Summary
Recent studies have revisited the roles of prime players in the immune response to tuberculosis (TB) and have highlighted novel functions of players. Specifically, immunoregulatory mechanisms mediated by IFNγ have been delineated as well as a novel role for neutrophils in promoting antigen presentation. New insights into the interaction between the bacterium and phagocyte indicate that the bacterium actively promotes phagocyte necrosis rather than apoptosis and that this impacts generation of the acquired response. There are also many new examples of how the phagocyte responds to the bacteria and how it mediates control. The phenotype of protective T cells is also being re-examined. These developments provide promise for improved vaccine design and highlight the complexity of this disease.
Introduction
Mycobacterium tuberculosis(Mtb) enters the lung via aerosol droplets that can penetrate to the alveolar tissue. This tissue is delicate and resists damage by elaborating several levels of anti-inflammatory mechanisms. Despite this regulated environment, inflammation occurs in response to infection with Mtb and it appears that virulent Mtb are very well equipped to manipulate the cellular immune response to promote accumulation of phagocytic cells while delaying activation of the acquired response. Once initiated, the antigen-specific cellular response is strong but as the cells accumulate within the infected tissue their ability to respond is limited. By this sequence of events, Mtb creates and resides within an immunologically privileged area while driving a strong immune response that generates the tissue damage required for transmission. Recent publications discussed below contribute new details to this model.
T cell activation
Upon arrival of Mtb in the lung there is very little stimulation of the acquired response, indeed it takes 7-9 days for bacteria to be delivered to the draining lymph node (DLN) and for antigen-specific T cells to respond (reviewed in [1]). While T cell responses can be initiated in the absence of DLN [2,3] the naive lung is a poor inducer of naive T cells and thus bacteria must traffic to the DLN. In addressing the role of DC’s in the dissemination of bacteria we identified a novel role for an alternatively spliced variant of the IL-12Rβ1. Specifically, the splice variant is induced in CD11c+ cells at the same time as the bacteria traffic to the DLN and its presence improves efficiency of DC migration and T cell activation [4]*. The issue of how the bacteria get into the motile DCs has been of interest and it appears that Mtb inhibits apoptosis of neutrophils thereby limiting the uptake apoptotic bodies containing Mtb by DCs [5]*. Depletion of neutrophils increases the number of directly infected DCs in the lung, however these cells are not responsive to lymph node homing chemokines whereas DC’s that have ingested apoptotic neutrophils remain responsive and are therefore better able to prime T cells in the DLN [6]*. In addition, by inhibiting PGE2, Mtb promotes necrosis in host cells which limits cross presentation of its antigens by DC and delays initiation of the T cell response [7]*. Other factors have been demonstrated to delay the initiation of the acquired cellular response including the induction of regulatory T cells at the same time as effector T cells [8]. Importantly, regulatory T cells in TB are dependent upon the Th1 transcription factor T-bet and IFNγ for their persistence [9]**. In a link to the increased susceptibility of individuals with diabetes to TB, it has been shown that induction of acquired immunity is delayed in diabetic mice and that this is associated with reduced innate responses in the lung following infection [10].
Innate/acquired bridge
While the acquired responses is required to stop Mtb growth, less classical lymphocytes are also capable of recognizing mycobacterial antigens and the role of these cells is being determined. Recent studies with CD1b tetramers demonstrate that CD4 T cells are the dominant CD1b binding cell in the blood of TB patients, indicating a larger pool of cells responding to these lipid than once thought [11]*. In examining the responsiveness of these cells in patients upon diagnosis it appears that mycolic acid can induce IL-2 and IFNγ in T cells in a CD1b dependent manner, these cells were found at sites of infection contracted upon treatment and were not detected in BCG vaccinees [12]*. CD1b can also present other molecules and lipomannan from M. smegmatis is recognized by CD1b-restricted cells whereas the larger more neutral lipomannan from Mtb is less stimulatory [13]. γδ T cells are also implicated in TB. In co-culture experiments γδ T cells (Vγ9Vδ2) and Mtb-infected DCs were found to mutually activate but Mtb-infected DCs drove only proliferation of the γδT cells without effector induction; addition of IL-15 restored differentiation of the γδ T cells and killing of the bacteria [14]. Finally a new cell type, the mucosal-associated invariant T cells (MAIT) has been implicated in antimycobacterial immunity. These cells have an invariant TCR alpha chain (Vα7.2), are restricted to the MHC related protein 1 (MR1) and are activated by cells infected with bacteria or yeast but not virus [15]*. These cells are lost from the blood of patients [15,16], appear in the lung during TB, respond to Mtb-infected lung epithelial cells [16]** and protect mice against M. abscessus [15].
