presenting cells (APCs) compared to other regions of the female genital tract [3]. In addition, both molecular and cellular antiviral events in the female genital tract are heavily affected by sex hormones, which is discussed extensively elsewhere [4,5].
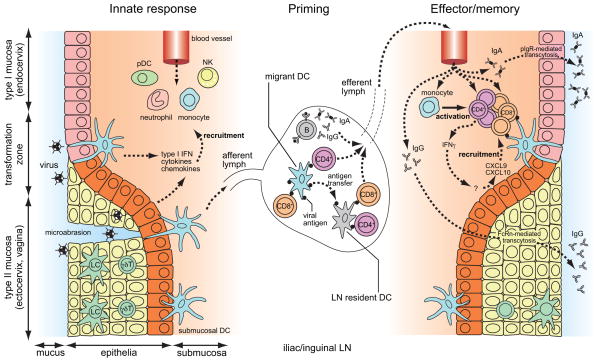
Figure 1. Antiviral adaptive immune responses in the cervical and vaginal mucosa.
Viral exposure is often thought to occur through the transformation zone or through microabrasion. At steady state, vaginal epithelial layer and the submucosa are surveyed by innate leukocytes and lymphocytes, but the recruitment of antigen-specific T and B cells to the vagina is restricted. Once infected, both epithelial cells and innate leukocytes produce type I IFNs, inflammatory cytokines and induce chemokines that recruit NK cells, monocytes, pDCs and neutrophils. Virions and viral antigens are taken up and processed by migrant submucosal DCs or by LN-resident DCs and presented to T cells. Activated effector T cells are recruited to the vagina and can persist for a long period. Vaginal epithelial cells lack polymeric Ig receptor (pIgR) for transport of sIgA. Instead, virus-specific IgG is transcytosed by FcRn into the vaginal lumen, and provides protection.
The epithelial surfaces of the female genital tract are covered with mucus. Mucus consists of mucin proteins that may inhibit viral entry and also contains secretory proteins that have microbicidal and antiviral activity [2]. In addition to mucus, the lower genital tract is populated with endogenous bacteria and fungi, with a predominant Lactobacillus species, that keep an acidic environment in the vagina. The acidic pH, epithelial barrier, mucus and innate immune responses triggered by the commensal flora act in concert to prevent virus infections in the female genital tract against pathogens including Haemophilus ducreyi, HSV—2 and Chlamydia trachomatis [6]. However, the influence of the vaginal microbiota on the adaptive immune responses to sexually transmitted viruses is unknown.
Inducible host immune responses to viral infection in the vagina
As in the other sites of the body, antiviral immune responses in the genital tract consist of four different phases – 1) recognition of the virus by invariant receptors of the innate immune system, leading to the activation of cytokines and antiviral response genes, 2) processing and presentation of the virus antigens by APCs to naïve lymphocytes leading to priming of adaptive immunity, 3) elimination of the virus by various effector mechanisms, and 4) establishing long-term memory (Figure 1). This review will use examples from human immunodeficiency virus 1 (HIV-1), herpes simplex viruses (HSV-1 and HSV-2) and human papilloma viruses (HPVs), which are clinically relevant sexually transmitted viruses in humans. Although kinetics of the immune response and viral elimination differs greatly between viruses, we here discuss general principles of these processes in the vagina.
Innate viral recognition
In general, pathogen recognition by the host innate immune system relies on receptors, known as pattern recognition receptors (PRRs) that recognize molecular patterns shared by different classes of pathogens [7]. In response to viral infection, genital epithelial cells produce pro-inflammatory cytokines, IFNβ and antimicrobial peptides such as defensins [5]. The tissue-resident macrophages, DCs and intraepithelial γδT cells also recognize the virus immediately after initial infection, and secrete antiviral factors such as type I IFNs and cytokines [2]. DCs have the capacity to link innate detection of viruses to the generation of adaptive immune responses. Nevertheless, virus infection is likely sensed by both the hematopoietic and non-hematopoietic compartments for the full induction of T cell-mediated adaptive immunity against genital HSV-2 infection [8].
