Abstract
Bone never forms without vascular interactions. This simple statement of fact does not adequately reflect the physiological and pharmacological implications of the relationship. The vasculature is the conduit for nutrient exchange between bone and the rest of the body. The vasculature provides the sustentacular niche for development of osteoblast progenitors, and is the conduit for egress of bone marrow cell products arising, in turn, from the osteoblast-dependent hematopoietic niche. Importantly, the second most calcified structure in humans after the skeleton is the vasculature. Once considered a passive process of dead and dying cells, vascular calcification has emerged as an actively regulated form of tissue biomineralization. Skeletal morphogens and osteochondrogenic transcription factors are elaborated by cells within the vessel wall, regulating the deposition of vascular calcium. Osteotropic hormones including parathyroid hormone regulate both vascular and skeletal mineralization. Cellular, endocrine, and metabolic signals flow bidirectionally between the vasculature and bone that are necessary for both bone health and vascular health. Dysmetabolic states including diabetes, uremia, and hyperlipidemia perturb the bone-vascular axis, giving rise to devastating vascular and skeletal disease. A detailed understanding of bone-vascular interactions is needed to address the unmet clinical needs of our increasingly aged and dysmetabolic population.
I. INTRODUCTION
Bone never forms without vascular interactions1, 2. This obvious and simple statement of fact does not adequately reflect the physiological and pharmacological implications of the relationship. The vasculature is the conduit for nutrient exchange between bone and the rest of the body; this is relevant not only to the rapid access to the skeletal calcium “bank” needed with urgent physiological demands3 –- be it for deposits or withdrawals -- but also the delivery of metabolic substrate to the basic multicellular unit (BMU) for bone–forming osteoblast functions4. The vasculature also provides the sustentacular niche for development of osteoblast progenitors5. Moreover, it is the conduit for egress of bone marrow cell products arising, in turn, from the osteoblast-dependent hematopoietic niche6. A detailed understanding of bone-vascular interactions during development and disease will be needed to address the unmet clinical needs of our increasingly aged and dysmetabolic population7.
Remarkably, the inextricable interdependence of vascular physiology, skeletogenesis, bone remodeling, and mineral metabolism has in general escaped widespread appreciation. Arteriosclerosis – the stiffening of conduit arteries from any cause -- including medial calcification and fibrosis in addition to atherosclerosis -- contributes to morbidity and mortality including musculoskeletal disease. The lower extremities bear the brunt of this disease burden, best manifested in hip fracture8, limb ischemia9, 10, and amputation risk9, 11, 12. In a preclinical model of critical limb ischemia, Ignarro et al have begun to capitalize upon this physiology, coupling parathyroid hormone (PTH) - mediated bone anabolism with granulocyte colony stimulating factor (G-CSF) treatment as a strategy to mobilize endothelial progenitor cells (ePCs) and improve limb recovery13. In humans, Khosla, Pignolo, and colleagues have demonstrated that marrow-derived progenitors with “osteogenic signatures” portend and participate in arterial function14, 15, valve calcification16, and fracture repair17. Anabolic cross-talk between osteoblasts and endothelial cells has been manipulated by Clemens and colleagues as a successful strategy to enhance bone regeneration following injury18, 19. Thus, a bone-vascular axis has emerged in which the vasculature supports musculoskeletal functions -- and bone-derived cell types and endocrine/paracrine cues impact vascular health.
In this brief manuscript, we provide an overview of the intricacies of the bone-vascular axis, emphasizing interactions during disease rather than during development (the reader is referred to two outstanding reviews that detail these interactions during skeletal morphogenesis2, 20). We emphasize dysmetabolic states such as diabetes, dyslipidemia, and uremia that compromise vascular health and thus skeletal health via heterogeneous arteriosclerotic calcification processes21, 22. We recount new data demonstrating that calciotropic hormones regulate vascular calcification23–26 – and that the dysmetabolic milieu impairs calciotropic hormone signals vital to the preservation of normal bone and vascular mineral homeostasis27–29. We relate the mechanisms whereby cellular, biochemical and hormonal cues elaborated by the skeleton systemically impact vascular health and vessel conduit function – and end by pointing to emerging therapeutic opportunities afforded by a better understanding of the bone-vascular axis.
II. THE CLINICAL SIGNIFICANCE OF ARTERIOSCLEROTIC CALCIFICATION
Vascular calcification has afflicted human beings for at least 5 millennia. Ötzi, the Tyrolean Ice Mummy who succumbed to homicide ~ 5300 years ago -- 500 years before Stonehenge was erected -- had significant deposits of arterial calcium in his abdominal aorta30. Most recently, with the enhanced longevity of modern humans the consequences of arterial calcification have become increasingly evident. Coronary artery calcium (CAC) scores identify those at greatest risk for progressive cardiovascular disease (CVD) in those with otherwise intermediate risk31. Tibial artery calcium (TAC) scores outperform ankle-brachial indices in portending amputation risk in patients with peripheral arterial disease (PAD)9. Using plain radiographs to assess patients with type II diabetes, the presence of arterial medial calcification was deemed to be a greater contributor to amputation risk than atherosclerotic calcification in this patient population12. The presence and extent of calcific aortic valve disease (CAVD) is the single best predictor of clinical progression in patients with asymptomatic, mild or moderate32- to- severe33 calcific aortic stenosis. Using plain pelvic and femoral radiographs to phenotype vascular calcification in setting of end stage renal disease (ESRD) requiring renal replacement therapy (RRT), London and colleagues identified that arterial calcification clearly portended mortality12. While the five-year Kaplan-Meier survival was under 50% for ESRD patients afflicted with atherosclerotic calcification or medial calcification, the fortunate 1/3rd lacking significant arterial calcification enjoyed ~90% survival during this same period34. After adjustment for age, dialysis vintage, gender, ethnicity, diabetes, non-dialysis CKD status,, hypertension, tobacco use, prior parathyroid surgery, and body mass index, atherosclerotic calcification and medial calcification conveyed 5- and 16-fold increases in the relative risk for mortality compared to those without vascular calcification12. Very recently, using CT-based volumetric scoring of carotid artery calcification load, intracranial carotid calcification was linked to the extent of MRI-detected CNS white matter lesions while extracranial carotid calcification was a harbinger of overt CNS infarcts35. Clearly, arterial calcific vasculopathy is a harbinger of cardiovascular disease.
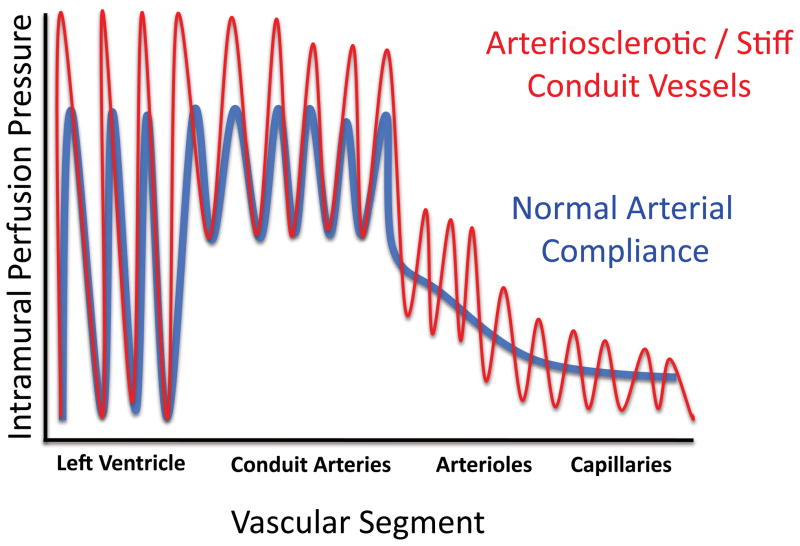
How can arterial calcification convey such clinically significant risks? Of course, thromboembolic events and fixed reductions in vessel lumen size induce tissue ischemia, and the calcified Stary type Vb plaque36, 37 characteristic of atherosclerotic calcification is a culprit in acute coronary syndromes37, 38. Aortic valve sclerosis distorts and stiffens valve leaflets, not only causing stenosis with attendant myocardial workload demand but also precluding the efficient valve leaflet coaptation that prevents regurgitant flow39, 40. Additionally, conduit vessel stiffening – arising from either atherosclerotic or medial mineralization41 – impairs Windkessel physiology42, which depends upon the rubbery elasticity of conduit vessels necessary for smooth distal tissue perfusion43 (Figure 1). With each cardiac cycle, a portion of the kinetic energy elaborated during ventricular contraction of systole is stored as potential energy throughout the vascular tree42; this energy is released during diastole and helps maintain uniformity of flow in distal capillary beds during the cardiac cycle43. Moreover, with arterial stiffening the attendant increase in pulse wave velocity interacts with impedance mismatch along the vascular tree to elevate systolic blood pressure, increasing myocardial workload as well as end-organ barotrauma43, 44 (Figure 1). Thus, the presence, extent and histoanatomic type of arterial calcification carries ominous predictions with respect to cardiovascular and CNS disease, mortality, and amputation risk22.
Figure 1. Consequences of arterial stiffening and impaired Windkessel physiology.
During systole, some kinetic energy is stored as potential energy in the elastic conduit arteries. This permits not only coronary perfusion but also smooth distal capillary perfusion during diastole (blue tracing). With arteriosclerotic stiffening (red tracing), less potential energy is stored during systole, giving rise to impaired, pulsatile and erratic flow during diastole (2/3rds of the cardiac cycle)209. Systolic blood pressure is also increased. See O’Rourke and Hashimoto81 for an excellent review and additional details.
At this point, it is useful to highlight the differences denoted when using the terms arteriosclerosis and atherosclerosis. Arteriosclerosis refers to arterial mechanical stiffening from any cause, be it from: (a) concentric medial and adventitial fibrosis with medial calcification, elastinolysis and mural thickening as occurs in type II diabetes; (b) eccentric intimal-medial atherosclerotic plaques with calcification, fibrosis, and cholesterol –laden lipoprotein deposition; or (c) neointima formation and mural fibrosis as occurs in chronic allograft vasculopathy in transplanted organs. Atherosclerosis specifically refers to the intimally oriented, eccentric, lumen-deforming processes initiating from subendothelial lipoprotein deposits with cholesterol-laden foam cell formation and subsequent matrix remodeling events that increase risk for atherothrombosis. While atherosclerosis decreases arterial compliance – i.e. causes arteriosclerosis – not all arteriosclerosis arises from atherosclerotic processes.
III. PATHOBIOLOGY OF VASCULAR CALCIFICATION
Vascular calcification was once considered only a passive process of dead and dying cells; however, data from a multitude of laboratories worldwide have clearly demonstrated that vascular calcification is an actively regulated form of extracellular matrix biomineralization21. Virchow’s initial pathological description of “atherosclerosis” presciently identified the contributions of perturbed lipid metabolism, inflammation, and osteo-fibrogenic differentiation to the biology of vascular calcium accrual45, 46. Of note, vascular ossification – true ectopic bone replete with marrow elements – can be seen in up to 15% of calcified arterial lesions47. At the molecular level, however, the signature of active osteogenic processes are found in virtually all calcified arterial segments48. Studies by Linda Demer, H. Clarke Anderson, and colleagues were the first to identify osteogenic “fingerprints” in calcifying atherosclerotic and medial lesions48. Bone alkaline phosphatase – a highly characteristic and important ectoenzyme required for bone mineralization49 – was localized to mineralizing arterial segments50, 51. Moreover, the powerful bone morphogen BMP2 was shown to be expressed in calcifying atherosclerotic plaques of human vessels52. Oxylipids derived from oxidized LDL (oxLDL) were subsequently identified as potent inducers of endothelial BMP253, 54, TNF55, and other macrophage-derived signals that drive mineralization of calcifying vascular cells (CVCs) in vitro and in vivo55 (vide infra). However, in atherosclerotic mice, inhibition of arterial BMP2 signaling with matrix Gla protein (MGP) reduces vascular calcium and lesion size56.