Different type of T cells induced
The function of T cells in TB is to regulate the inflammatory environment by activating phagocytes to kill bacteria and by regulating the innate response and limiting pathologic damage. The achieve these goals the T cells need to get into the inflamed site and see antigen in order to be stimulated to act. It has recently become clear that antigen-specific T cells exhibit only minor antigen-induced T cell arrest and polarized secretion of cytokine within granulomata suggesting limited availability or recognition of antigen [17**,18*]. This observation is surprising given the ability of mycobacteria to not only initiate strong immune responses but also by the fact that mycobacterially-infected phagocytes actively generate microvesicles and exosomes which contain antigen and can apparently stimulate T cells [19]. In addition, it has been shown that DC’s can migrate out of established granulomata and systemically activate T cells [20]*. Taken together with the observation that human T cell epitopes are highly conserved [21], it is clear that Mtb actively induces systemic T cell responsiveness while promoting a granulomatous environment that limits the efficacy of the T cells to respond.
In light of the ability of Mtb to manipulate the vertebrate immune response it is critical to define the type of T cells induced during TB while at the same time determine which T cell type may be best suited to mediating protection. In this regard a recent study that sorted CD4 T cells from Mtb-infected mice based on surface phenotype and returned them to congenically marked infected mice demonstrated that cells that were less polarized were better able to persist in the inflamed environment [22]**. In another study, the development of memory CD8 T cells in the TB lung was found to depend upon the ability of T cells to express the chemokine receptor CXCR3 [23]*. In its absence T cells had limited migration into the inflamed site, less access to antigen and differentiated less allowing greater development of memory cells [23]. In other studies, it was found that antigen-specific T cells that are expanded but remain plastic in their cytokine producing capacity are able to protect mice against Mtb but lose this ability if polarized to a Th17 phenotype [24,25]. Despite these studies, polarization remains an important aspect of the T cell response. In support of this, mycobacterial infection results in down regulation of the microRNA miR-29 that limits persistence of IFNγ mRNA in many lymphocytes [26]. Mice that express a “sponge” for miR-29 express increased IFNγ and an increased ability to combat a high dose challenge with Mtb [26]*. Finally, in humans multiparameter flow cytometry has allowed the phenotypes of responsive cells to be examined and multifunctional effector cells are associated with active disease, with triple cytokine producers being seen in a majority of those with disease and rarely in those with latent disease wherein double or single producers were evident [27,28].
Immunity
While the initiation and expansion of antigen-specific T cells appears critical for immunity, it is not these cells that express the anti-bacterial function. Control of bacterial growth is mediated by the infected phagocyte and the critical components of this response are being elucidated. Recent work has identified a critical role for the IFNγ-inducible 65-kD guanylate-binding proteins (Gbp) which represent 20% of IFNγ-inducible genes. In particular, Gbp1, Gbp6, Gbp7 and Gbp10 confer cell autonomous anti-bacterial activity in macrophages and the absence of Gbp1 results in profound susceptibility of mice to BCG [29]**. Detailed studies support a role for these proteins in promoting the oxidative burst, the process of autophagy and the action of anti-microbial peptides and autophagy effectors to kill intracellular bacteria [29]. In complementary studies, the genes regulated by Interferon Regulatory Factor 8 (IRF8), which is induced in Mtb-infected lungs, include multiple components of MHC class I and II antigen presentation machinery as well as the previously implicated immunity related GTPases (IRG) [30] and the newly implicated Gbps [31].
Recent studies with human phagocytes have highlighted the complexity of the phagocyte response. Autophagy is a normal cellular process however the autophagy-targeting molecule p62 in the autosome promotes anti-mycobacterial activity by delivering specific ribosomal and bulk ubiquinated proteins which become antibacterial peptides [32]*. An immunity related GTPase IRGM in humans has been related to autophagic defense against mycobacteria by promoting mitochondrial fission [33]*. Further, it was found that low vitamin D in peripheral blood cultures resulted in a blunted IFNγ-induced autophagic response as well as reduced phagosomal maturation and reduced production of anti-microbial peptides; delivery of vitamin D restored these responses and restored antimicrobial activity [34]*. It further appears that the miRNA, hsa-mir-21, directly down regulates TLR2/1-induced CYP27B1 (which generates the active form of vitamin D3) and IL1B as well as the vitamin-D dependent anti-microbial peptides CAMP and DEFB4A; knock down of this miRNA restored these anti-bacterial functions [35]*. Induction of miRNA seems to be a trait of Mtb as TNF biosynthesis is regulated at the level of RNA stability by differential induction of the destabilizing miRNA miR-125b relative to the stabilizing miR-155 [36]. The role of superoxide generation in human susceptibility was also recently clarified as newly identified mutations in CYBB (gp91(phox)) results in monocyte-derived macrophage specific defects in NADH oxidase assembly and this is associated with Medelian susceptibility to mycobacterial disease [37]. External forces can also limit phagocyte activation. Air pollution is associated with increased risk of TB and diesel exhaust particles reduce the human primary cell response to Mtb in terms of TNF, IL-1, IL-6 and IFNαβ production [38]. Co-infection can also have an impact as concomitant helminth infection results in increased IL-4R-dependent M2 profile for lesional phagocytes [39]*.