Following the first wave of responses by epithelial cells and tissue-resident leukocytes, more leukocytes such as neutrophils, monocytes, natural killer (NK) cells and pDCs are recruited to the vaginal mucosa, which, at least to some extent, was shown to be protective to the host during genital HSV infection in mice [4,9]. Of these cell types, pDCs are equipped with a robust capacity to respond to a variety of viruses through TLRs and provide a large burst of type I IFNs necessary for restricting virus infection [10]. Interestingly, however, neither pDCs nor type I IFNs are required for the development of adaptive immunity in genital HSV infection in mice [9,10].
Priming adaptive immunity
DCs present antigens most efficiently to naïve T cells and are thereby a key APC type that bridges innate and adaptive immunity. In both humans and mice, there are multiple DC subsets in the vaginal mucosa including intraepithelial Langerhans cells (LCs) and submucosal DC [11]. Unlike the skin, vaginal LCs in the mouse derive from circulating radiosensitive precursors at steady state [12]. Upon inflammation, other DC subsets are recruited including monocyte-derived DCs and pDCs [10,13]. Recent studies indicate differential roles of various DC subsets in priming, maintenance and execution phases of immune responses in the vaginal mucosa.
Immune priming by non-infected DCs
For non-viral antigens, DCs in the female genital tract are seemingly conditioned to be tolerogenic [14–17]. However, in the case of viral infection, DCs are capable of initiating robust T cell responses. Although genital epithelial cells may directly present antigens to T cells in some settings [4,18], direct viral antigen presentation by the infected epithelial cells is not likely to prime naïve lymphocytes, which preferentially circulate to secondary lymphoid tissues. In addition, directly infected DCs are incapable of presenting antigens as viruses are armed with molecules that inhibit their activation and function [19]. Accordingly, LCs are incapable of cross-presenting HSV antigens [20,21] or physically depleted by HPV E6 protein [22]. Furthermore, rather than priming T cells, LCs spread HIV infection beyond the site of viral entry by transmitting virions to CD4+T cells [23,24]. Thus, antigens are likely cross-presented by non-infected DCs.
Upon vaginal viral infection, virus-specific T cells are primed exclusively in vaginal draining lymph nodes (dLNs), which are iliac and inguinal LNs [1]. Once infection takes place in the vaginal mucosa, these dLNs undergo various adaptations to prime optimal immune responses. One such adaptation involves increasing naïve lymphocyte influx by enlargement of the feed arteriole [25,26]. Upon vaginal HSV-2 infection, only the migratory CD11b+ DCs, and not the lymph node-resident CD8a+ DCs effectively prime CD4+ T cells in the dLNs [21]. Another study demonstrated that intravaginal inoculation of ovalbumin mixed with the cholera toxin B subunit adjuvant leads to presentation of MHC class I epitopes by CD11b+ DCs, but not CD8+ DCs [27]. In contrast, vaginal HSV-1 infection results in CD4+ and CD8+ T cell priming by migrant CD11b+ and CD8α+ DCs [28]. How does this compare to antigen handling in the skin? Interestingly, while primary epicutaneous infection with HSV-1 results in antigen presentation by the LN-resident CD8α+ DCs [20,28,29], antigen presentation during recrudescence is handled almost exclusively by the CD103+ dermal DCs [30]. While similarities do exist between the skin and the vaginal mucosa in regard to inability of LCs and potential role for subepithelial DCs in cross-presenting antigens, further studies are needed to characterize vaginal DC subsets as counterparts for CD103+DCs have not been described for the vaginal submucosa [11].
Antiviral effector responses at the site of infection
Recent evidence indicates that effector responses are most efficient in exerting their protective function locally. Both humoral and T cell-mediated immunity against genital HSV infection are most efficiently induced by attenuated HSV when the vaccination is given intravaginally [31,32]. Here, we discuss the advantages of local effector responses and relate this to strategy for vaccine design.
