Abstract
Polymicrobial interactions on mucosal surfaces can influence inflammation, immunity, and disease outcome. Here, we review how host responses to colonization in the upper respiratory tract with the bacterial pathogen Streptococcus pneumoniae can be altered by co-infection. Recent advances provide a mechanistic understanding of how mucosal immunity can be subverted at distinct immunological time-points during pneumococcal colonization by other pathogens such as Haemophilus influenzae, influenza type A and Staphylococcus aureus. These examples use animal models of co-infection to highlight how otherwise effective host responses can be rendered ineffective by co-infection, and vice versa. The complex microbial ecology of mucosal sites must be considered to fully understand how immune responses in a natural setting influence the outcome of host-pathogen interactions.
Introduction
Our understanding of host-pathogen interactions has been achieved mainly by a reductionist approach. The power of reductionism is that it allows us to gain a precise, mechanistic understanding of how a specific pathogen interacts with a particular host response at the molecular level. Such insight can be especially valuable when studying how the host responds to invasive pathogens found in otherwise sterile sites, where they are the only pathogen and the host response is specifically directed against them. However, the reductionist approach can fall short in the context of host-pathogen interactions at non-sterile mucosal surfaces, where a given pathogen is just one member in a complex community of microbes. The microbiota of humans contains thousands of species, including many opportunistic pathogens, that can compete or cooperate to establish a niche in the host environment. Host responses elicited by a given pathogen can have far-reaching effects on other members of the mucosal community, including other pathogens. These off-target effects can be just as influential in the outcome of host-pathogen interactions as the pathogen-specific responses that are traditionally studied using a reductionist approach. There is a growing appreciation for the dynamic interactions among pathogens and how inflammation and immunity at mucosal surfaces can be influenced by polymicrobial interactions. This review highlights how co-infection with multiple pathogens can subvert mucosal immune responses and can alter disease outcome. As a model for these interactions, we focus on the bacterial pathogen Streptococcus pneumoniae and its primary ecological niche, the upper respiratory tract.
Modeling host-pathogen-pathogen interactions with S. pneumoniae in the upper respiratory tract
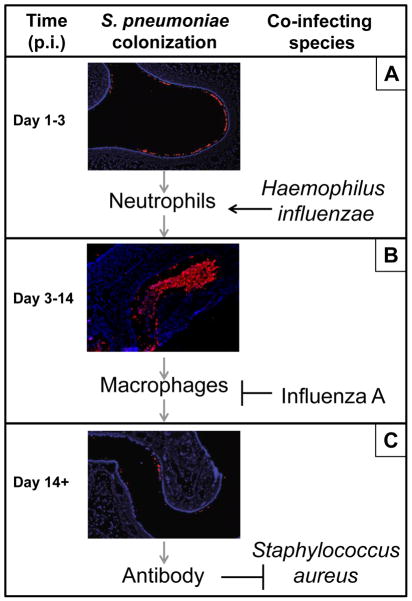
In the upper respiratory tract, the line between pathogen and commensal is often blurred, as many important human pathogens such as S. pneumoniae, Haemophilus influenzae, and Staphylococcus aureus can be found colonizing an average of 20–50% of healthy individuals [1–3]. Sequential and/or simultaneous colonization with more than one of these pathogens is common, especially during childhood. Colonization is the first step and a prerequisite for the development of disease [1]. Experimental human carriage studies have demonstrated that the acquisition of colonization is associated with mild inflammatory symptoms, including pharyngitis and rhinorrhea [4](M. Apicella, unpublished observations), but that these are insufficient to clear colonization which may last for weeks to months. A murine model of nasopharyngeal colonization with S. pneumoniae recapitulates these colonization dynamics and allows us to better characterize the time course of host responses [5] (Figure 1). Thirty minutes following atraumatic inoculation of pneumococci to the nares of mice, bacteria can be detected in the lumen of the nasal cavity. By one day post-inoculation, pneumococci have traversed the mucus layer and reached the epithelial surface of the nasopharynx where they establish colonization (Figure 1A). Bacterial contact with epithelial cells induces a TLR2-dependent signaling cascade that results in acute inflammation by day three, characterized by an influx of neutrophils [6,7]. However, these cells are insufficient to fully clear the pneumococcus which instead requires a gradual Th17-dependent monocyte/macrophage infiltrate over the course of weeks [6] (Figure 1B). During this time, a robust adaptive immune response is mounted to a diversity of pneumococcal antigens (Figure 1C), including antibody against the immunodominant capsular polysaccharide which contributes to protection against secondary exposure by promoting opsonophagocytic killing [8].
