1. Introduction
Marijuana (MJ) remains the most popular illicit drug among teens (Johnston et al., 2008). Marijuana initiation typically begins in adolescence, as youths aged 12-17 constitute approximately two thirds of the new MJ users (SAMHSA, 2004). Between 8th and 12th grade, illicit drug use prevalence increases from 8% to 22% (Squeglia et al., 2009a), highlighting the importance of understanding the impact of drug use during this age range. The adolescent brain undergoes significant regressive structural changes, including synaptic pruning of gray matter, in order to eliminate redundant or unnecessary neural connections (Sowell et al., 2004a). In addition to synaptic pruning of cortical and sub-cortical structures, the adolescent brain progressively increases its white matter fiber tract composition to promote efficiency of neural conductivity (Huppi and Dubois, 2006; Jernigan and Gamst, 2005; Lopez-Larson et al., 2010; Pfefferbaum et al., 1994; Sowell et al., 2004b). The effects of illicit drug exposure on adolescent brain development have not been fully described, although several studies have found abnormalities in white matter, cerebral electrophysiological functioning, neurotransmitters and brain metabolites (Bossong and Niesink, 2010; Dolmatova and Ivanets, 1995; Ennett et al., 1997; Garcia-Sevilla et al., 1997; Ozaita et al., 1998; Schweinsburg et al., 2008; Solowij et al., 1995; Squeglia et al., 2009b; Yamanouchi et al., 1995).
The performance of a motor act is facilitated by a complex integration of neural systems responsible for an array of cognitive processes, including planning, attention and execution. The network of structures engaged in these processes is likewise complex and extends throughout the brain, controlling everything from subtle to gross motor movements (Pillay et al., 2008). Cortical brain regions known to mediate motor function include Brodmann's area (BA) 4 (primary motor cortex), BA6 (supplementary motor area), the anterior cingulate (ACC), the parietal lobe, and the cerebellum (Jeannerod and Frak, 1999). Functional MRI studies utilizing a finger-tapping paradigm also report activation in these cortical brain regions (Bischoff-Grethe et al., 2004; Mostofsky et al., 2006; Rubia et al., 1999). For example, in a study assessing motor abnormalities in children with ADHD, performance of a sequential finger-tapping task revealed activation in the cerebellum, BA4 and BA6 (Mostofsky et al., 2006). In a similar subject group, Rubia et al. (1999) found activation in the ACC, as well as activation of mesial frontal gyrus and inferior prefrontal cortex during performance of a delay sequencing motor task. Using ROI analysis in a group of healthy adults, Horenstein, Lowe, Koenig and Phillips (2009) found significant activation of primary motor cortex (M1), primary sensory cortex (S1), dorsal premotor cortex (PMd), ventral premotor cortex (PMv), supplementary motor area (SMA), superior parietal lobule (SPL) (BA5/BA7), and inferior parietal lobule (IPL) (BA40) during completion of a complex finger tapping task. To further examine the structural and functional connectivity of the primary motor cortex, Guye and colleagues (2003) combined fMRI data of a finger-tapping paradigm with DTI/ tractography and found BA4 had the highest “likelihood” of connectivity to the pyramidal tracts, premotor areas, including BA6, parietal cortex, thalamus, cingulate motor areas and the cerebellum. These findings are consistent with the premise that the primary motor cortex (BA4) works in connection with the supplementary motor area (BA6) to coordinate and execute motor movements (Shibasaki et al., 1993; Shima and Tanji, 1998). Together with the cerebellum, BA6 is responsible for refining movements based on sensory input (Halsband et al., 1994; Jueptner et al., 1997). The cerebellum has also been shown to play a pivotal role in balance, psychomotor speed, visual tracking and coordination (Miall et al., 2000; Miall et al., 2001; Morton and Bastian, 2004; Moschner et al., 1999). The cingulate motor areas have dense connections to BA4 as well as to BA6 (Morecraft and Van Hoesen, 1992; Wang et al., 2001), and are thought to play an integral role in planning and executing bimanual motor movements Kermadi et al. (2000) and in directing attention to task-relevant events (Wenderoth et al., 2005). Taken together, the regions of the motor network work in concert to plan, coordinate, initiate, execute, and fine tune a movement based on input from the environment. The interaction and contribution of these cortical brain areas to motor movement affords the opportunity to study the effects of MJ on a specific brain network believed to be impacted by MJ use, which in turn may elucidate the altering effects of MJ on brain maturation.
The principal psychoactive component of marijuana, delta-9-tetrahydrocannabinol or THC, affects the brain via the cannabinoid receptor (CB1). This receptor is present throughout structures comprising the motor network, including striatal neurons of the basal ganglia, the cingulate gyrus, the cerebellum and the primary and secondary motor cortices (Glass et al., 1997; Herkenham, 1992; (Herkenham et al., 1990; Iversen, 2003; Mackie, 2008). The cingulate and the cerebellum may be particularly vulnerable to the effects of THC on CB1 receptors given that both brain regions are thought to mature throughout adolescence and early adulthood (Tamnes et al., 2010; Tiemeier et al., 2010). Furthermore, the negative impact of acute MJ use on cognition and psychomotor functions such as balance, psychomotor speed, visual tracking and coordination has been consistently reported (Liguori et al., 2002; Messinis et al., 2006; Stoller et al., 1976; Weinstein et al., 2008). Functional magnetic resonance imaging (fMRI) studies in adults, which utilized a motor paradigm, have reported reduced activation in BA4, BA6 and CG in MJ users compared to healthy controls (Pillay et al., 2008; Pillay et al., 2004). These activation differences were observed after 4-36 hours (Pillay et al., 2004) and following extended washout of 28 days (Pillay et al., 2008). While prior studies have not directly evaluated the impact of MJ use on the cerebellum during motor tasks, a number of studies have produced findings that suggest a vulnerability of this region to the effects of MJ (Mathew et al., 1998; Mathew et al., 2002; O'Leary et al., 2002; O'Leary et al., 2007; O'Leary et al., 2003; Volkow et al., 1996). For example, an early study by Volkow et al. (1996) used positron emission tomography (PET) to measure differences in glucose metabolism between 8 chronic marijuana abusers and 8 healthy controls at baseline and again during intoxication. At baseline, marijuana abusers showed lower relative cerebellar metabolism than healthy controls. Following administration of 2mg of MJ, researchers noted a relative increase in resting state cerebellar metabolism. In contrast, findings from a study assessing the non-acute effects of MJ use reported attenuated cerebellar activation during abstinence from MJ (Block et al., 2000). Taken together these findings summarize current evidence indicating a significant effect for MJ, both acute and non-acute, on regions critical to proper motor function and suggest that long-term exposure to THC may exert significant changes to these key structures. This is of particular concern in adolescent MJ users who may be at a heightened risk for these altering effects of MJ during a critical stage of neurodevelopment.
