Abstract
Spermatogenesis is a complex process that requires coordinated proliferation and differentiation of male germ cells. The molecular events that dictate this process are largely unknown, but are likely to involve highly regulated transcriptional control. In this study, we investigate the contribution of chromatin associated Sin3A in mouse germ cell lineage development. Genetic inactivation of Sin3A in the male germline leads to sterility that results from the early and penetrant apoptotic death observed in Sin3A-deleted germ cells, coincident with the reentry in mitosis. Sin3A-deleted testes exhibit a Sertoli-cell only phenotype, consistent with the absolute requirement for Sin3A in germ cells’ development and/or viability. Interestingly, transcripts analysis revealed that the expression program of Sertoli cells is altered upon inactivation of Sin3A in germ cells. These studies identified a central role for the mammalian Sin3-HDAC complex in the germ cell lineage, and point to an exquisite transcriptional crosstalk between germ cells and their niche to support fertility in mammals.
Keywords: Sin3A, testis, germ cells, transcription, Sertoli cells, knock-out mouse
Introduction
Throughout development and adult life, cell fate decisions are highly regulated events. Among the different layers of regulation that ensure proper cellular proliferation and differentiation, accurate gene expression is crucial and is achieved, in part, through precise transcriptional regulation. This level of regulation is accomplished via locus specific recruitment of factors that dictate the tethering and/or activity of the basal transcriptional machinery, in concert with coactivator or corepressor complexes. Recent technical advances have been used in genomewide studies to elucidate the molecular events dictating transcription machinery recruitment in embryonic stem cells and in somatic cells in culture (Young, 2011). By contrast, the molecular events dictating transcriptional regulation in the germ cell lineage remain largely elusive, in part due to the lack of an in vitro cellular system that faithfully recapitulates all aspects of mammalian germ cell proliferation and maturation (White-Cooper and Davidson, 2011).
Among co-factors that participate in the regulation of transcription, histone deacetylases (HDAC)- containing complexes, which function as co-repressor complexes, have been the subject of numerous studies in the past. Notably, HDAC inhibitors have proven efficient as anticancer agents in certain types of tumors (Dokmanovic and Marks, 2005); however, the molecular bases for these effects remain unknown. In addition, HDAC-containing complexes are highly conserved throughout evolution, pointing to central functions in cellular physiology (McDonel et al., 2009). Among the numerous HDAC containing complexes, the Sin3 co-repressor complex regulates a large number of transcriptional nodes in somatic cells (Silverstein and Ekwall, 2004). Mammalian Sin3 proteins, comprised of the large Sin3A protein and the closely related Sin3B protein, were first identified as essential co-repressors for several sequence specific transcription factors, including the Myc-antagonist Mad family of proteins (Ayer et al., 1995; Schreiber-Agus et al., 1995). Shortly after their identification, Sin3 proteins were found to be integral components of a large co-repressor complex, containing the class I histone deacetylases HDAC1 and HDAC2 (Alland et al., 1997; Hassig et al., 1997; Heinzel et al., 1997; Laherty et al., 1997). Biochemical approaches led to the delineation of a Sin3 core complex, conserved in eukaryotes and essential for the repression driven by a wide variety of sequence specific transcriptional repressors (Silverstein and Ekwall, 2004). Recently, we and others have engineered conditional alleles to delineate the function of the mammalian Sin3 and Sin3-associated proteins in mice (Cowley et al., 2005; Dannenberg et al., 2005; David et al., 2008; David et al., 2003). While Sin3A and Sin3B are expressed in all cell types examined so far, their genetic inactivation leads to divergent phenotypes. Sin3B deleted embryos develop until late gestation, but die around birth (David et al., 2008). By contrast, Sin3A null embryos are not found past the blastocyst stage, pointing to an early embryonic requirement for Sin3A. In agreement with the inability of Sin3A-null blastocysts to survive, acute somatic inactivation of Sin3A in embryonic fibroblasts produces rapid cell death, associated with defects in heterochromatin formation (Cowley et al., 2005; Dannenberg et al., 2005). Genetic inactivation of Sin3A in myotubes induces perinatal lethality, correlating with severe defects in sarcomeric structure (van Oevelen et al., 2010). In both fibroblasts and myotubes, the normal transcriptional program is widely altered upon Sin3A inactivation, suggesting that Sin3A regulates basic cellular functions. Together, these observations have led to the hypothesis that Sin3A is required for cellular viability in differentiated cells. Additionally, a recent study has pointed to a function of Sin3A in Sertoli cells in mice (Payne et al., 2010). Finally, it has been demonstrated that embryonic stem cells devoid of Sin3A undergo apoptosis due to unresolved double strand breaks, suggesting that Sin3A functions may be conserved in undifferentiated cells (McDonel et al., 2012).
Despite these observations, the contribution of Sin3A to germ cell biology has not been directly examined. To investigate the function of Sin3A in the germ cell lineage, we have genetically inactivated Sin3A in germ cells in the mouse, and analyzed the resulting phenotypes. Our results indicate that germ-cell expression of Sin3A is essential for spermatogenesis and male fertility. Additionally, our results uncover an aberrant gene expression program in Sertoli cells due to the absence of viable germ cells in the testis.
Materials and Methods
Tissue Processing
Testes are fixed overnight in 10% formalin then dehydrated prior to paraffin embedding; Tissue is rinsed in 50% EtOH then dehydrated 3× 20 min in 50% EtOH, 3× 20 min in 70% EtOH, 3× 20 min in 95% EtOH, 3× 20 min in 100% EtOH, 2× 10 min in Xylene then briefly dipped in melted paraffin and incubated in paraffin overnight at 60 degrees then embedded in paraffin blocks.