Inflammation
For a long time it has been appreciated that neutrophil accumulation is associated with poor outcome in TB and one of the most exciting developments in recent years is the definition of mechanisms whereby the host promotes mononuclear as opposed to granulocytic responses. Granulocytic inflammation can be induced by repeated antigen delivery and this is dependent upon IL-17 and IL-23 [40]. The absence of IFNγ receptor ligation on radioresistant cells results in the accumulation of IL-17-producing cells and thereby neutrophil recruitment [41]*. It appears that IFNγ production is the primary anti-inflammatory function of memory CD4 T cells as IFNγ-deficient memory cells can limit bacterial growth but cannot limit IL-17 production and concomitant neutrophil recruitment [25,42*]. Importantly, IFNγ also acts directly on neutrophils to limit survival suggesting that appearance of neutrophils indicates loss of IFNγ signaling [42]. The importance of neutrophils in human disease was identified in a comprehensive but unbiased analysis of transcripts from TB patients and controls. In these studies, a specific signature that reflected increased neutrophil-driven type I and II IFN signaling at both the cellular and gene transcription level was associated with active disease and this signature was lost upon treatment [43]**.
The ability of the innate response to limit damaging inflammation was recently suggested by the fact that the absence of CARD9, which integrates signals from multiple pattern recognition receptors, results in increased bacterial growth, pyogranulomatous pneumonia and increased inflammation. Importantly, neutralization of G-CSF and neutrophil depletion in this model prolonged survival [44]. It was also shown that mice with a natural mutation of the leptin receptor on non-hematopoietic cells have disorganized granulomata with neutrophilia [45]. Finally, circulating neutrophils from active TB patients have been shown to have high levels of the programmed cell death ligand-1 (PDL-1) which is implicated in immune regulation [46]. Upon infection, program cell death (PD-1) deficient mice develop focal necrotic areas in the lung with neutrophil infiltrates and excess proinflammatory cytokine expression [47]. It appears however that it is the absence of PD-1 on CD4 T cells that promotes this damage, as removal of CD4 T cells in PD-1 deficient mice alleviates the pathology [48].
Another area of recent interest is in the role of tissue proteases in mediating damage. One particular protease, matrix metalloproteinase (MMP)-1, is expressed by lung fibroblasts in an IL-1β and TNF dependent manner [49] and in microglia during CNS TB [50]. Key to the action of MMP-1 is that its regulators are down-regulated by Mtb [49,50] thus high levels of MMP-1 and low levels of inhibitors are found in the BAL of TB patients. Further, mice transgenically expressing MMP-1 develop more severe alveolar destruction and collagen breakdown during Mtb infection [51]**. It seems therefore that MMP-1 is a principal mediator of the lung damage required to promote dissemination of disease.
Some clarity regarding the role of IL-1 in TB has been achieved recently. IL-1β production is critical to control Mtb in the mouse model [52]*. Further, IL-1α and IL-1β are produced by macrophages and DCs and IFN α/β limits production from both cell types; IFNγ from CD4 T cells selectively blocks IL-1 from monocyte-macrophages [53]**. It has also been shown that the negative regulatory molecule TIM3 is expressed by Th1 cells, binds galectin-9 on Mtb-infected macrophages and limits bacterial growth by inducing IL-1β secretion [54]. Both Mtb and BCG induce IL-1βmRNA in human macrophages but only Mtb induces the inflammasome resulting in active IL-1β production. Mtb also limits IL-1β by inducing type I IFN which limits IL-1β mRNA stability, and also limits the inflammasome. These two pathways are not activated by RD1 deficient bacteria as these lack the ability to impact cytosolic pathways [55]*. Using a case-population study, polymorphisms significantly associated with increased TB were found in a region surrounding a negative regulator of TLR/IL-1R signaling; determining the extent to which these polymorphisms affect IL-1 signaling will be important to understanding TB [56].
Vaccination
In the search for vaccines it is necessary not only to define all potential epitopes but also to determine when they are available. In support of this, a sub unit vaccine containing early and latent (Rv2660c) antigens was effective prior to infection, protected against reactivation and lowered bacterial burden in mice already infected [57]**. Novel vaccine targets were detected by probing the response of latently infected people to DosR-regulon encoded antigens; effector memory phenotypes were detected supporting the potential of these antigens as targets for vaccine against latency [58]. In contrast, an unbiased bioinformatic approach for class I epitopes in humans was performed, 70 new Mtb epitopes were identified and the immunogenicity of 18 of them assessed with tetramers. There was broad IFN, IL-2 and TNF responsiveness to these antigens in cured TB patients suggesting that there is a much broader CD8 response than previously appreciated [59]. While the ESAT-6 and CFP-10 antigens (secreted via the secretion system esx-1) are immunodominant antigens, a related protein, esx-1 substrate protein C (Rv3615c), also appears to promote strong T cell responses in Mtb-exposed but not BCG vaccinated individuals [60]. Together these data suggest that there are many more epitopes available to be probed as vaccine targets. As a caution however, although specific epitope recognition can be induced by vaccination this responsiveness can be overridden by infection. Specifically, for CD8 T cells, immunodominance in Mtb is defined not by the epitope but by the availability of the epitope [61]. This is further complicated by the observation that the delivery vector can substantially alter the epitope recognition patterns as a result of targeting different Lamp positive compartments within the APC [62].
