Abstract
Head and neck cancer is a devastating disease that afflicts many individuals worldwide. Conventional therapies are successful in only a limited subgroup and often leave the patient with disfigurement and long lasting adverse effects on normal physiological functions. The field is in dire need of new therapies. Oncolytic viral as well as targeted therapies have shown some success in other malignancies and are attractive for the treatment of head and neck cancer. Recently, it has been shown that a subset of head and neck cancers is human papillomavirus (HPV) positive and that this subset of cancers is biologically distinct and more sensitive to chemoradiation therapies although the underlying mechanism is unclear. However, chemoresistance remains a general problem. One candidate mediator of therapeutic response, which is of interest for the targeting of both HPV-positive and -negative tumors is the human DEK proto-oncogene. DEK is upregulated in numerous tumors including head and neck cancers regardless of their HPV status. Depletion of DEK in tumor cells in culture results in sensitivity to genotoxic agents, particularly in rapidly proliferating cells. This suggests that tumors with high DEK protein expression may be correlated with poor clinical response to clastogenic therapies. Targeting molecules such as DEK in combination with new and/or conventional therapies, holds promise for novel future therapeutics for head and neck cancer.
Introduction
Head and Neck Cancer (HNC) is the sixth most common cancer worldwide and accounts for approximately 350,000 deaths per year (1, 2). Approximately 90% of HNCs are squamous cell carcinomas (HNSCCs). HNSCC continues to be difficult to treat and conventional treatment strategies result in a plethora of side effects that affect normal physiological functions including speech, swallowing and physical appearance. Moreover, aggressive therapy is often difficult secondary to co-morbidities common in HNSCC patients (3). Currently, treatment is based on site of disease and degree of invasion and metastases. Approximately 60% of HNSCC patients have locally advanced disease at presentation (4). Locally advanced disease is challenging to treat and often results in a high relapse rate. The decision to perform surgery vs. combined radiation and chemotherapy in this disease subset continues to be debated but often requires a multidisciplinary approach. Most standard treatment strategies include surgery, radiation and chemotherapy with or without biological and targeted therapies that require collaboration between several specialists. Even with these multimodality approaches, locally advanced disease is particularly difficult to cure. With current combination therapies, 35–55% of patients will develop locoregional or metastatic recurrence within two years. These statistics highlight the need for novel approaches for HNSCC treatment and a deeper understanding of disease pathogenesis.
Traditionally, HNC has been linked to tobacco and alcohol use. Recent developments have now also identified HPV infection as an etiologic contributor to a subset of HNC. HPV is highly prevalent in the US population. Seventy-five to eighty percent of American females are thought to become infected with HPV at some point before the age of 50 (5). Cervical cancer is a well known result of infection with high risk HPVs, most commonly types 16, 18 and 31. The viral DNA of high risk HPV subtypes is found integrated into the host genome in greater than 90% of cervical cancers; however, it is now known that viral DNA integration is not an absolute requirement for malignancy (6, 7). Interestingly, there is mounting evidence that HPV is also prevalent in HNC. Some reports have estimated that greater than 50% of oropharyngeal cancers are positive for HPV (8). In contrast to the multiple HPV types found in cervical cancer, HNC is almost exclusively associated with HPV16 which is present in 90–95% of cases (9). These findings, which indicate that HPV infection is associated with a sizable percentage of HNCs, may explain the increasing occurrence of HNC in young individuals who do not have classic risk factors of smoking and alcohol consumption but rather engage in sexual behaviors that put them at increased risk for HPV infection (10). Interestingly, HPV positive HNSCCs carry favorable clinical prognoses and are more sensitive to chemotherapy and radiation (11, 12). The mechanism underlying the increased sensitivity of HPV positive tumors to treatment remains poorly understood. In addition, new, multimodal treatments to improve patient survival for HPV negative (and positive) tumors and quality of life for all HNSCC patients remain as urgent as ever.
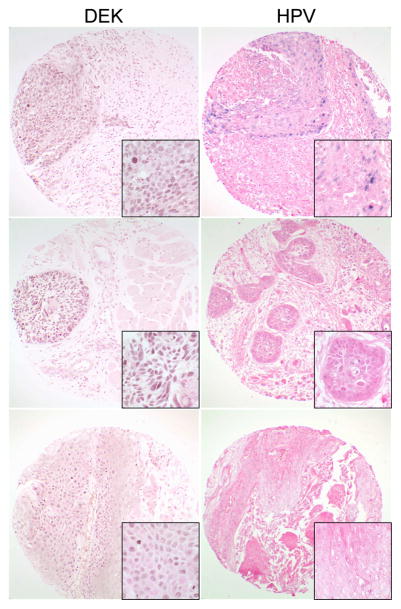
One attractive therapeutic target for HNC regardless of HPV status is the human DEK oncogene. DEK was initially discovered as a fusion protein, DEK-CAN, in acute myeloid leukemia (13). Since its discovery, DEK has been shown to be upregulated in several human tumors (14–20). DEK is an inhibitor of cell death and proliferation, and the knockdown of DEK results in apoptosis and senescence in cancer cells in culture and in preclinical animal models (21, 22). DEK is upregulated by the HPV E7 oncogene in cultured cells and in the epithelium of HPV16 E7 transgenic mice (22). Overexpression of DEK was recently shown to be a poor prognostic indicator in lung neuroendrocrine tumors (17). However, the potential of DEK as a possible therapeutic target has yet to be explored. Preliminary studies demonstrate DEK expression in 28/44 (64%) of HNC including 3/4 (75%) HPV positive tumors as assessed by immunohistochemistry on tissue microarrays from the head and neck cancer tissue array initiative which has been previously described (23) (see Figure 1). DEK staining was detected in the majority of tumor cells (>50%) in 27/28 (96%) cases with the staining intensity ranging from weak (12/28 (43%)) to strong (16/26 (57%)). In addition, DEK was detected in HNSCC from various sites including the oral cavity, oropharynx, larynx, salivary glands, nasal cavity/sinuses, and metastatic lymph nodes. Together, these data provide evidence that DEK is expressed in the majority of HNC and that DEK targeted therapy provides a potential novel approach to HNSCC treatment regardless of HPV status. Studies are currently ongoing to extend these analyses to a larger tumor database, correlate DEK expression with clinical parameters and determine the physiologic significance of DEK upregulation in HNC. Developing novel treatment strategies which target potential oncogenic candidates such as DEK will be paramount for improving therapeutic outcomes for patients with HNSCC.