Immunohistochemistry
Eight micrometer thick sections of testes are heated on charged slides at 60° C for 2 hours then deparaffinized in Xylene 2× 10 min and rehydrated in 100% EtOH 2× 5 min then 95% EtOH 2× 5 min and washed in running water for 10 min. Slides are microwaved at 900 watts for 20 min in Citrate Buffer (Sodium Citrate, pH 6.0, Tween 20) to retrieve antigens. Slides are washed in PBS, then endogenous peroxidase activity is quenched with 3% Hydrogen Peroxide for 15 min. Slides are washed in PBS, then blocked with 20% Normal Goat Serum for 30 min at room temperature in humid atmosphere. The slides are then incubated with Primary Antibody overnight at 4° C in humid atmosphere. Primary antibodies used in this study are anti-Sin3A K-20 (1:200, sc-994, Santa Cruz), anti-GCNA1 (1:250, gift from C. Payne), anti-GATA-1 N6 (1:100, sc-265, Santa Cruz), TRA98 (1:200 rat, ab82527, Abcam), Cleaved Caspase3 (Asp175; 5A1E) (1:800, 9664P, Cell Signaling) and anti-H2.AX (Ser139) (20E3) (1:480, 9718P, Cell Signaling). Slides are washed with PBS, and incubated with appropriate Secondary antibody for 30 min at room temp. Slides are washed with PBS, and then incubated with Streptavidin Peroxidase for 30 min at room temp. Slides are washed with PBS, and the stained with a DAB Kit (Vector Lab). Slides are rinsed in running water, counterstained with hematoxylin, rinsed in running water, then mounted using Crystal/Mount. Slides are mounted with Permount. Images were acquired with a Zeiss Axio Imager 2 microscope with Qcapture Pro 6.0 software. Images were processed with Photoshop CS5 (Adobe).
Transcript analysis
Whole testes are homogenized in Trizol reagent (Invitrogen) with a mechanical tissue homogenizer, and RNA is extracted according to the manufacturer’s instructions. Reverse transcription is done by using Moloney murine leukemia virus polymerase and oligo(dT) primers (Promega). Real-time PCR analyses are done using the SYBR Green method (Biorad). Primer sequences are available upon request. Results were reported as relative to the abundance of β2-microglobulin transcripts.
Results
Deletion of Sin3A in male germ cells results in sterility
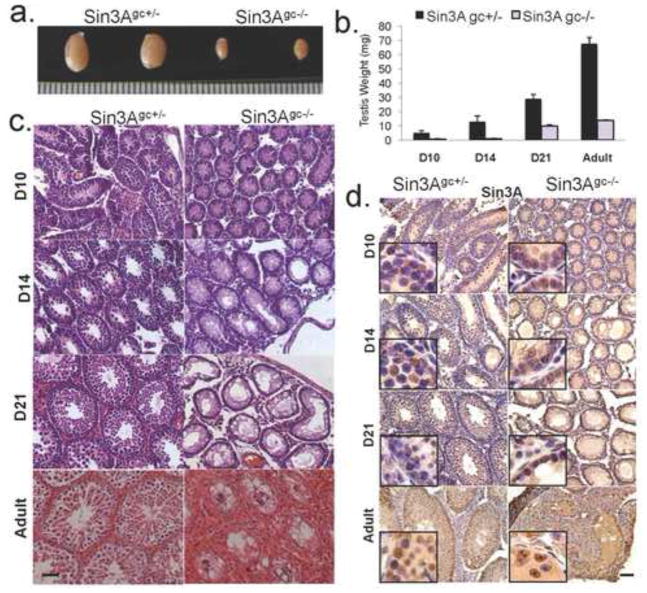
To examine the function of Sin3A in spermatogenesis, we conditionally inactivated Sin3A in the mouse germ cell lineage using the Vasa-Cre mouse transgenic strain. The Vasa-Cre transgene drives expression of the Cre recombinase specifically in gonads, between embryonic days e15 and e18 (Gallardo et al., 2007). Sin3A+/− Vasa-Cre+ males were crossed to females homozygous for the floxed Sin3A allele (Sin3AL/L) to generate Sin3AL/+ Vasa-Cre+ and Sin3AL/− Vasa-Cre+ mice. In these mice, the floxed allele is recombined specifically in germ cells during embryonic development, resulting in the excision of an essential exon within the Sin3A locus (Dannenberg et al., 2005). Sin3AL/+ Vasa-Cre+ and Sin3AL/− Vasa-Cre+ mice are hereafter referred to as Sin3Agc+/− (gc for germ cells) and Sin3Agc−/−, respectively. To determine the impact of Sin3A deletion on germ cells function and mouse fertility, 8-week-old Sin3Agc+/− and Sin3Agc−/− male mice were crossed with 8- to 16-week-old wildtype female mice. Notably, while Sin3Agc+/− males produced multiple litters of pups, Sin3Agc−/− males produced no offspring (n=4 for each genotype, data not shown), despite successful matings, as evidenced by the presence of vaginal plugs. These observations indicate that Vasa-Cre-mediated deletion of Sin3A results in male sterility. Testes from several independent Sin3Agc−/− mice and from their Sin3Agc+/− control littermates were isolated at post-natal day 10 (D10), D14, D21, and 24 weeks after birth (Adult) to document the phenotypic consequences of Sin3A deletion in germ cells. Strikingly, testis size and weight were drastically reduced in Sin3Agc−/− mice compared to their control littermates as early as D10 (Fig. 1a and b). Reduced weight in Sin3Agc−/− testes was observed throughout development and adulthood, culminating in a 79% reduction in adult testis weight in Sin3Agc−/− mice (Fig. 1b). Histological analysis of testis sections was performed, and as shown in figure 1c, hematoxylin and eosin staining indicated that germ cells may be absent in Sin3Agc−/− testes as early as day 10, as only a peripheral layer of cells, likely corresponding to Sertoli cells, was present. This phenotype has previously been referred to in several genetically modified and natural mutant mouse strains as a Sertoli-cell only testes phenotype (for example, see Chen et al., 2005; Costoya et al., 2004). To correlate the loss of Sin3A expression with the defects observed in Sin3A deleted testes, Sin3A mRNA abundance was documented