Abstract
Lumen-to-cell transport, cellular accumulation, and toxicity of L-cysteine (Cys), glutathione (GSH) and N-acetylcysteine (NAC) S-conjugates of methylmercury (CH3Hg+) were evaluated in isolated, perfused rabbit proximal tubular segments. When these conjugates were perfused individually through the lumen of S2 segments of the proximal tubule it was found that Cys-S-CH3Hg and GSH-S-CH3Hg were transported avidly, while NAC-S-CH3Hg was transported minimally. In addition, 95% of the 203Hg taken up by the tubular cells was associated with precipitable proteins of the tubular extract, while very little was found in the acid-soluble cytosol extract. No visual cellular pathological changes were observed during 30 min of study. Luminal uptake of Cys-S-CH3Hg was temperature-dependent and inhibited significantly by the amino acids L-methionine and L-cystine. Rates of luminal uptake of GSH-S-CH3Hg were twice as great as that of Cys-S-CH3Hg and uptake was inhibited significantly (74%) by the presence of acivicin. When 2,3-bis(sulfanyl)propane-1-sulfonate (DMPS) was added to the bathing or luminal fluid, luminal uptake of Cys-S-CH3Hg was diminished significantly. Overall, our data indicate that Cys-S-CH3Hg is likely a transportable substrate of one or more amino acid transporters (such as system B0,+ and system b0,+) involved in luminal absorption of L-methionine and L-cystine along the renal proximal tubule. In addition, GSH-S-CH3Hg appears to be degraded enzymatically to Cys-S-CH3Hg, which can then be taken up at the luminal membrane. By contrast NAC-S-CH3Hg and Cys-S-CH3Hg (in the presence of DMPS) are not taken up avidly at the luminal membrane of proximal tubular cells, thus promoting the excretion of CH3Hg+ into the urine.
Keywords: Thiol S-conjugates; Methylmercury; Membrane Transport; Renal Proximal Tubular Segments; Rabbit; Cystine; Cysteine; Methionine; 2, 3-bis(sulfanyl)propane-1-sulfonate (DMPS); amino acid transporters; molecular mimicry
INTRODUCTION
Methylmercury (CH3Hg+) is the predominant mercuric species that humans and other mammals are exposed to in various environmental settings. Its formation in the environment occurs following methylation of inorganic mercuric (Hg2+) ions by microorganisms in soil and water. Subsequently, CH3Hg+ accumulates widely in numerous species of animals in the food chain, especially in predatory fish. Consumption of animals and/or water contaminated with CH3Hg+ by humans can be significantly deleterious to their health, inasmuch as it is absorbed readily by the gastrointestinal tract and is delivered into the circulatory system (Kershaw, Clarkson et al. 1980; Clarkson 2002). Since methylmercuric ions have an extremely high affinity to bond to the reduced sulfur atom of thiol-containing molecules, one can assume that most, if not all, absorbed methylmercuric ions present in blood are in the form of some thiol S-conjugate (Fuhr and Rabenstein 1973; Zalups 2000). Some of the mercuric species believed to form in various compartments of the body include L-cysteine (Cys), homocysteine (Hcy), glutathione (GSH), N-acetylcysteine (NAC), hemoglobin and albumin S-conjugates of CH3Hg+ (Carty and Malone 1979).
The kidney plays an important role in reducing the body-burden of most heavy metals and their conjugates, including methylmercury. Within the kidney, the proximal tubule is the major site for methylmercury accumulation (Rodier and Kates 1988; Zalups 2000). A few transporters located in the basolateral membrane of the kidney including the organic anion transporter 1 (OAT1) (Zalups and Ahmad 2005; Zalups and Ahmad 2005; Zalups and Ahmad 2005) and system L (Simmons-Willis, Koh et al. 2002; Yin, Jiang et al. 2008) have been reported to transport some thiol S-conjugates of methylmercury. By contrast, little is known about the mechanisms involved in the absorptive transport of methylmercuric species at the luminal plasma membrane of epithelial cells lining the length of the proximal tubule.
System B0,+, by far, is the sole transporter that has been reported to be involved in the luminal uptake of certain thiol S-conjugates of CH3Hg+. Using Xenopus laevis oocytes, Bridges, et al. showed that system B0,+ is capable of the transporting the complexes Cys-S-CH3Hg and Hcy-S-CH3Hg, by using competitive inhibition experiments with substrates known to be transported by system B0,+ (Bridges and Zalups 2006). System B0,+ is a Na+/Cl−-dependent transporter for a variety of neutral and cationic amino acids including methionine (Met) (Sloan and Mager 1999; Nakanishi, Hatanaka et al. 2001), and is known to be localized in the luminal membrane of proximal tubular epithelial cells (Broer 2008).
Absorptive transport of thiol S-conjugates of CH3Hg+ has been explained by the putative mechanism involving molecular mimicry or homology (Zalups 2000; Broer 2008). Certain thiol S-conjugates of CH3Hg+ are similar structurally to some endogenous molecules, such as amino acids and amino acid derivatives, and thus, are believed to behave as mimics at the binding site(s) of selective transporters. Evidence for this theory has been provided by a few groups (Aschner and Clarkson 1988; Mokrzan, Kerper et al. 1995; Simmons-Willis, Koh et al. 2002), who demonstrated that the Cys S-conjugates of CH3Hg+ (Cys-S-CH3Hg) behave as mimics of L-Met at the transporter system L. Additionally, Cys-S-CH3Hg may compete with L-Met for transport by System B0,+. Moreover, it has also been demonstrated that Cys-S-CH3Hg may serve as a structural and/or functional mimic of L-cystine (Zalups 2000). L-cystine is known to be transported by system b0,+, which like B0,+ is located in the luminal membrane, but not the basolateral membrane of the renal proximal tubular epithelial cells (Furriols, Chillaron et al. 1993; Mora, Chillaron et al. 1996). Interestingly, system b0+ has been implicated in the luminal absorptive transport of the Cys S-conjugates of cadmium (Cys-Cd-Cys) in isolated perfused rabbit proximal tubular segments (Wang, Zalups et al. 2010). Since Hg and cadmium (Cd) are both group IIB metals, and both have a high binding affinity for sulfhydryl groups, it is possible that Cys-S-CH3Hg may also utilize system b0+ to enter proximal tubular cells.
The previous findings from our laboratory indicated that methylmercuric ions are avidly transported across the luminal membrane in isolated perfused proximal tubular segments (Zalups and Barfuss 1993). However, no data for the transport of Cys S-conjugates of CH3Hg+ by the luminal membrane of isolated perfused proximal tubular cells have been reported to date. The present study examined if methylmercuric ions (CH3Hg+) gain entry into proximal tubular epithelial cells at the luminal membrane when co-perfused with Cys, NAC or GSH in isolated perfused rabbit proximal tubular segments. It is presumed that the methylmercuric ions react with the co-perfused thiol compound forming the respective conjugates which are the transportable entities. In addition, we sought to obtain evidence implicating the luminal transporters system B0+ and system b0+ in the absorption of Cys-S-CH3Hg. Furthermore, the effect of the divalent metal complexing agent, 2,3-bis(sulfanyl)propane-1-sulfonate (known formerly as 2,3-dimercaptopropane-1-sulfonate, DMPS), on the luminal transport of Cys-S-CH3Hg was also evaluated. Our data are apparently the first attempt to characterize the transport of thiol S-conjugate of CH3Hg+ by the luminal membrane of renal proximal tubules.
MATERIALS AND METHODS
Animals
Female New Zealand rabbits (1–2kg) were used in the present study. All animals were allowed at least two days of acclimation prior to any experimentation. Water and a commercial laboratory diet for rabbits were provided ad libitum during all phases of the study.