Local humoral immunity
Generally, humoral immunity is thought to provide systemic coverage of various mucosal and visceral tissues through antibodies delivered via the circulation. However, recent studies highlight the importance of generating genital mucosa-resident B cell memory and plasmablast responses. In monkeys immunized against simian-HIV vaginally, virus-specific vaginal IgA and IgG, but not plasma IgG correlated with protection [33]. Another study on genital HSV infection in mice also demonstrated the presence of vagina-resident plasma cells that produce protective virus-specific IgG [32]. How are locally produced immunoglobulins transported to the vaginal lumen? Unlike in type I mucosa such as intestinal or uterine epithelia where IgA is abundant, antigen-specific antibodies in the vagina are dominated by IgG [2]. Recent studies showed that neonatal Fc receptor (FcRn) is responsible for transcytosis of IgG in the circulation to the apical surface of the vagina [34]. In contrast to basolateral-to-apical IgA transcytosis, FcRn-mediated IgG transcytosis is bidirectional and dependent on acidic pH [35]. Indeed, mucosal application of viral peptide fused with Fcγ leads to protective immunity against genital viral infection in an FcRn-dependent antigen transport [36,37].
Local effector T cell immunity
Although neutralizing antibodies are protective against infections with many viruses such as HPV [38], induction of T cell-mediated immunity, particularly antigen-specific CD4+ T cells, is critical for full protection in infections such as HIV and HSV. In murine genital HSV infection models, while B cells and CD8+ T cells may contribute to viral clearance, CD4+ T cells appear to be more critical in host protection [9]. If the CD8+ T cells are the executioner killer T cells, CD4+ T cells are the master regulator of innate and adaptive immune responses that go far beyond helping B cells and CD8+ T cells, and are themselves potent antiviral effectors during HSV infection [39]. In addition, CD4+ T cells are critical for effector CD8+ T cell mobilization into otherwise restricted tissues such as the vagina [40–42]. Requirement of CD4+ T cells for effector CD8+ T cell mobilization is organ-specific, as lung and intestine have been shown to be more permissive to effector CD8+ T cell infiltration [43]. CXCR3 expressing virus-specific effector CD8+ T cells enter HSV-infected vaginal mucosa via its ligands CXCL9 and CXCL10, which are produced locally in response to CD4+ T cell-derived but not NK cell-derived IFNγ [41]. Notably, such local production of IFNγ by CD4+ T cells requires infiltration of inflammatory monocyte-derived DCs in the vaginal mucosa [13]. Thus, CD4+ T cells provide many facets of local protective immunity by being a potent antiviral inducer and effector and by regulating traffic of other important cell types to the vagina. Nevertheless, cooperation between CD4+ and CD8+ T cells are particularly important, as suggested by the fact that the crucial role for CD8+T cell in controlling chronically-infected viruses including HIV relies on functionality of CD4+T cells [39].
Establishing local memory responses
In genital HSV infection, once established, both CD4+ and CD8+ effector T cells persist for a long period and form cluster-like structures in the vaginal tissue [44–46]. Evidence shows that tissue-resident HSV-specific memory CD8+ T cells are maintained locally, form a distinct subset from the circulating memory population and continuously monitor the neural endings to suppress reactivation of the virus from neuronal latency [47–50]. Indeed, the density of tissue-resident CD8+ T cells critically affects rates of reactivation in both mice and humans [51,52]. Stimulation of these tissue-resident memory CD8+ T cells is dependent on DCs and require CD4+ T cells in the elicitation phase [53]. In contrast, activation of tissue-resident memory CD4+ T cells require either DCs or B cells and is protective to the host against genital HSV-2 infection in an IFNγ-dependent manner [44] (Figure 1). Upon secondary infection, tissue resident memory T cells rapidly produce IFNγ, which serves as a predominant antiviral mechanism in recurrent herpetic lesions in humans [54].