by quantitative real-time PCR in RNA extracted from whole testes from mice at different time points (Supp Fig. 1). While Sin3A is detected at every time point analyzed (D10 to adult) in control testes, we observed a robust decrease of Sin3A expression (from 60 to 90% decrease) in Sin3Agc−/− testes, regardless of the time point analyzed. Immunohistochemical analysis of testis sections revealed that the Sin3A protein is detected throughout Sin3Agc+/− testes, in both germ cells and Sertoli cells, as assessed by the location of the positive cells within the seminiferous tubules (Fig. 1d). In Sin3Agc−/− tubules, most remaining cells express Sin3A, consistent with the fact that Vasa-Cre-driven deletion is germ cell specific and does not occur in somatic cells within the gonad (Gallardo et al., 2007). This observation is consistent with the residual Sin3A expression detected by RT-PCR on RNA extracted from whole testes (Supp Fig. 1). Together, these observations indicate that Sin3A is normally expressed throughout development in germ cells, and its depletion in this cell type correlates with impaired spermatogenesis.
Figure 1. Deletion of Sin3A in male germ cells results in Sertoli cell only phenotype.
(a.) Representative testes from adult (24-week old) Sin3Agc+/− and Sin3Agc−/− male mice. (b.) Weights (mg) of the testes from Sin3Agc+/− (black) and Sin3Agc−/− males (grey) at day 10 (D10, n=3 for each genotype, p<0.028), D14 (n=3, p<0.01), D21 day (n=6, p<0.008), adult (n=3, p<0.007). Error bars represent the standard deviation (c.) Representative Hematoxylin and eosin-stained cross sections of testes from D10, D14 and D21 and adult Sin3Agc+/− (left) or Sin3Agc−/− mice (right). Scale bar =50μm. (d.) Representative immunostaining with an anti-Sin3A antibody on cross sections from D10, D14, D21 and adult testis of the indicated genotype. Scale bar =50μm. Inset boxes correspond to higher magnification.
Sin3A is required for germ cell survival
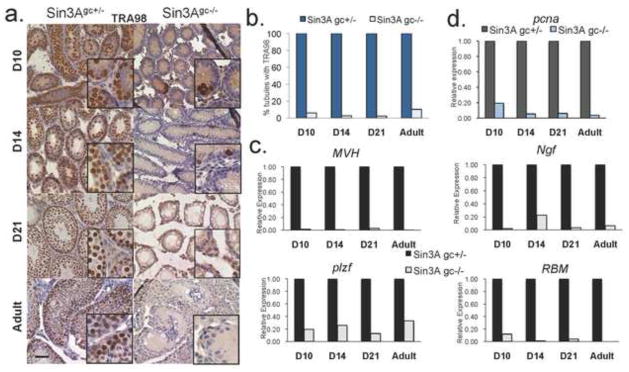
To further investigate the nature of the cellular defects observed in Sin3Agc−/− testes, the presence of TRA98, a marker for testicular germ cells, was documented by immunostaining (Tanaka et al., 1997). While virtually all seminiferous tubules contain cells expressing TRA98, as early as D10 and through adulthood in Sin3Agc+/− samples, few Sin3Agc−/− tubules contain TRA98-positive cells (Fig. 2a and 2b). Additionally, no staining was detected in Sin3Agc−/− testes with an antibody raised against GCNA1 (germ cell nuclear antigen), a marker of diploid germ cells (Enders and May, 1994), while control testes contain abundant GCNA1-positive cells as early as D10 (Supp Fig. 2). Analysis of testes from control or from Sin3Agc−/− mice at later time points (D14, D21 and Adult) revealed that, consistent with what is observed at D10, Vasa-Cre mediated deletion of Sin3A leads to an almost complete obliteration of germ cells, as evidenced by the lack of TRA98 or GCNA1 positive staining (Fig. 2a and Supp Fig. 2). Together, these results further confirm that Vasa-driven Cre deletion of Sin3A leads to an early loss of germ cells. Notably, rare cells stained positive for TRA98 in Sin3Agc−/− adult testes, likely corresponding to Sin3A-expressing cells (Supp Fig. 3). In agreement with the almost complete lack of germ cells in Sin3Agc−/− testes, transcripts analysis of whole testes revealed significantly reduced expression of various germ cell-associated markers in Sin3Agc−/− testes. Specifically, expression of mouse vasa homolog, MVH, is reduced to only 2% of the control levels in Sin3Agc−/− testis at D10 and remains almost undetectable hereafter (Fig. 2c). The expression levels of additional markers normally expressed in germ cells, including RBM (RNA Binding Motif) (Jarvis et al., 2005) and NGF (nerve growth factor) present specifically in round spermatids (Vidal et al., 2001), were strongly decreased in Sin3Agc−/− testes compared to control testes (Fig. 2c). Concordantly, the transcripts abundance for plzf (promyelocytic leukemia zinc-finger), required to maintain self-renewal in male germ cells (Buaas et al., 2004), is drastically reduced in Sin3Agc−/− testis (to 20–30% abundance compared to controls, from D10 to adult) (Fig. 2c). Altogether, these results strongly suggest that genetic inactivation of Sin3A is incompatible with cellular proliferation and/or viability in germ cells. Consistently, the abundance of transcripts corresponding to the proliferation marker pcna is dramatically decreased in Sin3Agc−/− testis (20%, 5%, 5% and 2% relative to controls in D10, D14, D21 and Adult testes, respectively) (Fig. 2d)
Figure 2. VasaCre-mediated deletion of Sin3A leads to a loss of germ cells.