Tubule Perfusion
Proximal tubular segments were dissected from isolated kidneys from female rabbits, mounted on a system of glass pipettes, and perfused and bathed in solutions similar to those described previously (Zalups and Barfuss 1993; Wang, Zalups et al. 2010) except for a few differences that are pointed out below. In general, 15 min after warming a tubular segment to 37°C, three collections of 30–50nL, at the flow rate of 7–10nL min−1, were made. Each perfused tubular segment was harvested and placed in 10μL of 3% trichloroacetic acid (TCA) solution for 20 sec to extract the accumulated 203Hg into acid-precipitable and non-precipitable fractions.
To evaluate the net absorption of each thiol S-conjugate of CH3Hg+ studied, a fresh perfusing solution (perfusate) was made, containing a 1:1 ratio of the thiol-containing molecule (L-Cys, L-glutathione, N-acetylcysteine or DMPS) to each molecular ion of CH3Hg+. The 1:1 ratio of thiol to CH3Hg+ was used to ensure the formation of a linear I coordinate covalent bond between each molecule of CH3Hg+ and the corresponding thiol. The vital dye FD&C Green 3 (809Da) was placed in the perfusate at a concentration of 250nM to visually determine any toxic effects of the thiol S-conjugates of CH3Hg+. L-[3H]-glucose (14.6Ci/mmol; American Radiolabeled Chemicals, Inc., St. Louis, MO, USA) was added to the perfusate as a volume marker.
In some experiments, L-methionine and L-cystine were added to the perfusate at the ratio of at least 10:1 (with respect to CH3Hg+) to promote potential inhibition of putative amino acid transporters possibly involved in the luminal uptake of CH3Hg+. All variants of perfusates were made fresh on the day of experiments.
The perfusate was identical to the bathing solution except that 2mM NaH2PO4 was replaced by 2mM HEPES because it was found that HPO4− or HPO42− caused precipitation of CH3Hg+ in aqueous solutions.
Generation of 203Hg2+ and CH3203Hg+
203Hg2+ was generated by the method described previously (Belanger, Westin et al. 2001; Bridges, Bauch et al. 2004). Briefly, 3 mg of mercuric oxide (HgO) were sealed in two quartz tubes, one inside the other, with an acetylene torch. Subsequently, the doubly-sealed sample of HgO was sent to the Missouri University Research Reactor (MURR) facility to be irradiated via neutron activation for four weeks. After we received the irradiated sample from the MURR facility, the solid content of mercury was dissolved in 1 N HCl and the radioactivity of 203Hg2+ was determined by standard isotopic methods using a Wallac Wizard 3 automatic gamma counter (Perkin Elmer, Gaithersburg, MD). The specific activities of the 203Hg2+ ranged, on average, between 6 to12 mCi/mg.
CH3203Hg+ was generated later by following a protocol adapted from Rouleau and Block (Rouleau 1997). Two mCi of 203Hg2+ were diluted in 40μL of deionized water. Subsequently, 670μL of 2 M acetate buffer and 2 mL of methylcobalamin were added to the solution. Methylcobalamin served as the methyl donor. This mixture was incubated for 24 h at room temperature in a fume hood. Following incubation, 16.7mL of 30% potassium chloride (KCl2) in 4% hydrochloric acid (HCl) were added. CH3203Hg+ was extracted with five washes of 8.3 mL of dichloromethane (DCM). The collected DCM was evaporated by bubbling nitrogen gas into the solution. Afterwards, the CH3203Hg+ was collected and stored at −20°C. The purity of the extracted CH3203Hg+ has been confirmed previously by thin layer chromatography (Rouleau 1997).
Calculations
Lumen-to-Cell Flux of 203Hg
Transport of 203Hg in lumen-to-cell transport experiments was determined by measuring the rate at which 203Hg disappeared from the luminal fluid. This disappearance flux (JD) (fmol min−1 (mm tubule length)−1) measurement was calculated by equation #1:
| 1) |
Where [203Hg]P and [203Hg]C are the concentrations (fmol nL−1) of 203Hg in the perfusate and collectate, respectively. [203Hg]P and [203Hg]C were determined from the specific activity of 203Hg. L is the length (mm) of the perfused tubular segment. VC is the collectate collection rate (nL min−1), which was measured from the time required to fill the constant volume pipette. VP is the perfusion rate (nl min−1) and was calculated by equation #2:
| 2) |
Where [VM]C and [VM]P are the concentrations (cpm nL−1) of the Volume Marker (3H-L-glucose) in the collectate and perfusate respectively. The final [VM]C was determined by adding the amount of [3H]-L-glucose (cpm min−1 x collectate collection time, min) that leaked into the bathing solution during the collection period to the collectate [3H]-L-glucose (cpm) then divided by the volume of the constant volume pipette, nL.
Tubular 203Hg Content
203Hg extracted from the tubule was divided into two fractions: precipitable and non-precipitable. The precipitable fraction is defined as the 203Hg that remained in the tubular structure that became rigid, white and opaque when place in the TCA solution. Presumably, the 203Hg is bound to cellular structures. The non-precipitable fraction is defined as the 203Hg that is extracted in the TCA fluid. Presumably, this 203Hg was free in the cell cytoplasm. Equation 3 was used to calculate both the precipitable fraction and the non-precipitable fraction.
| 3) |
Where HgPrecipitable or Non-precipitable (fmol (mm tubular length)−1) is the precipitable or non-precipitable amounts reported; cpmHg is the amount (cpm) of 203Hg in the precipitable or the non-precipitable fraction, respectively; while fmol cpm−1 is the specific activity of the luminal fluid 203Hg (perfusate). The total tubular content is the sum of the precipitable and the non-precipitable fractions, fmol min−1 (mm tubular length)−1.
Statistical Analyses
A minimum of five tubules were perfused under each experimental condition, with three samples per tubule. Data for each variable assessed were obtained from tubular segments isolated from at least two animals. For each perfused tubular segment, three measurements of disappearance flux (JD) for CH3Hg+ were averaged. The mean values for JD were used to compute the overall mean and standard error of the mean for each experimental condition. The same analytical sequence was used on the data for determining cellular concentration of mercuric ions in the perfused tubules.
Each set of data was first analyzed with the Smirnov-Kolmogorov test to assess normality and Levene’s test to assess for homogeneity of variance. Then, a one-way analysis of variance (ANOVA) was applied to all relevant data for each parameter being evaluated. When statistically significant (P<0.05) F-values were obtained by ANOVA, Tukey’s post-hoc test was used to determine statistically significant differences among all relevant pairs of means for each parameter. Values were assumed to be significantly different at P<0.05.