Given the importance of local memory T cells in providing protection against subsequent viral challenge, targeting and maintaining cellular immunity to genital mucosa is likely key for efficient vaccination. It has been shown that T cells that migrate to restrictive tissues such as the gut or skin express selective homing markers such as CCR9 and α4β7 or CCR10, respectively. Studies in vaginal non-viral infection models have shown that endothelial cells in infected genital mucosa upregulate ICAM-1, VCAM-1, MAdCAM-1 and E-selectin [55–58], while vagina-recruited T cells preferentially express LFA-1 (ICAM-1 ligand), α4β7 (MAdCAM-1 and VCAM-1 ligand) and αEβ7 integrins as well as cutaneous lymphocyte antigen (E-selectin ligand) [56,58–60]. Accordingly, naïve αE integrin-deficient mice have reduced numbers of vaginal intraepithelial leukocytes [61], and mice that lack E-selectin and T cells that lack β7 integrin were shown to have reduced vaginal recruitment and impaired bacterial clearance in genital Chlamydia infection [55]. In addition, similar to HSV-specific CD8+ T cells [41], entry of effector CD4+ T cells into genital mucosa in murine Chlamidya infection requires T cell-intrinsic expression of CXCR3 along with CCR5 [62]. To induce the expression of these homing markers, DCs responding to environmental vitamins imprint tissue tropism of effector T cells by altering expression levels of chemokine receptors and cell adhesion molecules. In the gut, DCs metabolize retinol (vitamin A) into retinoic acid that upregulates gut-tropic receptors CCR9 and α4β7 integrin in T cells [63], whereas in the skin DCs produce 1,25-dihydroxyvitamin D3 from inactive vitamin D3 to suppress CCR9 and α4β7 integrin and upregulate skin-tropic chemokine receptor CCR10 [64]. Interestingly, a recent study demonstrated that retinoic acid used as an adjuvant during immunization with adenovirus-encoded peptide upregulates mucosal homing receptors CCR9 and α4β7 and αEβ7 integrins in antigen-specific CD8+ T cells and successfully induces effector CD8+ T cells in mucosal organs including vagina [65]. However, a key question in this regard is what are the cellular and molecular requirements, such as endogenous environmental cues, that enable memory T cell recruitment and residency in the vagina? How do local DCs, if any, imprint vagina-tropic T cells? How is the formation of effector T cell clusters in the vagina controlled and what is the relevance of these clusters in antiviral protection? The answers to these questions will hold the key to designing effective vaccines against sexually transmitted viruses in the future.
Conclusion
Despite a great success in prophylactic HPV vaccine [38], no therapeutic vaccine has been made against any sexually transmitted viruses, nor is there an efficacious preventive vaccine against HIV-1 and HSV infection. While much has been learned from infection models in other mucosal tissues and skin, for a better vaccination strategy against sexually transmitted pathogens, it is critically important to understand cellular and molecular mechanisms of immune protection in the genital mucosa, and translate our basic understandings to clinically relevant outcome. Given the advances made in generating circulating virus-specific T cells and antibodies in humans, we must now focus our attention to developing long-term tissue-specific memory within the genital mucosa. We have recently proposed “prime-and-pull” vaccination strategy, in which antigen-specific lymphocytes are targeted to the genital mucosa by artificially-recruiting memory cells to the vagina [2]. Future studies are needed to test these and other ideas in preclinical and clinical settings.
Highlights.
Vagina is lined with stratified squamous epithelia with mucus and unique flora.
Vaginal epithelial cells and innate leukocytes provide the first line of cellular defense.
Local antibodies and T cells provide long-term protection in the vagina.
Vagina entry and residency by effector T cells require unique homing properties.
Long-term tissue-resident memory T cells are key for efficient vaccine.