(a.) Representative immunostaining with an anti-TRA98 antibody on cross sections from D10, D14, D21 day old and adult testis from Sin3Agc−/− and Sin3Agc+/− mice. Scale bar =50 μm. Inset boxes correspond to higher magnification. (b.) Percentage of seminiferous tubules with positive staining for TRA98. Shown is the average result from two independent experiments (c-d.) Relative expression determined by quantitative RT-PCR in of Sin3Agc+/− and Sin3Agc−/− testes at indicated time points for (c) germ cell specific transcripts MVH, plzf, RBM, and ngf and (d) proliferation marker pcna. Transcript abundance is expressed as relative to housekeeping B2M transcript abundance and normalized to the corresponding Sin3Agc+/− testes. Shown is the average result from two independent experiments performed in duplicate.
Sin3A prevents apoptosis of germ cells prior to the initiation of meiosis
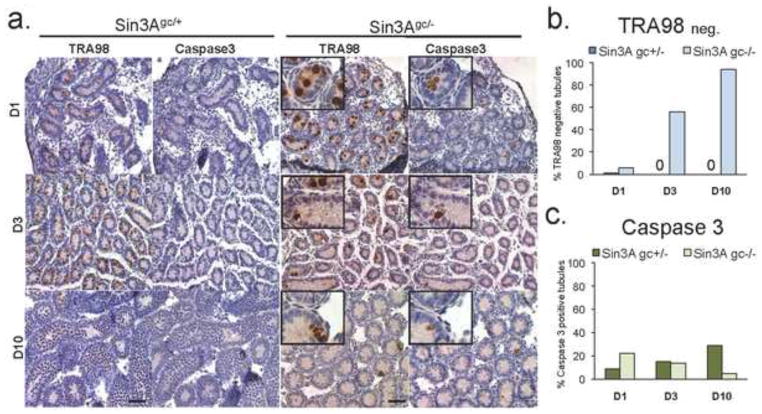
To gain further insights on the cellular events that lead to the elimination of Sin3A deleted germ cells, testis from Sin3Agc−/− and control littermates were collected and analyzed at earlier time points, specifically D1 and D3. Surprisingly, in both Sin3A deleted and control testes, germ cells were present in D1 seminiferous tubules, as indicated by the presence of TRA98-positive cells in almost all tubules (Fig. 3a and 3b). This observation indicates that during mid-to-late embryogenesis, Sin3A inactivation does not dramatically affect germ cells development, and that depletion of the germ cells elicited by Sin3A inactivation is likely to occur after birth. Subsequently, beginning at D3, the number of TRA98-positive cells per tubule decreases dramatically upon Vasa-Cre mediated deletion of Sin3A (Fig. 3a and 3b). By contrast, the number of TRA98-positive cells increases steadily between D1 and D10 in control testes, consistent with the first wave of mitosis in the germ cells at this stage (Fig. 3a). Concurrently, apoptosis, as detected by positive staining for cleaved Caspase 3, occurs at a significantly higher rate in D1 Sin3A deleted testes compared to controls (Fig. 3a and 3c). By contrast, apoptotic cells are found in close to 30% of tubules in control testes at D10, in agreement with previous reports (Ewen and Koopman, 2010). Importantly, staining of serial sections with antibodies to TRA98 and cleaved Caspase 3 indicate a frequent overlap between the presence of germ cells and apoptosis at D1 in Sin3A deleted testes (Fig. 3a). Together, these observations indicate that Sin3A deletion in germ cells leads to cellular death in the first days after birth. In addition, we detected substantial DNA damage in Sin3Agc−/− tubules at D1, as revealed by γH2AX positivity. By contrast, only a weak γH2AX signal was detected at D1 in the nuclei of the control testes (Supp Fig. 4). Interestingly, the timing of the emergence of DNA damage and apoptosis in Sin3A-deleted germ cells coincides with the reentry into mitosis, which occurs immediately after birth in the male mouse germline (Ewen and Koopman, 2010). This observation is reminiscent with the previously reported inability of Sin3A deleted fibroblasts to proliferate and the genomic instability that accompanies acute deletion of Sin3A (Dannenberg et al., 2005).
Figure 3. Early postnatal germ cells undergo apoptosis in the absence of Sin3A.