At this time, there are no studies that have evaluated the impact of MJ use on the cortico-cerebellar motor network and there are no studies that have examined the motor network in adolescents utilizing a finger-tapping fMRI paradigm. Therefore, the aim of the current study was to determine whether activation differences in key regions of the cortico-cerebellar network could be detected in adolescent MJ smokers. Based on previous studies we predicted BA4, BA6 and the ACC would show reduced activation. Furthermore, we hypothesized that cerebellum activation would also be abnormal. We planned to explore the association of age of onset of MJ use with cortico-cerebellar activation patterns in an attempt to address the question of whether or not early-onset of MJ use has a greater negative impact on motor circuit function. In order to test these hypotheses, we performed a bilateral finger-tapping sequence on 24 currently using MJ smoking adolescents and 24 HC.
2. METHODS
2.1 Subjects
The Institutional Review Board at the University of Utah approved this study. All subjects were recruited from the community via local advertisements and by word of mouth. Inclusion criteria for all subjects in this analysis were: age 16-19 years old. Inclusion criteria for MJ users included a self-report of heavy MJ use with at least 100 minimum smokes in the previous year. Healthy controls had no DSM-IV Axis I diagnosis based on structured and clinical interviews. Healthy controls had no first-degree family history of BPD, psychosis or any other psychiatric family history. Family history was obtained by clinical interview with participants and/or parents. Exclusion criteria for all subjects and HC were: major sensorimotor handicaps (e.g., deafness, blindness, paralysis); full scale IQ <70 or learning disabilities; history of claustrophobia, autism, schizophrenia, anorexia nervosa or bulimia; other drug or alcohol dependence (during 2 months prior to scan or total past history ≥12 months); active medical or neurological disease; history of ECT; metal fragments or implants; and current pregnancy or lactation. All subjects provided written assent, and their parents (or legal guardians) provided written informed consent for their adolescent's participation. All adolescents, including HC, underwent a clinical and diagnostic semi-structured interview by either a board-certified child psychiatrist (MLL) or a licensed clinical psychologist (EM). Adolescents under the age of 18 were administered the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Episode (K-SADS-PL) (Kaufman et al., 1997b) with additional mood onset and offset items derived from the WASH-U K-SADS (K-SADS-PL-W) (Geller et al., 2001). The K-SADS-PL is a semi-structured interview used to assess psychiatric disorders in children and adolescents. Since this instrument consists of the K-SADS-PL with supplemental items based on the WASH-U-KADS, we will refer to this instrument as the K-SADS-PL-W. For participants 18 and older, the Structured Clinical Interview for DSM-IV Patient Version (SCID-P) was used with the ADHD module from the K-SADS-PL-W (Kaufman et al., 1997a). All diagnoses were confirmed via a consensus (DYT, MLL, EM). Measures of current psychopathology were obtained using the Profile of Mood States (POMS) (McNair et al., 1992), the Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960) and the Hamilton Anxiety Scale (HAM-A) (Hamilton, 1959). The DSM-IV-TR Global Assessment of Functioning (GAF) (Endicott et al., 1976) was used to assess global functioning with a scale from 1 (worst) to 100 (best). All participants underwent a drug screen immediately prior to MRI scanning. Urine samples were subsequently analyzed to obtain urinary cannabinoid levels. In addition, information regarding age of first MJ use, age of regular use, and frequency of MJ use was obtained on all participants. Total lifetime MJ use was calculated by averaging number of smoking episodes per week multiplied by duration of use. Equality of groups on demographic and clinical variables was evaluated by t-tests for continuous variables and chi-square tests for categorical variables. All data are reported as means and standard deviations (SD) unless otherwise specified.
2.2 Data acquisition
Structural and functional imaging was performed at the Utah Center for Advanced Imaging Research (UCAIR) using a 3T Siemens Trio scanner. Structural acquisitions include a T1-weighted 3D MPRAGE grappa sequence acquired sagittally, with TE/TR/TI=3.38ms/2.0s/1.1s, 8° flip, 256×256 acquisition matrix, 256 mm2 FOV, 160 slices, 1.0 mm slice thickness. Each subject completed a standard bilateral fMRI finger tapping task during echo-planar imaging. A blood oxygen level dependent (BOLD) echo planar imaging (EPI) sequence, obtained axially, was used with a TR=2 seconds, TE=28 ms to central K-space, 64 × 64 matrix, parallel imaging with GRAPPA acceleration factor of 2, 40 slices at a thickness of 3 mm, FOV=220mm. The bilateral finger-tapping task was performed at the end of an imaging protocol that included both structural neuroimaging and 2 additional fMRI paradigms. Scanning blocks were 240 seconds in duration and each subject completed a self-paced bilateral finger tapping task for 20 seconds alternating with 20 seconds of rest. Participants completed 6 blocks of the finger tapping task and 6 blocks of rest. Participants were instructed to tap their thumb with each finger sequentially and continuously when prompted by the word “start” on the screen. The task was performed on both right and left hands and at the same time. The bilateral thumb to finger tap was self-paced and monitored via a camera to ensure participants were performing the task correctly. In addition, participants performed the task prior to entering the MRI scanner to ensure they were able to perform the task. The original imaging data were transferred from the scanner in the DICOM format and anonymized.
2.3 fMRI post-processing
FMRI images were analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, University College, London, UK) running in Matlab (MathWorks, Natick, MA, USA). Initially, blood oxygen level dependent (BOLD) fMRI data were corrected for motion in SPM5 using an intra-run realignment algorithm that uses the first image as a reference. A criterion of 2mm of head motion in any direction was used as an exclusionary criterion. The realigned images were then normalized to an EPI template in Montreal Neurological Institute (MNI) stereotactic space. Normalized images were re-sampled into 2 mm cubic voxels and then spatially smoothed using an isotropic Gaussian kernel with 8mm full width at half maximum (FWHM). Global scaling was not used, high-pass temporal filtering with a cut-off of 128s was applied, and serial autocorrelations were modeled with an AR(1) model in SPM5. Statistical parametric images were calculated individually for each subject and each task, using a general linear model (Friston et al., 1995a; Friston et al., 1995b).
We applied a region of interest approach as we had specific a priori hypotheses. Images were subsequently entered into a second level model, subjected to a voxel-wise contrast and t-test to assess statistical significance. The within-group analyses were done using one-sample t-tests for the MJ and HC groups. Using the two-sample t-test, we made direct comparisons between the heavy marijuana smokers and the non-marijuana smoking controls. Region of interest (ROI) masks were created using the Wake Forest University PickAtlas utility (Maldjian et al., 2003; 2004). These regions included the BA4, BA6, cingulate gyrus and the cerebellum. The statistical threshold for the ROIs was set at p<0.05, corrected, and k was set at 20 voxels. Finally, in order to determine the relationship between MJ use and discrete patterns of activity during finger tapping, regression analyses were completed between the BOLD signal data and the total number of smokes per week, age, age of onset, lifetime MJ use and urinary cannabinoid level for the smokers (p<0.001 and k=20). To examine whole brain analysis, the probability threshold was set at p<0.005, corrected, and a minimum cluster extent (k) of 20 contiguous voxels.