Acknowledgments
We thank Dr. Haina Shin for critical reading of this manuscript. The National Institutes of Health grants AI 054359, AI 081884, AI062428, AI 064705 and Midwest Center of Excellence in Biodefense and Emerging Infectious Disease Research (5U54 AI 057160) support the work performed in the laboratory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaushic C. The role of the local microenvironment in regulating susceptibility and immune responses to sexually transmitted viruses in the female genital tract. J Reprod Immunol. 2009;83:168–172. doi: 10.1016/j.jri.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 5.Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keele BF, Estes JD. Barriers to mucosal transmission of immunodeficiency viruses. Blood. 2011;118:839–846. doi: 10.1182/blood-2010-12-325860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 8.Sato A, Iwasaki A. Induction of antiviral immunity requires Toll-like receptor signaling in both stromal and dendritic cell compartments. Proc Natl Acad Sci U S A. 2004;101:16274–16279. doi: 10.1073/pnas.0406268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan T, Barra NG, Lee AJ, Ashkar AA. Innate and adaptive immunity against herpes simplex virus type 2 in the genital mucosa. J Reprod Immunol. 2011;88:210–218. doi: 10.1016/j.jri.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 11.Iijima N, Thompson JM, Iwasaki A. Dendritic cells and macrophages in the genitourinary tract. Mucosal Immunol. 2008;1:451–459. doi: 10.1038/mi.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iijima N, Linehan MM, Saeland S, Iwasaki A. Vaginal epithelial dendritic cells renew from bone marrow precursors. Proc Natl Acad Sci U S A. 2007;104:19061–19066. doi: 10.1073/pnas.0707179104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Iijima N, Mattei LM, Iwasaki A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc Natl Acad Sci U S A. 2011;108:284–289. doi: 10.1073/pnas.1005201108. This study shows that monocytes recruited to the HSV2-infected vagina provide critical signals to CD4+ effector T cells to produce IFNγ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Black CA, Rohan LC, Cost M, Watkins SC, Draviam R, Alber S, Edwards RP. Vaginal mucosa serves as an inductive site for tolerance. J Immunol. 2000;165:5077–5083. doi: 10.4049/jimmunol.165.9.5077. [DOI] [PubMed] [Google Scholar]
- 15.Marks E, Tam MA, Lycke NY. The female lower genital tract is a privileged compartment with IL-10 producing dendritic cells and poor Th1 immunity following Chlamydia trachomatis infection. PLoS Pathog. 2010;6:e1001179. doi: 10.1371/journal.ppat.1001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochiel DO, Ghosh M, Fahey JV, Guyre PM, Wira CR. Human uterine epithelial cell secretions regulate dendritic cell differentiation and responses to TLR ligands. J Leukoc Biol. 2010;88:435–444. doi: 10.1189/jlb.1009700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wira CR, Roche MA, Rossoll RM. Antigen presentation by vaginal cells: role of TGFbeta as a mediator of estradiol inhibition of antigen presentation. Endocrinology. 2002;143:2872–2879. doi: 10.1210/endo.143.8.8938. [DOI] [PubMed] [Google Scholar]
- 18.Ochiel DO, Rossoll RM, Schaefer TM, Wira CR. Effect of oestradiol and pathogen-associated molecular patterns on class II-mediated antigen presentation and immunomodulatory molecule expression in the mouse female reproductive tract. Immunology. 2012;135:51–62. doi: 10.1111/j.1365-2567.2011.03512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham AL, Donaghy H, Harman AN, Kim M, Turville SG. Manipulation of dendritic cell function by viruses. Curr Opin Microbiol. 2010;13:524–529. doi: 10.1016/j.mib.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews K, Leong CM, Baxter L, Inglis E, Yun K, Backstrom BT, Doorbar J, Hibma M. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-cadherin. J Virol. 2003;77:8378–8385. doi: 10.1128/JVI.77.15.8378-8385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballweber L, Robinson B, Kreger A, Fialkow M, Lentz G, McElrath MJ, Hladik F. Vaginal langerhans cells nonproductively transporting HIV-1 mediate infection of T cells. J Virol. 