Figure 1.
DEK expression in HPV positive and negative HNSCCs. DEK protein was detected in the majority of HNSCCs by immunohistochemistry on tissue microarrays from the head and neck cancer tissue array initiative (brown, left panel). Although several countries were represented in this tissue array, only tissues from the United States were used for this study. Sites included in this subset are as follows: Oral cavity, oropharynx, larynx, salivary glands, nasal cavity/sinuses, and metastatic lymph nodes. DEK staining patterns included strong diffuse staining (top and middle) and weak less uniform staining (bottom). DEK staining patterns and intensity were not associated with any specific site. HPV status was determined by in-situ hybridization using the Ventana system (blue, right panel). DEK expression was detected in both HPV positive (top) and negative (middle and bottom) HNSCCs. Original magnification: 200x, insets = 1000x.
Conventional treatment
As mentioned above, the treatment of locally advanced HNSCC often requires a multidisciplinary approach (24). Surgery plays an important role in early stage tumors, locally advanced oral cavity and laryngeal tumors, and as salvage therapy for tumor recurrence after radiotherapy. Surgery however is often disfiguring for the patient and may have undesirable effects including disruption of normal physiological functions. Surgical approaches have been developed which limit undesirable consequences. For instance, laryngeal preservation strategies have been shown to preserve natural speech without compromising survival. Laser-based procedures and transoral robotic surgery also serve to preserve natural physiological function and prevent disfigurement. Despite these advances however, novel treatment modalities will be critical for the prevention of recurrence while limiting side effects.
Locally advanced disease often requires radiation therapy. Conventional radiation therapy consists of 2 Gy/day given over single fraction 5 days/week for 7 weeks for a total of 70 Gy. Unfortunately, not all patients are able to complete a full course of radiation therapy because of the toxicity associated with the treatment. Examples of toxic complications include odynophagia, mucositis, xerostomia, and dysphagia requiring PEG tube placement. Other methods including altered fractionated schedules were developed in an effort to improve outcomes without worsening long term toxicity. Accelerated fractionation allows a reduction in the total treatment time and potentially inhibits cell repopulation between doses and has been shown to prolong overall survival (OS), decrease cancer related death, and improve locoregional control (25). Hyperfractionation is performed by administering two reduced-dose fractions per day to increase the total overall dose. The overall survival benefit was higher with hyperfractionation as compared to traditional approaches (25, 26). Intensity-modulated RT (IMRT) is a technique that focuses treatment to the tumor while avoiding surrounding viable tissue. This approach has been hypothesized to reduce late-toxicity effects with similar OS compared to the accelerated fractionation approach (27). IMRT is challenging however, and requires sophisticated techniques (24).
Chemotherapy in HNSCC is often limited to more advanced disease and is used either as combined with radiation as a chemo-sensitizing agent, adjuvant therapy, induction therapy or, in late stages, for palliation. Chemotherapy in conjunction with radiation (CRT) is recommended for patients that are at high risk for recurrence including those that have positive margins after surgery or extracapsular lymph node extension (28). It is also used in combination with radiation for locally advanced disease. Although chemotherapy in combination with radiation often improves locoregional control and OS and has become the standard of care, the incidence of side effects, including mucositis and xerostomia, is much higher than either treatment alone (29). The most commonly used chemotherapeutic agents are 5-fluorouracil (5-FU) and the platinum based drugs, carboplatin or cisplatin. However, in recurrent and metastatic disease taxol-based therapies and irinotecan are common options as well. All of these agents have a myriad of side effects including nausea and vomiting, neutropenia with increased rate of infection, diarrhea and renal failure.
Molecular target-based treatments
Because of the higher incidence of adverse outcomes with chemoradiation, other agents including biologics have been examined in clinical trials for HNSCC. The epidermal growth factor receptor (EGFR) is often expressed in HNSCC and its expression is associated with a poor outcome (30). Cetuximab, a monoclonal antibody targeted against EGFR, has been the most widely studied in HNSCC. In combination with radiotherapy, cetuximab offers greater locoregional control compared to radiotherapy alone in locally advanced tumors (31). In recurrent and metastatic disease, single agent cisplatin or carboplatin are often the first line treatment. The Eastern Cooperative Oncology Group study showed that when cetuximab was added to cisplatin in recurrent or metastatic disease, there was an increase in progression free survival, PFS, (4.2 vs. 2.7 months) and OS (8 vs. 9.2 months). However this difference was not statistically significant (32). Interestingly, the latter study showed that tumors with more than 80% of tumor cells positive had a lower response rate to cetuximab than those tumors that had less than 80% EGFR expression. This may be due to the inability of cetuximab to saturate the high density of receptors present in the former, but not the latter, tumors. The addition of cetuximab to 5-FU plus platin-based therapy has been shown to prolong the OS from 7.4 to 10.1 months without much added toxicity, although there was a statistically significant increase in sepsis (33). Currently there is no published data that directly compares combined chemoradiaton with combined cetuximab-radiation in locally advanced disease. The RTOG 0522 phase III study is investigating this question and the results of this study will be extremely important for the future treatment of this group of patients. These trials exemplify promise for targeted therapies in the treatment of HNSCC.
Although the addition of cetuximab to traditional therapies for HNSCC has been promising, it is known that many tumors ultimately become resistant to anti-EGFR therapy. Other anti-EGFR therapies including monoclonal antibodies such as zalutumumab, panitumumab and nimotuzumab, and Tyrosine Kinase Inhibitors (TKIs) which bind EGFR intracellularly inhibiting phosphorylation of downstream targets, are currently being investigated in clinical trials (for review see (34)). Unfortunately, most TKI studies have not shown significant benefit in survival or local control compared to chemoradiation alone thus far.
In order to overcome EGFR resistance, it will likely be important to target downstream and parallel pathways. Novel human epidermal receptor (HER) inhibitors have been developed including dual (HER-1/HER-2) and pan-HER inhibitors. Lapatinib is a dual EFGR (HER-1) and HER-2 inhibitor which has shown promise in combination with cisplatin and RT in a small study of locally advanced HNSCCs with an overall response rate of 81% (35). Randomized phase III studies have resulted and are currently ongoing. VEGF inhibitors including bevacizumab have also been studied in HNSCCs. Bevacizumab in combination with 5-FU, Hydroxyurea and RT was investigated in a phase II trial but unfortunately was discontinued early because of unexpected locoregional progression (36). Sunitinib was tested in recurrent and metastatic HNSCC but resulted in life-threatening bleeding suggesting that VEGF inhibitors need to be used with caution (37). Sorafenib was used as a single agent in two phase II studies with only modest response (38, 39). Combinations with other conventional and targeted treatments remain to be done.