3. RESULTS
3.1 Subject characteristics
We acquired data from 24 adolescents with heavy MJ use, ranging in age from 16 to 19 (mean 18.2 ± 0.7, 22 male, 2 female) and 24 non-MJ using healthy participants, ranging in age from 16 to 22 (mean 18.0 ± 1.9, 17 male, 7 female). All participants were right-handed. With the exception of the GAF, there were no significant differences between groups for age, sex, clinical correlates (HAM-A or HAM-D). Marijuana users were found to have higher verbal fluency scores than HC (MJ: 45.8 ± 9.6; HC: 37.5 ± 8.7; p = 0.03). All participants were currently enrolled in either high school or college, or had recently graduated high school with plans to attend college in the next 3 months. All participants reported average to above average success in academic achievement. Study participants also completed a psychomotor task, the Trail Making Test, as part of their clinical evaluation. MJ smokers did not show reduced motor speed or psychomotor function on this task.
No participants had any other past/present psychiatric or drug dependence disorders; however 2 MJ users also had a history of alcohol abuse that was less than 1 year in duration. MJ users reported an average age at first use of 15.3 ± 1.4 years and an average age at regular MJ use of 16 ± 1.0 years. The average frequency of MJ use was 10.3 ± 8.7 times/week and the total lifetime MJ use was 1500.6 ± 283.5. Twenty MJ participants reported their last MJ use was within 24 hours of MRI imaging. Three additional participants reported using MJ within 48 hours and one participant reported using MJ greater than 48 hours prior to MRI imaging. In order to overcome the inherent difficulty in obtaining accurate MJ use patterns, urine was collected on all MJ participants immediately prior to MRI imaging and evaluated for cannabinoid levels. The average cannabinoid urine level obtained on the day of MRI scanning was 408.1 ± 358.6ng/mL. For the MJ group, age of first alcohol use was 15.30 ± 1.29 years. Average frequency of alcohol use per week for these subjects was 0.87 ± 1.01. Additionally, 4 MJ participants meet criteria for current alcohol abuse and 2 participants had a past history of alcohol dependence that occurred over 1.5 years prior to participation in the study. For the MJ group, 4 reported current nicotine use (average duration: 1.31± 3.07 months) and 4 endorsed having a history of nicotine use (average duration: 1.47 ± 4.69 months). Demographic and clinical characteristics of the participants, including MJ use, are shown in Table 1.
Table 1.
Demographic and Clinical Description of Subject Sample
| MJ Users (n=24) | Controls (n=24) | |
|---|---|---|
| Age (years; mean, SD) | 18.2 ± 0.7 | 18.0 ± 1.9 |
| Sex | 22 male/2 female | 17 male/7 female |
| Handedness | 24 right handed | 24 right handed |
| Age at first MJ use (years; mean, SD) | 15.3 ± 1.4 | -- |
| Age at regular MJ use (years; mean, SD) | 16.0 ± 1.0 | -- |
| Frequency of MJ use/week (mean, SD) | 10.3 ± 8.7 | -- |
| Total lifetime MJ use* (mean, SD) | 1500.6 ± 283.5 | -- |
| Urinary THC level (ng/mL) (mean, SD) | 408.1 ± 358.6 | -- |
| GAF (mean, SD) | 84.7 ± 5.4** | 91.7 ± 2.4 |
| HAM-D (mean, SD) | 0.9 ± 1.7 | 1.0 ± 1.6 |
| HAM-A (mean, SD) | 1.5 ± 2.4 | 1.5 ± 1.8 |
| Trail making A (sec) (mean, SD) | 12.4 ± 6.9 | 13.8 ± 6.2 |
| Trail making B (sec) (mean, SD) | 20.3 ± 6.0 | 21.8 ± 7.4 |
| Trail making B-A (sec) (mean, SD) | 7.9 ± 6.5 | 8.6 ± 6.8 |
Total lifetime MJ use = Average number of smokes per week multiplied by duration of use
Significant at p<0.05; GAF = Global Assessment of Functioning; HAM-D = Hamilton Rating Scale for Depression; HAM-A = Hamilton Rating Scale for Anxiety
3.2 Whole-brain within group and between group analyses
Within group analysis of a standard bilateral finger-tapping task produced significant bilateral BOLD activation throughout various parts of the motor network, including BA4, BA6, cingulate, and cerebellum (See Figs. 1 and 2 and Table 2) in both HC and MJ users. Whole-brain between group analyses revealed significantly greater activation in the right cingulate gyrus (Tmax=4.44, k=541, p=0.005, corrected; Talairach coordinates: [-23, -27, 29]) for HC subjects compared to MJ smokers. Further, MJ smokers had a small region of increased activation in the right middle occipital gyrus compared to HC, but this difference only trended toward significance (Tmax= 4.45, k=184, p=0.01, uncorrected; Talairach coordinates: [35, -87, 16]).
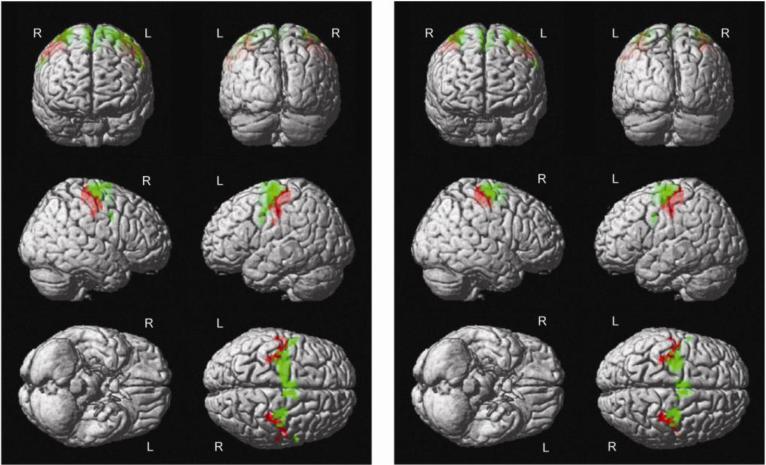
Figure 1.
Whole Brain Within-Group fMRI Results for Bilateral Finger Tapping Paradigm Showing Increased Activation in Brodmann Areas 4 and 6 for HC (Figure 1a) and Marijuana Users (Figure 1b). The color red and green indicates Brodmann Area 4 and 6, respectively.