A large number of vaccine studies have been initiated in recent years and the there is increased understanding of the outcomes of vaccination. By comparing the whole blood response of infants from Malawi and the United Kingdom to BCG vaccination, it was found that the Malawians had a higher inflammatory response compared to the infants from the United Kingdom, in contrast the UK children had a higher Th1 type responses [63]*. In another study to address the impact of natural exposure on vaccine efficacy, it was found that infants vaccinated 4.5 months after birth have a modest endogenous response prior to vaccination and also a lower response to vaccination. However by 9 months the response for those vaccinated at birth and at 4.5 months is equivalent [64]*. It is apparent that we still need more information on the impact of BCG vaccination. Pre-clinical vaccine studies have focused on mechanism. Using BCG to induce T cell responses IL-23-dependent IL-17 was shown to overcome BCG-induced IL-10 thereby promoting IL-12 which in turn promotes Th1 [65].
Manipulation of bacteria to improve vaccine efficacy has also been undertaken recently. In investigating the role of esx-3 in bacterial virulence it was noted that loss of esx-3 from M. smegmatis rendered it less virulent in a rag deficient mouse, and that insertion of the Mtb esx-3 resulted in the generation of a bacteria that was able to induce stronger anti-Mtb memory CD4 T cell responses [66]. By mutating the phoP response regulator in Mtb and using this mutant as a vaccine it was found that it protected better than BCG and that this was associated with increased frequency and persistence of antigen-specific central memory CD4 T cells [67]. The importance of long-term and targeted antigen delivery was assessed by the use of Mtb antigens delivered in carefully manipulated live recombinant Salmonella vaccine [68]. In this model, mice receiving an oral vaccine which had delayed lysis and regulated delayed antigen synthesis were better protected against TB.
Conclusion
It is clear that each of the immune system components that act during Mtb-infection have multiple roles and that the immune system response to such a persistent pathogen is complex. What is also clear is that Mtb is exquisitely able to manipulate this complex response to both provide a suitable environment for proliferation while promoting the immune-mediated damage required for transmission of disease.
Highlights.
There is a critical balance between immunity and immunopathology in tuberculosis
Key elements of antibacterial immunity also play a role in regulating immunopathology
Mycobacterium tuberculosis manipulates the immune response
By effectively driving a systemic acquired response and then limiting expression of that response at the site of infection Mycobacterium tuberculosis promotes development of the tissue damage required for transmission
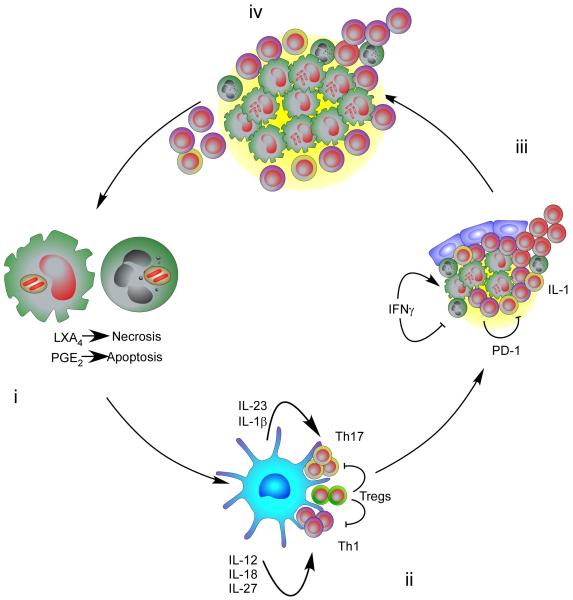
Figure.
(i) Exposure of lung phagocytes, both mononuclear and granulocytic, to invading Mtb results in the phagocytosis of the bacteria. Virulent Mtb inhibits apoptotic and promotes necrotic cell death of the host phagocyte which delays the transfer of Mtb bacteria and/or Mtb antigens to migratory DCs DLN and thereby slows the initiation of T cell responses.(ii) In the DLN the local expression of antigen and inflammatory cytokines defines the polarization of the T cells which then migrate to the Mtb manipulated inflammatory site. (iii) At this site the immune response is regulated by feedback mechanisms involving IFN and PD-1. (iv) Critical to the development of the immunopathology required for transmission is the strong induction of T cell responses which are then limited in function at the site of infection. T cells fail to penetrate fully the macrophage dominated site and also fail to express full functionality.