Proteosome inhibitors such as bortezomib have also been examined recently in HNSCC. These act on many pathways including nuclear factor-κB (NF-κB) resulting in anti-tumor activities. A small phase II study of 21 patients showed that this agent is well tolerated in combination with docetaxel in patients with recurrent or metastatic disease (40). Unfortunately the study was ended early due to a response rate that was lower than historical controls with docetaxel. Other clinical trials such as the ECOG trial with irinotecan and bortezomib will be important to evaluate the benefit of proteasome inhibitors when combined with other regimens.
Newer agents are in high demand in order to improve outcomes with reduced toxicity. Recent whole-exon sequencing studies identified known and novel genetic alterations in HNSCC (41, 42). Sequence information from HNSCC tumor cell DNA was compared with that from normal cells of the same patient. Mutations in genes such as p53 and Notch1 were identified which highlight a distinct set of proteins with broad functions in the suppression of HNSCC. These discoveries may yield insights into novel biomarkers, and will spur molecular studies to define therapeutically relevant oncogene activating and tumor suppressor inactivating pathways in HNSCC (43).
This genome-wide DNA sequencing effort also revealed non-overlapping activating mutations in PIK3CA and Ras, and inactivating mutations in PTEN (41, 42). These genetic alterations are all expected to converge on the activation of the Akt-mTOR pathway. Interestingly, there is remarkable activation of this signaling route in HNSCC, as judged by immunohistochemical analysis on HNSCC tissue microarrays using antibodies detecting the phosphorylated forms of Akt and ribosomal protein S6, which act upstream and downstream, respectively, from mTOR (23). The mTOR-regulated molecular network coordinates mitogenic signaling with nutrient-sensing pathways thereby controlling cell proliferation, and emerging information suggests that activation of mTOR represents one of the most frequent events in cancer (44). Extensive studies using HNSCC tumor xenografts in mice, as well as genetically-defined and chemically-induced HNSCC experimental cancer models support the pre-clinical efficacy of mTOR inhibitors for HNSCC (45–47). These studies provided the rationale for multiple open clinical trials in HNSCC using the mTOR allosteric inhibitors RAD-001 (everolimus), rapamycin (serolimus) and the rapamycin pro-drug, CCI-779 (temsirolimus), as single agents and as part of combination therapies. Currently there are ten open trials investigating mTOR inhibitors in HNSCC including NCT01195922 at the National Institute of Health which is investigating rapamycin in the neoadjuvant setting. The targeting of the mTOR pathway either alone or in combination with conventional and other novel therapies is promising and the outcome of these trials will be essential in guiding the future treatment of HNSCC.
Oncolytic viruses in the treatment of head and neck cancer
Previous studies have used replication-incompetent viruses genetically engineered to express a therapeutic gene that are successful in vitro but unfortunately, are not efficacious in vivo due to their inability to target every cancer cell (48). Oncolytic viruses are able to overcome this limitation: they are genetically engineered to facilitate viral replication specifically in tumor cells. This allows selective replication and killing of malignant cells without detriment to surrounding normal bystanders. Further modifications of these viruses are ongoing to better target specific tumors and tumor populations.
Oncolytic adenoviruses
Oncolytic adenoviruses are cytolytic in nature causing cell death of their natural host. Several oncolytic adenoviruses have been developed that selectively replicate in tumor cells. Modifications conferring tumor-selective replication include deletion of the p300/CBP-binding or the pRB-binding region of E1A (resulting in selective killing of tumor cells with defects in the Rb pathway), or deletion of partial or the entire E1B 55K gene resulting in the loss of p53 degradation and late viral RNA export (49–53). Treatment with different oncolytic viruses revealed variable responses on HNSCC cells in culture, with some viruses being less potent than the parent virus Ad5 (54). Capsid-modified oncolytic adenovirus, Ad5/3-delta24, was effective in inhibiting growth of laryngeal squamous cell carcinoma cells in vitro and in a xenograft model in vivo (55). Synergistic effects between this virus and conventional treatment with cisplatin and 5-flurouracil (5-FU) or radiation were achieved. In contrast, cetuximab had varying effects when combined with viral infection in these studies. The most impressive element of the study was that viral infection in combination with cetuximab, chemotherapy and radiation completely prevented tumor relapse in xenografts whereas this response was not achieved with viral infection plus cetuximab alone. The contribution of viral infection to the dramatic response was however difficult to discern since individual therapies were not studied separately. Oncolytic viruses were further genetically engineered to express a FCU1 protein which allows for selective conversion of 5-fluorocytosine to the active drug 5-fluorouridine in tumor cells (56). Increased apoptosis was observed in a laryngeal HNSCC xenograft model despite decreased viral DNA replication in the tumors. This suggests 5-FU effects can be limited to the tumor for added specificity.
Oncolytic adenoviruses have been examined in recurrent HNSCC in patients and had detectable but modest anti-tumoral activity. A small phase II study examined the oncolytic adenovirus, ONYX-015, as monotherapy in recurrent HNSCC either by single injections over five days or hyperfractioned injections over two days (57). This study found that this virus was safe with only mild toxicity (fever and injection site pain) and that hyperfractionation was beneficial for response. A Phase I study of the oncolytic adenovirus, KH901, which conditionally replicates in telomerase-positive cells and overexpresses GM-CSF showed that it was safe when administered to recurrent HNSCC by intratumoral injection (58). Resulting studies to determine the efficacy of oncolytic adenoviruses as anti-cancer treatments are ongoing.
Oncolytic herpes simplex viruses (HSV)
Oncolytic HSVs in combination with conventional therapies are already being pursued in clinical trials. HSV1716 is modified by a mutation in the RL1 gene encoding ICP 34.5 which co-opts ras signaling and allows replication in actively dividing, but not terminally differentiated cells. This virus has been tested for efficacy in head and neck cancer. HSV1716 alone and in combination with chemotherapy caused cytolysis and viral replication in HNSCC cell lines in vitro. However, when used at a low dose of 105 plaque forming units (PFU), there was no effect on viral burst in human oral surgical specimens (59). In the latter study, virus was injected into oral tumors 48–72 hours prior to scheduled surgical resection. Viral replication was not observed, but there were also no deleterious side effects noted in the patients. It will be important to test these viruses at higher titers to determine efficacy and side effects.