(a.) Representative immunostaining on cross sections of testes from D1, D3 and D10 mice of the indicated genotype with an anti-TRA98 or an anti-Cleaved Caspase 3 antibody. Inset boxes show higher magnification of the corresponding image. Scale bar =50 μm. (b-c.) Percentage of tubules with no positive staining for TRA98 (b.) and tubules with positive staining for cleaved Caspase 3 (c.) from testes of the indicated age and genotype. Note that no TRA98-megative tubules were detected in Sin3Agc/+ testes after D1. Shown is the average of two independent experiments performed in duplicate.
The loss of germ cells induced by Sin3A deletion affects Sertoli cells transcriptional program
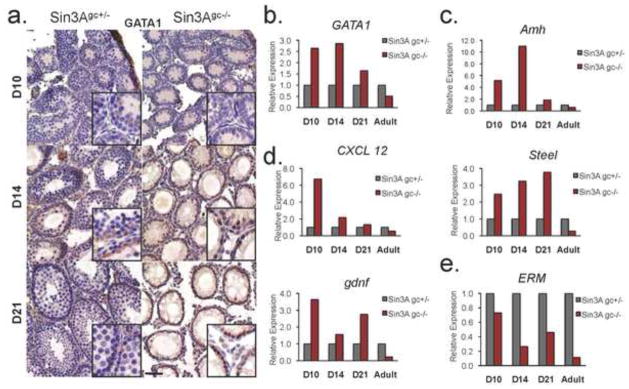
Molecular cross-talk between germ cells and Sertoli cells in the testis is crucial for the maintenance and maturation of progenitor cells, and by inference, for proper reproductive functions. We documented the presence and distribution of GATA-1 in Sin3Agc−/− testes. The expression of GATA-1, which is normally induced in Sertoli cells during the first wave of spermatogenesis, is repressed by the presence of germ cells at maturity (Yomogida et al., 1994). Immunohistochemistry analysis indicate that GATA-1 positive cells are uniformly distributed in control tubules during prepubertal testis development (D10 and D14), but are found only at the periphery of seminiferous tubules at later stages (D21), interspersed with germ cell progenitors, consistent with published reports (Yomogida et al., 1994) (Fig. 4a). By contrast, virtually all cells stain positive for GATA-1 in seminiferous tubules from Sin3Agc−/− testis, at D10, D14 and even D21. Consistently, GATA-1 expression is increased in Sin3Agc−/− testes, at D10, D14 and D21 compared to their control counterparts (Fig. 4b). Surprisingly, GATA-1 levels were significantly decreased in Sin3Agc−/− adult testes compared to control testes, indicating that, in this specific context, the absence of germ cells is not sufficient to fully obliterate the abovementioned repression of GATA-1 expression. Thus, we investigated whether the absence of germ cells, elicited by Sin3A deletion in germ cells, affects Sertoli cells expression program. Amh (Anti-Mullerian hormone) is normally expressed at high levels by Sertoli cells throughout embryonic development but its expression is repressed as germ cells develop (Munsterberg and Lovell-Badge, 1991). As shown in figure 4c, expression of Amh was significantly higher in Sin3Agc−/− testes than in control testes at D10 and D14, possibly reflecting the increased relative abundance of Sertoli cells in testes devoid of germ cells. Similarly, expression levels of CXCL12 (chemokine 12), Gdnf (Glial cell line-derived neurotrophic factor) and Steel are consistently higher in Sin3Agc−/− testes than in their heterozygotes counterparts at early time points (D10, D14 and D21) (Fig. 4d). Surprisingly, the expression levels of Amh, Steel, CXCL12 and gdnf are significantly lower in Sin3Agc−/− adult testes compared to testes from their control littermates, suggesting that, in this context, the absence of germ cells affects the expression program of Sertoli cells in vivo (Fig. 4c and d). Interestingly, chemically-induced depletion of germ cells does not significantly alter GATA-1 expression, nor does it lead to a decrease in Amh expression (O’Shaughnessy et al., 2008). These observations suggest that Sin3A depletion in germ cells leads to a specific alteration of the transcriptional program of Sertoli cells. In addition, expression data for Sertoli-specific transcription factor ERM, required for spermatogonial stem cell renewal (Chen et al., 2005), exhibits a distinctive pattern: As shown on figure 4e, ERM expression is lower throughout development and adulthood in Sin3Agc−/− testes as compared to controls, once again pointing to a deregulated transcription program in Sertoli cells in the absence of neighboring germ cells. Altogether, these observations indicate that lack of germ cells, elicited by Sin3A genetic inactivation, impairs the molecular behavior of Sertoli cells in a specific manner.
Figure 4. Sin3A mediated loss of germ cells affects Sertoli cells.
(a.) Representative immunostaining on cross sections of testes from D10, D14 and D21 mice of the indicated genotype with an anti-GATA1 antibody. Scale bar =50 μm. Inset boxes correspond to higher magnification (b.) Relative expression of Sertoli cell-specific transcripts, determined by quantitative RT-PCR in testis of Sin3Agc+/− and Sin3Agc−/− mice at indicated time points. Expression is relative to housekeeping gene B2M and normalized to corresponding Sin3Agc+/− testis. Shown is the average of two independent experiments performed on two independent mice per genotype in duplicate.