Acknowledgements
This work was supported by the Trudeau Institute, Inc. and by NIH grants AI046530, AI067723, AI069121 AI73564 and an ALA DeSouza grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cooper AM. Cell mediated immune responses in tuberculosis. Annu. Rev. Immunol. 2009;27:393–422. doi: 10.1146/annurev.immunol.021908.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khader S, Rangel-Moreno J, Fountain J, Martino C, Reiley W, Pearl J, Winslow G, Woodland D, Randall T. Cooper A: In a murine tuberculosis model, the absence of homeostatic chemokines delays granuloma formation and protective immunity. J. Immunol. 2009;183:8004–8014. doi: 10.4049/jimmunol.0901937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day TA, Koch M, Nouailles G, Jacobsen M, Kosmiadi GA, Miekley D, Kuhlmann S, Jörg S, Gamradt P, Mollenkopf H-J, et al. Secondary lymphoid organs are dispensable for the development of T-cell-mediated immunity during tuberculosis. Eur. J. Immunol. 2010;40:1663–1673. doi: 10.1002/eji.201040299. [DOI] [PubMed] [Google Scholar]
- *4.Robinson R, Khader S, Martino C, Fountain J, Teixeira-Coelho M, Pearl J, Smiley S, Winslow G, Woodland D, Walter M, et al. Mycobacterium tuberculosis infection induces il12rb1 splicing to generate a novel IL-12Rβ1 isoform that enhances DC migration. J. Exp. Med. 2010;207:591–605. doi: 10.1084/jem.20091085. This paper highlights a novel function for IL-12Rb1 and an alternative splice variant in migration of DCs from the lung to the DLN
- *5.Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J. Immunol. 2011;186:7110–7119. doi: 10.4049/jimmunol.1100001. [5 and 6] demonstrate that neutrophils do contribute to induction of early antigen specific T cell induction.
- *7.Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat. Immunol. 2010;11:751–758. doi: 10.1038/ni.1904. Demonstrates the importance of the early interaction of the bacteria with thehost pahgocytes and the impact of that interaction on T cell activation
- 8.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J. Exp. Med. 2010;207:1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **9.Koch M, Tucker-Heard G, Perdue N, Killebrew J, Urdahl K, Campbell D. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. Demonstrates the important of the environment in generating and maintaining regulatory T cells
- 10.Vallerskog T, Martens GW, Kornfeld H. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J. Immunol. 2010;184:6275–6282. doi: 10.4049/jimmunol.1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Kasmar AG, van Rhijn I, Cheng T-Y, Turner M, Seshadri C, Schiefner A, Kalathur RC, Annand JW, de Jong A, Shires J, et al. CD1b tetramers bind αβ T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. J. Exp. Med. 2011;208:1741–1747. doi: 10.1084/jem.20110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *12.Montamat-Sicotte DJ, Millington KA, Willcox CR, Hingley-Wilson S, Hackforth S, Innes J, Kon OM, Lammas DA, Minnikin DE, Besra GS, et al. A mycolic acid-specific CD1-restricted T cell population contributes to acute and memory immune responses in human tuberculosis infection. J. Clin. Invest. 2011;121:2493–2503. doi: 10.1172/JCI46216. [11 and 12] Together these studies begin to probe the function of CD1b responsive cells in human TB
- 13.Torrelles JB, Sieling PA, Arcos J, Knaup R, Bartling C, Rajaram MVS, Stenger S, Modlin RL, Schlesinger LS. Structural differences in lipomannans from pathogenic and nonpathogenic mycobacteria that impact CD1b-restricted T cell responses. J. Biol. Chem. 2011;286:35438–35446. doi: 10.1074/jbc.M111.232587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meraviglia S, Caccamo N, Salerno A, Sireci G, Dieli F. Partial and ineffective activation of Vγ9Vδ2 T cells by Mycobacterium tuberculosis-infected dendritic cells. J. Immunol. 2010;185:1770–1776. doi: 10.4049/jimmunol.1000966. [DOI] [PubMed] [Google Scholar]
- *15.Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, Lévy E, Dusseaux M, Meyssonnier V, Premel V, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- **16.Gold M, Cerri S, Smyk-Pearson S, Cansler M, Vogt T, Delepine J, Winata E, Swarbrick G, Chua W, Yu Y, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8:e1000407. doi: 10.1371/journal.pbio.1000407. [15 and 16] These papers identify a novel mucosal T cell and associate this cell with a role in mycobacterial disease in humans.
- **17.Egen JG, Rothfuchs AG, Feng CG, Horwitz MA, Sher A, Germain RN. Intravital imaging reveals limited antigen presentation and T cell effector function in mycobacterial granulomas. Immunity. 2011;34:807–819. doi: 10.1016/j.immuni.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *18.Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog. 2011;7:e1002063. doi: 10.1371/journal.ppat.1002063. [17 and 18] These papers show that T cells are not really performing within the granuloma and that this is associated with antigen availability.