In addition to ICP34.5 gene deletion, viruses with a deletion of ICP47 are being tested for tumor-selective viral replication and cell death. ICP47 expression blocks antigen presentation of MHC class I and II molecules via inhibition of the TAP1 and TAP2 transporters. These viruses are now further engineered to express genes which maximize cell killing abilities. One virus that is currently in clinical trials for Stage III/IV HNSCC in combination with radiation and chemotherapy is the JS1/34.5-/47-/GM-CSF which has the additional proposed function of immune activation to allow for tumor cell killing (60). This type of virus had shown promise in clinical studies in other tumors including subcutaneous squamous cell skin cancers and melanoma. This phase I/II study was designed to test safety and clinical activity and was performed by injecting the virus into metastatic cervical lymph nodes (the primary site for the metastases were HNSCC of the oropharynx) using a dose escalation schedule. Almost 50% of the patients had HPV positive disease. The patients were treated with conventional chemotherapy which included cisplatin for 3 cycles and 70 Gy radiation therapy over 7 weeks. Viral injections were performed concurrently with each cycle of cisplatin with an additional viral injection 2 weeks after the final cisplatin dose. Some individuals had a complete response after only 3 viral injections. The patients were then subjected to neck dissection at the completion of treatment. Several patients had adverse events, the majority of which were likely due to the chemotherapy. Pyrexia and fatigue were the only side effects considered to be related to viral infection. At the end of treatment, CT scanning showed a complete response in 23.5% of patients and a partial response in 58.8% of patients. All patients remained free of locoregional disease at the end of the study with a 70.5% OS and 82.4% disease-specific survival. These outcomes are clearly superior to other studies using radiation and chemotherapy alone (29, 61). These studies are promising for future investigation of genetically engineered viruses to stimulate immunity or tumor suppressor functions, or to target specific oncogenes with roles in HNC development and dissemination. However, there are many obstacles in utilizing oncolytic viruses for cancer treatment in humans (review (62)). Currently, oncolytic viruses in HNSCC are delivered intra-tumorally because intravenous administration often results in neutralizing antibodies and clearance of the virus. Although immune responses are thought to be critical for tumor clearance this is an obvious hindrance for viral replication. Plasmapharesis to clear anti-viral antibodies and the use of viral backbones that are not as immunogenic have been proposed to overcome this obstacle. Further understanding on manipulating the human immune system to the patient’s advantage will be paramount in the feasibility of these therapies in the future.
DEK oncogene addiction may be exploited for cancer therapy
The human DEK gene encodes a cellular molecule that is important for genome integrity, survival and proliferation, and is frequently transcriptionally upregulated in human tumors including HNC (17, 63–67). DEK is upregulated by human papillomavirus (HPV) E7 through Rb dependent pathways and has further been shown to be a direct target of E2F (22, 65). DEK inhibits apoptosis, senescence and differentiation depending upon the model system chosen, and DEK overexpression stimulates tumorigenesis in vitro and in vivo thus unequivocally defining this molecule as an oncogene (17, 18, 21, 22, 66, 68). Recent studies have demonstrated an additional role for DEK in DNA damage repair by promoting non-homologous end joining. Accordingly, DEK depletion resulted in increased DNA damage marked by increased γH2AX expression (69). This translated into increased cell death likely through DNA damage pathways, which was further exacerbated by several commonly used chemotherapeutics including hydroxyurea, camptothecin, chlorambucil, etoposide, cisplatin, and doxorubicin (16, 18, 69, 70). DEK knockdown in human keratinocytes and HPV positive cancer cells resulted in p53 dependent apoptosis (21). This effect was not seen in differentiated primary epithelial cells suggesting that apoptosis in response to DEK depletion was specific to rapidly dividing cells (68). Because of the broad overexpression and oncogenic activity of DEK in many human tumors, it has been proposed that DEK is an attractive target for novel cancer therapies (reviewed in detail in (71)).
As mentioned above, targeting DEK in tumors including HNSCC is attractive especially in combination with conventional treatments. Recent publications describing novel DEK functions and investigating cancer stem cells in HNSCC may indicate potential efficacy for this strategy. Cancer stem cells are defined as cells that have the capacity to self-renew and produce heterogenous progeny with the capacity to re-generate an entire tumor (72). Hoechst negative side-populations enriched for cancer stem cells were identified in HNC and are characterized by CD44 and CD133 surface marker expression (73). Cancer stem cells are relatively resistant to conventional chemotherapy treatments. More recently, relapse of HNSCC in mice originally treated with cetuximab, had enriched populations of CD44/CD133 positive cells suggesting that these cells are also resistant to cetuximab therapy (74). DEK was reported to contribute to carcinogenesis in neuroendocrine tumors of the lung by supporting growth of the stem cell population, perhaps through MLLT3 expression (17). In a leukemia model, DEK-CAN fusion protein could only promote leukemogenesis from immature cells with stem-like features (75). Furthermore, DEK was shown to contribute to breast cancer tumor growth, cancer cell enriched mammosphere formation and side populations, and cellular motility and invasion through β-catenin signaling (66). These recent data suggest that targeting DEK in the absence or presence of conventional therapies might inhibit HNSCC growth, metastasis and chemoresistance at least in part by targeting the cancer stem cell population. Additionally, the combination of DEK depletion and oncolytic tumor cell killing, perhaps in combination with chemotherapies, might result in synergistic effects with improved clinical outcomes for these patients.
The promise of DEK inhibition for cancer therapy does not come without cautionary considerations. There is intriguing evidence that DEK overexpression and secretion into the cellular microenvironment may result in a systemic immune response. DEK is a prominent autoantigen and is detected at high levels in the extracellular space in juvenile idiopathic arthritis. DEK protein may be secreted by activated human monocyte-derived macrophages or released by T lymphocytes undergoing apoptosis (70, 76). The latter results in inflammation and may stimulate immune-mediated killing during carcinogenesis. One might postulate that chemotherapy induced cell death may release high levels of DEK protein resulting in an immune response that supports eradication of surrounding tumor cells. In this case, depletion of DEK would be expected to minimize this effect. Furthermore, DEK has recently been shown to promote heterochromatin integrity and non-homologous end joining (69)(77). With this in mind, genome instability in response to DEK loss may lead to high mutational frequencies and therefore possibly the acquisition of pro-oncogenic mutations in surviving cell populations.