By histoanatomic and clinical criteria, at least 5 types of arterial calcification can be identified (Table I): atherosclerotic calcification, medial artery calcification, calcific aortic valve disease (a.k.a. calcific aortic stenosis), calcific uremic arteriolopathy (a.k.a. calciphylaxis) and the vascular calcification of ESRD (a.k.a. CKD-MBD; chronic kidney disease mineral and bone disorder). The reader is referred to recent review series for in-depth consideration22, 57. For the purposes of this review, only atherosclerotic calcification, medial calcification, and vascular calcification of CKD-MBD will be briefly discussed because of the immediate relevance to skeletal physiology and bone-vascular interactions.
TABLE I.
COMMON HISTOANATOMIC TYPES OF VASCULAR CALCIFICATION
| TYPE | CHARACTERISTICS/SETTINGS | PATHOBIOLOGY |
|---|---|---|
| Calcific aortic valve disease (CAVD) a.k.a. senile calcific aortic stenosis |
Calcification of aortic valve leaflets Advanced age, bicuspid aortic valvfe, hypercholesterolemia, metabolic syndrome, diabetes, hypertension, |
Fibrofatty expansion of valve fibrosa, splitting of elastic laminae, oxylipid deposition. Both osteogenic mineralization and amorphous calcium phosphate nodules deposited. True ectopic ossification-- woven bone formation -- in ~13% of specimens, but ectopic osteogenic interstitial cells (VICs) and circulating osteo-progenitors contribute to valve osteogenic cell populations. |
| Atherosclerotic intimal calcification (AIC) | Calcification of atherosclerotic plaques; eccentric, lumen-deforming, oriented by patchy intimal lipoprotein deposits. Same risk factors as CAVD. | Focal fibrofatty atherosclerotic plaque calcification with VSMC chondroid metaplasia and endochondral mineralization observed at base of lesions. Calcifying vascular cells (CVCs) related to pericytes also contribute. Driven by oxylipids from oxLDL. Lipid core and fibrous microcalcifications, focal elastinolysis noted. |
| Arterial medial calcification (AMC, sometimes MAC) a.k.a. Mönckeberg’s medial calcific sclerosis |
Calcification of the arterial tunica media; concentric, extensive, almost confluent. Common in type II diabetes, autonomic neuropathy, end stage renal disease / CKD5 | Circumferential calcification of the tunica media with lipidaceous matrix vesicles, elastinolysis, and early expression of membranous mineralization programs (later endochondral). Inflammation-driven adventitial –medial BMP-Wnt signaling directs initial osteogenic programming of vascular multipotent mesenchymal progenitors (pericytes, CVCs). |
| Vascular calcification of chronic kidney disease a.k.a. chronic kidney disease - mineral & bone disorder (CKD-MBD) |
CKD5 with any of the above. Major perturbations in calcium phosphate homeostasis, & reductions in serum fetuin, pyrophosphate. | VSMC apoptosis and osteochondrogenic metaplasia driven by hyperphosphatemia, worsened by iatrogenic hypoparathyroidism and low-turnover bone disease. Occurs most often in settings of antecedent medial and atherosclerotic disease processes that initiated prior to uremia |
| Calcific uremic arteriolopathy (CUA), a.k.a. calciphylaxis |
CKD4 or CKD5 with dermal arteriolar medial calcification and dermal fat necrosis, usually of pannus, buttocks, or lower extremities. Usually on warfarin. | Arteriolar (generally < 50 micron diameter) medial calcification with fibroproliferative occlusion leads to tissue necrosis. Dermal fat, lung, and mesentery most significantly affected. Warfarin impairs matrix Gla protein (MGP) gamma carboxylation and functions, including BMP2/4 inhibition & fetuin-dependent clearance of mineralizing matrix vesicles. |
III. A. Atherosclerotic calcification
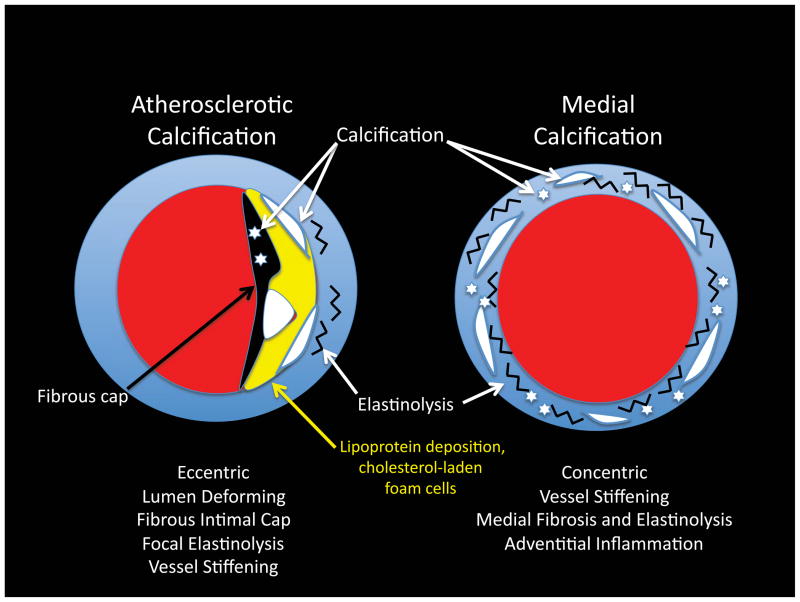
Atherosclerotic calcification represents the prototypic lesion described by Virchow45, 46. In the lexicon of cardiovascular pathology this is the type Vb plaque58 -- characterized by an eccentric, lumen deforming, outward remodeling lesion possessing a fibrous cap, cholesterol-laden macrophages and lipoprotein deposits, intensive focal inflammatory cell infiltration and localized elastinolysis37, 38 (Figure 2). Juxtaposed mineralizing apoptotic bodies and mineralizing matrix vesicles are elaborated by neighboring vascular smooth muscle cells (VSMCs) and CVCs undergoing chondroid metaplasia 37, 38. Stippled fibrous cap calcification is also noted, and such lesions appear to be precursors to the ruptured plaques of acute coronary syndromes37, 38. In bone, biomineralization occurs via either endochondral ossification or membranous ossification processes programmed by chondrocytes and osteoblasts59–61. Endochondral ossification is characterized by the mineralization of a chondrocyte-derived skeletal template, following chondrocyte hypertrophy, apoptosis, vascular invasion and concomitant osteoprogenitor recruitment with subsequent osteoblast-mediated bone formation. Of note, Runx2/Cbfa1 -- a master transcriptional regulator absolutely essential for both endochondral or membranous ossification processes62 –is upregulated in the vessel walls of apoE−/− mice undergoing atherosclerotic calcification63. Elegant studies by Giachelli and colleagues have recently demonstrated the “trans-differentiation” of arterial VSMCs to cells of the chondrocyte and osteoblast lineage during atherosclerotic calcification64–66 (Figure 3). Implementing SM22-Cre; Rosa26-LacZreporter; apoE−/− mice, they showed that cells originally derived from the VSMC lineage – “tagged” as blue with the Rosa locus LacZ reporter via VSMC-specific Cre activation – ended up as vascular chondrocytes and osteoblasts in the mineralizing segments of atherosclerotic plaques65. VSMC transdifferentiation in atherosclerotic apoE−/− mice occurred in the absence of augmented Msx2, Wnt, or Sp7/0sx signaling65.
Figure 2. Atherosclerotic vs. Medial Arterial Calcification.
Both atherosclerotic calcification and medial calcification stiffen arterial conduit vessels, impairing Windkessel physiology. The eccentric remodeling of atherosclerotic calcification also reduces lumen diameter, and predisposes to acute thrombosis.
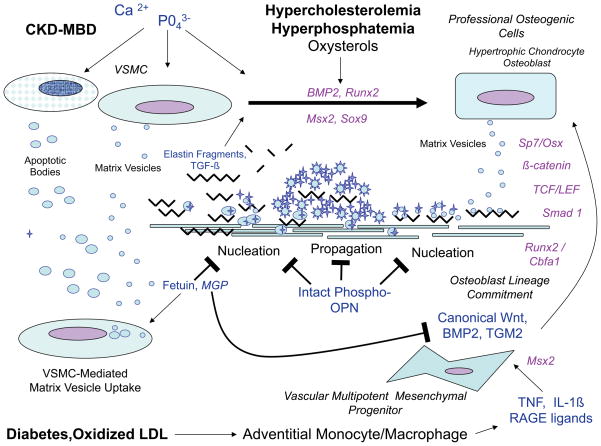
Figure 3. Vascular osteogenic cell origins, functions and phenotypes in arterial calcification.
Vascular mineralization is regulated by processes overlapping yet distinct form those that control skeletal bone formation. Osteogenic progenitors can arise from “transdifferentiation” of VSMCs, or osteogenic lineage allocation of multipotent mesenchymal progenitors. Health VSMCs also play an important role in limiting vascular calcium accrual via fetuin- and MGP- dependent pinocytotic uptake of matrix vesicles. Metabolic and inflammatory insults induce vascular changes that impair normal VSMC function/viability and induce osteogenic differentiation of vascular mesenchymal cells. Not shown are the circulating osteoprogenitors that may contribute to the “vascular ossification” – true bone formation replete with marrow elements – that can be seen in ~15% of specimens. Extracellular factors are lettered in blue, while intracellular transcriptional regulators are colored lavender. See text for details, adapted from Mizobuchi, Towler, and Slatopolsky108. TGM2, tissue transglutaminase210, 211.
What then triggers osteochondrogenic “transdifferentiation” of VSMCs? Oxidative stress signaling, oxylipids, and phosphate (see CKD-MBD below) play important pathophysiological roles67 (Figure 3). Oxysterols derived from oxidized LDL cholesterol (oxLDL) and hydrogen peroxide upregulate Runx2/Cbfa1 expression68 and osteo/chondrogenic trans-differentiation of VSMCs69. Activation of bone alkaline phosphatase in these VSMCs is critical to matrix mineralization responses elicited by oxLDL and peroxide, and inhibitors of bone alkaline phosphatase limit atherosclerotic calcification by VSMCs70. Intriguingly, as Demer and colleagues first demonstrated, hypercholesterolemia and oxylipids derived from oxLDL also suppress bone formation71, 72 and bone anabolic responses to PTH29, 73. Moreover, PTH responses were restored by administration of HDL-mimetics29, 73. These data implicate a metabolic milieu that can simultaneously engender atherosclerosis and osteoporosis. Thus, inflammatory oxylipids and oxLDL accumulating at sub-intimal venues drive atherosclerotic intimal calcification by activating osteogenic BMP2 and Runx2/Cbfa1 trans-differentiation of VSMCs67, 74. Recruitment of calcifying vascular cells (CVCs)75 – a mural multipotent mesenchymal cell related to the microvascular pericyte76 – provides an additional source of osteoprogenitors52.
III. B. Arterial medial calcification
Arterial medial calcification –sometimes called Monckebergs medial calcific sclerosis– is particularly common in the arteriosclerosis of type II diabetes10–12, 77. Medial calcification is a concentric process of the tunica media, associated with biomineralization initiating along mural elastin fibers78, 79 (Figure 2). Traditional thought was that medial calcification was clinically inconsequential -- succinctly articulated in the 3rd (1984) edition of the pathology text of Robbins et al on page 518 as follows80: “ This disorder is of relatively little clinical significance. It accounts for roentgenographic densities in the vessels of the extremities of aged individuals, but it is to be remembered that the lesions do not produce narrowing or occlusion of the vascular lumen80.” Yet, as subsequently highlighted, medial artery calcification impairs vessel mechanics and Windkessel physiology necessary for smooth distal tissue perfusion81 (Figure 1) – and best conveys risk for lower extremity amputation in patients with type II diabetes12.