RESULTS
Effect of L-cystine and L-methionine on the Lumen-to-Cell Transport of Cys-S-CH3Hg
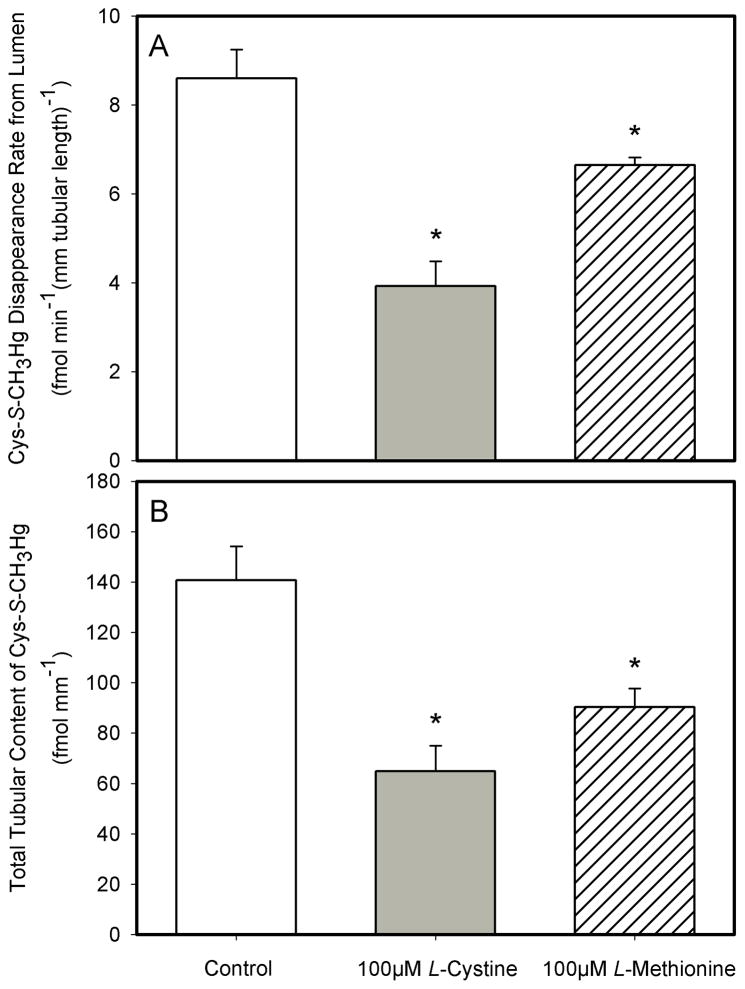
When 2 μM Cys-S-CH3Hg was perfused through the lumen of S2 segments of the rabbit proximal tubule for 30 minutes, the mean disappearance flux rate (JD) for Cys-S-CH3Hg was 8.6 ± 0.7 fmol min−1 (mm tubular length)−1) (Figure 1A). Addition of 100 μM L-cystine to the perfusate caused the mean JD in perfused S2 segments to be significantly lower (3.9 ± 0.6 fmol min−1 (mm tubular length)−1) than that in the control S2 segments perfused through the lumen with only 2 μM Cys-S-CH3Hg. On average, the JD was 54% less than that in the control S2 segments (Figure 1A). Addition of 100μM L-methionine to the perfusate containing 2μM Cys-S-CH3Hg also had a significantly effect on the net uptake of Cys-S-CH3Hg in S2 segments of the rabbit proximal tubule. On average, the JD for Cys-S-CH3Hg in the presence of 100μM methionine was approximately 22.7% less (8.6 ± 0.7 to 6.7 ± 0.2 fmol min−1 (mm tubular length)−1) than that in the S2 segments perfused with 2μM Cys-S-CH3Hg (Figure 1A).
Figure 1.
The effect of 100μM L-cystine and 100 μM L-methionine on the lumen-to-cell transport (A) and total tubular content (B) of Cys-S-CH3Hg in isolated S2 segments of the proximal tubule perfused through the lumen with 2 μM Cys-S-CH3Hg (at 37°C). Each value represents the mean ± S.E.M for a sample size of five or six. The “*” indicates a significant difference (P<0.05) from control S2 segments.
The mean total tubular content of Cys-S-CH3Hg that accumulated in the perfused S2 segments over the course of 30 minutes being perfused through the lumen with 2 μM Cys-S-CH3Hg was 140.6 ± 13.5 fmol (mm tubular length)−1 (Figure 1B). Addition of 100μM L-cystine to the perfusate caused a significant decrease in net accumulation of Cys-S-CH3Hg (a decrease to 64.9 ± 10.11 fmol (mm tubular length)−1). Interestingly, the net decrease in accumulation of 2 μM Cys-S-CH3Hg was also about 54% less than that in the control S2 segments perfused with Cys-S-CH3Hg (Figure 1B). In the S2 segments perfused with 100 μM L-methionine and 2 μM Cys-S-CH3Hg, the total tubular content of Cys-S-CH3Hg after 30 minutes of perfusion was 90.29 ± 7.4 fmol (mm tubular length)−1. This amount of accumulation of Cys-S-CH3Hg was about 35.8% less than that in the control S2 segments (Figure 1B).
Comparison of Transport of Cys-S-CH3Hg Among S1, S2 and S3 Segments of the Proximal Tubule
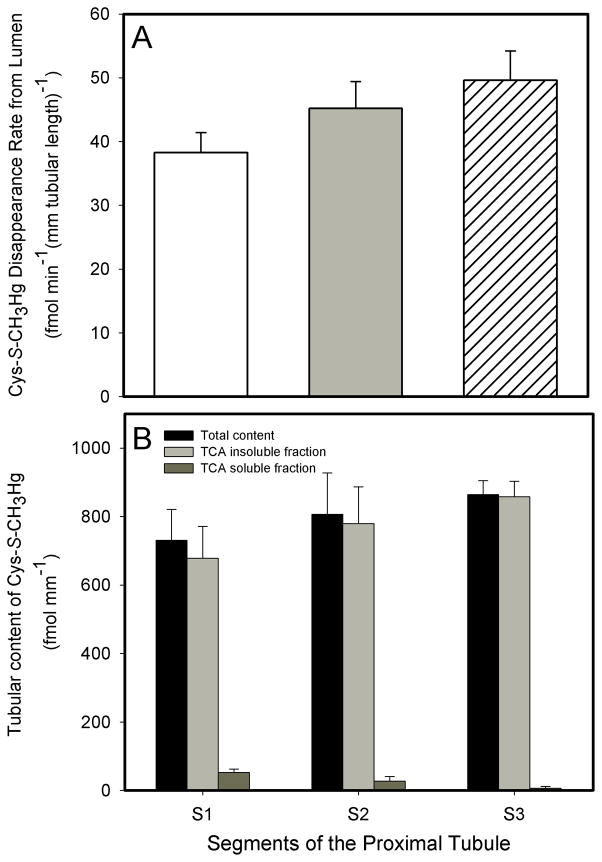
When a perfusate containing 20μM Cys-S-CH3Hg was perfused through the lumen of S1, S2, and S3 proximal tubular segments for 30 minutes, the following transport data were obtained: In S1 segments, the JD of Cys-S-CH3Hg was 38.2 ± 3.2 fmol min−1 (mm tubular length)−1, the total tubular content of Cys-S-CH3Hg was 730.5 ± 90.8 fmol (mm tubular length)−1, the TCA precipitable fraction of Cys-S-CH3Hg was 678.2 ± 93.3 fmol (mm tubular length)−1 and the TCA non-precipitable fraction of Cys-S-CH3Hg was 52.3 ± 10.3 fmol (mm tubular length)−1. In S2 segments, the JD of Cys-S-CH3Hg was 45.2 ± 4.2 fmol min−1 (mm tubular length)−1, the total tubular content of Cys-S-CH3Hg was 806.7 ± 120.5 fmol (mm tubular length)−1, the TCA precipitable fraction of Cys-S-CH3Hg was 778.9 ± 108.1 fmol (mm tubular length)−1 and the TCA non-precipitable fraction of Cys-S-CH3Hg was 27.8 ± 13.0 fmol (mm tubular length)−1. In S3 segments, the JD of Cys-S-CH3Hg was 49.6 ± 4.6 fmol min−1 (mm tubular length)−1, the total tubular content of Cys-S-CH3Hg was 864.3 ± 40.8 fmol (mm tubular length)−1, the TCA precipitable fraction is 857.8 ± 44.9 fmol (mm tubular length)−1 and the TCA non-precipitable fraction was 6.5 ± 5.4 fmol (mm tubular length)−1.
There were no significant differences in JD among the perfused S1, S2, and S3 segments (Figure 2A). Greater than 95% of the 203Hg+ were found in the TCA-precipitable fraction of the tubular extract in all experiments, shown in Figure 2B.