Availability of head and neck cancer models
Targeted therapies for HNSCC may hold promise for a currently devastating disease. Clinically relevant preclinical models are critical for comparing and contrasting novel treatment approaches. Many mouse models for HNSCC are currently available, each with distinct strengths and limitations. The ideal model system would entail i) maximal similarity to human disease pathogenesis, ii) ease of therapy administration, and iii) sufficient time frame to administer and monitor the effects of intervention. The most commonly used current mouse models include 4-nitroquinoline-1-oxide (4-NQO) administration, which mimics tobacco exposure, and human xenograft or orthotopic models either injected into the flank, floor of mouth or tongue (78). 4-NQO models allow for relatively natural development of murine HNSCC. When 4-NQO is brushed directly on the tongue, SCCs form after several weeks. This technique however can be challenging with regard to properly administering the agent repeatedly to the desired area and is time consuming (please refer to review for specific techniques (79)). 4-NQO can also be administered via the drinking water but this route of administration often leads to esophageal rather than oropharyngeal cancers. The mouse esophagus and proximal stomach is lined by squamous epithelium. This is in contrast to humans wherein squamous epithelium lines the upper esophagus which transitions to columnar epithelium with distal progression to the stomach. Consequently, many esophageal cancers of the lower segment of the esophagus are adenocarcinomas which develop from glandular cells at the gastro-esophageal junction. This is important to note, since in humans, esophageal squamous cell carcinomas (compared to esophageal adenocarcinomas) are generally treated as an extension of HNSCC and respond to therapies directed at HNSCCs. It follows that murine esophageal and HNSCCs can be considered a valid model for both human HNC and esophageal SCC. Alternatively, it has been shown that if 4-NQO is administered at lower doses, premalignant lesions often develop in the oral cavity which can transform into malignant HNSCC in the C57BL/6 mouse strain. This model may be ideal for testing preventative agents as was reported for rapamycin (80). Strain selection is critically important with regards to the therapy dosage. This requires optimization and thorough planning when developing genetic models. 4NQO is an excellent model for patients who develop HNSCC in response to tobacco exposure since this carcinogen induces similar genetic alterations. However, this may not mimic the natural occurrence of HPV induced carcinogenesis. Mouse models that express the HPV oncogene E7 under the control of the squamous epithelial cell specific K14 promoter may give insight into the pathogenesis of HPV related cancers. Interestingly, these genetically altered mice are uniquely susceptible to HNC formation when exposed to 4-NQO in the drinking water (81). These mice do not generate a high rate of tumors in the absence of carcinogens and thus perhaps best model human tumors arising in smokers with HPV infection. These animal models are excellent systems in which to test the effects of genetic alterations on carcinogenesis. It must be borne in mind however that murine cells and tumor microenvironments differ from those in humans and may not optimally reflect therapeutic and toxic side effects of targeted therapies in human patients.
Subcutaneous xenograft models are often used due to the ease of tumor cell injection, tumor growth monitoring, and intratumoral injection of promising therapeutic agents (82). Either tumor cell lines or isolated tumor tissue is directly injected subcutaneously, often into the flank, of the mouse. These models occur in immunodeficient mice, and therefore allow for human therapies to be tested on human tumors. Limitations of these models are that the systems do not reflect the natural growth of these tumors, do not consider critical immunological contributions, and are not ideal for studies of the tumor microenvironment. More similar microenvironments can be modeled in orthotopic xenografts where tumors are injected into organ specific sites in the mouse. This strategy often recapitulates the local metastatic potential of the tumor which is limited in the subcutaneous model (83). Tumors in the head and neck region can also cause significant morbidity and mortality which necessitates early animal euthanasia. Another hurdle in this endeavor is that HNSCC are often heterogeneous thus making definition of the site of origin in humans difficult to identify. Some HNC orthotopic models use injection into the anterior tongue which is easier for subsequent therapy administration but does not have the same lymph node drainage or blood supply as the base of the tongue where many oropharyngeal, especially HPV positive, tumors originate. Other models inject tumor cells into floor of the mouth given the high rate of metastasis when injecting into this site but again this may not mimic the original site of the human cancer (84). All orthotopic models of HNC are technically challenging and difficult to monitor quantitatively. In order to overcome this issue, tumor cells can be labeled with either luciferase or a fluorescent marker coupled with in vivo imaging to monitor progression (82, 85). Whether human tumors in the mouse environment mimic human cancers better than murine tumors is still incompletely defined. Can insights gained by studying base of tongue tumors injected into the floor of mouth increase our understanding of how a human HNC will respond to treatments? Can we use classical xenograft models for preclinical trials and agent safety studies even if the tumor microenvironment does not mimic that in the patient? Currently available techniques force extrapolation of data from animal models to humans for clinical trials but more optimal models will be critical in the future in order to better predict the feasibility and response of novel cancer therapeutics.
Concluding remarks
HNSCC is a widespread and devastating disease which requires novel therapeutics in order to improve patient outcomes. Individuals with HNSCC are limited in their treatment options due to multiple co-morbitities and suboptimal functional status. It is our hope that continued genomic and molecular studies of HNSCC will spur the development of improved multimodal approaches that include targeted therapies and oncolytic viruses.
Acknowledgments
Dr. Wise-Draper is supported by the Clinical Scientist Training Program at the University of Cincinnati. Dr. Wells is supported by Public Health Service grant, RO1 CA116316, from the NIH. Drs. Gutkind and Molinolo are supported by the Intramural Research Program, NIDCR, NIH. We would like to acknowledge David Witte and the CCHMC pathology core as well as the excellent technical support by Meredith Taylor for Ventana staining.