2011;85:13443–13447. doi: 10.1128/JVI.05615-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumamoto Y, Mattei LM, Sellers S, Payne GW, Iwasaki A. CD4+ T cells support cytotoxic T lymphocyte priming by controlling lymph node input. Proc Natl Acad Sci U S A. 2011;108:8749–8754. doi: 10.1073/pnas.1100567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soderberg KA, Payne GW, Sato A, Medzhitov R, Segal SS, Iwasaki A. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc Natl Acad Sci U S A. 2005;102:16315–16320. doi: 10.1073/pnas.0506190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luci C, Hervouet C, Rousseau D, Holmgren J, Czerkinsky C, Anjuere F. Dendritic cell-mediated induction of mucosal cytotoxic responses following intravaginal immunization with the nontoxic B subunit of cholera toxin. J Immunol. 2006;176:2749–2757. doi: 10.4049/jimmunol.176.5.2749. [DOI] [PubMed] [Google Scholar]
- 28.Lee HK, Zamora M, Linehan MM, Iijima N, Gonzalez D, Haberman A, Iwasaki A. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med. 2009;206:359–370. doi: 10.1084/jem.20080601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 31.Marks E, Helgeby A, Andersson JO, Schon K, Lycke NY. CD4 T-cell immunity in the female genital tract is critically dependent on local mucosal immunization. Eur J Immunol. 2011;41:2642–2653. doi: 10.1002/eji.201041297. [DOI] [PubMed] [Google Scholar]
- 32.Parr EL, Parr MB. Immunoglobulin G, plasma cells, and lymphocytes in the murine vagina after vaginal or parenteral immunization with attenuated herpes simplex virus type 2. J Virol. 1998;72:5137–5145. doi: 10.1128/jvi.72.6.5137-5145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Bomsel M, Tudor D, Drillet AS, Alfsen A, Ganor Y, Roger MG, Mouz N, Amacker M, Chalifour A, Diomede L, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity. 2011;34:269–280. doi: 10.1016/j.immuni.2011.01.015. This study demonstrated correlation of protection against simian-HIV with vaginal but not circulating virus-specific antibodies. [DOI] [PubMed] [Google Scholar]
- 34**.Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. This study demonstrates critical requirement of FcRn for transcytosing the circulating IgG to the vaginal lumen that is necessary for IgG-mediated protection against genital HSV2 infection in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 36*.Lu L, Palaniyandi S, Zeng R, Bai Y, Liu X, Wang Y, Pauza CD, Roopenian DC, Zhu X. A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol. 2011;85:10542–10553. doi: 10.1128/JVI.05441-11. These two papers show induction of protective humoral immunity in the genital mucosa by FcRn-based vaccination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Ye L, Zeng R, Bai Y, Roopenian DC, Zhu X. Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat Biotechnol. 2011;29:158–163. doi: 10.1038/nbt.1742. These two papers show induction of protective humoral immunity in the genital mucosa by FcRn-based vaccination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schiller JT, Castellsague X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26 (Suppl 10):K53–61. doi: 10.1016/j.vaccine.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4 T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70:8368–8377. doi: 10.1158/0008-5472.CAN-10-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong SB, Bos R, Sherman LA. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J Immunol. 2008;180:3122–3131. doi: 10.4049/jimmunol.180.5.3122. [DOI] [PubMed] [Google Scholar]
- 43.Lefrancois L, Puddington L. Intestinal and pulmonary mucosal T cells: local heroes fight to maintain the status quo. Annu Rev Immunol. 2006;24:681–704. doi: 10.1146/annurev.immunol.24.021605.090650. [DOI] [PubMed] [Google Scholar]
- 44.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang VA, Rosenthal KL. Intravaginal infection with herpes simplex virus type-2 (HSV-2) generates a functional effector memory T cell population that persists in the murine genital tract. J Reprod Immunol. 2010;87:39–44. doi: 10.1016/j.jri.2010.06.155. [DOI] [PubMed] [Google Scholar]