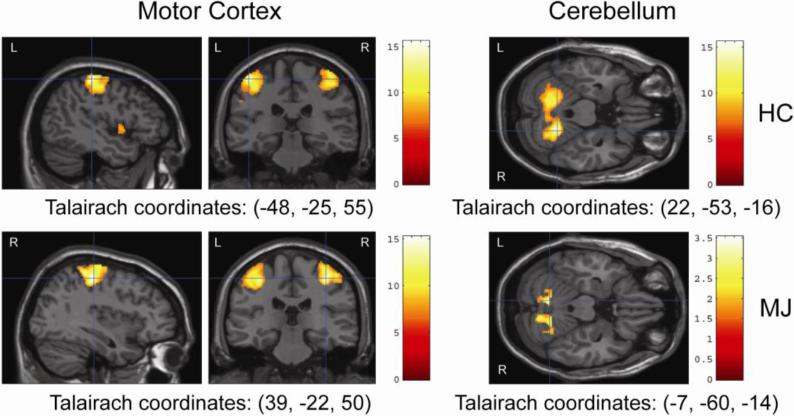
Figure 2.
Whole Brain Within-Group fMRI Results for Bilateral Finger Tapping Paradigm Showing Increased Activation in the Motor Cortex and the Cerebellum for HC and Marijuana Users.
Table 2.
Whole Brain Within-Group BOLD Activations for Healthy Controls and MJ Users During a Bilateral Finger-tapping Paradigm (reported in Talairach coordinates)
| Hemisphere | Region | Number of Voxels | T | Z | x | y | z | P |
|---|---|---|---|---|---|---|---|---|
| Healthy Controls | ||||||||
| Left | Primary Motor Cortex (BA4) | 2757 | 15.46 | 7.41 | -46 | -25 | 55 | <0.001 |
| Putamen/globus pallidus | 231 | 11.44 | 6.55 | -23 | -8 | 5 | <0.001 | |
| Precentral gyrus | 79 | 8.94 | 5.82 | -46 | 3 | 5 | <0.001 | |
| Cerebellum | 31 | 8.62 | 5.71 | 0 | -72 | -30 | <0.001 | |
| Thalamus | 22 | 7.73 | 5.37 | -11 | -22 | 10 | <0.001 | |
| Supplementary motor cortex (BA6) | 23 | 7.71 | 5.37 | -54 | 1 | 31 | <0.001 | |
| Right | Cerebellum | 2254 | 15.55 | 7.43 | 22 | -53 | -16 | <0.001 |
| Supplementary motor cortex (BA6) | 1236 | 12.60 | 6.83 | 35 | -13 | 63 | <0.001 | |
| Precentral gyrus | 33 | 9.70 | 6.06 | 49 | 0 | 6 | <0.001 | |
| Globus pallidus | 20 | 7.67 | 5.35 | 21 | -7 | 5 | <0.001 | |
| Marijuana Users | ||||||||
| Left | Primary Motor Cortex (BA4) | 1635 | 13.95 | 7.13 | -49 | -18 | 44 | <0.001 |
| Cerebellum | 1254 | 13.38 | 7.01 | -17 | -51 | -20 | <0.001 | |
| Postcentral gyrus | 31 | 8.25 | 5.75 | -51 | -20 | 19 | <0.001 | |
| <0.001 | ||||||||
| Right | Precentral gyrus | 1205 | 15.21 | 7.37 | 39 | -22 | 50 | <0.001 |
| Supplementary motor cortex (BA6) | 354 | 9.52 | 6.01 | 6 | 2 | 47 | <0.001 | |
3.3 Region of interest analyses
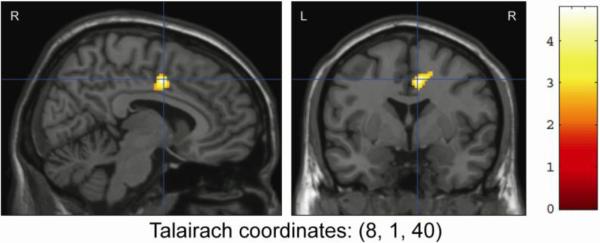
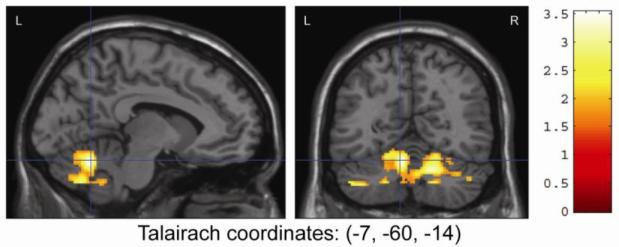
To test the study hypotheses, region of interest analyses were performed on a priori regions of interest (ROI), including the cerebellum, BA4, BA6, and cingulate. Compared to MJ users, HC subjects had significantly greater activation in the cingulate gyrus (Tmax = 4.79, k=238, p=0.05, corrected; Talairach coordinates: [8, 1, 40]) and cerebellum activation (Tmax=3.53, k=2702, p=0.002, corrected; Talairach coordinates: [-7, -60, -14]) (See Figs. 3 and 4). Further ROI analysis of the anterior and posterior lobes of the cerebellum found reduced activation in MJ users in the anterior lobe (Tmax =3.45, k=1207, p=0.034, corrected; Talairach coordinates [18, -57, -21]) and a trend toward reduced activation for MJ users in the posterior lobe (Tmax=3.53, k=958, p=0.011, uncorrected; Talairach coordinates [-7, -60, -14]). While small activation differences were noted between HC and MJ smokers in BA4 and BA6, these differences were not statistically significant. Marijuana smokers showed no increased activation for any ROI when compared to HC.
Figure 3.
Between-Group Region of Interest Analysis of Finger Tapping Results Showing Greater Activation of the Cingulate Gyrus in Healthy Controls Compared to Marijuana Smokers
Figure 4.
Between-Group Region of Interest Analysis of Finger Tapping Results Showing Greater Activation of the Cerebellum in Healthy Controls Compared to Marijuana Smokers
3.4 Regression analyses with marijuana clinical variables
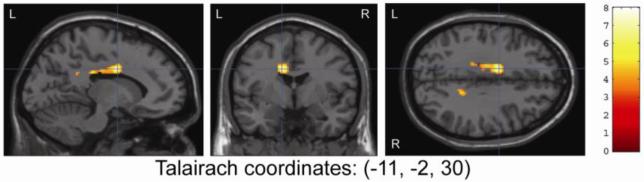
Whole brain and ROI regression analyses were performed to identify possible relationships between brain activation in the cerebellum and cingulate gyrus and indices of MJ use in the adolescent MJ group only. A significant negative regression was observed on both whole-brain (Tmax=7.96, k=532, p<0.001, corrected; Talairach coordinates: [-11, -2, 30]) and ROI (Tmax=7.96, k=296, p<0.001, corrected; Talairach coordinates [-11, -2, 30]) regression analysis for lifetime MJ use and brain activation in the cingulate gyrus (See Figure 5). Cerebellar ROI regression analysis also found a significant negative regression between total lifetime MJ use and activation in the cerebellum (Tmax = 4.72, k=51, p=0.04, uncorrected and p=0.08, corrected; Talairach coordinates: [18, -57, -23]). There were no other significant findings for age of onset of use or current urinary THC level for any region.