- 19.Ramachandra L, Qu Y, Wang Y, Lewis CJ, Cobb BA, Takatsu K, Boom WH, Dubyak GR, Harding CV. Mycobacterium tuberculosis synergizes with ATP to induce release of microvesicles and exosomes containing Major Histocompatibility Complex Class II molecules capable of antigen presentation. Infect. Immun. 2010;78:5116–5125. doi: 10.1128/IAI.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schreiber HA, Harding JS, Hunt O, Altamirano CJ, Hulseberg PD, Stewart D, Fabry Z, Sandor M. Inflammatory dendritic cells migrate in and out of transplanted chronic mycobacterial granulomas in mice. J. Clin. Invest. 2011;121:3902–3913. doi: 10.1172/JCI45113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, Ernst JD, Gagneux S. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat. Gene. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, Woodland DL. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA. 2010;107:19408–19413. doi: 10.1073/pnas.1006298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohlmeier JE, Reiley WW, Perona-Wright G, Freeman ML, Yager EJ, Connor LM, Brincks EL, Cookenham T, Roberts AD, Burkum CE, et al. Inflammatory chemokine receptors regulate CD8+ T cell contraction and memory generation following infection. J. Exp. Med. 2011;208:1621–1634. doi: 10.1084/jem.20102110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis MM, Rowell E, Shafiani S, Negash A, Urdahl KB, Wilson CB, Way SS. Fidelity of pathogen-specific CD4+ T cells to the Th1 lineage is controlled by exogenous cytokines, interferon-γ expression, and pathogen lifestyle. Cell Host Microbe. 2010;8:163–173. doi: 10.1016/j.chom.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infect. Immun. 2010;78:4187–4194. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, Hua M, Li N, Yao H, Cao X. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-[gamma] Nat. Immunol. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 27.Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, et al. Multifunctional CD4+ T cells correlate with active Mycobacterium tuberculosis infection. Eur. J. Immunol. 2010;40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland JS, de Jong BC, Jeffries DJ, Adetifa IM, Ota MOC. Production of TNF-α, IL-12(p40) and IL-17 can discriminate between active TB disease and latent infection in a West African cohort. PLoS One. 2010;5:e12365. doi: 10.1371/journal.pone.0012365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim B-H, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–721. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- 30.Tiwari S, Choi H-P, Matsuzawa T, Pypaert M, MacMicking JD. Targeting of the GTPase Irgm1 to the phagosomal membrane via PtdIns(3,4)P2 and PtdIns(3,4,5)P3 promotes immunity to mycobacteria. Nat Immunol. 2009;10:907–917. doi: 10.1038/ni.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, MacMicking JD. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012 doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- *32.Ponpuak M, Davis AS, Roberts EA, Delgado MA, Dinkins C, Zhao Z, Virgin HW, Iv, Kyei GB, Johansen T, Vergne I, et al. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010;32:329–341. doi: 10.1016/j.immuni.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *33.Singh SB, Ornatowski W, Vergne I, Naylor J, Delgado M, Roberts E, Ponpuak M, Master S, Pilli M, White E, et al. Human IRGM regulates autophagy and cell-autonomous immunity functions through mitochondria. Nat Cell Biol. 2010;12:1154–1165. doi: 10.1038/ncb2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Fabri M, Stenger S, Shin D-M, Yuk J-M, Liu PT, Realegeno S, Lee H-M, Krutzik SR, Schenk M, Sieling PA, et al. Vitamin D Is Required for IFN-γ-Mediated Antimicrobial Activity of Human Macrophages. Science Translational Medicine. 2011;3:104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Liu PT, Wheelwright M, Teles R, Komisopoulou E, Edfeldt K, Ferguson B, Mehta MD, Vazirnia A, Rea TH, Sarno EN, et al. MicroRNA-21 targets the vitamin D-dependent antimicrobial pathway in leprosy. Nat Med. 2012;18:267–273. doi: 10.1038/nm.2584. [32-35] These studies indentify novel mechanisms whereby human cell limit the survival of Mtb