Figure 1. Co-infection alters immune responses to S. pneumoniae colonization in the upper respiratory tract.
(A) S. pneumoniae establishes colonization on the mucosal epithelium of the upper respiratory tract at day 1 post inoculation (p.i.) into the nares. Pneumococcal colonization elicits a neutrophil response that peaks around day 3 p.i. but is insufficient to clear the pneumococcus. However, co-colonization with H. influenzae results in a synergistic amplification of the neutrophil response that leads to elimination of pneumococci from the nasopharynx at this time-point. These enhanced host responses also drive the evolution of opsonophagocytosis-resistance determinants in the pneumococcus that contribute to this organism’s pathogenicity. (B) During mono-inoculation with S. pneumoniae, a gradual monocyte/macrophage influx peaks around day 7 p.i. and is responsible for pneumococcal clearance. However, co-infection with influenza A results in a synergistic induction of type I interferon expression which inhibits macrophage recruitment and results in an increase in pneumococcal colonization density. Influenza-induced increases in S. pneumoniae in the nasopharynx lead to an increased risk of invasive pneumococcal disease and pneumococcal transmission. (C). A robust antibody response to a diversity of antigens is mounted by two weeks following S. pneumoniae colonization. Cross-reactive antibody could explain why S. aureus carriage is negatively associated with S. pneumoniae colonization in immunocompetent, but not immunocompromised, hosts. Immunofluorescent microscopy images generated and kindly shared by Dr. Aoife Roche (Univ. of Pennsylvania) and adapted from Ref.#5. Host cells, blue (DAPI); S. pneumoniae cells, red.
At critical points along the time-course of pneumococcal colonization, co-colonization or co-infection with other pathogen species can have dramatic consequences on disease progression and outcome. Here, we provide three mechanistic examples of subversion of mucosal immunity at distinct immunological stages during pneumococcal colonization. Specifically, we review how 1) H. influenzae co-colonization with S. pneumoniae enhances the acute neutrophil response, leading to enhanced clearance of S. pneumoniae and a selection for virulent serotypes; 2) influenza A co-infection with S. pneumoniae inhibits the late-stage macrophage influx via the expression of type I interferons, leading to increased pneumococcal colonization density and risk of invasive disease; and 3) S. aureus co-colonization with S. pneumoniae is inhibited in immunocompetent, but not immunocompromised, hosts, potentially via the generation of cross-reactive pneumococcal antibody.
Effect of co-colonization with H. influenzae
S. pneumoniae and H. influenzae share the nasopharynx as the site of colonization in humans [1]. Significantly high carriage rates (>50%) of both of these organisms in children suggest that co-colonization is common [9]. Competition between these organisms is likely due to the overlap in their site and frequency of colonization. S. pneumoniae possesses multiple mechanisms that provide a fitness advantage over H. influenzae during competition in vitro [10,11]. However, the outcome of competition is reversed during in vivo colonization in mice: co-colonization with H. influenzae results in a rapid depletion of pneumococci from the nasopharynx within three days post-inoculation and complete clearance by two weeks [12]. This drastic enhancement in clearance is in stark contrast to mono-colonization with S. pneumoniae which remains stable for over a month.
The effects of co-colonization with H. influenzae occur rapidly, are only observed in vivo, and thus implicate a modulation of the acute inflammatory response. Indeed, simultaneous colonization with S. pneumoniae and H. influenzae results in a synergistic proinflammatory response [13]. Similar responses are noted during co-stimulation of human airway epithelial cells, which results in a log10 increase in the production of the proinflammatory chemokine IL-8 over single-species stimulation with either organism [13]. The observed synergistic IL-8 response is independent of cell surface pattern recognition receptors such as TLR2 and TLR4 but dependent on NF-kB and the pneumococcal pore-forming toxin, pneumolysin [13]. Pneumolysin acts in two ways to enhance proinflammatory responses in this setting: 1) pore-formation activates p38 MAP kinase phosphorylation, which then stabilizes chemokine mRNA [14] and 2) the pore facilitates the diffusion of extracellular bacterial products into the intracellular milieu for recognition by cytoplasmic pattern recognition receptors [15]. Lysenko and colleagues demonstrate that peptidoglycan from H. influenzae but not S. pneumoniae is sufficient to induce enhanced pneumococcal clearance and that this stimulation requires the intracellular pattern recognition receptor Nod1 [12,16]. Nod1 recognizes the minimal structure γ-D-glutamyl-meso-diaminopimelic acid (meso-DAP) common in peptidoglycan from gram-negative but not gram-positive bacteria [17]. Synthetic meso-DAP is sufficient to recapitulate the immunomodulatory effects of co-colonization with live H. influenzae in a Nod1-dependent manner [16]. Taken together, these data describe a proinflammatory signaling cascade that is initiated by the pore-forming toxin pneumolysin, which allows peptidoglycan fragments from gram-negative bacteria to access their cytosolic receptor Nod1, thereby activating NF-kB-mediated expression of chemokines such as IL-8.