Figure 5.
Region of Interest Regression Analysis Showing Negative Regression of Lifetime MJ Use and Cingulate Activation
4. DISCUSSION
This is one of the first studies to evaluate regional differences in cortico-cerebellar circuits in a group of older adolescents with heavy MJ use utilizing a bilateral finger tapping fMRI task. We found reduced cingulate and cerebellar activation in adolescents with heavy MJ use compared to HC. No activation differences were observed between groups for BA4 or BA6. Both the cerebellum and cingulate activations were found to be negatively correlated with total lifetime MJ use. No correlations were found with age of onset of use or current urinary THC level for any region.
Our results are consistent with prior adult studies of motor function that found reduced cingulate activation in subjects with heavy MJ use. For example, Pillay and colleagues evaluated 9 MJ adult users, abstinent from MJ for 4-36 hours, and 16 HC on a 1.5T scanner utilizing a bilateral finger sequencing task and found significantly reduced activation in the cingulate (2004). Similar to the current study, the authors did not find significant activation differences for BA4, but in contrast the authors did find reduced activation in BA6 (Pillay et al., 2004). In a follow-up study by the same authors, 11 adult MJ smokers and 16 HC were examined utilizing a right and left finger-sequencing task. The authors compared fMRI data after 0, 7 and 28 days of abstinence. The authors found ipsilaterally diminished cingulate activation on Day 0, and contralaterally diminished cingulate activation on Day 0 and Day 28. For right-handed finger tapping, the authors found contralaterally diminished cingulate activation on Days 0, 7, and 28; and ipsilaterally diminished BA6 activation on Days 0, 7, and 28 (Pillay et al., 2008). Interestingly, we did not replicate the BA6 findings from Pillay (2004, 2008). The differences in findings in the studies by Pillay and colleagues as compared to the current study may be related to differences in the finger-tapping paradigm, image resolution associated with scanner strength (1.5T versus 3T), and the much older average participant's age (mean age = 37.3± 6.7 versus 18.2 ± 0.7). Additional differences between the current study and the two prior study samples include longer duration of use (> 21 years vs 2 years), larger number of total lifetime use (>16,700 times vs 1500 times) and higher rates of comorbid alcohol use and other substances of abuse.
To date, no other studies have directly assessed the effect of MJ on cerebellar activation and cortical brain regions within the motor network in adolescents utilizing a finger-tapping fMRI paradigm. Our findings of reduced cerebellar activation in adolescents with heavy MJ use are consistent with current literature regarding the vulnerability of the cerebellum to MJ. For example, a number of studies have found abnormal activation patterns in the cerebellum in acute (Mathew et al., 1998; Mathew et al., 2002; O'Leary et al., 2002; O'Leary et al., 2007; O'Leary et al., 2003; Volkow et al., 1996) and non-acute MJ administration (Block et al., 2000; Chang et al., 2006). Additionally, two studies reported increased bilateral resting state cerebellar blood flow (as measured by H2 15O-PET) in marijuana users following administration of THC (Mathew et al., 1998; Mathew et al., 2002). A series of more recent H2 15O-PET studies using a dichotic listening task and a self-paced counting task found increased acute cerebellar activation in MJ users when compared either to non-using controls or less frequently-using MJ users following a 20mg administration of THC (O'Leary et al., 2002; O'Leary et al., 2007; O'Leary et al., 2003). An fMRI study by Chang et al. (2006) compared activation patterns during a set of visual attention tasks in a group of 24 chronic marijuana users (12 abstinent and 12 active) and 19 well-matched control subjects. Chang and colleagues found that active marijuana users with positive urine tests for THC showed greater activation in the frontal and medial cerebellar regions than abstinent marijuana users.
Alterations of the cerebellum have also been reported in recent morphometric studies in adolescents with chronic MJ use (Jarvis et al., 2008; Medina et al., 2010). Jarvis and colleagues reported increased gray matter vermis volumes in adolescents with comorbid bipolar disorder and MJ use compared to bipolar alone (2008). Medina and colleagues further reported larger posterior vermis volumes in a group of adolescent, chronic MJ users who were scanned following 1 month of abstinence (2010). The larger vermal volumes in this study were associated with poorer executive functioning (Medina et al., 2010). Cognitive deficits including slower psychomotor processing speed, attentional deficits and executive dysfunction have been found in other studies of heavy MJ use in adolescents and adults (Bolla et al., 2002; Bolla et al., 2005; Lyons et al., 2004; Medina et al., 2007; Pope et al., 2003; Roser et al., 2009; Whitlow et al., 2004). Given the extensive connectivity of the cerebellum, changes in this region could impact a wide variety of brain functions. The cerebellum has been found to have reciprocal projections to cortical brain regions such as the prefrontal, parietal, temporal and limbic cortices via the thalamus. In turn, these same cortical brain regions send information back to the cerebellum via the pons (Schmahmann, 2010). These projections are arranged topographically in the pons and include not only motor information but also multimodal information relating to attention, executive function, language and emotion (Schmahmann, 1996, 2010; Schmahmann and Pandya, 1997). Therefore, the motor and cognitive dysfunction seen in individuals with heavy MJ use may be related to the reciprocal nature of the cortico-cerebellar circuits. Chronic MJ use is thought to lead to CB1 receptor desensitization and down-regulation (Gonzalez et al., 2005). This down-regulation or desensitization may impact the inhibitory connections of the Purkinje cells, the output neurons of the cerebellar cortex, to the deep cerebellar nuclei, which have an excitatory pathway through the thalamus to the motor cortex (Allen and Tsukahara, 1974) and other cortical areas, thus leading to impairments in several domains of functioning.