Figure 2.
Rate of the luminal disappearance (A) and total tubular content, TCA precipitable & TCA non-precipitable fractions (B) of Cys-S-CH3Hg (20 μM) in S1, S2, and S3 segments of the proximal tubule of the rabbit. Each value represents the mean ± S.E.M for a sample size of five or six.
Effect of Acivicin on the Lumen-to-Cell Transport of the GSH-S-CH3Hg Conjugate
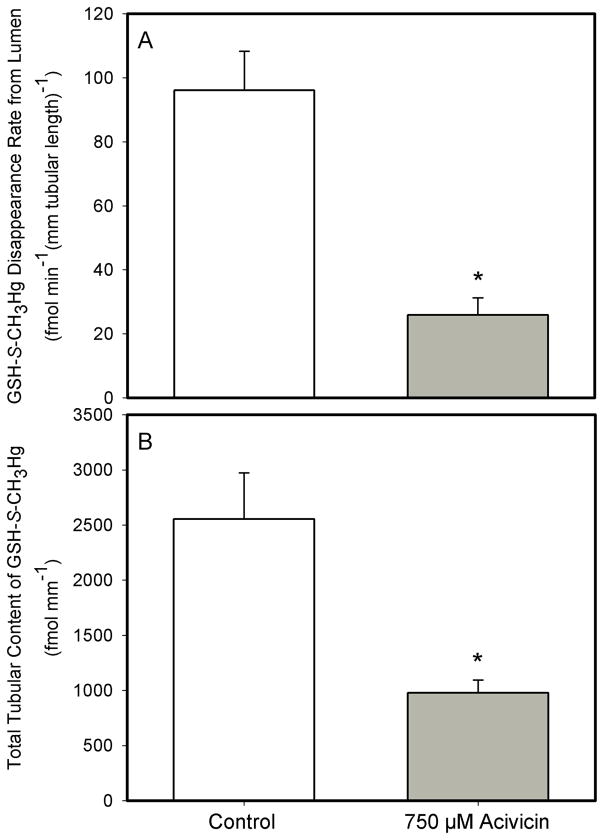
Addition of 750μM acivicin (a specific inhibitor of the luminal plasma membrane enzyme γ-glutamyltransferase) to a perfusate containing 20 μM of the GSH-S-conjugate of CH3Hg+ (GSH-S-CH3Hg) resulted in significant changes in transport of GSH-S-CH3Hg (Figure 3). More specifically, the JD of GSH-S-CH3Hg in tubules perfused through the lumen with 20 μM GSH-S-CH3Hg in the presence of 750 μM acivicin, was 73% lower (25.9 ± 5.4 fmol min−1 (mm tubular length)−1) than that (96.1 ± 12.2 fmol min−1 (mm tubular length)−1) in corresponding tubular segments not exposed to acivicin. Tubular accumulation of GSH-S-CH3Hg corresponded to the relative levels of JD under the two experimental conditions (2552.4 ± 519.5 fmol (mm tubular length)−1 in the absence of acivicin and 978.7 ± 113.6 fmol (mm tubular length)−1 in the presence of acivicin (Figure 3).
Figure 3.
The effect of 750μM acivicin on the lumen-to-cell transport (A) and total tubular content (B) of GSH-S-CH3Hg in isolated S2 segment of the proximal tubule of the rabbit perfused with 20 μM GSH-S-CH3Hg (at 37°C). Each value represents the mean ± S.E.M for a sample size of five or six. The “*” indicates a significant difference (P<0.05) from control S2 segments.
Comparison of Lumen-to-Cell Transport of the GSH-S-CH3Hg and Cys-S-CH3Hg Conjugates
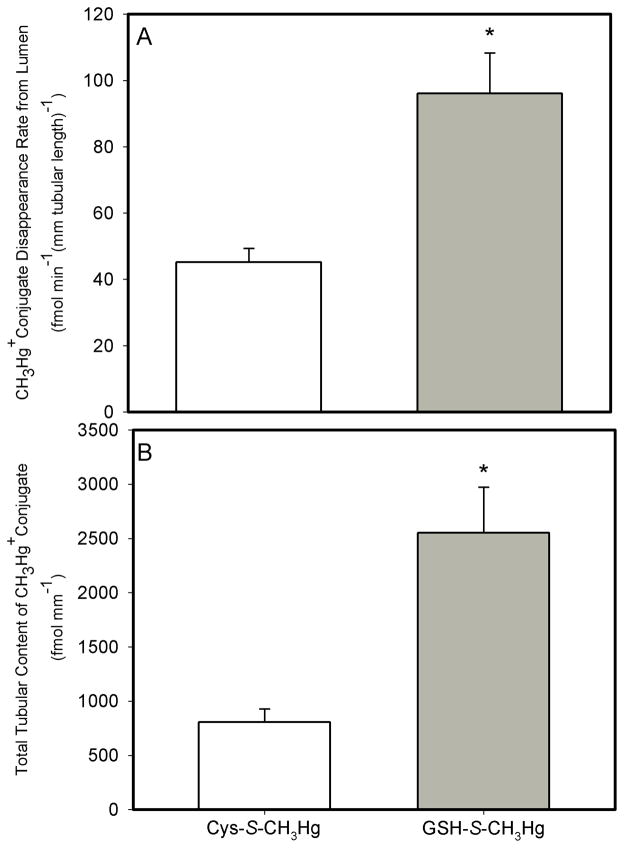
The JD of GSH-S-CH3Hg averaged 96.1 ± 12.2 fmol min−1 (mm tubular length)−1 in the tubular segments perfused with 20 μM GSH-S-CH3Hg. Interestingly, this value for JD of GSH-S-CH3Hg was 113% greater than that for that in the tubules perfused with Cys-S-CH3Hg. Moreover, the total tubular content of GSH-S-CH3Hg in the tubular segments perfused with GSH-S-CH3Hg only was 216% greater than that in the tubular segments perfused with Cys-S-CH3Hg only (Figure 4).
Figure 4.
Rate of luminal disappearance of both conjugates (A) and total tubular content (B) of when perfused with Cys-S-CH3Hg and GSH-S-CH3Hg in isolated S2 segments of the proximal tubule of the rabbit. The perfusate concentration was 20 μM for both solutes. Each value represents the mean ± S.E.M for a sample size of five or six.