Similar to the membranous ossification of craniofacial bone – where osteoblast-mediated biomineralization occurs in a type I collagen –based matrix without a preceding cartilage template61 – medial artery calcification does not involve overt chondrogenesis during disease initiation82 (chondrogenesis is sometimes seen in advanced disease with true ectopic ossification83). Electron microscopy first identified bone alkaline phosphatase-positive matrix vesicles associated with fragmented elastin in human arteries afflicted with medial artery calcification50. Additional insights into the pathobiology of disease initiation and progression have been forthcoming from detailed study of preclinical disease models22. When fed high fat diets (HFD) characteristic of westernized societies, male LDLR−/− develop obesity, type II diabetes, and dyslipidemia22 – with progressively severe medial artery calcification and subsequent atherosclerotic calcification as plaques begin to accumulate82. At the earliest phases of disease, arterial Msx2 and Msx1 – osteoblast homeodomain transcription factors absolutely necessary for membranous bone formation in the skull84 – are upregulated in the aortas of these animals85. Immunohistochemistry and in situ hybridization demonstrates the expression of Msx2 in aortic valve interstitial myofibroblasts, arterial adventitial myofibroblasts, and a subset of cells within the tunica media25, 48, 85. Data from our laboratory and others have characterized adventitial myofibroblasts as pericyte-like calcifying vascular cells (CVCs) with multi-lineage mesenchymal cell potential86 (Figure 3). As in atherosclerotic calcification, inflammation is again key22; diabetes with or without dyslipidemia upregulates adventitial TNF87 production by monocytes and macrophages 55 – -- which in turn induces pro-calcific Msx2 activity and paracrine Wnt signaling cascades that promote mural calcification and fibrosis82 (Figure 3). Along with BMP2, a powerful bone morphogen secreted by inflamed endothelial cells88, Msx-regulated Wnt family members elaborated by myofibroblasts support the osteogenic differentiation and collagenous matrix mineralization by mesenchymal progenitors89–91 – be it in bone 92 or within the arterial wall 25, 91. Thus, the paracrine polypeptide milieu of osteogenic morphogens activate osteoblast gene regulatory programs in multipotent mural mesenchymal cells such as CVCs and adventitial myofibroblasts93. Adventitial-to-medial biological signals drive concentric involvement of arterial vessels in diabetic medial calcification – quite distinct from the eccentric atherosclerotic calcification processes organized by subintimal oxylipid deposits (Figure 2). In diabetic arteriosclerosis, neoangiogenesis arising from the vasa vasorum in the inflamed adventitial-medial junction circumferentially upregulates mural BMP-Wnt signaling, and spawns additional adventitial myofibroblasts that can be allocated / programmed to elaborate osteogenic and fibrogenic phenotypes 94, 95 (Figure 3). Not surprisingly then, surgical stripping of arterial adventitia reduces medial calcification in preclinical disease models96.
Of note, recent data from Rajamannan, Miller, Heistad, and colleagues have demonstrated that similar processes contribute to aortic valve calcification directed by valve interstitial cells (VICs)97. Activation of Msx and Wnt signaling cascades is observed in calcifying human aortic valves98, and apoE−/− mice lacking the Wnt receptor LRP5 are protected from valve calcium accrual99. The behavior and molecular phenotype of the three calcifying vascular mesenchymal cell populations – CVCs, adventitial myofibroblasts, and VICs -- closely resembles that of the pericyte, the proliferative microvascular smooth muscle cell that maintains capillary integrity during neoangiogenesis100. Thus, as occurs in atherosclerotic calcification, medial calcification and valve calcium accrual is driven in great part by osteogenic cells derived from vascular residents21 (Figure 3). However, exciting new data from Pignolo et al has identified a circulating CD45+ osteocalcin+ osteoprogenitor (COP) cell that elaborates BMP family members and “homes” to sites of vascular injury16. As initially formulated by Khosla and colleagues in their elegant studies of human bone growth and fracture repair17, circulating osteoprogenitors may also participate in vascular mineralization processes in type II diabetes 101 as well as in true ectopic vascular ossification102. Therefore, strategies that inhibit vascular BMP-Wnt signaling or reduce COP cell populations may help alleviate the burden of arteriosclerotic disease in type II diabetes.
III. C. Vascular calcification of CKD / CKD-MBD: The “perfect storm”
In 2005, KGIDO (international group on Kidney Disease: Improving Global Outcomes) codified the clinical entity CKD-MBD, the mineral and bone disorder of chronic kidney disease that encompasses the vascular calcification of CKD103, 104. In CKD-MBD, the clinical link between bone disease and vascular disease arising from primary perturbations in calcium phosphate homeostasis is now formally recognized. Diabetes and hypertension – two diseases that independently promote arteriosclerotic calcification – are responsible for approximately 60% of ESRD patients requiring RRT (renal replacement therapy)105; however, the vast majority of patients with CKD from any cause experience increased cardiovascular mortality and calcific vasculopathy34, 106. While antecedent diabetes, hypertension, dyslipidemia, and metabolic syndrome continue to contribute to arteriosclerosis107, hyperphosphatemia, reduced Klotho expression, and impaired soft tissue calcification defenses are key pathophysiological components of CKD-MBD108, 109. Indeed, even in the setting of normal renal function, serum phosphate levels track severity and extent of coronary artery calcium110, 111. Hyperphosphatemia and hypercalcemia increase the elaboration of VMSC matrix vesicles -- ~100 nm diameter phosphatidylserine- and annexin- rich, bilaminate spheroids resembling the mineralizing vesicles of chondroyctes112. Shanahan and colleagues demonstrated that VSMCs elaborate these matrix vesicles as one mechanism to clear extracellular matrix calciprotein complexes112, 113 (Figure 3). However, these same matrix vesicles can serve to nucleate and propagate extracellular matrix mineralization in the absence of cell-mediated pinocytotic uptake113. Matrix vesicle uptake by VSMCs requires serum fetuin113, a hepatocyte-derived calcium binding protein that maintains calcium solubility in the supersaturated serum and interstitial fluid compartments114. Dialysis reduces serum fetuin levels as does inflammation115. In addition to increasing matrix vesicle release, phosphate also increases BMP2 elaboration by VSMCs and upregulates VSMC Runx2/Cbfa1 and Msx2 via SLC20A1 signaling65, 116. BMP2 in turn further enhances phosphate uptake and osteogenic programming of VSMCs in a feed-forward vicious cycle117. Thus, like oxLDL and peroxide, elevated serum phosphate can reprogram the VMSC phenotype to support osteogenic mineral deposition66. Furthermore, sustained hyperphosphatemia simultaneously induces VSMC apoptosis, removing the first-line cell-mediated mechanism for clearing vascular calciprotein complexes118. The ensuing vascular mineral deposition is an exuberant medial calcification that is almost always superimposed on antecedent atherosclerotic and medial calcification arising from diabetes, dyslipidemia, and other causes. Finally, with the massive increase in mural extracellular matrix calcium, the second-line defenses for handling vascular extracellular matrix calcium – viz., cells of the monocyte/macrophage lineage – are recruited119. Vascular calcium phosphate deposition elicits innate immune responses by monocyte/macrophage cells120, increasing the production of TNF121 and down-stream activation of osteogenic BMP-Msx -Wnt and Runx2/Cbfa1 signaling116, 121 – yet another, feed-forward vicious cycle in vascular mineralization. Thus, patients with CKD suffer the “perfect storm” of vascular calcification.
Perhaps not surprisingly, then, statin-based strategies focused upon LDL cholesterol reduction have generally failed to reduce cardiovascular morbidity and mortality in end stage renal disease (ESRD)122. This past year, a combined approach using implementing a statin with the cholesterol absorption inhibitor ezetimibe reduced overall major atherosclerotic disease endpoints including non-hemorrhagic stroke by ca. 20% -- but failed to significantly reduce myocardial infarction or associated death123. Strategies that emphasize phosphate binders as a primary approach to control hyperphosphatemia have met with early successes – as long as the binding is not calcium based124–126. As highlighted by Raggi et al, the use of calcium based phosphate binders significantly contribute to vascular calcium load in ESRD127. This may be directly related the impaired capacity of the uremic skeleton to rapidly handle serum calcium transients. Indeed, London demonstrated that patients with the most extensive vascular calcification exhibited the lowest levels of bone formation on histomorphometric evaluation, reflected in inappropriately normal or low PTH values128. As compared to sevelamer – the first phosphate binder that does not contain calcium or aluminum -- calcium based phosphate binders suppress PTH, reduce bone mass, and increase vascular calcium load in ESRD127. Because of the biphasic relationships between PTH, vitamin D, and vascular health in ESRD, a rigorous focus upon the role and endocrine physiology of calciotropic hormones and calcium phosphate homeostasis should provide new hope to patients afflicted with CKD129.
IV. REGULATION OF VASCULAR CALCIFICATION BY CALCIOTROPIC HORMONES
While vascular calcification is clearly an actively regulated form of extracellular calcified matrix metabolism, remarkably few studies have been undertaken with respect to control by calciotropic hormones130. The prototypic calciotropic hormones are parathyroid hormone (PTH), vitamin D and its metabolites, parathyroid hormone related polypeptide (PTHrP), calcitonin, and estrogens including estradiol. Estrogen signaling via non-genotropic signaling mechanisms acutely activates endothelial nitric oxide synthase in caveolae – an acute vasodilatory response that is theoretically protective131. Estrogen exposure in women was shown to reduce the incidence of arterial calcification as revealed from mammography132. In the Rancho Bernardo Study, estrogen use in post-menopausal women was associated with reduced coronary artery calcification133; however, estrogen replacement therapy worsened cardiovascular endpoints in older women in the Womens Health Initiative134 – and estrogens including low-dose oral contraceptives are associated with PAD135. No published studies have examined the actions of calcitonin on arteriosclerosis, but the calcitonin gene - related polypeptide, encoded by the calcitonin gene, is a vasodilator136.
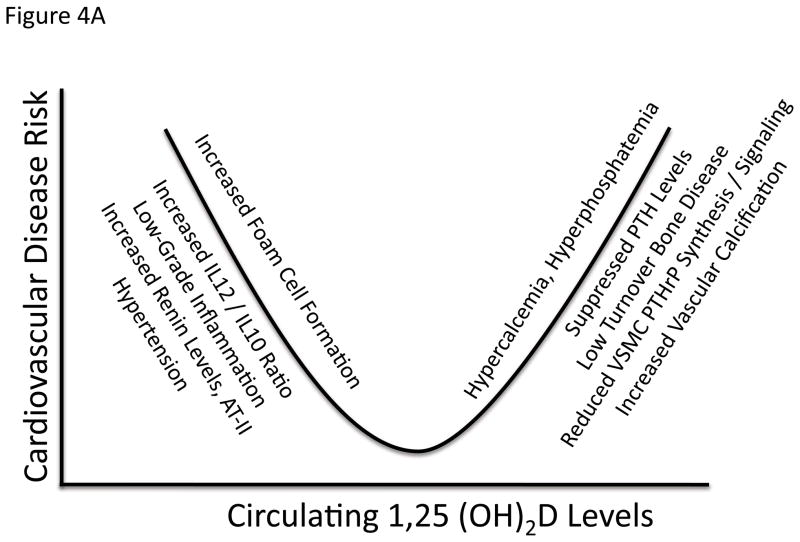
Vitamin D insufficiency is associated with PAD137 as well as other cardiovascular diseases including congestive heart failure138. Additionally, vitamin D inhibits foam cell formation and macrophage activation in patients with diabetes139. However, vitamin D replacement has not been shown to improve any primary cardiac endpoint – although the largest studies have not used uniform preparations of vitamin D140. In ESRD, a biphasic U-shaped bimodal response to calcitriol levels has clearly emerged with respect to vascular disease141 (Figure 4a). While vitamin D insufficiency and reduced 1,25(OH2)D levels in ESRD engenders more severe secondary hyperparathyroidism, excessive 1,25(OH2)D dosing can induce low-turnover bone disease, hypoparathyroidism, and increase vascular calcium deposition. As London first established, ESRD patients with the most extensive and severe arterial calcification exhibited the lowest bone formation rates and PTH levels128. Vitamin D intoxication induces widespread vascular calcification and nephrocalcinosis – and is a commonly used rodent model of vascular calcification potentially relevant to the bimodal relationship in ESRD142, 143. The calcium sensing receptor is expressed in VSMCs as well as in parathyroid glands144, and signaling with this agonist to control secondary hyperparathyroidism (sHPT) in uremic rat models results in reduced vascular calcium accrual145. Of note, in cultured VSMCs calcitriol induces calcification in part by suppressing PTHrP production and inducing alkaline phosphatase146. Via paracrine actions, PTHrP relaxes smooth muscle cells, inhibits fibroproliferative responses, prevents alkaline phosphatase induction and inhibits calcium deposition by signaling via the PTH/PTHrP receptor (PTH1R)146. Indeed, adenoviral delivery of the obligatory paracrine form of PTHrP inhibits neointima formation in a porcine model of stent-induced coronary restenosis147, 148. The role of VSMC PTHrP-PTH1R signals in cardiovascular arteriosclerosis has not been exhaustively studied to date in part because PTH1R-null mice die in utero due to massive cardiomyocyte apoptosis149. Nevertheless, the post-natal vasculopathy arising from vitamin D intoxication may involve lesioning of protective paracrine PTHrP-PTH1R signals in VSMCs in addition to hyperphosphatemia and hypercalcemia145, 146.