Data regarding structural MRI changes in the cingulate and frontal areas associated with motor functions are limited. One preliminary DTI study of lighter smoking MJ users found no differences on measures of white matter integrity between users and HC (Delisi et al., 2006), while another pilot study of ten MJ users found trends toward decreased white matter integrity in users compared to HC (Gruber and Yurgelun-Todd, 2005). Findings from the latter study also utilized fMRI to measure brain activation for MJ users and HC, and found significant activation differences in key brain regions such as the ACC and dorsolateral prefrontal cortex despite demonstrating similar behavioral performance (Gruber and Yurgelun-Todd 2005). This is consistent with a recent study from Padula, Schweinsburg and Tapert (2007), who found different brain activation patterns in abstinent adolescent marijuana users compared to controls during performance of a spatial working memory task. Although groups did not differ on behavioral measures of task performance, marijuana users showed significantly more activation in left ACC as well as left and middle temporal gyri. In a PET study in abstinent marijuana users utilizing a modified version of the Stroop task, Eldreth and colleagues found hypoactivity of the left perigenual ACC and the lateral prefrontal cortex and hyperactivity of the hippocampus bilaterally in the marijuana group compared to healthy controls (Eldreth et al., 2004). Again, these activation differences were observed despite similarities in task performance. The ACC is a region thought to be critical to error processing, and a diminished ability to detect errors has been associated with clinical symptoms including loss of insight, delusions and perseverative behavior. In order to observe associations between brain activation and task performance and error awareness, Hester, Nestor and Garavan (2009) administered a Go/No-go response inhibition task to 16 MJ users (mean age: 24.6 ± 1.5) and 16 matched controls (mean age: 25.2 ± 1.3). Despite a lack of differences between users and HC on inhibitory control performance, MJ users showed a significant deficit in awareness of commission errors. This diminished capacity for behavior monitoring was associated with hypoactivity in the ACC and right insula. Additionally, increased levels of hypoactivity in both the ACC and right insula were significantly correlated with error-awareness rates in the MJ group but not in controls. Together these findings suggest that MJ may exert modulatory effects on brain systems responsible for the execution of cognitive tasks (Roberts and Garavan, 2010). However, these activation differences are not limited to cognitive or psychomotor tasks. Functional MRI abnormalities in the cingulate of MJ users have also been observed during tasks of emotion processing (Fusar-Poli et al., 2009). In summary, previous findings highlight the central role for the ACC in cognitive performance, response regulation and behavioral awareness. They further indicate that MJ use may lead to alterations in ACC functional neural networks.
There is growing evidence that age of onset of MJ use is a critical variable in understanding the effects of MJ on structural, functional and neurocognitive measures. Previous studies have found that MJ users with an age of onset prior to 16 or 17 years of age have more deficits in cognitive and emotional processes than users with a later onset of use. For example, early-onset MJ use appears to be related to a permanent deficit in visual scanning ability (Ehrenreich et al., 1999; Huestegge et al., 2002), verbal working memory (Becker et al., 2010), and verbal IQ (Pope 2003). Other research has shown that individuals who use MJ before age 18 are more likely to develop substance abuse or dependence than are those who use MJ when they are 18 or older (von Sydow et al., 2001). Wilson and colleagues (2000) reported smaller whole brain volumes and abnormal gray and white matter content in adolescents with an onset of use prior to age 17 compared to adolescents who began using later. Furthermore, earlier onset of MJ use may result in atypical cortical gray matter development and abnormal gyrification patterns than later onset use (Lopez-Larson et al., 2011; Mata et al., 2010). These studies demonstrate that an early age of onset of MJ use may have long-term effects on brain development. Although we did not detect an association between activation patterns in cingulate or cerebellar regions with age of onset of MJ use, we did find that cerebellum and cingulate activation was negatively correlated with total lifetime MJ use. Total lifetime MJ use was calculated by averaging the number of smokes per week multiplied by duration of use, which suggests that both early age of onset (duration) and severity of use are important contributors to the negative sequelae of MJ use. Furthermore, our findings suggest that the cingulate and the cerebellum maybe particularly sensitive to the effects of THC on CB1 receptors given that both brain regions are thought to mature throughout adolescence and early adulthood (Lebel et al., 2008; Tamnes et al., 2010; Tiemeier et al., 2010). In adolescent rats, developmental differences in the expression and function of the CB1 cannabinoid receptor have also been found in limbic/association areas such as the cingulate but not in sensorimotor cortical areas (Heng et al., 2010). Furthermore, in a study by Wallace and colleagues, the cerebellum was noted to have less heritability than other brain regions which may confer an increased risk from environmental exposures such as cannabinoids (2006).
Our current findings should be interpreted with care given the modest sample size, cross-sectional nature of the study and the inclusion of youths who were currently using MJ, with presumably different usage patterns. Furthermore, we utilized verbal fluency as an estimate of IQ. Prior studies have found modest to moderate correlations between verbal fluency and estimates of intelligence (Strauss et al., 2006). Interestingly, our MJ subjects had higher verbal fluency scores than non-using control participants. If intellectual function were influencing our results, we would expect that our group differences would be less robust than if the groups were matched for verbal fluency. Strengths of this study include the narrow age range of the adolescents and the rigorous clinical assessment of study subjects resulting in the inclusion of adolescents with limited comorbid substance abuse disorders and free of psychiatric comorbidity or history of psychotropic medication use.
Our findings suggest that heavy MJ use appears to have an impact on functioning in key regions of the cingulo-cerebellar circuits of the developing brain. Further connectivity analyses (dynamic causal modeling) are currently being performed to examine whether heavy MJ use leads to abnormalities within the motor network or independently impacts cingulate and cerebellar brain regions. These brain regions have high levels of cannabinoid receptors and alterations of these receptors during development may alter brain developmental trajectories. In adolescents, the cerebellum and the cognitive/attentional component of the motor network (cingulate) appear to be significantly affected in heavy MJ use, which may lead to impairments in motor function, cognition and mood. The greater the lifetime severity of MJ use the more impaired the activation patterns of the cingulo-cerebellar nodes of the motor network were (at least acutely). Further study of the clinical and neuroanatomical correlates of cingulo-cerebellar motor function in MJ using adolescents is needed.