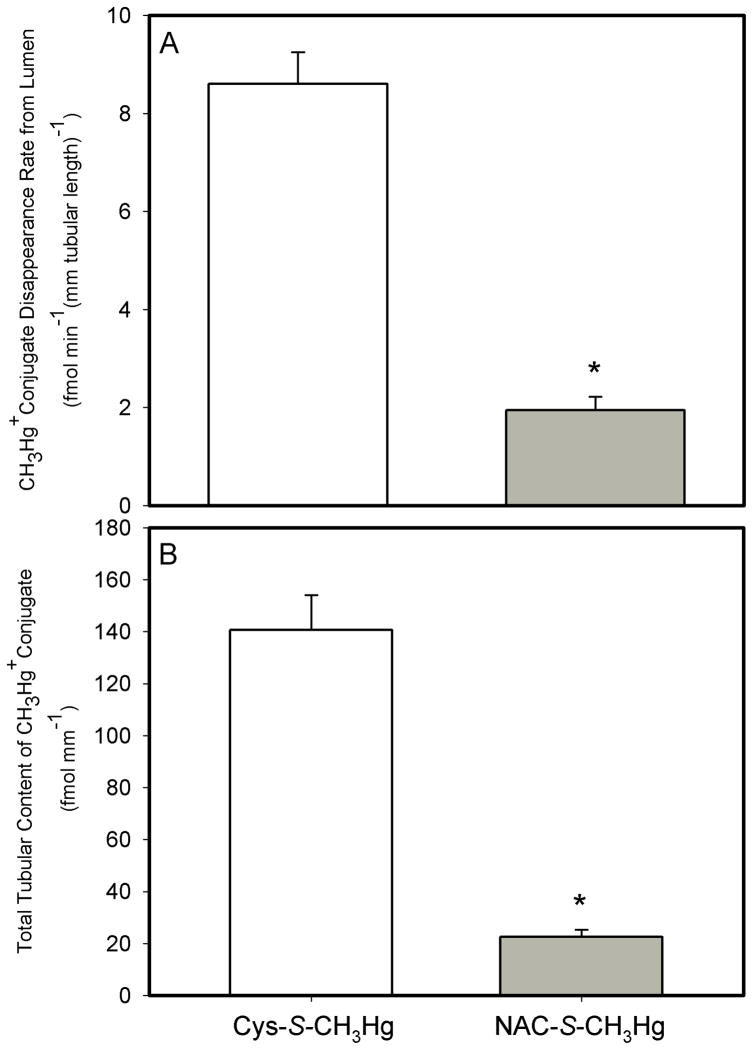
Lumen-to-Cell Transport of the NAC-S-CH3Hg Conjugate
By contrast, when 2μM of the N-acetylcysteine (NAC) S-conjugate of CH3Hg+ (NAC-S- CH3Hg) was perfused through the lumen of S2 segments of the rabbit proximal tubule, the JD of NAC-S-CH3Hg averaged 1.9 ± 0.2 fmol min−1 (mm tubular length)−1 and the total tubular content of NAC-S- CH3Hg was 22.6 ± 2.7 fmol (mm tubular length)−1 (Figure 5). This JD of NAC-S-CH3Hg was significantly lower (by approximately 77%) than those perfused with 2μM Cys-S-CH3Hg only. The total tubular content of NAC-S-CH3Hg was also significantly (84%) lower than the Cys-S-CH3Hg control tubules (Figure 5).
Figure 5.
Rate of luminal disappearance of the conjugates (A) and total tubular content (B) of Cys-S-CH3Hg and GSH-S-CH3Hg in isolated S2 segments of the proximal tubule of the rabbit. The perfusate concentration was 2 μM for both solutes. Each value represents the mean ± S.E.M for a sample size of five or six.
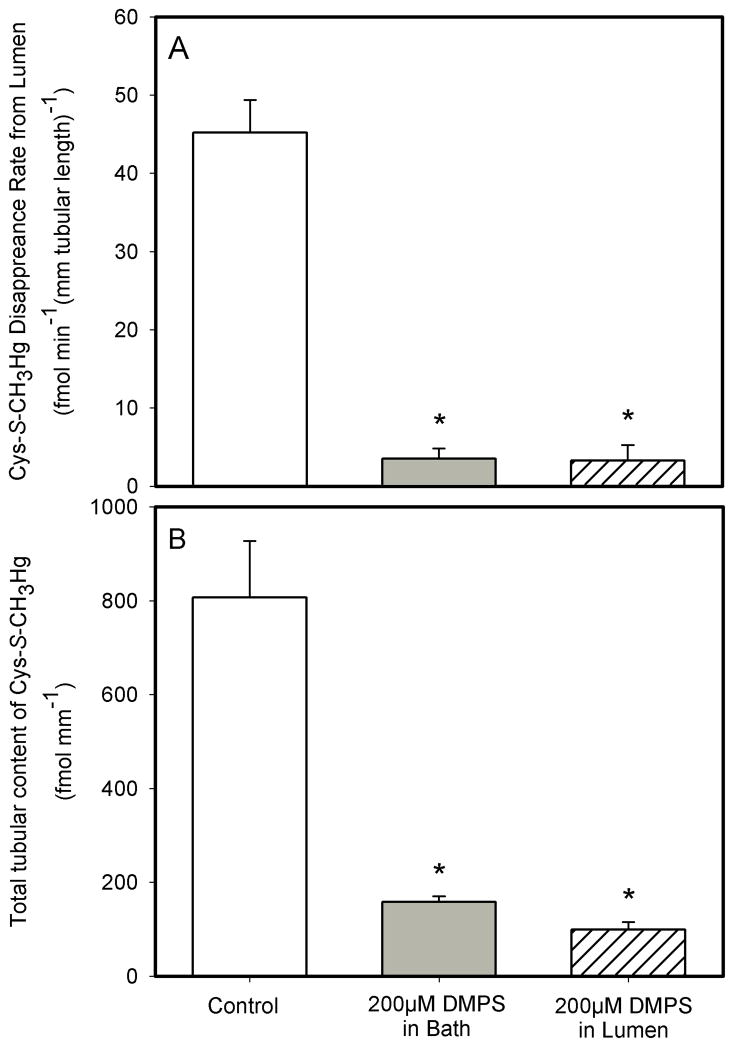
Effect of DMPS on the Lumen-to-Cell Transport of Cys-S-CH3Hg Conjugate
Flux experiments with DMPS were carried out under three conditions. Under control conditions, S2 segments of the proximal tubule were perfused through the lumen with 20 μM Cys-S-CH3Hg, with no DMPS in either the perfusate or bath. Under the second set of experimental conditions, 200 μM DMPS was added only to the solution bathing the basolateral surface of the perfused tubular segments. In the third experimental condition, 200 μM DMPS was added to the perfusate which also contained 20 μM Cys-S-CH3Hg.
When 200 μM DMPS was added to the bath only, the JD of Cys-S-CH3Hg was about 92% less (dropping from 45.2 ± 4.2 fmol min−1 (mm tubular length)−1 to 3.5 ± 1.3 fmol min−1 (mm tubular length)−1) than that in the tubular segments not exposed to DMPS (Control) (Figure 6A). In the corresponding tubular segments exposed to DMPS in the bath only, the total net tubular content of Cys-S-CH3Hg was 158.4 ± 11.8 fmol (mm tubular length)−1, which was 80% less than that in the control tubules (806.7 ± 120.5 fmol (mm tubular length)−1) (Figure 6B).
Figure 6.
The effect of 200 μM DMPS on the luminal rate of disappearance (A) and total tubular content (B) of Cys-S-CH3Hg (20 μM) in isolated S2 segments of the rabbit proximal tubule perfused with DMPS in the bathing solution only or co-perfused with Cys-S-CH3Hg (at 37°C). Each value represents the mean ± S.E.M for a sample size of five or six. The “*” indicates a significant difference (P<0.05) from control S2 segments.
When 200 μM DMPS was co-perfused with Cys-S-CH3Hg in the lumen, the JD of Cys-S-CH3Hg (3.25 ± 1.99 fmol min−1 (mm tubular length)−1) was very low, and it averaged about 93% less than that in control tubules. Accordingly, the total tubular content of Cys-S-CH3Hg was also very low (99.1 ± 16.5 fmol (mm tubular length)−1), averaging about 87% less than that in control tubules, Cys-S-CH3Hg only (Figure 6B).
