Abstract
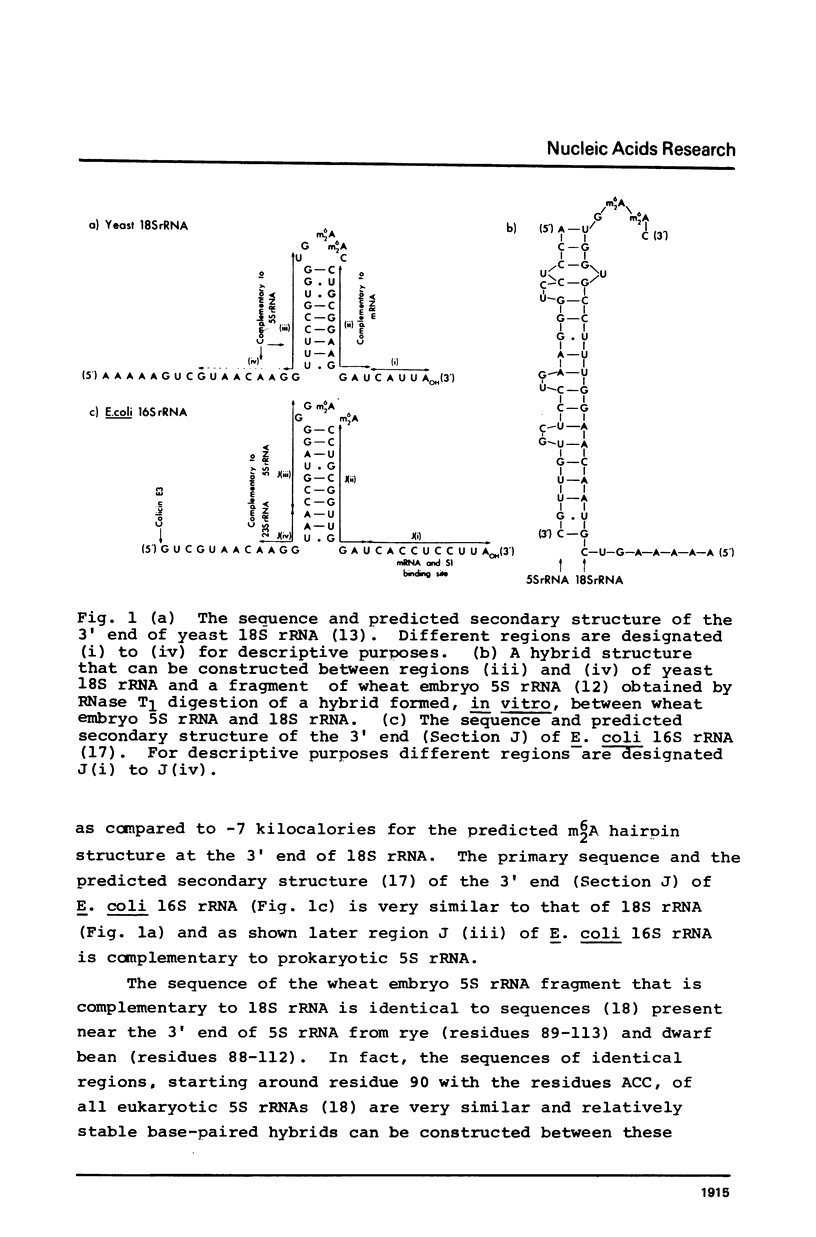
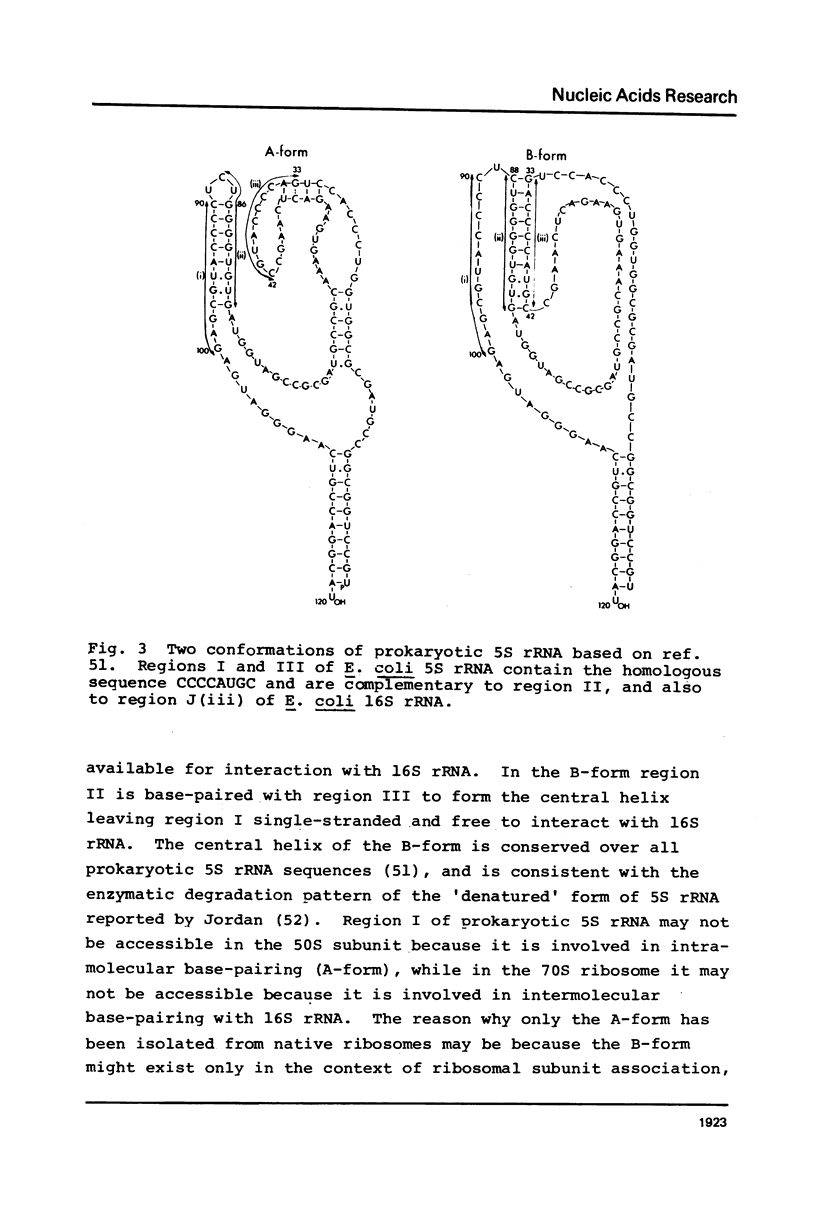
Highly conserved sequences present at an identical position near the 3' ends of eukaryotic and prokaryotic 5S rRNAs are complementary to the 5' strand of the m2(6)A hairpin structure near the 3' ends of 18S rRNA and 16S rRNA, respectively. The extent of base-pairing and the calculated stabilities of the hybrids that can be constructed between 5S rRNAs and the small ribosomal subunit RNAs are greater than most, if not all, RNA-RNA interactions that have been implicated in protein synthesis. The existence of complementary sequences in 5S rRNA and small ribosomal subunit RNA, along with the previous observation that there is very efficient and selective hybridization in vitro between 5S and 18S rRNA, suggests that base-pairing between 5S rRNA in the large ribosomal subunit and 18S (16S) rRNA in the small ribosomal subunit might be involved in the reversible association of ribosomal subunits. Structural and functional evidence supporting this hypothesis is discussed.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty H., Raba M., Gross H. J. Isolation from rat liver and sequence of a RNA fragment containing 32 nucleotides from position 5 to 36 from the 3' end of ribosomal 18S RNA. Nucleic Acids Res. 1978 Feb;5(2):425–434. doi: 10.1093/nar/5.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avadhani N. G., Buetow D. E. A proposed role for 5S ribosomal RNA. Biochem Biophys Res Commun. 1973 Jan 23;50(2):443–451. doi: 10.1016/0006-291x(73)90860-7. [DOI] [PubMed] [Google Scholar]
- Azad A. A., Deacon N. J. Base-paired interaction, in vitro, between hen globin 9S mRNA and eukaryotic ribosomal RNAs. Biochem Biophys Res Commun. 1979 Feb 14;86(3):568–576. doi: 10.1016/0006-291x(79)91751-0. [DOI] [PubMed] [Google Scholar]
- Azad A. A. Hybridization between 5S rRNA and 18S rRNA from barley embryos and mouse sarcoma 180 ascites cells. Biochem Biophys Res Commun. 1978 Jul 14;83(1):259–265. doi: 10.1016/0006-291x(78)90425-4. [DOI] [PubMed] [Google Scholar]
- Azad A. A., Lane B. G. A possible role for 5 S rRNA as a bridge between ribosomal subunits. Can J Biochem. 1973 Dec;51(12):1669–1672. doi: 10.1139/o73-224. [DOI] [PubMed] [Google Scholar]
- Azad A. A., Lane B. G. Wheat-embryo ribonucleates. IV. Factors that influence the formation and stability of a complex between 5S rRNA and 18S rRNA. Can J Biochem. 1975 Mar;53(3):320–327. doi: 10.1139/o75-045. [DOI] [PubMed] [Google Scholar]
- Baan R. A., Hilbers C. W., Van Charldorp R., Van Leerdam E., Van Knippenberg P. H., Bosch L. High-resolution proton magnetic resonance study of the secondary structure of the 3'-terminal 49-nucleotide fragment of 16S rRNA from Escherichia coli. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1028–1031. doi: 10.1073/pnas.74.3.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baan R. A., Naaktgeboren N., van Charldorp R., van Knippenberg P. H., Bosch L. Consequences of a specific cleavage in situ of 16-S ribosomal RNA for polypeptide chain initiation. Eur J Biochem. 1978 Jun 1;87(1):131–136. doi: 10.1111/j.1432-1033.1978.tb12358.x. [DOI] [PubMed] [Google Scholar]