Acknowledgements
This work was supported by research grants from an NIH:1R01 DA020269-01 to DYT and training awards through the American Psychiatric Association's Program for Minority Research Training in Psychiatry (5T32 MH19126)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen GI, Tsukahara N. Cerebrocerebellar communication systems. Physiological Reviews. 1974;54:957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Progress in Neuropsychopharmacology & Biological Psychiatry. 2010;34:837–845. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Bischoff-Grethe A, Goedert KM, Willingham DT, Grafton ST. Neural substrates of response-based sequence learning using fMRI. Journal of Cognitivie Neuroscience. 2004;16:127–138. doi: 10.1162/089892904322755610. [DOI] [PubMed] [Google Scholar]
- Block RI, O'Leary DS, Hichwa RD, Augustinack JC, Ponto LL, Ghoneim MM, Arndt S, Ehrhardt JC, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Cerebellar hypoactivity in frequent marijuana users. Neuroreport. 2000;11:749–753. doi: 10.1097/00001756-200003200-00019. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Progress in Neurobiology. 2010;92:370–385. doi: 10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Delisi LE, Bertisch HC, Szulc KU, Majcher M, Brown K, Bappal A, Ardekani BA. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduction Journal. 2006;3:17. doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmatova LS, Ivanets TA. [Change in the level of prostaglandins E and glutathione-S-transferase in leukocytes and plasma of hashish addicts]. Voprosy meditsinkoi khimii. 1995;41:57–60. [PubMed] [Google Scholar]
- Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, Gigerenzer G, Hoehe MR. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berlin) 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Ennett ST, Flewelling RL, Lindrooth RC, Norton EC. School and neighborhood characteristics associated with school rates of alcohol, cigarette, and marijuana use. Journal of Health and Social Behavior. 1997;38:55–71. [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995a;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995b;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, Seal M, Surguladze SA, O'Carrol C, Atakan Z, Zuardi AW, McGuire PK. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Archives of General Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Garcia-Sevilla JA, Ventayol P, Busquets X, La Harpe R, Walzer C, Guimon J. Marked decrease of immunolabelled 68 kDa neurofilament (NF-L) proteins in brains of opiate addicts. Neuroreport. 1997;8:1561–1565. doi: 10.1097/00001756-199705060-00003. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. Journal of the American Acadamey of Child and Adolescent Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacology Biochemistry and Behavior. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Research Cognitive Brain Research. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Guye M, Parker GJ, Symms M, Boulby P, Wheeler-Kingshott CA, Salek-Haddadi A, Barker GJ, Duncan JS. Combined functional MRI and tractography to demonstrate the connectivity of the human primary motor cortex in vivo. Neuroimage. 2003;19:1349–1360. doi: 10.1016/s1053-8119(03)00165-4. [DOI] [PubMed] [Google Scholar]
- Halsband U, Matsuzaka Y, Tanji J. Neuronal activity in the primate supplementary, pre-supplementary and premotor cortex during externally and internally instructed sequential movements. Neuroscience Research. 1994;20:149–155. doi: 10.1016/0168-0102(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2010;65:278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horenstein C, Lowe MJ, Koenig KA, Phillips MD. Comparison of unilateral and bilateral complex finger tapping-related activation in premotor and primary motor cortex. Human Brain Mapping. 2009;30:1397–1412. doi: 10.1002/hbm.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestegge L, Radach R, Kunert HJ, Heller D. Visual search in long-term cannabis users with early age of onset. Progress in Brain Research. 2002;140:377–394. doi: 10.1016/S0079-6123(02)40064-7. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Dubois J. Diffusion tensor imaging of brain development. Seminars in Fetal and Neonatal Medicine. 2006;11:489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jarvis K, DelBello MP, Mills N, Elman I, Strakowski SM, Adler CM. Neuroanatomic comparison of bipolar adolescents with and without cannabis use disorders. Journal of Child and Adolescent Psychopharmacology. 2008;18:557–563. doi: 10.1089/cap.2008.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M, Frak V. Mental imaging of motor activity in humans. Current Opinion in Neurobiology. 1999;9:735–739. doi: 10.1016/s0959-4388(99)00038-0. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC. Changes in volume with age--consistency and interpretation of observed effects. Neurobiology of Aging. 2005;26:1271–1274. doi: 10.1016/j.neurobiolaging.2005.05.016. discussion 1275-1278. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman HG, Schulenberg JE. Secondary School Students. Vol. 1. National Institute on Drug Abuse; Bethesda, MD: 2008. Monitoring the future national survey results on drug use, 1975-2007. p. 707. [Google Scholar]
- Jueptner M, Ottinger S, Fellows SJ, Adamschewski J, Flerich L, Muller SP, Diener HC, Thilmann AF, Weiller C. The relevance of sensory input for the cerebellar control of movements. Neuroimage. 1997;5:41–48. doi: 10.1006/nimg.1996.0249. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Kiddie-Schedule for Affective Disorders and Schizophrenia-Present and Lifetime version, (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997a:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997b;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Liu Y, Rouiller EM. Do bimanual motor actions involve the dorsal premotor (PMd), cingulate (CMA) and posterior parietal (PPC) cortices? Comparison with primary and supplementary motor cortical areas. Somatosensory and Motor Research. 2000;17:255–271. doi: 10.1080/08990220050117619. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Liguori A, Gatto CP, Jarrett DB. Separate and combined effects of marijuana and alcohol on mood, equilibrium and simulated driving. Psychopharmacology (Berlin) 2002;163:399–405. doi: 10.1007/s00213-002-1124-0. [DOI] [PubMed] [Google Scholar]
- Lopez-Larson M, Breeze JL, Kennedy DN, Hodge SM, Tang L, Moore C, Giuliano AJ, Makris N, Caviness VS, Frazier JA. Age-related changes in the corpus callosum in early-onset bipolar disorder assessed using volumetric and cross-sectional measurements. Brain Imaging and Behavior. 2010;4:220–231. doi: 10.1007/s11682-010-9101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behavioural Brain Research. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MJ, Bar JL, Panizzon MS, Toomey R, Eisen S, Xian H, Tsuang MT. Neuropsychological consequences of regular marijuana use: a twin study. Psychological Medicine. 2004;34:1239–1250. doi: 10.1017/s0033291704002260. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. Journal of Neuroendocrinology 20 Suppl. 2008;1:10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Mata I, Perez-Iglesias R, Roiz-Santianez R, Tordesillas-Gutierrez D, Pazos A, Gutierrez A, Vazquez-Barquero JL, Crespo-Facorro B. Gyrification brain abnormalities associated with adolescence and early-adulthood cannabis use. Brain Research. 2010;1317:297–304. doi: 10.1016/j.brainres.2009.12.069. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Coleman RE. Cerebellar activity and disturbed time sense after THC. Brain Research. 1998;797:183–189. doi: 10.1016/s0006-8993(98)00375-8. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Hawk TC, Coleman RE, DeGrado TR, Provenzale J. Time course of tetrahydrocannabinol-induced changes in regional cerebral blood flow measured with positron emission tomography. Psychiatry Research. 2002;116:173–185. doi: 10.1016/s0925-4927(02)00069-0. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppeman L. Profile of Mood States Manual. Educational and Industrial Testing Service; San Diego, CA: 1992. [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Research. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P. Neuropsychological deficits in long-term frequent cannabis users. Neurology. 2006;66:737–739. doi: 10.1212/01.wnl.0000201279.83203.c6. [DOI] [PubMed] [Google Scholar]