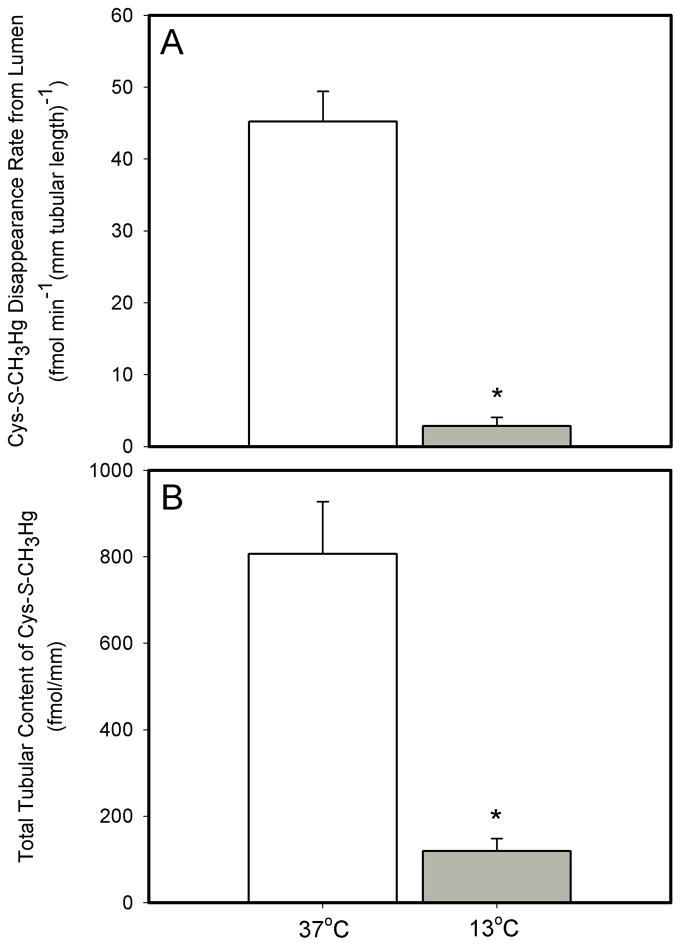
Effect of Temperature on the Transport of Cys-S-CH3Hg Conjugate
To assess whether the transport of Cys-S-CH3Hg (when presented to the luminal compartment as Cys-S-CH3Hg) is modulated by temperature, S2 segments of proximal tubules were perfused with 20μM Cys-S-CH3Hg at 37°C and 13°C. The JD of Cys-S-CH3Hg at these temperatures was 45.2 ± 4.2 and 2.8 ± 1.2 fmol min−1 (mm tubular length)−1, respectively. The tubular content of Cys-S-CH3Hg in the corresponding S2 segments was 806.7 ± 120.5 and 119.2 ± 29.1 fmol (mm tubular length)−1. Significant decreases in JD (94% reduction at 13°C), tubular content (85.2% reduction at 13°C) of Cys-S-CH3Hg were observed in response to these reductions in temperature (Figure 7).
Figure 7.
The effect of temperature on the lumen-to-cell transport (A) and total tubular content (B) of Cys-S-CH3Hg in isolated S2 segments of the rabbit proximal tubule perfused with 20 μM CH3Hg-Cys (at 37°C). Each value represents the mean ± S.E.M for a sample size of five or six. The “*” indicates a significant difference (P<0.05) from control S2 segments.
No Acute Cellular Intoxicated of Tubular Segments
No visual evidence of acute cellular toxicity, such as cellular swelling, blebbing of the luminal membrane, or uptake of the cellular vital dye (FD&C green), was noted in any of the S1, S2 and S3 segments used in the experiments of this study. In addition, intercellular leak of the volume marker ([3H]-L-glucose) did not increase under any of the experimental conditions studied.
DISCUSSION
Findings from previous studies have suggested that thiol S-conjugates of CH3Hg+ may be the primary transportable forms of CH3Hg+ in the kidney (Carty and Malone 1979; Zalups 2000). The present study tested indirectly the hypothesis that luminal membrane transport of three thiol S-conjugates of CH3Hg+, namely Cys-S-CH3Hg, GSH-S-CH3Hg, and NAC-S-CH3Hg, are absorbed along the three segments (S1, S2 and S3) of the proximal tubule via carrier-mediated processes. The data indicate that Cys-S-CH3Hg is likely absorbed avidly by the S2 segments of the proximal tubule, while NAC-S-CH3Hg is not as avidly transported.
Absorptive transport of Cys-S-CH3Hg has been demonstrated recently to be mediated by Xenopus Laevis oocytes expressing B0+ (Bridges and Zalups 2006). Moreover, it was demonstrated that this transport could be inhibited by L-methionine, suggesting that system B0+ may participate in the luminal absorptive transport of Cys-S-CH3Hg in renal proximal tubules (Bridges and Zalups 2006). To provide additional support for this hypothesis, we studied the luminal transport of Cys-S-CH3Hg in perfused proximal tubular segments in the presence of L-methionine. We also demonstrate that L-methionine can inhibit significantly the absorptive transport of Cys-S-CH3Hg in pars recta segments of the proximal tubule (Figure. 1), indicating that an amino acid transporter involved in the uptake of methionine likely participates in the luminal uptake of Cys-S-CH3Hg.
Based on the hypothesis that Cys-S-CH3Hg may have chemical morphology similar to the amino acid, L-cystine, we measured the absorptive transport of Cys-S-CH3Hg in the presence of L-cystine. The L-cystine transporter, system b0,+ like system B0+, is localized almost exclusively in the luminal membrane of proximal tubular epithelial cells (Furriols, Chillaron et al. 1993; Mora, Chillaron et al. 1996), and has been implicated in the renal cellular uptake of the Cys S-conjugate of the divalent forms of inorganic mercury (Hg2+), Cys-S-Hg-S-Cys (Bridges, Bauch et al. 2004), and cadmium (Cd2+), Cys-S-Cd-S-Cys (Wang, Zalups et al. 2010). Interestingly, we demonstrate in the present study that L-cystine does indeed inhibit significantly the transport of Cys-S-CH3Hg (Figure 1). This finding lends support to the hypothesis that system b0,+ may also be involved in the luminal uptake of Cys-S-CH3Hg in proximal tubular cells.
Possible axial heterogeneity in the transport of Cys-S-CH3Hg among all three segments of the proximal tubule, i.e. S1, S2, and S3 segments, was also evaluated in this study. Our data show, however, that there are no significant differences in disappearance flux (JD) of Cys-S-CH3Hg and the cellular distribution among these three segments (Figure 2). The absence of a difference in luminal uptake and cellular accumulation of Cys-S-CH3Hg may relate to the distribution of transporters capable of transporting Cys-S-CH3Hg along the length of the proximal tubule. For example, expression of system B0,+ occurs primarily in the S1 segment and decreases gradually from S1 to S3 segments (Fleck, Schwertfeger et al. 2003). In contrast, the expression of system b0+ is minimal in the S1 segment, but increases progressively from the S1 to the S3 segments (Fleck, Schwertfeger et al. 2003). Due to this reciprocal axial distribution of these two transporters along the proximal tubule, the combined transport of these two transporters may result in similar levels of absorptive transport of Cys-S-CH3Hg along the length of the entire proximal tubule. These observations also support our hypotheses that Cys-S-CH3Hg may utilize more than one transport system in its luminal uptake.
GSH-S-CH3Hg has also been suggested to be a transportable form of CH3Hg+ in tissues and organs such as brain and liver (Dutczak and Ballatori 1994). GSH is a metabolic reservoir for L-cysteine and is more abundant than free L-cysteine in mammalian cells. γ-Glutamyltransferase and cysteinylglycinase, enzymes that metabolize GSH to L-cysteine are present in great abundance in the luminal plasma membrane of proximal tubular cells (Zalups 2000). When these enzymes act on GSH-S-CH3Hg, the methyl mercuric ion remains bonded to L-Cys during the catabolism of GSH to Cys (Naganuma, Oda-Urano et al. 1988). Therefore, Cys-S-CH3Hg is likely the primary transportable form of CH3Hg+, and not GSH-S-CH3Hg. Use of the inhibitory alkylator of γ-glutamyltransferase, acivicin, provides additional support for this notion (Figure 3). Our findings are consistent with those of previous studies showing that pretreatment with acivicin decreases renal accumulation and increases urinary excretion of CH3Hg+ (Berndt, Baggett et al. 1985; Gregus, Stein et al. 1987; Naganuma, Oda-Urano et al. 1988; de Ceaurriz and Ban 1990). Interestingly, rates of GSH-S-CH3Hg transport were about twice that of Cys-S-CH3Hg (98 vs 45 fmol min−1 (mm tubular length)−1) at a luminal concentration 20 μM (Figure 4) and there was not 100% inhibition of GSH-S-CH3Hg transport by acivicin (98 to 25 fmol min−1 (mm tubular length)−1). Both of these observations indicate that perhaps some GSH-S-CH3Hg may be transported across the luminal membrane intact.