IL-8 in humans and the equivalent in the mouse, MIP-2, are known to be potent inducers of neutrophil recruitment. In mice, a synergistic amplification of MIP-2 production can be measured following co-colonization with S. pneumoniae and H. influenzae and correlates with a robust migration of polymorphonuclear cells into the lumen of the nasopharynx one day following inoculation [12,13]. These cells are essential effectors of the observed immunomodulation since their depletion completely abrogates the inhibitory effect of H. influenzae on pneumococcal colonization regardless of similarly elevated MIP-2 levels [12]. A similar requirement for active complement in this system suggests that the mechanism of enhanced pneumococcal clearance is by neutrophil-mediated opsonophagocytic killing [12]. At this early time-point during primary colonization, antibody is neither present nor required for opsonization of pneumococci, which instead occurs via the alternative pathway of complement activation and recognition by complement receptor 3 (CR3; also known as Mac-1 or CD11b/CD18) [16].
Immunomodulation by co-colonization with H. influenzae results in a complete reversal in the outcome of early-stage interactions between the host and S. pneumoniae. To do so, H. influenzae induces a host-mediated inflammatory response to which itself is resistant that clears its niche of susceptible competitors. To survive this additional selective pressure, successful bacteria must evolve ways of resisting opsonophagocytic killing in order to establish colonization in the presence of competing H. influenzae. In this way, within-host colonization competition between microbes drives the selection for innate immune evasion mechanisms that in other settings contribute to virulence and allow for invasive disease. Pneumococci defend themselves against neutrophil-mediated killing by expressing an anti-opsonic capsular polysaccharide [5]. Over 90 distinct capsule types have been identified and each contributes to opsonophagocytic resistance in varying degrees [18]. Resistance to opsonophagocytic killing by capsule is the major virulence mechanism in the pathogenesis of S. pneumoniae disease. Recently, it was demonstrated that capsule type dictates whether S. pneumoniae are sensitive or resistant to the immunomodulation of H. influenzae colonization [19]. This effect was independent of the genetic background of pneumococcal strains, since mutant strains expressing heterologous capsule types maintained the phenotype of the capsule regardless of strain genotype [19]. Therefore, when pneumococci of different capsule types compete for niche-space within the nasopharynx, innate immune pressure by co-colonizing H. influenzae selects for colonization by more opsonophagocytosis-resistant, and thus more virulent strains. In this way, immunomodulation by H. influenzae not only influences the outcome of pneumococcal disease progression within a host but also influences the outcome of future host-pathogen interactions by promoting the evolution of virulence traits in an otherwise commensal organism.
Effect of co-infection with influenza A
Co-morbidity of influenza and S. pneumoniae infection has been reported widely throughout the past century and has been recently reviewed elsewhere [20]. The majority of deaths during influenza pandemics are due to secondary bacterial infections from prevalent upper-respiratory tract bacteria, such as S. pneumoniae [21]. Seasonal influenza has also been temporally associated with an elevated incidence of invasive pneumococcal disease [22]. Many studies have described mechanisms of synergistic virulence based on mouse models wherein influenza and S. pneumoniae are simultaneously inoculated directly into the otherwise sterile lower respiratory tract, the ultimate site of fatality-inducing pathology [20]. However, it may be equally important to understand the initial stages of co-infection in the more physiologically-relevant setting of the upper respiratory tract, where the influenza virus first interacts with the pneumococcus in its natural reservoir.
During the natural course of influenza infection, the virus replicates in the mucosal epithelial cells of the upper respiratory tract [23,24] and is temporally associated with significant increases in the numbers of colonizing pneumococci in children [25,26] and in mice [24]. Elevated bacterial load in the nasopharynx due to viral co-infection significantly increases the risk of transmission of pneumococci into normally sterile anatomical sites, resulting in increased rates of invasive disease and death [24]. Similarly, greater densities of pneumococcal colonization increases the risk of transmission from host to host, resulting in increased spread within human populations [26] and animal models [27]. Thus, infection with influenza during upper respiratory tract colonization with pneumococci dramatically alters the outcome of pneumococcal host-pathogen interactions, independent of the effects of co-infection in the lung.