Discussion
Using genetic inactivation of Sin3A in the mouse male germline, we have demonstrated here that Sin3A is essential for fertility and spermatogenesis. In the mouse, Primordial Germ Cells (PGCs) exit the cell cycle between e12.5–14.5 and remain arrested in G1/G0 until after birth, when they undergo mitosis, preceding meiosis before puberty (Ewen and Koopman, 2010). Vasa-Cre-mediated deletion of a floxed reporter allele occurs as early as e15 (Gallardo et al., 2007). In the present study, acute deletion of Sin3A in male germ cells, driven by the Vasa-Cre transgene, is therefore believed to take place as germ cells have undergone cell cycle exit and are quiescent. As Sin3A-deleted germ cells resume mitosis, they undergo apoptosis, concurrent with the accumulation of DNA damage. Within ten days, virtually no germ cell is found in Sin3Agc−/− testes. As a consequence, Vasa-Cre driven deletion of Sin3A leads to a Sertoli cell only phenotype hereafter. Strikingly, the transcriptional profile of Sertoli cells in Sin3AGC−/− adult testes differs from the one reported in other models of Sertoli-cells only testes (O’Shaughnessy et al., 2008), suggesting that Sin3A deletion in germ cells affects the transcriptional program of Sertoli cells in a specific manner. Our results indicate that the loss of germ cells upon Sin3A deletion occurs during the post-natal wave of mitosis, before the initiation of meiosis. A pre-meiosis loss of germ cells is not regularly studied. Indeed, the documented loss of germ cells in several mouse models where a Sertoli cell-only phenotype is observed occurs after the first wave of meiosis. For example, genetic disruption of PLZF, GDNF or ERM leads to a depletion of germ cell reserves and subsequent Sertoli cell-only phenoype in post-meiotic seminiferous tubules (Chen et al., 2005; Costoya et al., 2004; Meng et al., 2000). While germ cells obliteration does not appear to disrupt Sertoli cells development, our results indicate that it leads to significant alterations in Sertoli cells gene expression program. Interestingly, a recent study demonstrated that genetic inactivation of Sin3A in Sertoli cells specifically blocks spermatogenesis (Payne et al., 2010). Along with the present study, these results re-enforce the notion of a transcriptional crosstalk between germ cells and their niche, and identify Sin3A as a master regulator of transcriptional control in germ cells differentiation.
The molecular mechanism underlying germ cell depletion upon Sin3A inactivation remains elusive, but our results indicate that at the onset of mitosis, Sin3A deleted germ cells accumulate DNA damage, that culminates in cell death by apoptosis. Presently, whether the double strand breaks detected in D1 Sin3A-deleted testes only reflect the DNA fragmentation that accompanies apoptosis, or alternatively, actively contribute to the apoptotic elimination of germ cells is unknown. However, based on the previous report of genomic instability in dividing Sin3A deleted cells and on the timing of apparition of γH2AX in Sin3A deleted testes, we favor the latter explanation (Dannenberg et al., 2005). Consistent with what we have previously reported in mouse embryonic fibroblasts and lymphocytes, a recent study showed that genetic inactivation of Sin3A in mouse embryonic stem cells leads to unresolved DNA damage and apoptosis (Dannenberg et al., 2005; McDonel et al., 2012). How loss of Sin3A triggers DNA damage is currently unknown. We have previously shown that an integral mammalian Sin3 complex is required for proper pericentric heterochromatinization, and genetic inactivation of Sin3A or of the associated Sds3 protein leads to chromosomal missegregation, which may then result in chromosome breakage (Dannenberg et al., 2005; David et al., 2003). Similarly, it is possible that the silencing of repeat sequences, such as retrotransposons, is abolished in the absence of Sin3A in germ cells, which are exquisitely sensitive to mobilization of these elements. Altogether, our results demonstrate for the first time that Sin3A is a central regulator of germ cell differentiation, potentially revealing a novel actor of epigenetic modulation of gene expression in this process. In this regard, it is important to note that genetic inaction of several chromatin modifiers that participate in the heterochromatinization and silencing of repeat sequences, including the H3K9 histone methyltransferase Suv39h or the DNMT3L proteins, affect meiosis during germ cell maturation (Bourc’his and Bestor, 2004; Peters et al., 2001). Alternatively, the cell death elicited by Sin3A deletion in germ cells could be the result of impaired transcriptional regulation. Indeed, we and others have previously demonstrated that Sin3A deletion impairs numerous transcriptional programs in somatic cells (Cowley et al., 2005; Dannenberg et al., 2005; van Oevelen et al., 2010). Importantly, we had also previously shown that Sin3A and PLZF physically interact, and that Sin3A is required for PLZF-mediated transcriptional repression (David et al., 1998). However, based on the different timing of testis phenotype onset, it is unlikely that the phenotypes observed upon germ-cell deletion of Sin3A are solely due to defects in PLZF-mediated repression (Chen et al., 2005; Costoya et al., 2004; Meng et al., 2000). Impaired transcriptional repression driven by numerous additional transcription factors could explain the lack of germ cells in the absence of Sin3A. Interestingly, a recent study has revealed an important interplay between Sin3A and c-myc in skin stem cells homeostasis (Nascimento et al., 2011). Based on the known requirement for controlled c-myc levels during spermatogenesis (Suzuki et al., 1996), it is tempting to speculate that Sin3A inactivation leads to aberrant c-myc activity in testis, leading to germ cell death. Finally, H4 acetylation was found markedly increased in human testes from patients with testicular cancer exhibiting a Sertoli-cell only phenotype. The global increase of H4 acetylation may result from a disruption of germ cell-Sertoli cell communication (Faure et al., 2003). While histone acetylation has been shown to play an important role in meiotic germ cell differentiation and post-meiostic events, very little is know of the role of acetylation in PGC development. Data mining of expression libraries revealed that Sin3A is highly expressed in male PGC during early embryonic development (data not shown). Upon Sin3A deletion in germ cells, the absence of a functional Sin3-HDAC complex may disrupt a deacetylation step necessary for PGC maintenance. Whether the Sin3A pathway is disrupted in the PGC of such patients remain to be investigated.