- Bellemare G., Jordan B. R., Rocca-Serra J., Monier R. Accessibility of Escherichia coli 5S RNA base residues to chemical reagents. Influence of chemical alterations on the affinity of 5S RNA for the 50S subunit structure. Biochimie. 1972;54(11):1453–1466. doi: 10.1016/s0300-9084(72)80087-7. [DOI] [PubMed] [Google Scholar]
- Bowman C. M., Sidikaro J., Nomura M. Specific inactivation of ribosomes by colicin E3 in vitro and mechanism of immunity in colicinogenic cells. Nat New Biol. 1971 Dec 1;234(48):133–137. doi: 10.1038/newbio234133a0. [DOI] [PubMed] [Google Scholar]
- Carbon P., Ehresmann C., Ehresmann B., Ebel J. P. The sequence of Escherichia coli ribosomal 16 S RNA determined by new rapid gel methods. FEBS Lett. 1978 Oct 1;94(1):152–156. doi: 10.1016/0014-5793(78)80926-0. [DOI] [PubMed] [Google Scholar]
- Chapman N. M., Noller H. F. Protection of specific sites in 16 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1977 Jan 5;109(1):131–149. doi: 10.1016/s0022-2836(77)80049-1. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., Kurland C. G., Stöffler G. 30S ribosomal proteins associated with the 3'-terminus of 16S RNA. FEBS Lett. 1975 Oct 15;58(1):281–284. doi: 10.1016/0014-5793(75)80279-1. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dahlberg J. E. Binding of ribosomal protein S1 of Escherichia coli to the 3' end of 16S rRNA. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2940–2944. doi: 10.1073/pnas.72.8.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgarno L., Shine J. Conserved terminal sequence in 18SrRNA may represent terminator anticodons. Nat New Biol. 1973 Oct 31;245(148):261–262. doi: 10.1038/newbio245261a0. [DOI] [PubMed] [Google Scholar]
- De Jonge P., Klootwijk J., Planta R. J. Sequence of the 3'-terminal 21 nucleotides of yeast 17S ribosomal RNA. Nucleic Acids Res. 1977 Oct;4(10):3655–3663. doi: 10.1093/nar/4.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delihas N., Dunn J. J., Erdmann V. A. The reaction of 5S RNA in 70S ribosomes with kethoxal. FEBS Lett. 1975 Oct 15;58(1):76–80. doi: 10.1016/0014-5793(75)80229-8. [DOI] [PubMed] [Google Scholar]