The influenza-induced increase in pneumococcal colonization density in mice occurs one week following inoculation, co-incident with the peak macrophage influx normally required to clear the pneumococcus during mono-inoculation [6]. Indeed, co-infection in the nasopharynx results in an inhibition of macrophage recruitment at this time point, correlating with a synergistic increase in type I interferon (IFN) expression and a resulting decrease in expression of the macrophage recruitment cytokine CCL2 [24]. These immunomodulatory effects of co-infection are abrogated during co-infection of mice with a targeted deletion in the type I IFN receptor (Ifnar−/−), suggesting that type I IFN signaling is required for the observed increases in pneumococcal colonization density [24]. Moreover, boosting type I IFN expression by the synthetic TLR3 ligand poly-ICLC during mono-inoculation of pneumococci recapitulates the synergistic effects of co-infection with influenza, indicating that type I IFN signaling is necessary and sufficient to increase pneumococcal burden in the upper respiratory tract [24]. Synergistic increases in type I IFN expression were dependent on the bacterial toxin, pneumolysin, and the cytosolic pattern recognition receptor for peptidoglycan, Nod2, as bacteria and mice deficient for these factors, respectively, no longer mounted elevated type I IFN levels in response to co-infection [24]. Together, these data support a mechanism wherein co-induction of type I IFN by influenza and pneumolysin-expressing pneumococci signaling through Nod2 attenuates the CCL2 response and blocks the macrophage recruitment that is necessary to clear pneumococcal colonization. Although it is well established that type I IFNs are central to the antiviral host response [28], the study of immunomodulation during co-infection reveals how type I IFN expression in response to viral co-infection can negatively impact anti-pneumococcal host defenses in the upper respiratory tract.
Effect of co-colonization with S. aureus
The primary ecological niche for the opportunistic pathogen Staphylococcus aureus is the anterior squamous epithelium of the upper respiratory tract [3]. Nasal colonization is a major risk factor for disease since approximately 80% of S. aureus infections are due to the host’s colonizing strain [29,30]. Very little is known about what determines whether a human host will be permissive for nasal carriage. However, epidemiological studies have revealed that one subpopulation with a significantly reduced risk of S. aureus carriage are children who are colonized with S. pneumoniae [31-39]. These nine independent cohorts, which include over 10,000 subjects across 5 continents, have reproducibly demonstrated that immunocompetent pneumococcal carriers are approximately 50% less likely to be colonized with S. aureus that non-pneumococcal carriers. Conversely, a decline in pneumococcal colonization due to vaccination has been associated with an increased incidence of S. aureus-induced otitis media in children [40].
The mechanism underlying the observed protection against S. aureus by S. pneumoniae colonization is unknown. Similar to the studies of H. influenzae/S. pneumoniae antagonism described above, in vitro co-culture experiments implicated hydrogen peroxide-mediated competition between these two species [41–44]. However, neither hydrogen peroxide secretion by S. pneumoniae nor hydrogen peroxide sensitivity of S. aureus is predictive of co-colonization patterns in children [45,46] or animal models [47], suggesting that this mechanism may not be relevant in vivo. Moreover, since S. aureus and S. pneumoniae do not share the same microniche in the upper respiratory tract [48,49], a direct competitive mechanism is unlikely.
Rather, we and others [50] hypothesize that an immunological mechanism may be involved because the inhibitory effect of pneumococcal colonization on S. aureus carriage is abolished in HIV-positive immunocompromised hosts [33,34,51]. Generally, antibody functions to block the natural acquisition of bacterial pathogens in the upper respiratory tract [52]. During pneumococcal colonization in immunocompetent hosts, a robust antibody response is mounted to a diversity of proteins, however this is ineffective against the pneumococcus itself due to its anti-opsonic polysaccharide capsule. In contrast, anti-pneumococcal antibody responses and opsonophagocytosis are significantly ablated in HIV-positive individuals [53,54], who have impaired opsonic function of IgG isolated from their respiratory mucosa [55]. Data from co-colonization in animal models in our lab suggest that antibody elicited by pneumococcal colonization cross-reacts with the surface of S. aureus and is necessary and sufficient to inhibit the acquisition of S. aureus nasal colonization (RS Lijek et al., submitted). If this antibody response provides cross-protection against the acquisition of S. aureus carriage in children, then its absence in hosts lacking a well-orchestrated adaptive response could explain the observed reduced incidence of S. aureus carriage in HIV-negative but not HIV-positive children.
Interspecies cross-protection by antibody is not novel. For example, cross-reactive antibody is the basis for the development of natural immunity to H. influenzae type b [56]. Just as this cross-reactivity led to an understanding of immune protection against H. influenzae type b, a mechanistic understanding of the immunological inhibition of S. aureus carriage by S. pneumoniae colonization may inform future vaccine development against S. aureus, a public health priority.