Footnotes
All authors have disclosed that they do not have any financial or personal relationship with organizations that could potentially be perceived as influencing this review and understand the journal’s policy on disclosure of potential conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005 Mar-Apr;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Muir CS. Cancer incidence in five continents. comparability and quality of data. IARC Sci Publ. 1992;120(120):45–173. [PubMed] [Google Scholar]
- 3.Hall SF, Groome PA, Rothwell D. The impact of comorbidity on the survival of patients with squamous cell carcinoma of the head and neck. Head Neck. 2000 Jul;22(4):317–22. doi: 10.1002/1097-0347(200007)22:4<317::aid-hed1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Forastiere AA, Ang KK, Brizel D, Brockstein BE, Burtness BA, et al. National Comprehensive Cancer Network. Head and neck cancers. J Natl Compr Canc Netw. 2008 Aug;6(7):646–95. doi: 10.6004/jnccn.2008.0051. [DOI] [PubMed] [Google Scholar]
- 5.Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006 Aug 4;55(RR-11):1–94. [PubMed] [Google Scholar]
- 6.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002 Apr;55(4):244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pett M, Coleman N. Integration of high-risk human papillomavirus: A key event in cervical carcinogenesis? The Journal of pathology. 2007 Aug;212(4):356–67. doi: 10.1002/path.2192. [DOI] [PubMed] [Google Scholar]
- 8.Fakhry C, Gillison ML. Clinical implications of human papillomavirus in head and neck cancers. J Clin Oncol. 2006 Jun 10;24(17):2606–11. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev. 2005 Feb;14(2):467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 10.Scully C. Oral squamous cell carcinoma; from an hypothesis about a virus, to concern about possible sexual transmission. Oral oncology. 2002 Apr;38(3):227–34. doi: 10.1016/s1368-8375(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 11.Psyrri A, DiMaio D. Human papillomavirus in cervical and head-and-neck cancer. Nature clinical practice. 2008 Jan;5(1):24–31. doi: 10.1038/ncponc0984. [DOI] [PubMed] [Google Scholar]
- 12.Vidal L, Gillison ML. Human papillomavirus in HNSCC: Recognition of a distinct disease type. Hematology/oncology clinics of North America. 2008 Dec;22(6):1125, 42, vii. doi: 10.1016/j.hoc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 13.von Lindern M, Fornerod M, van Baal S, Jaegle M, de Wit T, Buijs A, et al. The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol Cell Biol. 1992 Apr;12(4):1687–97. doi: 10.1128/mcb.12.4.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orlic M, Spencer CE, Wang L, Gallie BL. Expression analysis of 6p22 genomic gain in retinoblastoma. Genes Chromosomes Cancer. 2006 Jan;45(1):72–82. doi: 10.1002/gcc.20263. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q, Hoffmann MJ, Hartmann FH, Schulz WA. Amplification and overexpression of the ID4 gene at 6p22.3 in bladder cancer. Mol Cancer. 2005 May 5;4(1):16. doi: 10.1186/1476-4598-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Secchiero P, Voltan R, di Iasio MG, Melloni E, Tiribelli M, Zauli G. The oncogene DEK promotes leukemic cell survival and is downregulated by both nutlin-3 and chlorambucil in B-chronic lymphocytic leukemic cells. Clin Cancer Res. 2010 Mar 15;16(6):1824–33. doi: 10.1158/1078-0432.CCR-09-3031. [DOI] [PubMed] [Google Scholar]
- 17.Shibata T, Kokubu A, Miyamoto M, Hosoda F, Gotoh M, Tsuta K, et al. DEK oncoprotein regulates transcriptional modifiers and sustains tumor initiation activity in high-grade neuroendocrine carcinoma of the lung. Oncogene. 2010 Aug 19;29(33):4671–81. doi: 10.1038/onc.2010.217. [DOI] [PubMed] [Google Scholar]
- 18.Khodadoust MS, Verhaegen M, Kappes F, Riveiro-Falkenbach E, Cigudosa JC, Kim DS, et al. Melanoma proliferation and chemoresistance controlled by the DEK oncogene. Cancer Res. 2009 Aug 15;69(16):6405–13. doi: 10.1158/0008-5472.CAN-09-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Q, Li Z, Lin H, Han L, Liu S, Lin Z. DEK overexpression in uterine cervical cancers. Pathology international. 2008 Jun;58(6):378–82. doi: 10.1111/j.1440-1827.2008.02239.x. [DOI] [PubMed] [Google Scholar]
- 20.Abba MC, Sun H, Hawkins KA, Drake JA, Hu Y, Nunez MI, et al. Breast cancer molecular signatures as determined by SAGE: Correlation with lymph node status. Mol Cancer Res. 2007 Sep;5(9):881–90. doi: 10.1158/1541-7786.MCR-07-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wise-Draper TM, Allen HV, Jones EE, Habash KB, Matsuo H, Wells SI. Apoptosis inhibition by the human DEK oncoprotein involves interference with p53 functions. Mol Cell Biol. 2006 Oct;26(20):7506–19. doi: 10.1128/MCB.00430-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wise-Draper TM, Allen HV, Thobe MN, Jones EE, Habash KB, Munger K, et al. The human DEK proto-oncogene is a senescence inhibitor and an upregulated target of high-risk human papillomavirus E7. J Virol. 2005 Nov;79(22):14309–17. doi: 10.1128/JVI.79.22.14309-14317.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molinolo AA, Hewitt SM, Amornphimoltham P, Keelawat S, Rangdaeng S, Meneses Garcia A, et al. Dissecting the Akt/mammalian target of rapamycin signaling network: Emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res. 2007 Sep 1;13(17):4964–73. doi: 10.1158/1078-0432.CCR-07-1041. [DOI] [PubMed] [Google Scholar]
- 24.Ang KK. Multidisciplinary management of locally advanced SCCHN: Optimizing treatment outcomes. Oncologist. 2008 Aug;13(8):899–910. doi: 10.1634/theoncologist.2007-0157. [DOI] [PubMed] [Google Scholar]
- 25.Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet. 2006 Sep 2;368(9538):843–54. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 26.Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, et al. A radiation therapy oncology group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000 Aug 1;48(1):7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 27.Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: Promises and pitfalls. J Clin Oncol. 2006 Jun 10;24(17):2618–23. doi: 10.1200/JCO.2005.04.7225. [DOI] [PubMed] [Google Scholar]