- Dube S. K. Recognition of tRNA by the ribosome. A possible role of 5 S RNA. FEBS Lett. 1973 Oct 1;36(1):39–42. doi: 10.1016/0014-5793(73)80332-1. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Mackie G. A., Zimmermann R. A., Ebel J. P., Fellner P. Primary sequence of the 16S ribosomal RNA of Escherichia coli. Nucleic Acids Res. 1975 Feb;2(2):265–278. doi: 10.1093/nar/2.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A. Collection of published 5S and 5.8S ribosomal RNA sequences. Nucleic Acids Res. 1978 Jan;5(1):r1–r13. [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A., Fahnestock S., Higo K., Nomura M. Role of 5S RNA in the functions of 50S ribosomal subunits. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2932–2936. doi: 10.1073/pnas.68.12.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A., Sprinzl M., Pongs O. The involvement of 5S RNA in the binding of tRNA to ribosomes. Biochem Biophys Res Commun. 1973 Oct 1;54(3):942–948. doi: 10.1016/0006-291x(73)90785-7. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Woese C. R. 5S RNA secondary structure. Nature. 1975 Aug 7;256(5517):505–507. doi: 10.1038/256505a0. [DOI] [PubMed] [Google Scholar]
- Fox J. W., Wong K. P. Changes in the conformation and stability of 5 S RNA upon the binding of ribosomal proteins. J Biol Chem. 1978 Jan 10;253(1):18–20. [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Change in methylation of 16S ribosomal RNA associated with mutation to kasugamycin resistance in Escherichia coli. Nat New Biol. 1971 Sep 1;233(35):12–14. doi: 10.1038/newbio233012a0. [DOI] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- Herr W., Chapman N. M., Noller H. F. Mechanism of ribosomal subunit association: discrimination of specific sites in 16 S RNA essential for association activity. J Mol Biol. 1979 Jun 5;130(4):433–449. doi: 10.1016/0022-2836(79)90433-9. [DOI] [PubMed] [Google Scholar]
- Herr W., Noller H. F. Protection of specific sites in 23 S and 5 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1979 Jun 5;130(4):421–432. doi: 10.1016/0022-2836(79)90432-7. [DOI] [PubMed] [Google Scholar]
- Hochkeppel H. K., Craven G. R. Significant changes in 16 S RNA conformation accompanying assembly of the 30 S ribosome in vitro. J Mol Biol. 1977 Jul 15;113(4):623–634. doi: 10.1016/0022-2836(77)90226-1. [DOI] [PubMed] [Google Scholar]
- Jordan B. R. Studies on 5 s RNA conformation by partial ribonuclease hydrolysis. J Mol Biol. 1971 Feb 14;55(3):423–439. doi: 10.1016/0022-2836(71)90327-5. [DOI] [PubMed] [Google Scholar]
- Kolb A., Hermoso J. M., Thomas J. O., Szer W. Nucleic acid helix-unwinding properties of ribosomal protein S1 and the role of S1 in mRNA binding to ribosomes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2379–2383. doi: 10.1073/pnas.74.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebbing D., Liarakos C. D. Nucleotide sequence at the 5' end of ovalbumin messenger RNA from chicken. Nucleic Acids Res. 1978 Jul;5(7):2253–2266. doi: 10.1093/nar/5.7.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter L. C., Zeichhardt H., Kurland C. G. Ribosomal protein neighborhoods. I. S18 and S21 as well as S5 and S8 are neighbors. Mol Gen Genet. 1972;119(4):357–366. [PubMed] [Google Scholar]