We also examined the transport of the NAC S-conjugate of CH3Hg+ (NAC-S-CH3Hg), which is a polar negatively charged species. Our data are consistent with previous findings showing that NAC-S-CH3Hg is transported poorly by Xenopus Laevis oocytes expressing the luminal transporter system B0+ (Bridges and Zalups 2006). The very low rate of uptake of NAC-S-CH3Hg at the luminal plasma membrane of S2 segments is likely related to the net negative charge of this molecule. Interestingly, luminal secretion of NAC-S-CH3Hg was first noted when NAC was observed to cause transient increases in urinary excretion of methylmercury (Aremu, Madejczyk et al. 2008). Current literature indicates that the organic anion transporter 1 (OAT1) in the basolateral plasma membrane and multidrug resistance protein 2 (MRP2) in the luminal plasma membrane are involved in the enhanced urinary excretion of CH3Hg+ mediated by administration of NAC. Accordingly, NAC-S-CH3Hg in blood appears to be a substrate taken up into the proximal tubular epithelial cells by OAT1 at the basolateral membrane (Koh, Simmons-Willis et al. 2002; Zalups and Ahmad 2005). Subsequently, intracellular NAC-S-CH3Hg is extracted or eliminated by the epithelial cells by secretion into tubular lumen via the actions of the ATP-binding cassette protein, MRP2, (Madejczyk, Aremu et al. 2007). It is possible that the NAC-S-CH3Hg which gained access into the epithelial cells lining the S2 segments of the proximal tubule in the present study would have been made available for secretion back into the tubular lumen by the actions of MRP2 (Ballatori 2002). This could also serve as an explanation for the low rate in net absorption and tubular content of 203Hg that we observed (Figure 5).
DMPS is a metal complexing agent known to increase the renal clearance and urinary excretion of CH3Hg+ (Aposhian 1983; Aposhian, Maiorino et al. 1992). These effects of DMPS have been assumed to be due to unimpeded filtration of DMPS S-conjugates of CH3Hg+ at renal glomeruli and secretion of CH3Hg+ from within proximal tubular epithelial cells, subsequent to the formation of transportable DMPS S-conjugates of CH3Hg+. A series of recent studies have demonstrated the proximal tubular extraction of DMPS S-conjugates of CH3Hg+ is mediated by the multi-drug resistance protein, MRP2 (Zalups and Bridges 2009). It had been reported that DMPS S-conjugates of CH3Hg+ may be substrates for the organic anion transporter 1 (OAT1) (Koh, Simmons-Willis et al. 2002), also definitive proof of this transport remains lacking. Overall, there is sufficient evidence indicating that there are likely transport-mechanisms involved in the secretion of DMPS S-conjugates of CH3Hg+ by the epithetical cells of the proximal tubule. In the present study, when DMPS was added to the luminal fluid, absorptive transport and accumulation of Cys-S-CH3Hg was reduced to very low to negligible levels (Figure 6). These findings suggest that once formed in the lumen, the DMPS S-conjugate of Cys-S-CH3Hg or CH3-Hg+ is not transported into proximal tubular epithelial cells at the luminal membrane. Similar findings were obtained when DMPS was placed in the basolateral compartment while tubules were perfused with Cys-S-CH3Hg. In this case, the low levels of net absorptive transport are likely due to avid basolateral uptake of DMPS and subsequent binding to Cys-S-CH3Hg or CH3-Hg+ intracellularly and/or in the lumen. Once the conjugate enters or is formed in the lumen, it becomes a molecular species not readily taken up by proximal tubular epithelial cells. It can only be speculated as to what conjugate is formed in these circumstances, DMPS-HgCH3-S-Cys or DMPS-S-HgCH3.
It is note-worthy to mention that greater than 95% of the mercuric ions of conjugated-S-CH3203Hg associated with the perfused proximal tubular segments were present in a TCA-precipitable fraction of the tubular extract in all experiments (Figure 2B). This is in contrast to our findings from proximal tubular segments tubules perfused with non-conjugated CH3Hg+ (Zalups and Barfuss 1993) where the majority of the metal ions was found in the TCA- or acid-soluble fraction of the tubule extract. It is presumed that TCA extracts and precipitates large cellular proteins because the tubule retains its overall shape, and becomes rigid, white, and opaque after exposure to TCA. The non-precipitable fraction is presumed to be acid-soluble cytosolic contents. There is no current explanation as to why CH3Hg+ conjugates and free CH3Hg+ are distributed differently between these extracts.
The temperature-dependent experiments indicate strongly that the luminal uptake of Cys-S-CH3Hg involves specific carrier proteins, and that the uptake is not due to non-specific binding and/or paracellular leakage (Figure 7). The leak properties of tight junctions remained unchanged since the leak rate of the volume marker was not altered when temperature was altered.
In conclusion, the current studies show that Cys-S-CH3Hg and GSH-S-CH3Hg appear to be transportable forms of CH3Hg+ conjugates at the luminal membrane of proximal tubular epithelial cells. Cys-S-CH3Hg appears to utilize amino acid transporters involved in the transport of L-methionine and L-cystine. GSH-S-CH3Hg appears to be degraded to Cys-S-CH3Hg by exo-enzymes in the luminal membrane, which is subsequently transported into proximal tubular epithelial cells, and transported intact. NAC-S-CH3Hg and the DMPS conjugate of Cys-S-CH3Hg (or DMPS-HgCH3?) are not transported efficiently, if at all, at the luminal membrane of proximal tubular epithelial cells.
Luminal transport of thiol conjugates of CH3Hg+ were studied for the first time
CH3Hg+ conjugates of Cys and GSH are transported avidly
CH3Hg+ conjugates of NAC and DMPS are transported minimally
Transport of Cys-S-CH3Hg is inhibited by L-methionine and L-cystine
Transport of GSH-S-CH3Hg was only partially inhibited by acivicin
Acknowledgments
This work was supported by the National Institutes of Environmental Health Sciences (Grants ES05980 to R.K.Z. and D.W.B, ES05157 to R.K.Z.). We would like to thank Mr. Brandon Smith for his suggestions and technical help on this project.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rudolfs K. Zalups, Email: zalups_rk@mercer.edu.
Delon W. Barfuss, Email: dbarfuss@gsu.edu.