Conclusions
The outcome of host-pathogen interactions at mucosal sites is a composite of the complex microbial ecology in those sites and the immunological pressures exerted by the host in response to colonization and infection. We describe the model of S. pneumoniae colonization in the upper respiratory tract and how the introduction of even one additional player – eg. H. influenzae, influenza A or S. aureus – can drastically influence disease progression. These examples highlight that otherwise effective host responses can be rendered ineffective by co-infection, and vice versa. While it is widely understood that successful pathogens are resistant to the immune responses they induce, pathogens are not necessarily resistant to immune responses induced by other organisms. “Off-target” immune responses can alter the microbial landscape by removing competing species from a host niche, driving the acquisition of virulence characteristics, enhancing or inhibiting pathogen clearance, and preventing pathogen acquisition. This can occur at myriad points along the time-course of host-pathogen interactions and can include components of both the innate and adaptive immune response. An important conclusion from these examples is that the triad of pathogen-host-pathogen interactions is not the sum of its individual pairings. Indeed, results from in vitro bacterial competition experiments that lack the host or in vivo analyses with a single pathogen may be misleading. The inclusion of additional species and variables into controlled experiments can be challenging [57]. However, understanding how co-infection subverts mucosal immunity may better inform us of the underlying immunological mechanisms in a natural setting.
Highlights.
Host responses elicited by a pathogen affect other microbes including other pathogens
Effective host responses can be rendered ineffective by co-infection, and vice versa
H. influenzae enhances PMN clearance of S. pneumoniae colonization but promotes virulence
Influenza increases pneumococcal carriage levels and risk of invasive disease via IFN
S. pneumoniae may reduce the risk of S. aureus carriage via adaptive immunity
Acknowledgments
This work was supported by a Commonwealth Universal Research Enhancement (CURE) Grant from the Commonwealth of Pennsylvania Department of Health and by the U.S. Public Health Service (AI-055400).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiser J. The pneumococcus: why a commensal misbehaves. Journal of Molecular Medicine. 2010;88:97–102. doi: 10.1007/s00109-009-0557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gessner BD, Adegbola RA. The impact of vaccines on pneumonia: Key lessons from Haemophilus influenzae type b conjugate vaccines. Vaccine. 2008;26(Supplement 2):B3–B8. doi: 10.1016/j.vaccine.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 3.Wertheim HFL, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infectious Diseases. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 4.McCool TL, Cate TR, Moy G, Weiser JN. The immune response to pneumococcal proteins during experimental human carriage. Journal of Experimental Medicine. 2002;195:359–365. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nature Reviews Microbiology. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 6*.Zhang Z, Clarke TB, Weiser JN. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. The Journal of Clinical Investigation. 2009;119:1899–1909. doi: 10.1172/JCI36731. Defines the inflammatory responses elicited by pneumococcal colonization and determines which are necessary for pneumococcal clearance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthias KA, Roche AM, Standish AJ, Shchepetov M, Weiser JN. Neutrophil-toxin interactions promote antigen delivery and mucosal clearance of Streptococcus pneumoniae. The Journal of Immunology. 2008;180:6246–6254. doi: 10.4049/jimmunol.180.9.6246. [DOI] [PubMed] [Google Scholar]
- 8.Dagan R, Givon-Lavi N, Fraser D, Lipsitch M, Siber GR, Kohberger R. Serum Serotype-Specific Pneumococcal Anticapsular Immunoglobulin G Concentrations after Immunization with a 9-Valent Conjugate Pneumococcal Vaccine Correlate with Nasopharyngeal Acquisition of Pneumococcus. Journal of Infectious Diseases. 2005;192:367–376. doi: 10.1086/431679. [DOI] [PubMed] [Google Scholar]
- 9.Neto AS, Lavado P, Flores P, Dias R, Pessanha MA, Sousa E, Palminha JM, Caniça M, Esperança-Pina J. Risk factors for the nasopharyngeal carriage of respiratory pathogens by Portuguese children: phenotype and antimicrobial susceptibility of Haemophilus influenzae and Streptococcus pneumoniae. Microbial Drug Resistance. 2003;9:99–108. doi: 10.1089/107662903764736409. [DOI] [PubMed] [Google Scholar]