- Nichols J. L., Wijesinghe W. Identification of the 5S RNA binding site in intermolecular complexes of wheat embryo ribosomal 5S and 18S RNA. Can J Biochem. 1978 Jul;56(7):760–764. doi: 10.1139/o78-114. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Herr W. Letters to the editor: Accessibility of 5 S RNA in 50 S ribosomal subunits. J Mol Biol. 1974 Nov 25;90(1):181–184. doi: 10.1016/0022-2836(74)90266-6. [DOI] [PubMed] [Google Scholar]
- Oakden K. M., Azad A. A., Lane B. G. Wheat embryo ribonucleates. VII. Rapid, efficient and selective formation of 5S-18S and 5.8S-26S hybrids in an aqueous solution of the four ribosomal polynucleotides, and the results of a search for the corresponding hybrids in wheat embryo ribosomes. Can J Biochem. 1977 Jan;55(1):99–109. doi: 10.1139/o77-016. [DOI] [PubMed] [Google Scholar]
- Payne P. I., Dyer T. A. Evidence for the nucleotide sequence of 5-S rRNA from the flowering plant Secale cereale (Rye). Eur J Biochem. 1976 Dec;71(1):33–38. doi: 10.1111/j.1432-1033.1976.tb11086.x. [DOI] [PubMed] [Google Scholar]
- Politz S. M., Glitz D. G. Ribosome structure: localization of N6,N6-dimethyladenosine by electron microscopy of a ribosome-antibody complex. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1468–1472. doi: 10.1073/pnas.74.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards E. G. 5S RNA. An analysis of possible base pairing schemes. Eur J Biochem. 1969 Aug;10(1):36–42. doi: 10.1111/j.1432-1033.1969.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Santer M., Shane S. Area of 16S ribonucleic acid at or near the interface between 30S and 50S ribosomes of Escherichia coli. J Bacteriol. 1977 May;130(2):900–910. doi: 10.1128/jb.130.2.900-910.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J. P., Hsiung N., Cantor C. R. Fluorescence studies of the accessibility of the 3' ends of the ribosomal RNAs in Escherichia coli ribosomes and subunits. Nucleic Acids Res. 1979 Jan;6(1):181–193. doi: 10.1093/nar/6.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior B. W., Holland I. B. Effect of colicin E3 upon the 30S ribosomal subunit of Escherichia coli. Proc Natl Acad Sci U S A. 1971 May;68(5):959–963. doi: 10.1073/pnas.68.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A. R., Davis B. D. Activity of initiation factor F3 in dissociating Escherichia coli ribosomes. Nature. 1970 Dec 26;228(5278):1273–1275. doi: 10.1038/2281273a0. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Colicin E3 inhibits rabbit globin synthesis. FEBS Lett. 1978 May 1;89(1):121–125. doi: 10.1016/0014-5793(78)80536-5. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Wallace B. J., Davis B. D. Actions of aurintricarboxylate, kasugamycin, and pactamycin on Escherichia coli polysomes. Biochemistry. 1973 Feb;12(4):616–620. doi: 10.1021/bi00728a008. [DOI] [PubMed] [Google Scholar]