References
- Aposhian HV. DMSA and DMPS--water soluble antidotes for heavy metal poisoning. Annu Rev Pharmacol Toxicol. 1983;23:193–215. doi: 10.1146/annurev.pa.23.040183.001205. [DOI] [PubMed] [Google Scholar]
- Aposhian HV, Maiorino RM, et al. Human studies with the chelating agents, DMPS and DMSA. J Toxicol Clin Toxicol. 1992;30(4):505–528. doi: 10.3109/15563659209017938. [DOI] [PubMed] [Google Scholar]
- Aremu DA, Madejczyk MS, et al. N-acetylcysteine as a potential antidote and biomonitoring agent of methylmercury exposure. Environ Health Perspect. 2008;116(1):26–31. doi: 10.1289/ehp.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Clarkson TW. Uptake of methylmercury in the rat brain: effects of amino acids. Brain Res. 1988;462(1):31–39. doi: 10.1016/0006-8993(88)90581-1. [DOI] [PubMed] [Google Scholar]
- Ballatori N. Transport of toxic metals by molecular mimicry. Environ Health Perspect. 2002;110(Suppl 5):689–694. doi: 10.1289/ehp.02110s5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger M, Westin A, et al. Some health physics aspects of working with 203Hg in university research. Health Phys. 2001;80(2 Suppl):S28–30. [PubMed] [Google Scholar]
- Berndt WO, Baggett JM, et al. Renal glutathione and mercury uptake by kidney. Fundam Appl Toxicol. 1985;5(5):832–839. doi: 10.1016/0272-0590(85)90166-6. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Bauch C, et al. Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0,+): implications of molecular mimicry. J Am Soc Nephrol. 2004;15(3):663–673. doi: 10.1097/01.ASN.0000113553.62380.F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. System b0,+ and the transport of thiol-s-conjugates of methylmercury. J Pharmacol Exp Ther. 2006;319(2):948–956. doi: 10.1124/jpet.106.109371. [DOI] [PubMed] [Google Scholar]
- Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008;88(1):249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- Carty AJ, Malone SJ. The chemistry of mercury in biological systems. In: Nriagu JO, editor. The Biogeochemistry of Mercury in the Environment. Vol. 3. Elsevier/North Holand Biomedical Press; Amsterdam: 1979. pp. 433–479. [Google Scholar]
- Clarkson TW. The three modern faces of mercury. Environ Health Perspect. 2002;110(Suppl 1):11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ceaurriz J, Ban M. Role of gamma-glutamyltranspeptidase and beta-lyase in the nephrotoxicity of hexachloro-1,3-butadiene and methyl mercury in mice. Toxicol Lett. 1990;50(2–3):249–256. doi: 10.1016/0378-4274(90)90017-g. [DOI] [PubMed] [Google Scholar]
- Dutczak WJ, Ballatori N. Transport of the glutathione-methylmercury complex across liver canalicular membranes on reduced glutathione carriers. J Biol Chem. 1994;269(13):9746–9751. [PubMed] [Google Scholar]
- Fleck C, Schwertfeger M, et al. Regulation of renal amino acid (AA) transport by hormones, drugs and xenobiotics - a review. Amino Acids. 2003;24(4):347–374. doi: 10.1007/s00726-002-0316-6. [DOI] [PubMed] [Google Scholar]
- Fuhr BJ, Rabenstein DL. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX. The binding of cadmium, zinc, lead, and mercury by glutathione. J Am Chem Soc. 1973;95(21):6944–6950. doi: 10.1021/ja00802a013. [DOI] [PubMed] [Google Scholar]
- Furriols M, Chillaron J, et al. rBAT, related to L-cysteine transport, is localized to the microvilli of proximal straight tubules, and its expression is regulated in kidney by development. J Biol Chem. 1993;268(36):27060–27068. [PubMed] [Google Scholar]
- Gregus Z, Stein AF, et al. Effect of inhibition of gamma-glutamyltranspeptidase on biliary and urinary excretion of glutathione-derived thiols and methylmercury. J Pharmacol Exp Ther. 1987;242(1):27–32. [PubMed] [Google Scholar]
- Kershaw TG, Clarkson TW, et al. The relationship between blood levels and dose of methylmercury in man. Arch Environ Health. 1980;35(1):28–36. doi: 10.1080/00039896.1980.10667458. [DOI] [PubMed] [Google Scholar]
- Koh AS, Simmons-Willis TA, et al. Identification of a mechanism by which the methylmercury antidotes N-acetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: transport by the renal organic anion transporter-1. Mol Pharmacol. 2002;62(4):921–926. doi: 10.1124/mol.62.4.921. [DOI] [PubMed] [Google Scholar]
- Madejczyk MS, Aremu DA, et al. Accelerated urinary excretion of methylmercury following administration of its antidote N-acetylcysteine requires Mrp2/Abcc2, the apical multidrug resistance-associated protein. J Pharmacol Exp Ther. 2007;322(1):378–384. doi: 10.1124/jpet.107.122812. [DOI] [PubMed] [Google Scholar]
- Mokrzan EM, Kerper LE, et al. Methylmercury-thiol uptake into cultured brain capillary endothelial cells on amino acid system L. J Pharmacol Exp Ther. 1995;272(3):1277–1284. [PubMed] [Google Scholar]
- Mora C, Chillaron J, et al. The rBAT gene is responsible for L-cystine uptake via the b0,(+)-like amino acid transport system in a “renal proximal tubular” cell line (OK cells) J Biol Chem. 1996;271(18):10569–10576. doi: 10.1074/jbc.271.18.10569. [DOI] [PubMed] [Google Scholar]
- Naganuma A, Oda-Urano N, et al. Possible role of hepatic glutathione in transport of methylmercury into mouse kidney. Biochem Pharmacol. 1988;37(2):291–296. doi: 10.1016/0006-2952(88)90731-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Hatanaka T, et al. Na+- and Cl−-coupled active transport of carnitine by the amino acid transporter ATB(0,+) from mouse colon expressed in HRPE cells and Xenopus oocytes. J Physiol. 2001;532(Pt 2):297–304. doi: 10.1111/j.1469-7793.2001.0297f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM, Kates B. Histological localization of methylmercury in mouse brain and kidney by emulsion autoradiography of 203Hg. Toxicol Appl Pharmacol. 1988;92(2):224–234. doi: 10.1016/0041-008x(88)90382-1. [DOI] [PubMed] [Google Scholar]
- Rouleau CaBM. Fast and high yield synthesis of radioactive CH3203 Hg (II) Appl Organomet Chem. 1997;2:751–753. [Google Scholar]
- Simmons-Willis TA, Koh AS, et al. Transport of a neurotoxicant by molecular mimicry: the methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem J. 2002;367(Pt 1):239–246. doi: 10.1042/BJ20020841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan JL, Mager S. Cloning and functional expression of a human Na(+) and Cl(−)-dependent neutral and cationic amino acid transporter B(0+) J Biol Chem. 1999;274(34):23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zalups RK, et al. Potential mechanisms involved in the absorptive transport of cadmium in isolated perfused rabbit renal proximal tubules. Toxicol Lett. 2010;193(1):61–68. doi: 10.1016/j.toxlet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Jiang H, et al. The methylmercury-L-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J Neurochem. 2008;107(4):1083–1090. doi: 10.1111/j.1471-4159.2008.05683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev. 2000;52(1):113–143. [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Handling of cysteine S-conjugates of methylmercury in MDCK cells expressing human OAT1. Kidney Int. 2005;68(4):1684–1699. doi: 10.1111/j.1523-1755.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Handling of the homocysteine S-conjugate of methylmercury by renal epithelial cells: role of organic anion transporter 1 and amino acid transporters. J Pharmacol Exp Ther. 2005;315(2):896–904. doi: 10.1124/jpet.105.090530. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Transport of N-acetylcysteine s-conjugates of methylmercury in Madin-Darby canine kidney cells stably transfected with human isoform of organic anion transporter 1. J Pharmacol Exp Ther. 2005;314(3):1158–1168. doi: 10.1124/jpet.105.086645. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Barfuss DW. Transport and toxicity of methylmercury along the proximal tubule of the rabbit. Toxicol Appl Pharmacol. 1993;121(2):176–185. doi: 10.1006/taap.1993.1143. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Bridges CC. MRP2 involvement in renal proximal tubular elimination of methylmercury mediated by DMPS or DMSA. Toxicol Appl Pharmacol. 2009;235(1):10–17. doi: 10.1016/j.taap.2008.11.003. [DOI] [PubMed] [Google Scholar]