- Miall RC, Imamizu H, Miyauchi S. Activation of the cerebellum in co-ordinated eye and hand tracking movements: an fMRI study. Experimental Brain Research. 2000;135:22–33. doi: 10.1007/s002210000491. [DOI] [PubMed] [Google Scholar]
- Miall RC, Reckess GZ, Imamizu H. The cerebellum coordinates eye and hand tracking movements. Nature Neuroscience. 2001;4:638–644. doi: 10.1038/88465. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Van Hoesen GW. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. The Journal of Comparative Neurology. 1992;322:471–489. doi: 10.1002/cne.903220403. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist. 2004;10:247–259. doi: 10.1177/1073858404263517. [DOI] [PubMed] [Google Scholar]
- Moschner C, Crawford TJ, Heide W, Trillenberg P, Kompf D, Kennard C. Deficits of smooth pursuit initiation in patients with degenerative cerebellar lesions. Brain. 1999;122(Pt 11):2147–2158. doi: 10.1093/brain/122.11.2147. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Rimrodt SL, Schafer JG, Boyce A, Goldberg MC, Pekar JJ, Denckla MB. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biological Psychiatry. 2006;59:48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, Ponto LB, Watkins GL, Hurtig RR, Hichwa RD. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26:802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Block RI, Koeppel JA, Schultz SK, Magnotta VA, Ponto LB, Watkins GL, Hichwa RD. Effects of smoking marijuana on focal attention and brain blood flow. Human Psychopharmacology. 2007;22:135–148. doi: 10.1002/hup.832. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Block RI, Turner BM, Koeppel J, Magnotta VA, Ponto LB, Watkins GL, Hichwa RD, Andreasen NC. Marijuana alters the human cerebellar clock. Neuroreport. 2003;14:1145–1151. doi: 10.1097/00001756-200306110-00009. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Escriba PV, Ventayol P, Murga C, Mayor F, Jr., Garcia-Sevilla JA. Regulation of G protein-coupled receptor kinase 2 in brains of opiate-treated rats and human opiate addicts. Journal of Neurochemistry. 1998;70:1249–1257. doi: 10.1046/j.1471-4159.1998.70031249.x. [DOI] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of Addictive Behaviors. 2007;21:478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Rogowska J, Kanayama G, Gruber S, Simpson N, Pope HG, Yurgelun-Todd DA. Cannabis and motor function: fMRI changes following 28 days of discontinuation. Experimental and Clinical Psychopharmacology. 2008;16:22–32. doi: 10.1037/1064-1297.16.1.22. [DOI] [PubMed] [Google Scholar]
- Pillay SS, Rogowska J, Kanayama G, Jon DI, Gruber S, Simpson N, Cherayil M, Pope HG, Yurgelun-Todd DA. Neurophysiology of motor function following cannabis discontinuation in chronic cannabis smokers: an fMRI study. Drug and Alcohol Dependence. 2004;76:261–271. doi: 10.1016/j.drugalcdep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug and Alcohol Dependence. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Roberts GM, Garavan H. Evidence of increased activation underlying cognitive control in ecstasy and cannabis users. Neuroimage. 2010;52:429–435. doi: 10.1016/j.neuroimage.2010.04.192. [DOI] [PubMed] [Google Scholar]
- Roser P, Gallinat J, Weinberg G, Juckel G, Gorynia I, Stadelmann AM. Psychomotor performance in relation to acute oral administration of Delta9-tetrahydrocannabinol and standardized cannabis extract in healthy human subjects. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:284–292. doi: 10.1007/s00406-009-0868-5. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Bullmore ET. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. American Journal of Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2003 National Survey on Drug Use and Health: National Findings. Substance Abuse and Mental Health Service Administration; Rockville, MD: 2004. [Google Scholar]
- Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychology Review. 2010;20:236–260. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. International Review of Neurobiology. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Reviews. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H, Sadato N, Lyshkow H, Yonekura Y, Honda M, Nagamine T, Suwazono S, Magata Y, Ikeda A, Miyazaki M, et al. Both primary motor cortex and supplementary motor area play an important role in complex finger movement. Brain. 1993;116(Pt 6):1387–1398. doi: 10.1093/brain/116.6.1387. [DOI] [PubMed] [Google Scholar]
- Shima K, Tanji J. Both supplementary and presupplementary motor areas are crucial for the temporal organization of multiple movements. Journal of Neurophysiology. 1998;80:3247–3260. doi: 10.1152/jn.1998.80.6.3247. [DOI] [PubMed] [Google Scholar]
- Solowij N, Grenyer BF, Chesher G, Lewis J. Biopsychosocial changes associated with cessation of cannabis use: a single case study of acute and chronic cognitive effects, withdrawal and treatment. Life Sciences. 1995;56:2127–2134. doi: 10.1016/0024-3205(95)00198-f. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004a;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004b;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clinical EEG And Neuroscience: Official Journal Of The EEG And Clinical Neuroscience Society (ENCS) 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller K, Swanson GD, Bellville JW. Effects on visual tracking of delta9-tetrahydrocannabinol and pentobarbital. Journal of Clinical Pharmacology. 1976;16:271–275. doi: 10.1002/j.1552-4604.1976.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests : administration, norms, and commentary. 3rd ed Oxford University Press; Oxford ; New York: 2006. [Google Scholar]
- Tamnes CK, Ostby Y, Walhovd KB, Westlye LT, Due-Tonnessen P, Fjell AM. Neuroanatomical correlates of executive functions in children and adolescents: a magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 2010;48:2496–2508. doi: 10.1016/j.neuropsychologia.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2010;49:63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Research. 1996;67:29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- von Sydow K, Lieb R, Pfister H, Hofler M, Sonntag H, Wittchen HU. The natural course of cannabis use, abuse and dependence over four years: a longitudinal community study of adolescents and young adults. Drug and Alcohol Dependence. 2001;64:347–361. doi: 10.1016/s0376-8716(01)00137-5. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Eric Schmitt J, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Molloy EA, Clasen LS, Kendler KS, Neale MC, Giedd JN. A pediatric twin study of brain morphometry. Journal of Child Psychology and Psychiatry. 2006;47:987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shima K, Sawamura H, Tanji J. Spatial distribution of cingulate cells projecting to the primary, supplementary, and pre-supplementary motor areas: a retrograde multiple labeling study in the macaque monkey. Neuroscience Research. 2001;39:39–49. doi: 10.1016/s0168-0102(00)00198-x. [DOI] [PubMed] [Google Scholar]
- Weinstein A, Brickner O, Lerman H, Greemland M, Bloch M, Lester H, Chisin R, Mechoulam R, Bar-Hamburger R, Freedman N, Even-Sapir E. Brain imaging study of the acute effects of Delta9-tetrahydrocannabinol (THC) on attention and motor coordination in regular users of marijuana. Psychopharmacology (Berlin) 2008;196:119–131. doi: 10.1007/s00213-007-0940-7. [DOI] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. European Journal of Neuroscience. 2005;22:235–246. doi: 10.1111/j.1460-9568.2005.04176.x. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug and Alcohol Dependence. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. Journal of Addictive Diseases. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]
- Yamanouchi N, Okada S, Kodama K, Hirai S, Sekine H, Murakami A, Komatsu N, Sakamoto T, Sato T. White matter changes caused by chronic solvent abuse. American Journal of Neuroradiology. 1995;16:1643–1649. [PMC free article] [PubMed] [Google Scholar]