- 36.Rajaram MVS, Ni B, Morris JD, Brooks MN, Carlson TK, Bakthavachalu B, Schoenberg DR, Torrelles JB, Schlesinger LS. Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2 (MK2) and microRNA miR-125b. Proc. Natl. Acad. Sci. USA. 2011;108:17408–17413. doi: 10.1073/pnas.1112660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, Grant AV, Marchal CC, Hubeau M, Chapgier A, et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. 2011;12:213–221. doi: 10.1038/ni.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar S, Song Y, Sarkar S, Kipen HM, Laumbach RJ, Zhang J, Ohman Strickland PA, Gardner CR, Schwander S. Suppression of the NF-κB pathway by diesel exhaust particles impairs human antimycobacterial Immunity. J. Immunol. 2012;188:2778–2793. doi: 10.4049/jimmunol.1101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *39.Potian JA, Rafi W, Bhatt K, McBride A, Gause WC, Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J. Exp. Med. 2011;208:1863–1874. doi: 10.1084/jem.20091473. This paper carefully addresses a long standing question in TB, whether there is potnential for co-infection to impact specific pathways in control of disease
- 40.Cruz A, Fraga A, Fountain J, Rangel-Moreno J, Torrado E, Saraiva M, Pereira D, Randall T, Pedrosa J, Cooper A, et al. Pathological role of Interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J. Exp. Med. 2010;207:1609–1616. doi: 10.1084/jem.20100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Desvignes L, Ernst JD. Interferon-γ-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974–985. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *42.Nandi B, Behar SM. Regulation of neutrophils by interferon-γ limits lung inflammation during tuberculosis infection. J. Exp. Med. 2011;208:2251–2262. doi: 10.1084/jem.20110919. [41 and 42] Together these papers redfine the role of IFN in control of immunopathology during TB
- **43.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. Outstanding study that changed the way we think about neutrophils and IFN in human TB
- 44.Dorhoi A, Desel C, Yeremeev V, Pradl L, Brinkmann V, Mollenkopf H-J, Hanke K, Gross O, Ruland J, Kaufmann SHE. The adaptor molecule CARD9 is essential for tuberculosis control. J. Exp. Med. 2010;207:777–792. doi: 10.1084/jem.20090067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemos MP, Rhee KY, McKinney JD. Expression of the leptin receptor outside of bone marrow-derived cells regulates tuberculosis control and lung macrophage MHC expression. J. Immunol. 2011;187:3776–3784. doi: 10.4049/jimmunol.1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNab FW, Berry MPR, Graham CM, Bloch SAA, Oni T, Wilkinson KA, Wilkinson RJ, Kon OM, Banchereau J, Chaussabel D, et al. Programmed death ligand 1 is over-expressed by neutrophils in the blood of patients with active tuberculosis. Eur. J. Immunol. 2011;41:1941–1947. doi: 10.1002/eji.201141421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR., Jr. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc. Natl. Acad. Sci. USA. 2010;107:13402–13407. doi: 10.1073/pnas.1007394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barber DL, Mayer-Barber KD, FC G. Sher A: CD4 T cells promote rather than control tuberculosis in the absence of PD-1 mediated inhibition. J. Immunol. 2010;186:1598–1607. doi: 10.4049/jimmunol.1003304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Kane CM, Elkington PT, Jones MD, Caviedes L, Tovar M, Gilman RH, Stamp G, Friedland JS. STAT3, p38 MAPK, and NF-κB drive unopposed monocyte-dependent fibroblast MMP-1 secretion in tuberculosis. Am J Respir Cell Mol Biol. 2010;43:465–474. doi: 10.1165/rcmb.2009-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green JA, Elkington PT, Pennington CJ, Roncaroli F, Dholakia S, Moores RC, Bullen A, Porter JC, Agranoff D, Edwards DR, et al. Mycobacterium tuberculosis upregulates microglial matrix metalloproteinase-1 and -3 expression and secretion via NF-κB- and activator protein-1-dependent monocyte networks. J. Immunol. 2010;184:6492–6503. doi: 10.4049/jimmunol.0903811. [DOI] [PubMed] [Google Scholar]
- **51.Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA, Walker NF, Saraiva L, Pedersen B, Mauri F, Lipman M, et al. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J. Clin. Invest. 2011;121:1827–1833. doi: 10.1172/JCI45666. Identifies a key mediator of tissue damage during mycobacterial infection
- *52.Mayer-Barber K, Barber D, Shenderov K, White S, Wilson MS, Cheever A, Kugler D, Hieny S, Caspar P, Núñez G, et al. Cutting Edge: Caspase-1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **53.Mayer-Barber KD, Andrade BB, Barber DL, Hieny S, Feng CG, Caspar P, Oland S, Gordon S, Sher A. Innate and adaptive interferons suppress IL-1α and IL-1β production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. Two careful pieces of work that begin the process of determining exactly what IL1 is doing during TB