- Thammana P., Cantor C. R. Studies on ribosome structure and interactions near the m62Am62A sequence. Nucleic Acids Res. 1978 Mar;5(3):805–823. doi: 10.1093/nar/5.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnowsky F., Drews J., Eich F., Högenauer G. In vitro inactivation of ascites ribosomes by colicin E 3. Biochem Biophys Res Commun. 1973 May 1;52(1):327–334. doi: 10.1016/0006-291x(73)90991-1. [DOI] [PubMed] [Google Scholar]
- Van Duin J., Kurland C. G., Dondon J., Grunberg-Mangago M., Branlant C., Ebel J. P. New aspects of the IF3-ribosome interaction. FEBS Lett. 1976 Feb 15;62(2):111–114. doi: 10.1016/0014-5793(76)80030-0. [DOI] [PubMed] [Google Scholar]
- Vigne R., Jordan B. R. Partial enzyme digestion studies on Escherichia coli, Pseudomonas, Chlorella, Drosophila, HeLa and yeast 5S RNAs support a general class of 5S RNA models. J Mol Evol. 1977 Sep 20;10(1):77–86. doi: 10.1007/BF01796136. [DOI] [PubMed] [Google Scholar]
- Weidner H., Yuan R., Crothers D. M. Does 5S RNA function by a switch between two secondary structures? Nature. 1977 Mar 10;266(5598):193–194. doi: 10.1038/266193a0. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E., Zablen L., Uchida T., Bonen L., Pechman K., Lewis B. J., Stahl D. Conservation of primary structure in 16S ribosomal RNA. Nature. 1975 Mar 6;254(5495):83–86. doi: 10.1038/254083a0. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H. Reticulocyte 5 S ribosomal RNA inhibition of cell-free protein synthesis. Novel responses in ribosomal behaviour. FEBS Lett. 1978 Oct 1;94(1):145–151. doi: 10.1016/0014-5793(78)80925-9. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H. The role of ribosomal RNA in protein synthesis. Inhibition of translation by reticulocyte 5 S ribosomal RNA. FEBS Lett. 1978 Oct 1;94(1):139–144. doi: 10.1016/0014-5793(78)80924-7. [DOI] [PubMed] [Google Scholar]
- Yu R. S., Wittmann H. G. The structural basis for functional inactivity of reconstituted 50-S ribosomal subunits of Escherichia coli. Biochim Biophys Acta. 1973 Sep 7;319(3):388–400. doi: 10.1016/0005-2787(73)90179-2. [DOI] [PubMed] [Google Scholar]
- Zimmermann R. A., Mackie G. A., Muto A., Garrett R. A., Ungewickell E., Ehresmann C., Stiegler P., Ebel J. P., Fellner P. Location and characteristics of ribosomal protein binding sites in the 16S RNA of Escherichia coli. Nucleic Acids Res. 1975 Feb;2(2):279–302. doi: 10.1093/nar/2.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duin J., Kurland C. G., Dondon J., Grunberg-Manago M. Near neighbors of IF3 bound to 30S ribosomal subunits. FEBS Lett. 1975 Nov 15;59(2):287–290. doi: 10.1016/0014-5793(75)80394-2. [DOI] [PubMed] [Google Scholar]
- van Knippenberg P. H. A possible role of the 5' terminal sequence of 16S ribosomal RNA in the recognition of initiation sequences for protein synthesis. Nucleic Acids Res. 1975 Jan;2(1):79–85. doi: 10.1093/nar/2.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]