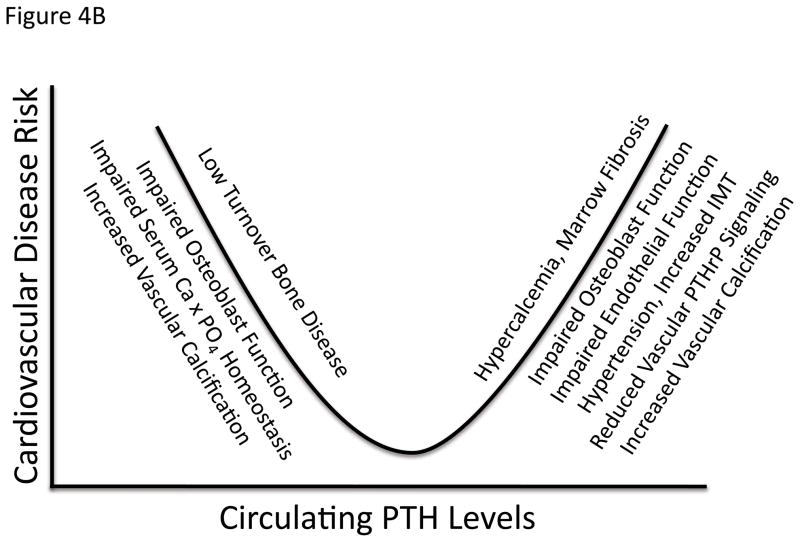
Figure 4. The biphasic relationship between cardiovascular disease and calciotropic hormones.
As in all key endocrine systems, there also is a “sweet spot” that represents the optimal set point for calciotropic hormones levels and vascular health. Panel A, both calcitriol excess and deficiency have been associated with cardiovascular disease141, 212, recently confirmed in children with CKD213. Panel B, similar cardiovascular problems arise with either both excesses196 and insufficiencies127, 128 in PTH. See text for details.
This past decade, we examined the impact of PTH/PTHrP receptor signaling on arteriosclerotic calcification in the LDLR−/− model23–25. Bone anabolic responses that increased skeletal calcium accrual were accompanied by reductions in aortic calcium accrual23, 24. Additionally, arterial expression of osteogenic genes were down-regulated while skeletal expression of these same genes were increased24, 25. The PTH1R is highly expressed in VSMCs --- and is very susceptible to homologous desensitization upon tonic exposure to PTH or PTHrP150. Sustained pharmacologic vascular exposure to either PTHrP or PTH – mimicking the setting of hyperparathyroidism – induces arterial tachyphylaxis to acute PTH/PTHrP agonist administration150. In order to address the potential role for VSMC-autonomous role for PTH1R signaling in arteriosclerotic vascular responses, we generated and evaluated SM22-PTH1R(H223R); LDLR−/− transgenic mice23. In these animals, a ligand-independent constitutively active form of the PTH1R, PTH1R(H223R)151, is expressed in VSMC from a VSMC-specific promoter23. As compared to non-transgenic male siblings, male SM22-PTH1R(H223R);LDLR−/− exhibited reduced aortic calcification, aortic fibrosis, and aortic oxidative stress23. Arteriosclerotic Wnt/beta-catenin signaling and type I collagen gene expression was concomitantly reduced23. Aortic wall thickness was also decreased, and ex vivo vessel mechanical compliance (distensibility) was increased. Importantly, Friedman and colleagues independently demonstrated that pulsatile administration of PTH(1–34) reduces arterial mineralization in a rat model of uremia, although fibrosis and vessel mechanics were not evaluated26.
Our in vivo data with the SM22-PTH1R(H223R) transgene demonstrate that sustained VSMC PTH1R activation actually reduces vascular oxidative stress and pro-calcific and pro-fibrotic signals that drive arteriosclerotic disease23. Recall that dysmetabolic states associated with macrovascular disease -- such as diabetes, dyslipidemia, and uremia -- induce tissue resistance to PTH1R activation27, 29, 152. These findings converge to prompt a major shift in our thinking as concerns the pathobiology of arteriosclerosis in type II diabetes and other dysmetabolic states, including primary and secondary hyperparathyroidism (pHPT and sHPT, respectively). How so? The negative impact of dysmetabolic states (dyslipidemia, diabetes, uremia) on PTH1R signaling may become to be viewed in part as acquired insufficiencies of endocrine paracrine PTHrP actions that help preserve vascular health. The increase in carotid IMT associated with pHPT153 might be viewed in part as being the consequence of homologous vascular desensitization150, 154 occurring in response to (a) tonically elevated PTH levels; and (b) impaired capacity of paracrine VSMC PTHrP production to restrain proliferative155 and calcific146 arteriosclerotic responses (Figure 4b). However, via its bone anabolic actions PTH signaling upregulates circulating intact OPN24 – an inhibitor of vascular mineralization – and supports the hematopoietic niche including cellular elements such as ePCs that program vascular healing responses. Furthermore, PTH upregulates the expression of matrix Gla protein (MGP)156, 157, an important negative regulator of matrix mineralization and BMP2/4 signaling in the vasculature56. Whether MGP participates in PTH inhibition of vascular myofibroblast BMP2 signaling25 remains to be evaluated. The relative contributions of direct vs. indirect actions of PTH1R on vascular health are undergoing additional scrutiny and evaluation.
V. THE IMPACT OF ARTERIOSCLEROSIS ON SKELETAL HEALTH AND FUNCTION
Atherosclerosis, calcification, mural hypertrophy and fibrosis, and elastin matrix senescence cause arteriosclerosis, the age-associated vascular stiffening that impairs Windkessel physiology necessary for smooth distal tissue perfusion. With aging, vascular remodeling processes can increase wall thickness and regionally reduce conduit artery lumen patency, thus worsening arterial compliance and its clinical impact43. In the musculoskeletal system, lower extremities experience the brunt of arteriosclerotic disease. Claudication and amputation are the most salient manifestations that reduce mobility and increase morbidity, but hip fracture is also increased with peripheral arterial disease (PAD) 8. Multiple studies have now established that the presence and extent of arteriosclerotic calcification conveys lower extremity amputation risk. Medial arterial calcification of type II diabetes conveys a 3-fold increased risk for amputation12. Recently, implementing CT scanning and the Agatston method to quantify tibial artery calcification (TAC), Guzman established that TAC scoring outperforms ankle-brachial indices (ABI) in predicting lower extremity amputation risk9. Applying the conventional ABI method to characterize peripheral artery disease (PAD), Cummings and colleagues showed that PAD increased the rate of femoral neck bone loss and increased the risk for hip fracture in men9. They concluded that “Further research should examine the biological mechanisms underlying the association between reduced limb blood flow and fractures.” 9 The increasing prevalence of type II diabetes and associated PAD will contribute to the future arteriosclerotic disease burden in our society.
Atherosclerotic deposits can and do impact bone marrow arteries. However, the physiological responses of bone to arteriosclerosis are multifactorial. In 1985, Brenneise and Squier examined the relationships between atherosclerotic disease and blood flow in rhesus monkeys maintained on high fat atherogenic diets158. These diets induced extensive calcified atherosclerotic carotid plaques and as well as atherosclerotic changes in arteries perfusing skeletal muscle. However, atherogenic diets failed to induce significant atherosclerotic changes in the principal nutrient arteries of maxillary and mandibular bone --- and did not alter osseous lumen area, intraosseous vessel wall thickness, or vessel lumen area / tissue area158. Nevertheless, atherosclerosis reduced osseous blood flow by 80% in the anterior mandible, posterior mandible, and maxilla158. How can this occur? These seminal findings indicated that macrovascular Windkessel function and endothelial function necessary for regulating bone tissue perfusion are severely impaired with atherosclerosis and arteriosclerosis81. Recently, we described and characterized a novel murine model of arteriosclerotic vascular stiffening -- the osteopontin (OPN)-null mouse on the LDLR-deficient background. Even in the absence of atherogenic diets, male OPN−/−;LDLR−/− mice exhibit aortic adventitial fibrosis and vascular stiffening. Using fluorescence microsphere perfusion assays, we demonstrated significantly reduced lower extremity bone (femur) blood flow in arteriosclerotic OPN−/−;LDLR−/− animals159. By contrast, blood flow to the kidneys was not significantly altered as assessed in this assay. Thus, arteriosclerosis and atherosclerosis impair skeletal blood flow158, 159.
At this point it should be noted that in healthy young long bone, diaphyseal blood flow is primarily centrifugal, with flow originating from marrow compartment (supplied via nutrient artery) to trabeculae and bone cortex160 (Figure 5). However, with aging flow becomes increasingly centripetal, with the greatest extent of diaphyseal cortex being perfused by periosteal arteries161. This age-dependent change in the “vector” of blood flow may become more exaggerated due to the enhanced sensitivity of nutrient artery to vasoconstrictors as compared to vasodilators162. With aging the vasodilator response in bone nutrient arteries becomes increasingly reduced163. Blood flow is a critical determinant of the bone formation rate at the BMU within the adult skeleton4. The interactions between age-dependent changes in this cortical bone flow vector and arteriosclerotic conduit vessel stiffening have not been systematically examined. Since osteoblast-mediated bone formation is directly related to perfusion4, these age-dependent changes are predicted to contribute to reduced skeletal anabolism in the absence of intervention.
Figure 5. Age-dependent changes in cortical blood flow of long bones.
Left panel, healthy cancellous bone has a marrow flow of about 20 cc/min/100 grams via the nutrient, ascending and descending medullary arteries; this helps maintain a relatively high intramedullary pressure that drives centrifugal flow through cortical bone (~ 5 cc/min/gram)160. Right panel, with aging 161 and arteriosclerosis158, 159 perfusion is altered, with blood supply to the aging cortex provided primarily from periosteal conduit vessels161. Age- and exercise - related changes in vasodilatation responses of nutrient arteries may impact the extent of centrifugal vs. centripetal cortical blood flow163, 214 since the nutrient arteries are more responsive to vasocontrictors162.
To be sure, perfusion- and diffusion – dependent nutrient supplies must be rate-limiting contributors to the impairment of bone physiology with arteriosclerotic disease. Indeed, Prisby et al highlighted that it is that the proximity of the bone-forming multicellular unit (BMU) to the microvasculature – not the mass of the skeletal microvasculature – that is critical in maintaining bone anabolism164. Vascular endothelial growth factor (VEGF), a prototypic angiogenic factor produced by osteoblasts, was shown to be critically important164. Moreover, the sub-intimal vascular accumulation of oxidized LDL in bone impairs anabolic responses to PTH27. However, it has become increasingly evident that the vasculature itself provides paracrine and juxtacrine cues that regulate osteoblast function functions165. Ephrin B2, BMP2, RANKL, and nitric oxide are but a few of the potent osteotropic signals elaborated by endothelial cells165–171. Furthermore, the vasculature also provides the sustentacular niche for osteoblast progenitors172 --- and is the conduit for egress of bone marrow – derived formed elements from the osteoblast-regulated hematopoietic niche173. By coupling enhanced PTH-mediated bone anabolism with granulocyte colony stimulating factor (G-CSF) treatment as a strategy to mobilize endothelial progenitor cells (ePCs), Ignarro has demonstrated the capacity to implement endocrine pharmacotherapy to enhance ischemic limb recovery in mice13. Thus, multiple bone-vascular interactions may contribute to impaired skeletal anabolism with arteriosclerosis. The important preclinical “proof of principle” provided by Ignarro et al13 supplies compelling pharmacologic and physiological evidence for an emerging bone-vascular axis that regulates cardiovascular and skeletal health.