- 54.Jayaraman P, Sada-Ovalle I, Beladi S, Anderson AC, Dardalhon V, Hotta C, Kuchroo VK, Behar SM. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J. Exp. Med. 2010;207:2343–2354. doi: 10.1084/jem.20100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *55.Novikov A, Cardone M, Thompson R, Shenderov K, Kirschman KD, Mayer-Barber KD, Myers TG, Rabin RL, Trinchieri G, Sher A, et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1β production in human macrophages. J. Immunol. 2011;187:2540–2547. doi: 10.4049/jimmunol.1100926. Nice study of the interaction between Mtb and human cells and how this results in altered expression of IL-1
- 56.Horne DJ, Randhawa AK, Chau TTH, Bang ND, Yen NTB, Farrar JJ, Dunstan SJ, Hawn TR. Common polymorphisms in the PKP3-SIGIRR-TMEM16J gene region are associated with susceptibility to tuberculosis. J. Inf. Dis. 2012;205:586–594. doi: 10.1093/infdis/jir785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **57.Aagaard C, Hoang T, Dietrich J, Cardona P-J, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 2011;17:189–194. doi: 10.1038/nm.2285. Important demonstration that delivery of latency associated antigens can promote an altered outcome
- 58.Commandeur S, Lin MY, van Meijgaarden KE, Friggen AH, Franken KLMC, Drijfhout JW, Korsvold GE, Oftung F, Geluk A, Ottenhoff THM. Double- and monofunctional CD4+ and CD8+ T-cell responses to Mycobacterium tuberculosis DosR antigens and peptides in long-term latently infected individuals. Eur. J. Immunol. 2011;41:2925–2936. doi: 10.1002/eji.201141602. [DOI] [PubMed] [Google Scholar]
- 59.Tang ST, van Meijgaarden KE, Caccamo N, Guggino G, Klein MR, van Weeren P, Kazi F, Stryhn A, Zaigler A, Sahin U, et al. Genome-based in silico identification of new Mycobacterium tuberculosis antigens activating polyfunctional CD8+ T cells in human tuberculosis. J. Immunol. 2011;186:1068–1080. doi: 10.4049/jimmunol.1002212. [DOI] [PubMed] [Google Scholar]
- 60.Millington KA, Fortune SM, Low J, Garces A, Hingley-Wilson SM, Wickremasinghe M, Kon OM, Lalvani A. Rv3615c is a highly immunodominant RD1 (Region of Difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA. 2011;108:5730–5735. doi: 10.1073/pnas.1015153108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodworth JS, Shin D, Volman M, Nunes-Alves C, Fortune SM, Behar SM. Mycobacterium tuberculosis directs immunofocusing of CD8+ T cell responses despite vaccination. J. Immunol. 2011;186:1627–1637. doi: 10.4049/jimmunol.1002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Billeskov R, Grandal MV, Poulsen C, Christensen JP, Winter N, Vingsbo-Lundberg C, Hoang TTKT, van Deurs B, Song Y-H, Aagaard C, et al. Difference in TB10.4 T-cell epitope recognition following immunization with recombinant TB10.4, BCG or infection with Mycobacterium tuberculosis. Eur. J. Immunol. 2010;40:1342–1354. doi: 10.1002/eji.200939830. [DOI] [PubMed] [Google Scholar]
- *63.Lalor MK, Floyd S, Gorak-Stolinska P, Ben-Smith A, Weir RE, Smith SG, Newport MJ, Blitz R, Mvula H, Branson K, et al. BCG vaccination induces different cytokine profiles following infant BCG vaccination in the UK and Malawi. J. Inf. Dis. 2011;204:1075–1085. doi: 10.1093/infdis/jir515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *64.Burl S, Adetifa UJ, Cox M, Touray E, Ota MO, Marchant A, Whittle H, McShane H, Rowland-Jones SL, Flanagan KL. Delaying Bacillus Calmette-Guérin vaccination from birth to 4 1/2 months of age reduces postvaccination Th1 and IL-17 responses but leads to comparable mycobacterial responses at 9 months of age. J. Immunol. 2010;185:2620–2628. doi: 10.4049/jimmunol.1000552. [63 and 64]These types of careful studies in vaccinated humans are essential to understanding of how BCG works (and doesn’t work)
- 65.Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M, Kalinski P, Khader SA. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur. J. Immunol. 2012;42:364–373. doi: 10.1002/eji.201141569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sweeney KA, Dao DN, Goldberg MF, Hsu T, Venkataswamy MM, Henao-Tamayo M, Ordway D, Sellers RS, Jain P, Chen B, et al. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat. Med. 2011;17:1261–1268. doi: 10.1038/nm.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nambiar JK, Pinto R, Aguilo JI, Takatsu K, Martin C, Britton WJ, Triccas JA. Protective immunity afforded by attenuated, PhoP-deficient Mycobacterium tuberculosis is associated with sustained generation of CD4+ T-cell memory. Eur. J. Immunol. 2012;42:385–392. doi: 10.1002/eji.201141903. [DOI] [PubMed] [Google Scholar]
- 68.Juárez-Rodríguez MD, Arteaga-Cortés LT, Kader R, Curtiss R, Clark-Curtiss JE. Live attenuated Salmonella vaccines against Mycobacterium tuberculosis with antigen delivery via the type III secretion system. Infect. Immun. 2012;80:798–814. doi: 10.1128/IAI.05525-11. [DOI] [PMC free article] [PubMed] [Google Scholar]