- 10.Shakhnovich EA, King SJ, Weiser JN. Neuraminidase expressed by Streptococcus pneumoniae desialylates the lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae: a paradigm for interbacterial competition among pathogens of the human respiratory tract. Infection and Immunity. 2002;70 :7161–7164. doi: 10.1128/IAI.70.12.7161-7164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pericone CD, Overweg K, Hermans PWM, Weiser JN. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infection and Immunity. 2000;68 :3990–3997. doi: 10.1128/iai.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Lysenko ES, Ratner AJ, Nelson AL, Weiser JN. The Role of Innate Immune Responses in the Outcome of Interspecies Competition for Colonization of Mucosal Surfaces. PLoS Pathogens. 2005;1:e1. doi: 10.1371/journal.ppat.0010001. With Refs 13, 16, provides mechanistic evidence of synergistic immune responses during H. influenzae and S. pneumoniae co-colonization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13**.Ratner AJ, Lysenko ES, Paul MN, Weiser JN. Synergistic proinflammatory responses induced by polymicrobial colonization of epithelial surfaces. Proceedings of the National Academy of Sciences. 2005;102:3429–3434. doi: 10.1073/pnas.0500599102. See Ref. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguilar JL, Kulkarni R, Randis TM, Soman S, Kikuchi A, Yin Y, Ratner AJ. Phosphatase-dependent regulation of epithelial mitogen-activated protein kinase responses to toxin-induced membrane pores. PLoS ONE. 2009;4:e8076. doi: 10.1371/journal.pone.0008076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke TB, Weiser JN. Intracellular sensors of extracellular bacteria. Immunological Reviews. 2011;243:9–25. doi: 10.1111/j.1600-065X.2011.01039.x. [DOI] [PubMed] [Google Scholar]
- 16**.Lysenko ES, Clarke TB, Shchepetov M, Ratner AJ, Roper DI, Dowson CG, Weiser JN. Nod1 Signaling Overcomes Resistance of S. pneumoniae to Opsonophagocytic Killing. PLoS Pathogens. 2007;3:e118. doi: 10.1371/journal.ppat.0030118. See Ref. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girardin S, Boneca I, Carneiro L, Antignac A, Jehanno M, Viala J, Tedin K, Taha M, Labigne A, Zähringer U, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 18.Hyams C, Yuste J, Bax K, Camberlein E, Weiser JN, Brown JS. Streptococcus pneumoniae resistance to complement-mediated immunity is dependent on the capsular serotype. Infection and Immunity. 2010;78:716–725. doi: 10.1128/IAI.01056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Lysenko ES, Lijek RS, Brown SP, Weiser JN. Within-Host Competition Drives Selection for the Capsule Virulence Determinant of Streptococcus pneumoniae. Current Biology. 2010;20:1222–1226. doi: 10.1016/j.cub.2010.05.051. Uses theoretical modeling and experimental animal models to demonstrate that immunomodulation by H. influenzae drives selection of virulent pneumococcal capsule types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCullers JA. Insights into the Interaction between Influenza Virus and Pneumococcus. Clinical Microbiology Reviews. 2006;19:571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. Journal of Infectious Diseases. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim PE, Musher DM, Glezen WP, Barradas MCR, Nahm WK, Wright CE. Association of invasive pneumococcal disease with season, atmospheric conditions, air pollution, and the isolation of respiratory viruses. Clinical Infectious Diseases. 1996;22:100–106. doi: 10.1093/clinids/22.1.100. [DOI] [PubMed] [Google Scholar]
- 23.Hatta M, Hatta Y, Kim JH, Watanabe S, Shinya K, Nguyen T, Lien PS, Le QM, Kawaoka Y. Growth of H5N1 Influenza A Viruses in the Upper Respiratory Tracts of Mice. PLoS Pathogens. 2007;3:e133. doi: 10.1371/journal.ppat.0030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. The Journal of Clinical Investigation. 2011;121:3657–3665. doi: 10.1172/JCI57762. Provides mechanistic evidence of a synergy between influenza and S. pneumoniae during co-infection in the upper respiratory tract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vu HTT, Yoshida LM, Suzuki M, Nguyen HAT, Nguyen CDL, Nguyen ATT, Oishi K, Yamamoto T, Watanabe K, Vu TD, et al. Association Between Nasopharyngeal Load of Streptococcus pneumoniae, Viral Coinfection, and Radiologically Confirmed Pneumonia in Vietnamese Children. The Pediatric Infectious Disease Journal. 2011;30:11–18. doi: 10.1097/INF.0b013e3181f111a2. [DOI] [PubMed] [Google Scholar]