VI. SKELETAL FUNCTION AND VASCULAR HEALTH: THE BONE-VASCULAR AXIS
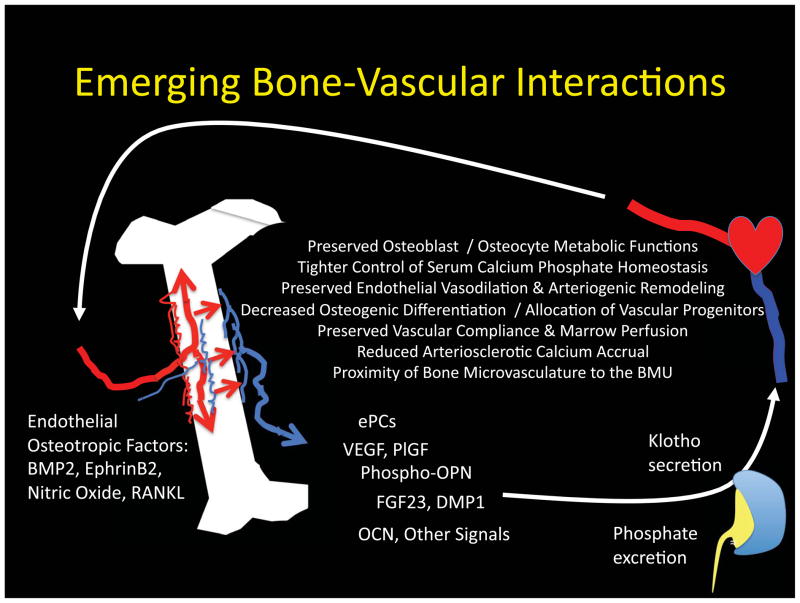
In the preceding sections, we’ve emphasized the impact of vascular disease on skeletal function. However, in the past decade it has became clear that bone is in fact an endocrine organ174, capable of elaborating phosphaturic hormones such as DMP1 and FGF23 as relevant to the pathobiology of vascular disease175. FGF23 (fibroblast growth factor 23) is an osteocyte-derived hormone that enhances phosphate excretion by the kidney via down-regulation of proximal tubule sodium phosphate symporters Slc34A1/NaPi-2a and Slc34A3/ NaPi-2c.176 Hyperphosphatemia and vascular calcification were observed in FGF23 null mice -- mitigated by simultaneous reductions in the vitamin D 1-alpha-hydroxylase CYP27B1177. Of note, FGF23 directly suppresses CYP27B1178. Thus, the vascular toxicity arising from deficiency in this osteoblast- and osteocyte- derived hormone arises from endogenous calcitriol intoxication with hyperphosphatemia179. With declining renal function, post-prandial elevations in FGF23 are amongst the earliest changes observed180, and likely serve as a bone-derived defense mechanism for limiting the toxicities of hyperphosphatemia in conjunction with PTH175. With progressive uremia, end-organ responses to FGF23 are diminished due to reduced Klotho co-factor expression by the kidney and parathyroid glands 181–185. Of note, both FGF23/Klotho185, 186 and DMP1187 signaling have been demonstrated to exert direct, beneficial vascular actions. Thus, the kidney emerges as a particularly important intermediary in the bone-vascular axis via hormonally regulated phosphate excretion and Klotho production (Figure 6). Osteopontin (OPN), a very potent inhibitor of matrix mineralization in its phosphorylated form188, is secreted into the circulation by skeletal osteoblasts and increases in response to bone anabolic stimuli such as PTH24. Since the osteoblast-derived OPN is highly phosphorylated189 and stable to proteolysis190, this circulating pool of OPN may serve as an important defense as well to vascular mineral accrual as first posited by Giachelli188. Studies by Ducy and Karsenty have highlighted the important role of bone-derived osteocalcin (OCN) as a Gla-dependent hormone controlling beta-cell longevity191. With respect to arteriosclerosis, the capacity of OCN to support adiponectin production by adipocytes may be of particular importance191. Adiponectin suppresses the inflammatory responses elicited by TNF192, 193 – an important stimulus for vascular calcification as highlighted above82. Moreover, adiponectin – null mice develop arterial calcification that is mitigated by adenoviral – mediated vascular restoration194. Recently, Monsonego-Ornan have provided new evidence that OCN may in also directly enhance osteochondrogenic mineralization of VSMCs143; this has yet to be confirmed in atherosclerotic disease models. Nevertheless, given that bone marrow-derived cell types can either promote or limit vascular calcium accrual, two arms of the bone-vascular axis clearly emerge -- one being cell-mediated via the paracrine actions of osteoblasts on hematopoietic niche and cell egress, and the other being humoral via osteoblast- and osteocyte-derived endocrine signals that regulate renal phosphate excretion, PTH production, and vascular proximity to the BMU (Figure 6).
Figure 6. Clinical promises and pitfalls of the emerging bone-vascular axis.
A bidirectional endocrine / metabolic relationship exists between bone and the vasculature that mutually benefits bone and vascular health. The kidney is an important intermediary via regulation of phosphate excretion182 and the elaboration of Klotho109, 199. Importantly, PTH/PTHrP receptor signaling (a) maintains bone formation, sustains hematopoietic niche function215 and ePC mass13; (b) promotes intact osteoblast OPN24 and osteocyte FGF23197, 199 secretion; (c) supports renal Klotho production199; and (d) suppresses aortic osteo-fibrogenic Wnt/β-catenin signaling23, 146 and vascular calcium accrual23, 26. PTH/PTHrP receptor signaling also reduces aortic23 and skeletal216 oxidative stress, and maintains the proximity of the microvasculature to the BMU during bone formation164. Declining renal function and tissue resistance to PTH/PTHrP receptor signaling represent key features in the perturbation of the bone-vascular axis with disease. P1GF, placental growth factor. RANKL, receptor activator for NF-KappaB Ligand. BMU, basic multicellular unit. See text for details.
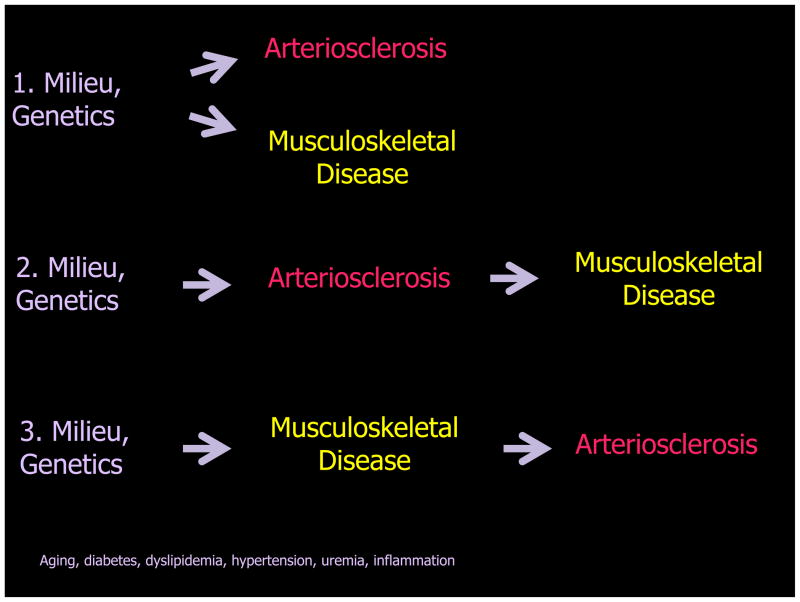
Thus, with respect to diseases of the bone-vascular axis, three relationships can now be envisioned (Figure 7). Most certainly, metabolic milieu and genetics can independently cause arteriosclerotic disease and bone disease. Additionally, primary changes in arteriosclerotic vessel functions arising from milieu and genetics can cause bone disease via alterations in perfusion. However, given emerging nature of the bone-vascular axis, primary disease changes in bone and bone marrow function arising from milieu and genetics may cause vascular disease. Under this latter and more novel view, a healthy skeleton and marrow is a good thing for maintenance of vascular health. The relative contributions of bone-derived cellular vs. endocrine signals to changes in vascular physiology may be particularly important with uremia, aging, and diabetes195. A better understanding of how bone-derived endocrine cues and marrow-derived cell types interact to regulated vascular health is clearly necessary.
Figure 7. Metabolic milieu, genetics, arteriosclerosis, and musculoskeletal disease.
Oxylipids simultaneously drive arteriosclerotic calcification, suppress bone formation, and increase osteoclastogenesis; parallel progression arteriosclerosis and musculoskeletal disease ensues (schema 1). However, via vessel stiffening and reductions in endothelium-dependent control of bone perfusion, arteriosclerosis can negatively impact bone anabolic responses necessary for skeletal homeostasis and fracture repair (schema 2). Finally, since osteoblasts, osteocytes, and a bone marrow elaborate cellular elements and hormonal cues that prevent arteriosclerotic remodeling and preserve vascular health, primary disease of bone may give rise to or at least exacerbate arteriosclerotic disease (schema 3).
VIII. CONCLUSIONS AND FUTURE DIRECTIONS
Preclinical and clinical studies performed over the past two decades have converged to highlight the presence of and critical role for a bone –vascular regulatory axis in human health. Not unlike the famous legend of the three blind men describing the elephant, the perspectives of experts in endocrinology, cardiology, developmental biology, orthopedics, biochemistry, genetics, pathology, engineering and hematology often emphasize different features of the bone-vascular axis. Fortunately, the picture emerging from the assembly of these multiple viewpoints is providing an increasingly sharper image. Calciotropic hormones have direct and indirect vasculotropic actions deserving consideration; both excesses196 and insufficiencies128 in calciotropic hormones such as PTH engender vascular disease. The inability to tightly regulate calcium phosphate homeostasis results in vascular toxicity. Cellular and endocrine signals arising from bone and marrow impact vascular physiology and function – and are maintained in part by PTH-dependent signals13, 182, 197. Age- and disease-dependent changes in vascular physiology impact bone health and fracture risk. Inflammation and oxidative stress / oxylipid signals reciprocally regulate bone and vascular mineralization – and impair PTH/PTHrP receptor signaling important to bone and vascular health29, 67, 198. The kidney is an important intermediary in the bone-vascular axis via hormonally regulated phosphate expression and Klotho expression182, 199. Declining renal function and tissue resistance to PTH/PTHrP receptor signaling represent key features in the perturbation of the bone-vascular axis with disease (Figure 6). Endocrine regulation of the bone-vascular axis is feasible; for example, strategies that modulate PTH/PTHrP receptor signaling, end organ responsiveness, and calcium phosphate homeostasis offer opportunities to improve bone health and preserve vascular health in patients with diabetes, dyslipidemia, and uremia PAD13, 23, 29, 127, 200, 201.
On a cautionary note, however, our understanding of the relationships between bone and vascular mineral homeostasis is rudimentary. As clinicians, we are coming to appreciate the reciprocal relationships between bone mass / osteoporosis and atherosclerosis as detected by vascular calcification202–205 – but the endocrine regulation of this relationship may change with age. For example, aminobisphosphonate therapy for osteoporosis decreases the risk for aortic valve and thoracic aorta calcification in women > 75 years old – but INCREASES risk in women under age 55206. Furthermore, although helpful to bone in some contexts, oral calcium supplementation in those with renal insufficiency127 and advanced age207, 208 may have down-side impacts on cardiovascular health. As our understanding of the bone-vascular axis continues to improve, so too will our capacity to meet the clinical needs of our patients afflicted with musculoskeletal and vascular diseases.
Acknowledgments
Supported by NIH grants HL88651, HL69299, and HL81138 to DAT., and the Barnes-Jewish Hospital Foundation.