Supplementary Material
Supplemental Figure 1: Relative abundance of Sin3A transcripts, determined by quantitative RT-PCR in whole testes from Sin3Agc+/− and Sin3Agc−/− mice of the indicated age. Expression is relative to housekeeping gene B2M and normalized to the corresponding Sin3Agc+/− testis.
Supplemental Figure 2: Immunostaining on mouse cross sections from testes from mice of the indicated genotype at D10, D14 or D21 with an antibody to GCNA1. Scale Bar =50μm
Supplemental Figure 3: Immunostaining on mouse serial cross sections from testes from an adult Sin3Agc−/− mouse with an antibody to TRA98 (left) or Sin3A (right). Shown is a rare tubule where TRA98-positive cells were found. Note the positive Sin3A signal in the corresponding cells on the adjacent serial section.
Supplemental Figure 4: Immunostaining on cross sections of mouse testis of the indicated genotype at D1 with an antibody to phosphorylated H2AX.
Highlights.
Deletion of Sin3A in the germ cell lineage leads to male sterility.
Sin3A-deleted testes acquire a Sertoli cell only phenotype.
Sin3A prevents apoptosis in male germ cells resuming mitosis at birth.
Germ cell loss triggered by Sin3A deletion affects the Sertoli cells expression program.
Acknowledgments
We are grateful to all members of the David laboratory for helpful discussions during the preparation of the manuscript. We thank Dr. Christopher Payne and Dr. Robert Braun for the generous gift of GCNA1 antibody. This work was funded by the American Cancer Society (115014-RSG-08-054-01-GMC to GD), the National Institute of Health (5R01CA148639 to GD) and the Irma T. Hirschl Charitable Trust (GD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho RA. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- Ayer DE, Lawrence QA, Eisenman RN. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell. 1995;80:767–776. doi: 10.1016/0092-8674(95)90355-0. [DOI] [PubMed] [Google Scholar]
- Bourc’his D, Bestor TH. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature. 2004;431:96–99. doi: 10.1038/nature02886. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, Cheng AM, Hassell JA, Chandrashekar V, Hofmann MC, Hess RA, Murphy KM. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nature genetics. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- Cowley SM, Iritani BM, Mendrysa SM, Xu T, Cheng PF, Yada J, Liggitt HD, Eisenman RN. The mSin3A chromatin-modifying complex is essential for embryogenesis and T-cell development. Mol Cell Biol. 2005;25:6990–7004. doi: 10.1128/MCB.25.16.6990-7004.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg JH, David G, Zhong S, van der Torre J, Wong WH, Depinho RA. mSin3A corepressor regulates diverse transcriptional networks governing normal and neoplastic growth and survival. Genes Dev. 2005;19:1581–1595. doi: 10.1101/gad.1286905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Alland L, Hong SH, Wong CW, DePinho RA, Dejean A. Histone deacetylase associated with mSin3A mediates repression by the acute promyelocytic leukemia-associated PLZF protein. Oncogene. 1998;16:2549–2556. doi: 10.1038/sj.onc.1202043. [DOI] [PubMed] [Google Scholar]
- David G, Grandinetti KB, Finnerty PM, Simpson N, Chu GC, Depinho RA. Specific requirement of the chromatin modifier mSin3B in cell cycle exit and cellular differentiation. Proc Natl Acad Sci U S A. 2008;105:4168–4172. doi: 10.1073/pnas.0710285105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Turner GM, Yao Y, Protopopov A, DePinho RA. mSin3-associated protein, mSds3, is essential for pericentric heterochromatin formation and chromosome segregation in mammalian cells. Genes Dev. 2003;17:2396–2405. doi: 10.1101/gad.1109403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokmanovic M, Marks PA. Prospects: histone deacetylase inhibitors. Journal of cellular biochemistry. 2005;96:293–304. doi: 10.1002/jcb.20532. [DOI] [PubMed] [Google Scholar]
- Enders GC, May JJ., 2nd Developmentally regulated expression of a mouse germ cell nuclear antigen examined from embryonic day 11 to adult in male and female mice. Dev Biol. 1994;163:331–340. doi: 10.1006/dbio.1994.1152. [DOI] [PubMed] [Google Scholar]
- Ewen KA, Koopman P. Mouse germ cell development: from specification to sex determination. Mol Cell Endocrinol. 2010;323:76–93. doi: 10.1016/j.mce.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Faure AK, Pivot-Pajot C, Kerjean A, Hazzouri M, Pelletier R, Peoc’h M, Sele B, Khochbin S, Rousseaux S. Misregulation of histone acetylation in Sertoli cell-only syndrome and testicular cancer. Mol Hum Reprod. 2003;9:757–763. doi: 10.1093/molehr/gag101. [DOI] [PubMed] [Google Scholar]
- Gallardo T, Shirley L, John GB, Castrillon DH. Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis. 2007;45:413–417. doi: 10.1002/dvg.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassig CA, Fleischer TC, Billin AN, Schreiber SL, Ayer DE. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- Heinzel T, Lavinsky RM, Mullen TM, Soderstrom M, Laherty CD, Torchia J, Yang WM, Brard G, Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfeld MG. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature. 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- Jarvis S, Elliott DJ, Morgan D, Winston R, Readhead C. Molecular markers for the assessment of postnatal male germ cell development in the mouse. Hum Reprod. 2005;20:108–116. doi: 10.1093/humrep/deh565. [DOI] [PubMed] [Google Scholar]