- 26.Diavatopoulos DA, Short KR, Price JT, Wilksch JJ, Brown LE, Briles DE, Strugnell RA, Wijburg OL. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. The FASEB Journal. 2010;24:1789–1798. doi: 10.1096/fj.09-146779. [DOI] [PubMed] [Google Scholar]
- 27.McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. Journal of Infectious Diseases. 2010;202:1287–1295. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I Interferons (α/β) in immunity and autoimmunity. Annual Review of Immunology. 2005;23:307–335. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 29.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal Carriage as a Source of Staphylococcus aureus Bacteremia. New England Journal of Medicine. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 30.Wertheim HFL, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JAJW, van Keulen PHJ, Vandenbroucke-Grauls CMJE, Meester MHM, Verbrugh HA. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. The Lancet. 2004;364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 31*.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rümke HC, Verbrugh HA, Hermans PWM. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. The Lancet. 2004;363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. With Ref. 36, the first epidemiological studies to report that healthy pneumococcal carriers are 50% less likely to be colonized with S. aureus than non-pneumococcal carriers. [DOI] [PubMed] [Google Scholar]
- 32.Kwambana B, Barer M, Bottomley C, Adegbola R, Antonio M. Early acquisition and high nasopharyngeal co-colonisation by Streptococcus pneumoniae and three respiratory pathogens amongst Gambian newborns and infants. BMC Infectious Diseases. 2011;11:175. doi: 10.1186/1471-2334-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Madhi SA, Kuwanda L, Cutland C, Holm A, Käyhty H, Klugman KP. Quantitative and qualitative antibody response to pneumococcal conjugate vaccine among African human immunodeficiency virus-infected and uninfected children. The Pediatric Infectious Disease Journal. 2005;24:410–416. doi: 10.1097/01.inf.0000160942.84169.14. 33. With Ref 34, epidemiological studies demonstrating that inhibitory effect of pneumococcal colonization on S. aureus carriage is lost in immunocompromised hosts. [DOI] [PubMed] [Google Scholar]
- 34*.McNally LM, Jeena PM, Gajee K, Sturm AW, Tomkins AM, Coovadia HM, Goldblatt D. Lack of association between the nasopharyngeal carriage of Streptococcus pneumoniae and Staphylococcus aureus in HIV-infected South African children. The Journal of Infectious Diseases. 2006;194:385–390. doi: 10.1086/505076. See Ref. 33. [DOI] [PubMed] [Google Scholar]
- 35.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. Microbial interactions during upper respiratory tract infections. Emerging Infectious Diseases. 2008;14 :1584–1591. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Regev-Yochay G, Dagan R, Raz M, Carmeli Y, Shainberg B, Derazne E, Rahav G, Rubinstein E. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. JAMA. 2004;292:716–720. doi: 10.1001/jama.292.6.716. See Ref. 31. [DOI] [PubMed] [Google Scholar]
- 37.Regev-Yochay G, Lipsitch M, Basset A, Rubinstein E, Dagan R, Raz M, Malley R. The Pneumococcal Pilus Predicts the Absence of Staphylococcus aureus Co- Colonization in Pneumococcal Carriers. Clinical Infectious Diseases. 2009;48 :760–763. doi: 10.1086/597040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Gils EJM, Hak E, Veenhoven RH, Rodenburg GD, Bogaert D, Bruin JP, van Alphen L, Sanders EAM. Effect of seven-valent pneumococcal conjugate vaccine on Staphylococcus aureus colonisation in a randomised controlled trial. PLoS ONE. 2011;6:e20229. doi: 10.1371/journal.pone.0020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watson K, Carville K, Bowman J, Jacoby P, Riley TV, Leach AJ, Lehmann D. Upper respiratory tract bacterial carriage in aboriginal and non-aboriginal children in a semi-arid area of Western Australia. The Pediatric Infectious Disease Journal. 2006;25:782–790. doi: 10.1097/01.inf.0000232705.49634.68. [DOI] [PubMed] [Google Scholar]
- 40.Veenhoven R, Bogaert D, Uiterwaal C, Brouwer C, Kiezebrink H, Bruin J, Ijzerman E, Hermans P, de Groot R, Zegers B, et al. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. The Lancet. 2003;361:2189–2195. doi: 10.1016/S0140-6736(03)13772-5. [DOI] [PubMed] [Google Scholar]