References
- 1.Zelzer E, et al. Skeletal defects in VEGF(120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129:1893–904. doi: 10.1242/dev.129.8.1893. [DOI] [PubMed] [Google Scholar]
- 2.Maes C, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–44. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qing H, et al. PTHR1 in Osteocytes Plays a Major role in Perilacunar Remodeling through the Activation of “Osteoclastic” Genes in Osteocytes. J Bone Miner Res. 2010;25(Suppl 1) Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=fbaf7531-6ce4-43fc-a854-93cb42413eaf. [Google Scholar]
- 4.Reeve J, et al. Skeletal blood flow, iliac histomorphometry, and strontium kinetics in osteoporosis: a relationship between blood flow and corrected apposition rate. J Clin Endocrinol Metab. 1988;66:1124–31. doi: 10.1210/jcem-66-6-1124. [DOI] [PubMed] [Google Scholar]
- 5.Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117:5281–8. doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]
- 6.Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–31. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Towler DA. The osteogenic-angiogenic interface: novel insights into the biology of bone formation and fracture repair. Curr Osteoporos Rep. 2008;6:67–71. doi: 10.1007/s11914-008-0012-x. [DOI] [PubMed] [Google Scholar]
- 8.Collins TC, et al. Peripheral arterial disease is associated with higher rates of hip bone loss and increased fracture risk in older men. Circulation. 2009;119:2305–12. doi: 10.1161/CIRCULATIONAHA.108.820993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman RJ, et al. Tibial artery calcification as a marker of amputation risk in patients with peripheral arterial disease. J Am Coll Cardiol. 2008;51:1967–74. doi: 10.1016/j.jacc.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soor GS, Vukin I, Leong SW, Oreopoulos G, Butany J. Peripheral vascular disease: who gets it and why? A histomorphological analysis of 261 arterial segments from 58 cases. Pathology. 2008;40:385–91. doi: 10.1080/00313020802036764. [DOI] [PubMed] [Google Scholar]
- 11.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 12.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–83. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 13.Napoli C, et al. Therapeutic targeting of the stem cell niche in experimental hindlimb ischemia. Nat Clin Pract Cardiovasc Med. 2008;5:571–9. doi: 10.1038/ncpcardio1214. [DOI] [PubMed] [Google Scholar]
- 14.Gossl M, Modder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008;52:1314–25. doi: 10.1016/j.jacc.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gossl M, et al. Coronary endothelial dysfunction in humans is associated with coronary retention of osteogenic endothelial progenitor cells. Eur Heart J. 2010;31:2909–14. doi: 10.1093/eurheartj/ehq373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egan KP, Kim JH, Mohler ER, 3rd, Pignolo RJ. Role for Circulating Osteogenic Precursor Cells in Aortic Valvular Disease. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.234724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eghbali-Fatourechi GZ, et al. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–66. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 18.Shen X, et al. Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. J Orthop Res. 2009;27:1298–305. doi: 10.1002/jor.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan C, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A. 2008;105:686–91. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zelzer E, Olsen BR. Multiple roles of vascular endothelial growth factor (VEGF) in skeletal development, growth, and repair. Curr Top Dev Biol. 2005;65:169–87. doi: 10.1016/S0070-2153(04)65006-X. [DOI] [PubMed] [Google Scholar]
- 21.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–48. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao JS, Cheng SL, Sadhu J, Towler DA. Inflammation and the osteogenic regulation of vascular calcification: a review and perspective. Hypertension. 2010;55:579–92. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng SL, et al. Activation of vascular smooth muscle parathyroid hormone receptor inhibits Wnt/beta-catenin signaling and aortic fibrosis in diabetic arteriosclerosis. Circ Res. 2010;107:271–82. doi: 10.1161/CIRCRESAHA.110.219899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao JS, Cheng SL, Charlton-Kachigian N, Loewy AP, Towler DA. Teriparatide (human parathyroid hormone (1–34)) inhibits osteogenic vascular calcification in diabetic low density lipoprotein receptor-deficient mice. J Biol Chem. 2003;278:50195–202. doi: 10.1074/jbc.M308825200. [DOI] [PubMed] [Google Scholar]
- 25.Shao JS, et al. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–20. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sebastian EM, Suva LJ, Friedman PA. Differential effects of intermittent PTH(1–34) and PTH(7–34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone. 2008;43:1022–30. doi: 10.1016/j.bone.2008.07.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang MS, et al. Hyperlipidemia impairs osteoanabolic effects of PTH. J Bone Miner Res. 2008;23:1672–9. doi: 10.1359/JBMR.080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang MS, et al. Atherogenic phospholipids attenuate osteogenic signaling by BMP-2 and parathyroid hormone in osteoblasts. J Biol Chem. 2007;282:21237–43. doi: 10.1074/jbc.M701341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sage AP, et al. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res. 2011;26:1197–206. doi: 10.1002/jbmr.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy WA, Jr, et al. The iceman: discovery and imaging. Radiology. 2003;226:614–29. doi: 10.1148/radiol.2263020338. [DOI] [PubMed] [Google Scholar]
- 31.Polonsky TS, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. Jama. 2010;303:1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenhek R, et al. Mild and moderate aortic stenosis. Natural history and risk stratification by echocardiography. Eur Heart J. 2004;25:199–205. doi: 10.1016/j.ehj.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Rosenhek R, et al. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–7. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 34.London GM, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–40. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 35.Bos D, et al. Calcification in Major Vessel Beds Relates to Vascular Brain Disease. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.232728. [DOI] [PubMed] [Google Scholar]
- 36.Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20:1177–8. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 37.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 38.Ward MR, Pasterkamp G, Yeung AC, Borst C. Arterial remodeling. Mechanisms and clinical implications. Circulation. 2000;102:1186–91. doi: 10.1161/01.cir.102.10.1186. [DOI] [PubMed] [Google Scholar]
- 39.Rajamannan NM. Calcific aortic stenosis: lessons learned from experimental and clinical studies. Arterioscler Thromb Vasc Biol. 2009;29:162–8. doi: 10.1161/ATVBAHA.107.156752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajamannan NM, Bonow RO, Rahimtoola SH. Calcific aortic stenosis: an update. Nat Clin Pract Cardiovasc Med. 2007;4:254–62. doi: 10.1038/ncpcardio0827. [DOI] [PubMed] [Google Scholar]
- 41.Greenwald SE. Ageing of the conduit arteries. J Pathol. 2007;211:157–72. doi: 10.1002/path.2101. [DOI] [PubMed] [Google Scholar]
- 42.Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Compute. 2009;47:131–41. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 43.O’Rourke MF. Arterial aging: pathophysiological principles. Vasc Med. 2007;12:329–41. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- 44.Safar ME, Boudier HS. Vascular development, pulse pressure, and the mechanisms of hypertension. Hypertension. 2005;46:205–9. doi: 10.1161/01.HYP.0000167992.80876.26. [DOI] [PubMed] [Google Scholar]
- 45.Virchow R, Chance F. Twenty lectures delivered in the Pathological institute of Berlin during the months of February, March and April, 1858. R. M. De Witt; New York: 1860. Cellular pathology, as based upon physiological and pathological histology. [Google Scholar]
- 46.Virchow R. Cellular pathology. As based upon physiological and pathological histology. Lecture XVI-Atheromatous affection of arteries. 1858. Nutr Rev. 1989;47:23–5. doi: 10.1111/j.1753-4887.1989.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 47.Mohler ER, 3rd, et al. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–8. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 48.Tyson KL, et al. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–94. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 49.Hessle L, et al. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanimura A, McGregor DH, Anderson HC. Calcification in atherosclerosis. I. Human studies. J Exp Pathol. 1986;2:261–73. [PubMed] [Google Scholar]
- 51.Tanimura A, McGregor DH, Anderson HC. Calcification in atherosclerosis. II. Animal studies. J Exp Pathol. 1986;2:275–97. [PubMed] [Google Scholar]
- 52.Bostrom K, et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–9. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang M, et al. Atorvastatin downregulates BMP-2 expression induced by oxidized low-density lipoprotein in human umbilical vein endothelial cells. Circ J. 2008;72:807–12. doi: 10.1253/circj.72.807. [DOI] [PubMed] [Google Scholar]
- 54.Cola C, Almeida M, Li D, Romeo F, Mehta JL. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Bio chem Biophys Res Commun. 2004;320:424–7. doi: 10.1016/j.bbrc.2004.05.181. [DOI] [PubMed] [Google Scholar]
- 55.Tintut Y, et al. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105:650–5. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 56.Yao Y, et al. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–94. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Towler DA, Demer LL. Thematic series on the pathobiology of vascular calcification: an introduction. Circ Res. 2011;108:1378–80. doi: 10.1161/CIRCRESAHA.110.234419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stary HC, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–74. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 59.Chung UI, Kawaguchi H, Takato T, Nakamura K. Distinct osteogenic mechanisms of bones of distinct origins. J Orthop Sci. 2004;9:410–4. doi: 10.1007/s00776-004-0786-3. [DOI] [PubMed] [Google Scholar]
- 60.Cohen MM., Jr The new bone biology: pathologic, molecular, and clinical correlates. Am J Med Genet A. 2006;140:2646–706. doi: 10.1002/ajmg.a.31368. [DOI] [PubMed] [Google Scholar]
- 61.Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007;134:3133–44. doi: 10.1242/dev.002709. [DOI] [PubMed] [Google Scholar]
- 62.Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–96. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- 63.Aikawa E, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–86. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 64.Steitz SA, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89:1147–54. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 65.Speer MY, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–41. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Speer MY, Li X, Hiremath PG, Giachelli CM. Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J Cell Bio chem. 2010;110:935–47. doi: 10.1002/jcb.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Demer L, Tintut Y. The roles of lipid oxidation products and receptor activator of nuclear factor-kappaB signaling in atherosclerotic calcification. Circ Res. 2011;108:1482–93. doi: 10.1161/CIRCRESAHA.110.234245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Richardson JA, et al. Oxysterol-induced osteoblastic differentiation of pluripotent mesenchymal cells is mediated through a PKC- and PKA-dependent pathway. J Cell Biochem. 2007;100:1131–45. doi: 10.1002/jcb.21112. [DOI] [PubMed] [Google Scholar]
- 69.Byon CH, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–27. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Narisawa S, et al. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res. 2007;22:1700–10. doi: 10.1359/jbmr.070714. [DOI] [PubMed] [Google Scholar]
- 71.Parhami F, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 72.Parhami F, et al. Atherogenic high-fat diet reduces bone mineralization in mice. J Bone Miner Res. 2001;16:182–8. doi: 10.1359/jbmr.2001.16.1.182. [DOI] [PubMed] [Google Scholar]
- 73.Sage AP, Lu J, Tintut Y, Demer LL. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int. 2011;79:414–22. doi: 10.1038/ki.2010.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–36. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tintut Y, et al. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–10. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 76.Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–8. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 77.Nelson RG, et al. Lower-extremity amputations in NIDDM. 12-yr follow-up study in Pima Indians. Diabetes Care. 1988;11:8–16. doi: 10.2337/diacare.11.1.8. [DOI] [PubMed] [Google Scholar]