- Laherty CD, Yang WM, Sun JM, Davie JR, Seto E, Eisenman RN. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/s0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- McDonel P, Costello I, Hendrich B. Keeping things quiet: roles of NuRD and Sin3 co-repressor complexes during mammalian development. The international journal of biochemistry & cell biology. 2009;41:108–116. doi: 10.1016/j.biocel.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonel P, Demmers J, Tan DW, Watt F, Hendrich BD. Sin3a is essential for the genome integrity and viability of pluripotent cells. Dev Biol. 2012;363:62–73. doi: 10.1016/j.ydbio.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, Pichel JG, Westphal H, Saarma M, Sariola H. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Munsterberg A, Lovell-Badge R. Expression of the mouse anti-mullerian hormone gene suggests a role in both male and female sexual differentiation. Development. 1991;113:613–624. doi: 10.1242/dev.113.2.613. [DOI] [PubMed] [Google Scholar]
- Nascimento EM, Cox CL, MacArthur S, Hussain S, Trotter M, Blanco S, Suraj M, Nichols J, Kubler B, Benitah SA, Hendrich B, Odom DT, Frye M. The opposing transcriptional functions of Sin3a and c-Myc are required to maintain tissue homeostasis. Nature cell biology. 2011;13:1395–1405. doi: 10.1038/ncb2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Hu L, Baker PJ. Effect of germ cell depletion on levels of specific mRNA transcripts in mouse Sertoli cells and Leydig cells. Reproduction. 2008;135:839–850. doi: 10.1530/REP-08-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CJ, Gallagher SJ, Foreman O, Dannenberg JH, Depinho RA, Braun RE. Sin3a is required by sertoli cells to establish a niche for undifferentiated spermatogonia, germ cell tumors, and spermatid elongation. Stem Cells. 2010;28:1424–1434. doi: 10.1002/stem.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AH, O’Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, Sibilia M, Jenuwein T. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- Schreiber-Agus N, Chin L, Chen K, Torres R, Rao G, Guida P, Skoultchi AI, DePinho RA. An amino-terminal domain of Mxi1 mediates anti-Myc oncogenic activity and interacts with a homolog of the yeast transcriptional repressor SIN3. Cell. 1995;80:777–786. doi: 10.1016/0092-8674(95)90356-9. [DOI] [PubMed] [Google Scholar]
- Silverstein RA, Ekwall K. Sin3: a flexible regulator of global gene expression and genome stability. Curr Genet. 2004 doi: 10.1007/s00294-004-0541-5. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Abe K, Yoshinaga K, Obinata M, Furusawa M. Specific arrest of spermatogenesis caused by apoptotic cell death in transgenic mice. Genes to cells : devoted to molecular & cellular mechanisms. 1996;1:1077–1086. doi: 10.1046/j.1365-2443.1996.d01-228.x. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Pereira LA, Nozaki M, Tsuchida J, Sawada K, Mori H, Nishimune Y. A germ cell-specific nuclear antigen recognized by a monoclonal antibody raised against mouse testicular germ cells. Int J Androl. 1997;20:361–366. doi: 10.1046/j.1365-2605.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- van Oevelen C, Bowman C, Pellegrino J, Asp P, Cheng J, Parisi F, Micsinai M, Kluger Y, Chu A, Blais A, David G, Dynlacht BD. The mammalian Sin3 proteins are required for muscle development and sarcomere specification. Mol Cell Biol. 2010;30:5686–5697. doi: 10.1128/MCB.00975-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal F, Lopez P, Lopez-Fernandez LA, Ranc F, Scimeca JC, Cuzin F, Rassoulzadegan M. Gene trap analysis of germ cell signaling to Sertoli cells: NGF-TrkA mediated induction of Fra1 and Fos by post-meiotic germ cells. J Cell Sci. 2001;114:435–443. doi: 10.1242/jcs.114.2.435. [DOI] [PubMed] [Google Scholar]
- White-Cooper H, Davidson I. Unique aspects of transcription regulation in male germ cells. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yomogida K, Ohtani H, Harigae H, Ito E, Nishimune Y, Engel JD, Yamamoto M. Developmental stage- and spermatogenic cycle-specific expression of transcription factor GATA-1 in mouse Sertoli cells. Development. 1994;120:1759–1766. doi: 10.1242/dev.120.7.1759. [DOI] [PubMed] [Google Scholar]
- Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Relative abundance of Sin3A transcripts, determined by quantitative RT-PCR in whole testes from Sin3Agc+/− and Sin3Agc−/− mice of the indicated age. Expression is relative to housekeeping gene B2M and normalized to the corresponding Sin3Agc+/− testis.
Supplemental Figure 2: Immunostaining on mouse cross sections from testes from mice of the indicated genotype at D10, D14 or D21 with an antibody to GCNA1. Scale Bar =50μm
Supplemental Figure 3: Immunostaining on mouse serial cross sections from testes from an adult Sin3Agc−/− mouse with an antibody to TRA98 (left) or Sin3A (right). Shown is a rare tubule where TRA98-positive cells were found. Note the positive Sin3A signal in the corresponding cells on the adjacent serial section.
Supplemental Figure 4: Immunostaining on cross sections of mouse testis of the indicated genotype at D1 with an antibody to phosphorylated H2AX.