- 41.McLeod JW, Gordon J. Production of hydrogen peroxide by bacteria. Biochemical Journal. 1922;16:499–506. doi: 10.1042/bj0160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park B, Nizet V, Liu GY. Role of Staphylococcus aureus catalase in niche competition against Streptococcus pneumoniae. Journal of Bacteriology. 2008;190:2275–2278. doi: 10.1128/JB.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regev-Yochay G, Trzcinski K, Thompson CM, Malley R, Lipsitch M. Interference between Streptococcus pneumoniae and Staphylococcus aureus: In vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. Journal of Bacteriology. 2006;188:4996–5001. doi: 10.1128/JB.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selva L, Viana D, Regev-Yochay G, Trzcinski K, Corpa JM, Lasa i, Novick RP, Penades JR. Killing niche competitors by remote-control bacteriophage induction. Proceedings of the National Academy of Sciences. 2009;106:1234–1238. doi: 10.1073/pnas.0809600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melles DC, Bogaert D, Gorkink RFJ, Peeters JK, Moorhouse MJ, Ott A, van Leeuwen WB, Simons G, Verbrugh HA, Hermans PWM, et al. Nasopharyngeal co-colonization with Staphylococcus aureus and Streptococcus pneumoniae in children is bacterial genotype independent. Microbiology. 2007;153:686–692. doi: 10.1099/mic.0.2006/002279-0. [DOI] [PubMed] [Google Scholar]
- 46.Regev-Yochay G, Malley R, Rubinstein E, Raz M, Dagan R, Lipsitch M. In vitro bactericidal activity of Streptococcus pneumoniae and bactericidal susceptibility of Staphylococcus aureus strains isolated from cocolonized versus noncocolonized children. Journal of Clinical Microbiology. 2008;46:747–749. doi: 10.1128/JCM.01781-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Margolis E. Hydrogen peroxide-mediated interference competition by Streptococcus pneumoniae has no significant effect on Staphylococcus aureus nasal colonization of neonatal rats. Journal of Bacteriology. 2009;191:571–575. doi: 10.1128/JB.00950-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crook DW, Brueggemann AB, Sleeman KL, Peto TEA. Pneumococcal carriage. In: Tuomanen E, editor. The Pneumococcus. ASM Press; 2004. [Google Scholar]
- 49.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clinical Microbiology Reviews. 1997;10:505. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regev-Yochay G, Bogaert D, Malley R, Hermans PWM, Veenhoven RH, Sanders EAM, Lipsitch M, Rubinstein E. Does Pneumococcal Conjugate Vaccine Influence Staphylococcus aureus Carriage in Children? Clinical Infectious Diseases. 2008;47:289–291. doi: 10.1086/589573. [DOI] [PubMed] [Google Scholar]
- 51.Bogaert D, Nouwen J, Hermans P, van Belkum A. Lack of interference between Streptococcus pneumoniae and Staphylococcus aureus in HIV-infected individuals? The Journal of Infectious Diseases. 2006;194:1617–1618. doi: 10.1086/508886. [DOI] [PubMed] [Google Scholar]
- 52.Dagan R, Givon-Lavi N, Zamir O, Sikuler-Cohen M, Guy L, Janco J, Yagupsky P, Fraser D. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. Journal of Infectious Diseases. 2002;185:927–936. doi: 10.1086/339525. [DOI] [PubMed] [Google Scholar]
- 53.Janoff E, Fasching C, Ojoo J, O'Brian J, Gilks C. Responsiveness of human immunodeficiency virus type 1-infected Kenyan women with or without prior pneumococcal disease to pneumococcal vaccine. Journal of Infectious Diseases. 1997;175:975–978. doi: 10.1086/514004. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi H, Oishi K, Yoshimine H, Kumatori A, Moji K, Watanabe K, Nalwoga H, Tugume SB, Kebba A, Mugerwa R, et al. Decreased Serum Opsonic Activity against Streptococcus pneumoniae in Human Immunodeficiency Virus-Infected Ugandan Adults. Clinical Infectious Diseases. 2003;37:1534–1540. doi: 10.1086/379511. [DOI] [PubMed] [Google Scholar]
- 55.Eagan R, Twigg HL, III, French N, Musaya J, Day RB, Zijlstra EE, Tolmie H, Wyler D, Molyneux ME, Gordon SB. Lung Fluid Immunoglobulin from HIV-Infected Subjects Has Impaired Opsonic Function against Pneumococci. Clinical Infectious Diseases. 2007;44:1632–1638. doi: 10.1086/518133. [DOI] [PubMed] [Google Scholar]
- 56.Schneerson R, Robbins JB. Induction of serum Haemophilus influenzae type B capsular antibodies in adult volunteers fed cross-reacting Escherichia coli 075:K100:H5. New England Journal of Medicine. 1975;292:1093–1096. doi: 10.1056/NEJM197505222922103. [DOI] [PubMed] [Google Scholar]
- 57.Bakaletz LO. Developing animal models for polymicrobial diseases. Nature Reviews Microbiology. 2004;2:552–568. doi: 10.1038/nrmicro928. [DOI] [PMC free article] [PubMed] [Google Scholar]