- 78.Leskinen Y, Salenius JP, Lehtimaki T, Huhtala H, Saha H. The prevalence of peripheral arterial disease and medial arterial calcification in patients with chronic renal failure: requirements for diagnostics. Am J Kidney Dis. 2002;40:472–9. doi: 10.1053/ajkd.2002.34885. [DOI] [PubMed] [Google Scholar]
- 79.Towler DA. Vascular Calcification: A Perspective On An Imminent Disease Epidemic. IBMS BoneKEy. 2008;5:41–58. doi: 10.1138/20080298. [DOI] [Google Scholar]
- 80.Robbins SL, Cotran RS, Kumar V. Pathologic basis of disease. Saunders; Philadelphia: 1984. [Google Scholar]
- 81.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 82.Al-Aly Z, et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–96. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 83.Qiao JH, Mertens RB, Fishbein MC, Geller SA. Cartilaginous metaplasia in calcified diabetic peripheral vascular disease: morphologic evidence of enchondral ossification. Hum Pathol. 2003;34:402–7. doi: 10.1053/hupa.2003.72. [DOI] [PubMed] [Google Scholar]
- 84.Satokata I, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet. 2000;24:391–5. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- 85.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem. 1998;273:30427–34. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 86.Canfield AE, et al. Role of pericytes in vascular calcification: a review. Z Kardiol. 2000;89 (Suppl 2):20–7. doi: 10.1007/s003920070096. [DOI] [PubMed] [Google Scholar]
- 87.Zhang L, et al. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108:472–8. doi: 10.1161/01.CIR.0000080378.96063.23. [DOI] [PubMed] [Google Scholar]
- 88.Csiszar A, et al. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H202, and high intravascular pressure. Circulation. 2005;111:2364–72. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- 89.Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–53. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 90.Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem. 2003;278:45969–77. doi: 10.1074/jbc.M306972200. [DOI] [PubMed] [Google Scholar]
- 91.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: lessons learned from the aorta. Arterioscler Thromb Vasc Biol. 2006;26:1423–30. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 92.Cheng SL, Shao JS, Cai J, Sierra OL, Towler DA. Msx2 exerts bone anabolism via canonical Wnt signaling. J Biol Chem. 2008;283:20505–22. doi: 10.1074/jbc.M800851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bostrom KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res. 2011;109:564–77. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orrico C, et al. Dysfunctional vasa vasorum in diabetic peripheral artery obstructive disease with critical lower limb ischaemia. Eur J Vasc Endovasc Surg. 2010;40:365–74. doi: 10.1016/j.ejvs.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 95.Hayden MR, Tyagi SC. Vasa vasorum in plaque angiogenesis, metabolic syndrome, type 2 diabetes mellitus, and atheroscleropathy: a malignant transformation. Cardiovasc Diabetol. 2004;3:1. doi: 10.1186/1475-2840-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bujan J, et al. Modifications induced by atherogenic diet in the capacity of the arterial wall in rats to respond to surgical insult. Atherosclerosis. 1996;122:141–52. doi: 10.1016/0021-9150(95)05727-7. [DOI] [PubMed] [Google Scholar]
- 97.Miller JD, et al. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–50. doi: 10.1016/j.jacc.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caira FC, et al. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–12. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rajamannan NM. The role of Lrp5/6 in cardiac valve disease: Experimental hypercholesterolemia in the ApoE(−/−) /Lrp5(−/−) mice. J Cell Bio chem. 2011 doi: 10.1002/jcb.23221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103–14. doi: 10.1007/978-3-540-78281-0_7. [DOI] [PubMed] [Google Scholar]
- 101.Fadini GP, et al. Widespread increase in myeloid calcifying cells contributes to ectopic vascular calcification in type 2 diabetes. Circ Res. 2011;108:1112–21. doi: 10.1161/CIRCRESAHA.110.234088. [DOI] [PubMed] [Google Scholar]
- 102.Pignolo RJ, Shore EM. Circulating osteogenic precursor cells. Crit Rev Eukaryot Gene Expr. 2010;20:171–80. doi: 10.1615/critreveukargeneexpr.v20.i2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hruska KA, Choi ET, Memon I, Davis TK, Mathew S. Cardiovascular risk in chronic kidney disease (CKD): the CKD-mineral bone disorder (CKD-MBD) Pediatr Nephrol. 2009 doi: 10.1007/s00467-009-1337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moe SM, Drueke T, Lameire N, Eknoyan G. Chronic kidney disease-mineral-bone disorder: a new paradigm. Adv Chronic Kidney Dis. 2007;14:3–12. doi: 10.1053/j.ackd.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 105.Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18:2644–8. doi: 10.1681/ASN.2007020220. [DOI] [PubMed] [Google Scholar]
- 106.London GM. Cardiovascular calcifications in uremic patients: clinical impact on cardiovascular function. J Am Soc Nephrol. 2003;14:S305–9. doi: 10.1097/01.asn.0000081664.65772.eb. [DOI] [PubMed] [Google Scholar]
- 107.Ishimura E, et al. Different risk factors for vascular calcification in end-stage renal disease between diabetics and nondiabetics: the respective importance of glycemic and phosphate control. Kidney Blood Press Res. 2008;31:10–5. doi: 10.1159/000112542. [DOI] [PubMed] [Google Scholar]
- 108.Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453–64. doi: 10.1681/ASN.2008070692. [DOI] [PubMed] [Google Scholar]
- 109.Moe SM, et al. The pathophysiology of early stage chronic kidney disease-mineral bone disorder (CKD-MBD) and response to phosphate binders in the rat. J Bone Miner Res. 2011 doi: 10.1002/jbmr.485. [DOI] [PubMed] [Google Scholar]
- 110.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphate and left ventricular hypertrophy in young adults: the coronary artery risk development in young adults study. Kidney Blood Press Res. 2009;32:37–44. doi: 10.1159/000203348. [DOI] [PubMed] [Google Scholar]
- 111.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reynolds JL, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–67. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 113.Reynolds JL, et al. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–30. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 114.Jahnen-Dechent W, Heiss A, Schafer C, Ketteler M. Fetuin-A regulation of calcified matrix metabolism. Circ Res. 2011;108:1494–509. doi: 10.1161/CIRCRESAHA.110.234260. [DOI] [PubMed] [Google Scholar]
- 115.Ciaccio M, et al. Changes in serum fetuin-A and inflammatory markers levels in end-stage renal disease (ESRD): effect of a single session haemodialysis. Clin Chem Lab Med. 2008;46:212–4. doi: 10.1515/CCLM.2008.041. [DOI] [PubMed] [Google Scholar]
- 116.Villa-Bellosta R, Levi M, Sorribas V. Vascular smooth muscle cell calcification and SLC20 inorganic phosphate transporters: effects of PDGF, TNF-alpha, and Pi. Pflugers Arch. 2009;458:1151–61. doi: 10.1007/s00424-009-0688-5. [DOI] [PubMed] [Google Scholar]
- 117.Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199:271–7. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shroff RC, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–57. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 119.Nadra I, et al. Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ Res. 2005;96:1248–56. doi: 10.1161/01.RES.0000171451.88616.c2. [DOI] [PubMed] [Google Scholar]
- 120.Bostrom K. Proinflammatory vascular calcification. Circ Res. 2005;96:1219–20. doi: 10.1161/01.RES.0000172407.20974.e5. [DOI] [PubMed] [Google Scholar]
- 121.Koleganova N, et al. Arterial calcification in patients with chronic kidney disease. Nephrol Dial Transplant. 2009;24:2488–96. doi: 10.1093/ndt/gfp137. [DOI] [PubMed] [Google Scholar]
- 122.Fellstrom BC, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 123.Baigent C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–41. doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 125.Moe SM, Chertow GM. The case against calcium-based phosphate binders. Clin J Am Soc Nephrol. 2006;1:697–703. doi: 10.2215/CJN.00560206. [DOI] [PubMed] [Google Scholar]
- 126.Raggi P, Vukicevic S, Moyses RM, Wesseling K, Spiegel DM. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin J Am Soc Nephrol. 2010;5 (Suppl 1):S31–40. doi: 10.2215/CJN.05880809. [DOI] [PubMed] [Google Scholar]
- 127.Raggi P, et al. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J Bone Miner Res. 2005;20:764–72. doi: 10.1359/JBMR.041221. [DOI] [PubMed] [Google Scholar]
- 128.London GM, et al. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15:1943–51. doi: 10.1097/01.asn.0000129337.50739.48. [DOI] [PubMed] [Google Scholar]
- 129.Demer L, Tintut Y. The bone-vascular axis in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:349–53. doi: 10.1097/MNH.0b013e32833a3d67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Findlay DM, Martin TJ. Receptors of calciotropic hormones. Horm Metab Res. 1997;29:128–34. doi: 10.1055/s-2007-979005. [DOI] [PubMed] [Google Scholar]
- 131.Chambliss KL, et al. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 132.Ferreira JA, Pompei LM, Fernandes CE, Azevedo LH, Peixoto S. Breast arterial calcification is a predictive factor of cardiovascular disease in Brazilian postmenopausal women. Climacteric. 2009;12:439–44. doi: 10.1080/13697130902957287. [DOI] [PubMed] [Google Scholar]
- 133.Barrett-Connor E, Laughlin GA. Hormone therapy and coronary artery calcification in asymptomatic postmenopausal women: the Rancho Bernardo Study. Menopause. 2005;12:40–8. doi: 10.1097/00042192-200512010-00009. [DOI] [PubMed] [Google Scholar]
- 134.Rossouw JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 135.Van Den Bosch MA, et al. The RATIO study: oral contraceptives and the risk of peripheral arterial disease in young women. J Thromb Haemost. 2003;1:439–44. doi: 10.1046/j.1538-7836.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 136.Bukoski RD, Kremer D. Calcium-regulating hormones in hypertension: vascular actions. Am J Clin Nutr. 1991;54:220S–226S. doi: 10.1093/ajcn/54.1.220S. [DOI] [PubMed] [Google Scholar]
- 137.Holick MF. Sunlight and vitamin D: both good for cardiovascular health. J Gen Intern Med. 2002;17:733–5. doi: 10.1046/j.1525-1497.2002.20731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brewer LC, Michos ED, Reis JP. Vitamin D in atherosclerosis, vascular disease, and endothelial function. Curr Drug Targets. 2011;12:54–60. doi: 10.2174/138945011793591617. [DOI] [PubMed] [Google Scholar]
- 139.Oh J, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–98. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Neuhouser ML, et al. Multivitamin use and risk of cancer and cardiovascular disease in the Women’s Health Initiative cohorts. Arch Intern Med. 2009;169:294–304. doi: 10.1001/archinternmed.2008.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Querfeld U, Mak RH. Vitamin D deficiency and toxicity in chronic kidney disease: in search of the therapeutic window. Pediatr Nephrol. 2010;25:2413–30. doi: 10.1007/s00467-010-1574-2. [DOI] [PubMed] [Google Scholar]
- 142.Price PA, Williamson MK, Nguyen TM, Than TN. Serum levels of the fetuin-mineral complex correlate with artery calcification in the rat. J Biol Chem. 2004;279:1594–600. doi: 10.1074/jbc.M305199200. [DOI] [PubMed] [Google Scholar]
- 143.Idelevich A, Rais Y, Monsonego-Ornan E. Bone Gla Protein Increases HIF-1{alpha}-Dependent Glucose Metabolism and Induces Cartilage and Vascular Calcification. Arterioscler Thromb Vasc Biol. 2011;31:e55–71. doi: 10.1161/ATVBAHA.111.230904. [DOI] [PubMed] [Google Scholar]
- 144.Alam MU, et al. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res. 2009;81:260–8. doi: 10.1093/cvr/cvn279. [DOI] [PubMed] [Google Scholar]
- 145.Henley C, et al. 1,25-Dihydroxyvitamin D3 but not cinacalcet HCl (Sensipar/Mimpara) treatment mediates aortic calcification in a rat model of secondary hyperparathyroidism. Nephrol Dial Transplant. 2005;20:1370–7. doi: 10.1093/ndt/gfh834. [DOI] [PubMed] [Google Scholar]
- 146.Jono S, Nishizawa Y, Shioi A, Morii H. Parathyroid hormone-related peptide as a local regulator of vascular calcification. Its inhibitory action on in vitro calcification by bovine vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1997;17:1135–42. doi: 10.1161/01.atv.17.6.1135. [DOI] [PubMed] [Google Scholar]
- 147.Fiaschi-Taesch N, et al. Mutant parathyroid hormone-related protein, devoid of the nuclear localization signal, markedly inhibits arterial smooth muscle cell cycle and neointima formation by coordinate up-regulation of p15Ink4b and p27kip1. Endocrinology. 2009;150:1429–39. doi: 10.1210/en.2008-0737. [DOI] [PubMed] [Google Scholar]
- 148.Fiaschi-Taesch N, Takane KK, Masters S, Lopez-Talavera JC, Stewart AF. Parathyroid-hormone-related protein as a regulator of pRb and the cell cycle in arterial smooth muscle. Circulation. 2004;110:177–85. doi: 10.1161/01.CIR.0000134483.30849.B7. [DOI] [PubMed] [Google Scholar]
- 149.Qian J, et al. Midgestational lethality in mice lacking the parathyroid hormone (PTH)/PTH-related peptide receptor is associated with abrupt cardiomyocyte death. Endocrinology. 2003;144:1053–61. doi: 10.1210/en.2002-220993. [DOI] [PubMed] [Google Scholar]