- 46.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, et al. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med. 2009;15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 48.Himmelein S, St Leger AJ, Knickelbein JE, Rowe A, Freeman ML, Hendricks RL. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae. 2011;2:5. doi: 10.1186/2042-4280-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. Rates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cells. J Virol. 2007;81:8157–8164. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Schiffer JT, Abu-Raddad L, Mark KE, Zhu J, Selke S, Koelle DM, Wald A, Corey L. Mucosal host immune response predicts the severity and duration of herpes simplex virus-2 genital tract shedding episodes. Proc Natl Acad Sci U S A. 2010;107:18973–18978. doi: 10.1073/pnas.1006614107. Through a combination of biopsy sampling and mathematical modeling, this study shows close correlation between the local effector/memory CD8+T cell density and the severity of recurrent herpetic lesions in human genital mucosa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 54.Peng T, Zhu J, Klock A, Phasouk K, Huang ML, Koelle DM, Wald A, Corey L. Evasion of the mucosal innate immune system by herpes simplex virus type 2. J Virol. 2009;83:12559–12568. doi: 10.1128/JVI.00939-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly KA, Chan AM, Butch A, Darville T. Two different homing pathways involving integrin beta7 and E-selectin significantly influence trafficking of CD4 cells to the genital tract following Chlamydia muridarum infection. Am J Reprod Immunol. 2009;61:438–445. doi: 10.1111/j.1600-0897.2009.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly KA, Rank RG. Identification of homing receptors that mediate the recruitment of CD4 T cells to the genital tract following intravaginal infection with Chlamydia trachomatis. Infect Immun. 1997;65:5198–5208. doi: 10.1128/iai.65.12.5198-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kelly KA, Walker JC, Jameel SH, Gray HL, Rank RG. Differential regulation of CD4 lymphocyte recruitment between the upper and lower regions of the genital tract during Chlamydia trachomatis infection. Infect Immun. 2000;68:1519–1528. doi: 10.1128/iai.68.3.1519-1528.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wormley FL, Jr, Chaiban J, Fidel PL., Jr Cell adhesion molecule and lymphocyte activation marker expression during experimental vaginal candidiasis. Infect Immun. 2001;69:5072–5079. doi: 10.1128/IAI.69.8.5072-5079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hawkins RA, Rank RG, Kelly KA. Expression of mucosal homing receptor alpha4beta7 is associated with enhanced migration to the Chlamydia-infected murine genital mucosa in vivo. Infect Immun. 2000;68:5587–5594. doi: 10.1128/iai.68.10.5587-5594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kelly KA, Wiley D, Wiesmeier E, Briskin M, Butch A, Darville T. The combination of the gastrointestinal integrin (alpha4beta7) and selectin ligand enhances T-Cell migration to the reproductive tract during infection with Chlamydia trachomatis. Am J Reprod Immunol. 2009;61:446–452. doi: 10.1111/j.1600-0897.2009.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 62**.Olive AJ, Gondek DC, Starnbach MN. CXCR3 and CCR5 are both required for T cell-mediated protection against C. trachomatis infection in the murine genital mucosa. Mucosal Immunol. 2011;4:208–216. doi: 10.1038/mi.2010.58. By using Chlamydia-specific TCR-transgenic CD4+T cells, this study demonstrated requirement of CCR5 and CXCR3 for the recruitment of effector CD4+T cells to the genital mucosa and host protection upon C. trachomatis infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 64.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 65**.Tan X, Sande JL, Pufnock JS, Blattman JN, Greenberg PD. Retinoic acid as a vaccine adjuvant enhances CD8+ T cell response and mucosal protection from viral challenge. J Virol. 2011;85:8316–8327. doi: 10.1128/JVI.00781-11. This study shows that the use of retinoic acid as an adjuvant enhances recruitment of effector/memory CD8+T cells to mucosal tissues including vagina. [DOI] [PMC free article] [PubMed] [Google Scholar]