- 28.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: A comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005 Oct;27(10):843–50. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 29.Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, et al. Final results of the 94–01 french head and neck oncology and radiotherapy group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004 Jan 1;22(1):69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Chung CH, Ely K, McGavran L, Varella-Garcia M, Parker J, Parker N, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol. 2006 Sep 1;24(25):4170–6. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 31.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010 Jan;11(1):21–8. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 32.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA Eastern Cooperative Oncology Group. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: An eastern cooperative oncology group study. J Clin Oncol. 2005 Dec 1;23(34):8646–54. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 33.Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007 Oct 25;357(17):1695–704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 34.Machiels JP, Schmitz S. Molecular-targeted therapy of head and neck squamous cell carcinoma: Beyond cetuximab-based therapy. Curr Opin Oncol. 2011 May;23(3):241–8. doi: 10.1097/CCO.0b013e328344f581. [DOI] [PubMed] [Google Scholar]
- 35.Del Campo JM, Hitt R, Sebastian P, Carracedo C, Lokanatha D, Bourhis J, et al. Effects of lapatinib monotherapy: Results of a randomised phase II study in therapy-naive patients with locally advanced squamous cell carcinoma of the head and neck. Br J Cancer. 2011 Aug 23;105(5):618–27. doi: 10.1038/bjc.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salama JK, Haraf DJ, Stenson KM, Blair EA, Witt ME, Williams R, et al. A randomized phase II study of 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy compared with bevacizumab plus 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy for intermediate-stage and T4N0-1 head and neck cancers. Ann Oncol. 2011 Oct;22(10):2304–9. doi: 10.1093/annonc/mdq736. [DOI] [PubMed] [Google Scholar]
- 37.Machiels JP, Henry S, Zanetta S, Kaminsky MC, Michoux N, Rommel D, et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J Clin Oncol. 2010 Jan 1;28(1):21–8. doi: 10.1200/JCO.2009.23.8584. [DOI] [PubMed] [Google Scholar]
- 38.Williamson SK, Moon J, Huang CH, Guaglianone PP, LeBlanc M, Wolf GT, et al. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: Southwest oncology group study S0420. J Clin Oncol. 2010 Jul 10;28(20):3330–5. doi: 10.1200/JCO.2009.25.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elser C, Siu LL, Winquist E, Agulnik M, Pond GR, Chin SF, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007 Aug 20;25(24):3766–73. doi: 10.1200/JCO.2006.10.2871. [DOI] [PubMed] [Google Scholar]
- 40.Chung CH, Aulino J, Muldowney NJ, Hatakeyama H, Baumann J, Burkey B, et al. Nuclear factor-kappa B pathway and response in a phase II trial of bortezomib and docetaxel in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2010 Apr;21(4):864–70. doi: 10.1093/annonc/mdp390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011 Aug 26;333(6046):1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011 Aug 26;333(6046):1154–7. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brakenhoff RH. Cancer another NOTCH for cancer. Science. 2011 Aug 26;333(6046):1102–3. doi: 10.1126/science.1210986. [DOI] [PubMed] [Google Scholar]
- 44.Zoncu R, Efeyan A, Sabatini DM. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011 Jan;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev Res (Phila) 2009 Jan;2(1):27–36. doi: 10.1158/1940-6207.CAPR-08-0147. [DOI] [PubMed] [Google Scholar]
- 46.Patel V, Marsh CA, Dorsam RT, Mikelis CM, Masedunskas A, Amornphimoltham P, et al. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res. 2011 Nov 15;71(22):7103–12. doi: 10.1158/0008-5472.CAN-10-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raimondi AR, Molinolo A, Gutkind JS. Rapamycin prevents early onset of tumorigenesis in an oral-specific K-ras and p53 two-hit carcinogenesis model. Cancer Res. 2009 May 15;69(10):4159–66. doi: 10.1158/0008-5472.CAN-08-4645. [DOI] [PubMed] [Google Scholar]
- 48.McCormick F. Cancer gene therapy: Fringe or cutting edge? Nat Rev Cancer. 2001 Nov;1(2):130–41. doi: 10.1038/35101008. [DOI] [PubMed] [Google Scholar]
- 49.O’Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004 Dec;6(6):611–23. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the rb pathway produces anti-glioma effect in vivo. Oncogene. 2000 Jan 6;19(1):2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 51.Howe JA, Demers GW, Johnson DE, Neugebauer SE, Perry ST, Vaillancourt MT, et al. Evaluation of E1-mutant adenoviruses as conditionally replicating agents for cancer therapy. Mol Ther. 2000 Nov;2(5):485–95. doi: 10.1006/mthe.2000.0206. [DOI] [PubMed] [Google Scholar]
- 52.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996 Oct 18;274(5286):373–6. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 53.Shen Y, Kitzes G, Nye JA, Fattaey A, Hermiston T. Analyses of single-amino-acid substitution mutants of adenovirus type 5 E1B-55K protein. J Virol. 2001 May;75(9):4297–307. doi: 10.1128/JVI.75.9.4297-4307.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Zeeburg HJ, Huizenga A, Brink A, van den Doel PB, Zhu ZB, McCormick F, et al. Comparison of oncolytic adenoviruses for selective eradication of oral cancer and pre-cancerous lesions. Gene Ther. 2010 Dec;17(12):1517–24. doi: 10.1038/gt.2010.99. [DOI] [PubMed] [Google Scholar]
- 55.Dias JD, Guse K, Nokisalmi P, Eriksson M, Chen DT, Diaconu I, et al. Multimodal approach using oncolytic adenovirus, cetuximab, chemotherapy and radiotherapy in HNSCC low passage tumour cell cultures. Eur J Cancer. 2010 Feb;46(3):625–35. doi: 10.1016/j.ejca.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 56.Dias JD, Liikanen I, Guse K, Foloppe J, Sloniecka M, Diaconu I, et al. Targeted chemotherapy for head and neck cancer with a chimeric oncolytic adenovirus coding for bifunctional suicide protein FCU1. Clin Cancer Res. 2010 May 1;16(9):2540–9. doi: 10.1158/1078-0432.CCR-09-2974. [DOI] [PubMed] [Google Scholar]
- 57.Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001 Jan 15;19(2):289–98. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 58.Chang J, Zhao X, Wu X, Guo Y, Guo H, Cao J, et al. A phase I study of KH901, a conditionally replicating granulocyte-macrophage colony-stimulating factor: Armed oncolytic adenovirus for the treatment of head and neck cancers. Cancer Biol Ther. 2009 Apr;8(8):676–82. doi: 10.4161/cbt.8.8.7913. [DOI] [PubMed] [Google Scholar]