- 150.Nyby MD, et al. Desensitization of vascular tissue to parathyroid hormone and parathyroid hormone-related protein. Endocrinology. 1995;136:2497–504. doi: 10.1210/endo.136.6.7750471. [DOI] [PubMed] [Google Scholar]
- 151.Schipani E, Kruse K, Juppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995;268:98–100. doi: 10.1126/science.7701349. [DOI] [PubMed] [Google Scholar]
- 152.Langub MC, et al. Administration of PTH-(7–84) antagonizes the effects of PTH-(1–84) on bone in rats with moderate renal failure. Endocrinology. 2003;144:1135–8. doi: 10.1210/en.2002-221026. [DOI] [PubMed] [Google Scholar]
- 153.Walker MD, et al. Carotid vascular abnormalities in primary hyperparathyroidism. J Clin Endocrinol Metab. 2009;94:3849–56. doi: 10.1210/jc.2009-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maeda S, et al. Targeted overexpression of parathyroid hormone-related protein (PTHrP) to vascular smooth muscle in transgenic mice lowers blood pressure and alters vascular contractility. Endocrinology. 1999;140:1815–25. doi: 10.1210/endo.140.4.6646. [DOI] [PubMed] [Google Scholar]
- 155.Song GJ, et al. EBP50 inhibits the anti-mitogenic action of the parathyroid hormone type 1 receptor in vascular smooth muscle cells. J Mol Cell Cardiol. 2010;49:1012–21. doi: 10.1016/j.yjmcc.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suttamanatwong S, Franceschi RT, Carlson AE, Gopalakrishnan R. Regulation of matrix Gla protein by parathyroid hormone in MC3T3-E1 osteoblast-like cells involves protein kinase A and extracellular signal-regulated kinase pathways. J Cell Biochem. 2007;102:496–505. doi: 10.1002/jcb.21314. [DOI] [PubMed] [Google Scholar]
- 157.Gopalakrishnan R, Suttamanatwong S, Carlson AE, Franceschi RT. Role of matrix Gla protein in parathyroid hormone inhibition of osteoblast mineralization. Cells Tissues Organs. 2005;181:166–75. doi: 10.1159/000091378. [DOI] [PubMed] [Google Scholar]
- 158.Brenneise CV, Squier CA. Blood flow in maxilla and mandible of normal and atherosclerotic rhesus monkeys. J Oral Pathol. 1985;14:800–8. doi: 10.1111/j.1600-0714.1985.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 159.Shao JS, et al. Vascular Calcification and Aortic Fibrosis: A Bifunctional Role for Osteopontin in Diabetic Arteriosclerosis. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.230011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McCarthy I. The physiology of bone blood flow: a review. J Bone Joint Surg Am. 2006;88 (Suppl 3):4–9. doi: 10.2106/JBJS.F.00890. [DOI] [PubMed] [Google Scholar]
- 161.Bridgeman G, Brookes M. Blood supply to the human femoral diaphysis in youth and senescence. J Anat. 1996;188 ( Pt 3):611–21. [PMC free article] [PubMed] [Google Scholar]
- 162.Brinker MR, Lippton HL, Cook SD, Hyman AL. Pharmacological regulation of the circulation of bone. J Bone Joint Surg Am. 1990;72:964–75. [PubMed] [Google Scholar]
- 163.Prisby RD, et al. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res. 2007;22:1280–8. doi: 10.1359/jbmr.070415. [DOI] [PubMed] [Google Scholar]
- 164.Prisby R, et al. Intermittent PTH 1–84 is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone forming sites. Journal of Bone and Mineral Research. 2011 doi: 10.1002/jbmr.459. [DOI] [PubMed] [Google Scholar]
- 165.Bouletreau PJ, et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plast Reconstr Surg. 2002;109:2384–97. doi: 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- 166.Zhao C, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006;4:111–21. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 167.Bochenek ML, Dickinson S, Astin JW, Adams RH, Nobes CD. Ephrin-B2 regulates endothelial cell morphology and motility independently of Eph-receptor binding. J Cell Sci. 2010;123:1235–46. doi: 10.1242/jcs.061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kanazawa I, et al. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol. 2007;8:51. doi: 10.1186/1471-2121-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Armour KE, et al. Defective bone formation and anabolic response to exogenous estrogen in mice with targeted disruption of endothelial nitric oxide synthase. Endocrinology. 2001;142:760–6. doi: 10.1210/endo.142.2.7977. [DOI] [PubMed] [Google Scholar]
- 170.Collin-Osdoby P, et al. Receptor activator of NF-kappa B and osteoprotegerin expression by human microvascular endothelial cells, regulation by inflammatory cytokines, and role in human osteoclastogenesis. J Biol Chem. 2001;276:20659–72. doi: 10.1074/jbc.M010153200. [DOI] [PubMed] [Google Scholar]
- 171.Dominguez JM, 2nd, Prisby RD, Muller-Delp JM, Allen MR, Delp MD. Increased nitric oxide-mediated vasodilation of bone resistance arteries is associated with increased trabecular bone volume after endurance training in rats. Bone. 2010;46:813–9. doi: 10.1016/j.bone.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bianco P, Sacchetti B, Riminucci M. Osteoprogenitors and the hematopoietic microenvironment. Best Pract Res Clin Haematol. 2011;24:37–47. doi: 10.1016/j.beha.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 173.Brunner S, et al. Parathyroid hormone effectively induces mobilization of progenitor cells without depletion of bone marrow. Exp Hematol. 2008;36:1157–66. doi: 10.1016/j.exphem.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 174.Fukumoto S, Martin TJ. Bone as an endocrine organ. Trends Endocrinol Metab. 2009;20:230–6. doi: 10.1016/j.tem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 175.Gutierrez O, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–15. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 176.Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91–104. doi: 10.1146/annurev.med.051308.111339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sitara D, et al. Genetic ablation of vitamin D activation pathway reverses biochemical and skeletal anomalies in Fgf-23-null animals. Am J Pathol. 2006;169:2161–70. doi: 10.2353/ajpath.2006.060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fukumoto S, Yamashita T. FGF23 is a hormone-regulating phosphate metabolism--unique biological characteristics of FGF23. Bone. 2007;40:1190–5. doi: 10.1016/j.bone.2006.12.062. [DOI] [PubMed] [Google Scholar]
- 179.Stubbs JR, et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18:2116–24. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 180.Isakova T, et al. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol. 2008;19:615–23. doi: 10.1681/ASN.2007060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Shimizu H, et al. Indoxyl sulfate downregulates renal expression of Klotho through production of ROS and activation of nuclear factor-kB. Am J Nephrol. 2011;33:319–24. doi: 10.1159/000324885. [DOI] [PubMed] [Google Scholar]
- 182.Farrow EG, White KE. Recent advances in renal phosphate handling. Nat Rev Nephrol. 2010;6:207–17. doi: 10.1038/nrneph.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Canalejo R, et al. FGF23 fails to inhibit uremic parathyroid glands. J Am Soc Nephrol. 2010;21:1125–35. doi: 10.1681/ASN.2009040427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Adijiang A, Niwa T. An oral sorbent, AST-120, increases Klotho expression and inhibits cell senescence in the kidney of uremic rats. Am J Nephrol. 2010;31:160–4. doi: 10.1159/000264634. [DOI] [PubMed] [Google Scholar]
- 185.Hu MC, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–36. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Saito Y, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998;248:324–9. doi: 10.1006/bbrc.1998.8943. [DOI] [PubMed] [Google Scholar]
- 187.Wacker M, et al. Dentin Matrix Protein 1 Null Mice, a Model of Human Autosomal Recessive Hyphosphatemic Rickets, Exhibit Decreases in Cardiac, Skeletal, and Vascular Smooth Muscle Functon. J Bone Miner Res. 2010;25 Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=f749d1b2-a7fe-4502-ac65–6df9f1cb81fa. [Google Scholar]
- 188.Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- 189.Christensen B, et al. Cell type-specific post-translational modifications of mouse osteopontin are associated with different adhesive properties. J Biol Chem. 2007;282:19463–72. doi: 10.1074/jbc.M703055200. [DOI] [PubMed] [Google Scholar]
- 190.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–9. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 191.Lee NK, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–80. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 193.Son BK, et al. Adiponectin antagonizes stimulatory effect of tumor necrosis factor-alpha on vascular smooth muscle cell calcification: regulation of growth arrest-specific gene 6-mediated survival pathway by adenosine 5′-monophosphate-activated protein kinase. Endocrinology. 2008;149:1646–53. doi: 10.1210/en.2007-1021. [DOI] [PubMed] [Google Scholar]
- 194.Luo XH, et al. Development of arterial calcification in adiponectin-deficient mice: adiponectin regulates arterial calcification. J Bone Miner Res. 2009;24:1461–8. doi: 10.1359/jbmr.090227. [DOI] [PubMed] [Google Scholar]
- 195.Lomonte C, et al. Serum parathyroid hormone and phosphate influence the levels of circulating CD34+ cells in uremia. J Nephrol. 2010;23:693–8. [PubMed] [Google Scholar]
- 196.Yu N, et al. Increased mortality and morbidity in mild primary hyperparathyroid patients. The Parathyroid Epidemiology and Audit Research Study (PEARS) Clin Endocrinol (Oxf) 2010;73:30–4. doi: 10.1111/j.1365-2265.2009.03766.x. [DOI] [PubMed] [Google Scholar]
- 197.Rhee Y, et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49:636–43. doi: 10.1016/j.bone.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sage AP, et al. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res. 2010 doi: 10.1002/jbmr.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lopez I, et al. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011;80:475–82. doi: 10.1038/ki.2011.107. [DOI] [PubMed] [Google Scholar]
- 200.Kosch M, et al. Impaired flow-mediated vasodilation of the brachial artery in patients with primary hyperparathyroidism improves after parathyroidectomy. Cardiovasc Res. 2000;47:813–8. doi: 10.1016/s0008-6363(00)00130-9. [DOI] [PubMed] [Google Scholar]
- 201.Teng M, et al. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349:446–56. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 202.Golestani R, et al. Abdominal aortic calcification detected by dual X-ray absorptiometry: A strong predictor for cardiovascular events. Ann Med. 2010;42:539–45. doi: 10.3109/07853890.2010.515604. [DOI] [PubMed] [Google Scholar]
- 203.Sennerby U, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302:1666–73. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- 204.von Muhlen D, Allison M, Jassal SK, Barrett-Connor E. Peripheral arterial disease and osteoporosis in older adults: the Rancho Bernardo Study. Osteoporos Int. 2009;20:2071–8. doi: 10.1007/s00198-009-0912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bagger YZ, et al. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int. 2007;18:505–12. doi: 10.1007/s00198-006-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Elmariah S, et al. Bisphosphonate Use and Prevalence of Valvular and Vascular Calcification in Women MESA (The Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2010;56:1752–9. doi: 10.1016/j.jacc.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bolland MJ, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. Bmj. 2008;336:262–6. doi: 10.1136/bmj.39440.525752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yoshimura T, et al. Impaired peripheral circulation in lower-leg arteries caused by higher arterial stiffness and greater vascular resistance associates with nephropathy in type 2 diabetic patients with normal ankle-brachial indices. Diabetes Res Clin Pract. 2008;80:416–23. doi: 10.1016/j.diabres.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 210.Johnson KA, Polewski M, Terkeltaub RA. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102:529–37. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Faverman L, Mikhaylova L, Malmquist J, Nurminskaya M. Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett. 2008;582:1552–7. doi: 10.1016/j.febslet.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 212.Towler DA. Calciotropic hormones and arterial physiology: “D”-lightful insights. J Am Soc Nephrol. 2007;18:369–73. doi: 10.1681/ASN.2006121367. [DOI] [PubMed] [Google Scholar]
- 213.Shroff R, et al. A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol. 2008;19:1239–46. doi: 10.1681/ASN.2007090993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Behnke BJ, et al. Influence of ageing and physical activity on vascular morphology in rat skeletal muscle. J Physiol. 2006;575:617–26. doi: 10.1113/jphysiol.2006.108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kronenberg HM. PTH regulates the hematopoietic stem cell niche in bone. Adv Exp Med Biol. 2007;602:57–60. doi: 10.1007/978-0-387-72009-8_7. [DOI] [PubMed] [Google Scholar]
- 216.Jilka RL, et al. Decreased oxidative stress and greater bone anabolism in the aged, when compared to the young, murine skeleton with parathyroid hormone administration. Aging Cell. 2010;9:851–67. doi: 10.1111/j.1474-9726.2010.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]