- 59.Mace AT, Ganly I, Soutar DS, Brown SM. Potential for efficacy of the oncolytic herpes simplex virus 1716 in patients with oral squamous cell carcinoma. Head Neck. 2008 Aug;30(8):1045–51. doi: 10.1002/hed.20840. [DOI] [PubMed] [Google Scholar]
- 60.Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010 Aug 1;16(15):4005–15. doi: 10.1158/1078-0432.CCR-10-0196. [DOI] [PubMed] [Google Scholar]
- 61.Isles MG, McConkey C, Mehanna HM. A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol. 2008 Jun;33(3):210–22. doi: 10.1111/j.1749-4486.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- 62.Wong HH, Lemoine NR, Wang Y. Oncolytic viruses for cancer therapy: Overcoming the obstacles. Viruses. 2010 Jan;2(1):78–106. doi: 10.3390/v2010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanchez-Carbayo M, Socci ND, Lozano JJ, Li W, Charytonowicz E, Belbin TJ, et al. Gene discovery in bladder cancer progression using cDNA microarrays. Am J Pathol. 2003 Aug;163(2):505–16. doi: 10.1016/S0002-9440(10)63679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wise-Draper TM, Mintz-Cole RA, Morris TA, Simpson DS, Wikenheiser-Brokamp KA, Currier MA, et al. Overexpression of the cellular DEK protein promotes epithelial transformation in vitro and in vivo. Cancer research. 2009 Mar 1;69(5):1792–9. doi: 10.1158/0008-5472.CAN-08-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carro MS, Spiga FM, Quarto M, Di Ninni V, Volorio S, Alcalay M, et al. DEK expression is controlled by E2F and deregulated in diverse tumor types. Cell Cycle. 2006 Jun;5(11):1202–7. doi: 10.4161/cc.5.11.2801. [DOI] [PubMed] [Google Scholar]
- 66.Privette Vinnedge LM, McClaine R, Wagh PK, Wikenheiser-Brokamp KA, Waltz SE, Wells SI. The human DEK oncogene stimulates beta-catenin signaling, invasion and mammosphere formation in breast cancer. Oncogene. 2011 Jun 16;30(24):2741–52. doi: 10.1038/onc.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kappes F, Khodadoust MS, Yu L, Kim DS, Fullen DR, Markovitz DM, et al. DEK expression in melanocytic lesions. Hum Pathol. 2011 Jul;42(7):932–8. doi: 10.1016/j.humpath.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wise-Draper TM, Morreale RJ, Morris TA, Mintz-Cole RA, Hoskins EE, Balsitis SJ, et al. DEK proto-oncogene expression interferes with the normal epithelial differentiation program. The American journal of pathology. 2009 Jan;174(1):71–81. doi: 10.2353/ajpath.2009.080330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kavanaugh GM, Wise-Draper TM, Morreale RJ, Morrison MA, Gole B, Schwemberger S, et al. The human DEK oncogene regulates DNA damage response signaling and repair. Nucleic Acids Res. 2011 Jun 7; doi: 10.1093/nar/gkr454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kappes F, Fahrer J, Khodadoust MS, Tabbert A, Strasser C, Mor-Vaknin N, et al. DEK is a poly(ADP-ribose) acceptor in apoptosis and mediates resistance to genotoxic stress. Molecular and cellular biology. 2008 May;28(10):3245–57. doi: 10.1128/MCB.01921-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riveiro-Falkenbach E, Soengas MS. Control of tumorigenesis and chemoresistance by the DEK oncogene. Clin Cancer Res. 2010 Jun 1;16(11):2932–8. doi: 10.1158/1078-0432.CCR-09-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001 Nov 1;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 73.Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol. 2008 Jun 10;26(17):2871–5. doi: 10.1200/JCO.2007.15.1613. [DOI] [PubMed] [Google Scholar]
- 74.Dias JD, Guse K, Nokisalmi P, Eriksson M, Chen DT, Diaconu I, et al. Multimodal approach using oncolytic adenovirus, cetuximab, chemotherapy and radiotherapy in HNSCC low passage tumour cell cultures. Eur J Cancer. 2010 Feb;46(3):625–35. doi: 10.1016/j.ejca.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Oancea C, Ruster B, Henschler R, Puccetti E, Ruthardt M. The t(6;9) associated DEK/CAN fusion protein targets a population of long-term repopulating hematopoietic stem cells for leukemogenic transformation. Leukemia. 2010 Nov;24(11):1910–9. doi: 10.1038/leu.2010.180. [DOI] [PubMed] [Google Scholar]
- 76.Mor-Vaknin N, Punturieri A, Sitwala K, Faulkner N, Legendre M, Khodadoust MS, et al. The DEK nuclear autoantigen is a secreted chemotactic factor. Mol Cell Biol. 2006 Dec;26(24):9484–96. doi: 10.1128/MCB.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kappes F, Waldmann T, Mathew V, Yu J, Zhang L, Khodadoust MS, et al. The DEK oncoprotein is a su(var) that is essential to heterochromatin integrity. Genes Dev. 2011 Apr 1;25(7):673–8. doi: 10.1101/gad.2036411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang XH, Knudsen B, Bemis D, Tickoo S, Gudas LJ. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004 Jan 1;10(1 Pt 1):301–13. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- 79.Vitale-Cross L, Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. Chemical carcinogenesis models for evaluating molecular-targeted prevention and treatment of oral cancer. Cancer Prev Res (Phila) 2009 May;2(5):419–22. doi: 10.1158/1940-6207.CAPR-09-0058. [DOI] [PubMed] [Google Scholar]
- 80.Czerninski R, Amornphimoltham P, Patel V, Molinolo AA, Gutkind JS. Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev Res (Phila) 2009 Jan;2(1):27–36. doi: 10.1158/1940-6207.CAPR-08-0147. [DOI] [PubMed] [Google Scholar]
- 81.Strati K, Pitot HC, Lambert PF. Identification of biomarkers that distinguish human papillomavirus (HPV)-positive versus HPV-negative head and neck cancers in a mouse model. Proc Natl Acad Sci U S A. 2006 Sep 19;103(38):14152–7. doi: 10.1073/pnas.0606698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sano D, Myers JN. Xenograft models of head and neck cancers. Head Neck Oncol. 2009 Aug 13;1:32. doi: 10.1186/1758-3284-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: Better than commonly perceived-but they can be improved. Cancer Biol Ther. 2003 Jul-Aug;2(4 Suppl 1):S134–9. [PubMed] [Google Scholar]
- 84.Simon C, Nemechek AJ, Boyd D, O’Malley BW, Jr, Goepfert H, Flaitz CM, et al. An orthotopic floor-of-mouth cancer model allows quantification of tumor invasion. Laryngoscope. 1998 Nov;108(11 Pt 1):1686–91. doi: 10.1097/00005537-199811000-00018. [DOI] [PubMed] [Google Scholar]
- 85.Reddy NP, Miyamoto S, Araki K, Liu T, Feldman M, O’Malley BW, Jr, et al. A novel orthotopic mouse model of head and neck cancer with molecular imaging. Laryngoscope. 2011 Jun;121(6):1202–7. doi: 10.1002/lary.21794. [DOI] [PubMed] [Google Scholar]