Abstract
Mechanisms that govern cell-fate specification within developing epithelia have been intensely investigated, with many of the critical intercellular signaling pathways identified, and well characterized. Much less is known, however, about downstream events that drive the morphological differentiation of these cells, once their fate has been determined. In the Drosophila wing-blade epithelium, two cell types predominate: vein and intervein. After cell proliferation is complete and adhesive cell-cell contacts have been refined, the vast majority of intervein cells adopt a hexagonal morphology. Within vein territories, however, cell-shape refinement results in trapezoids. Signaling events that differentiate between vein and intervein cell fates are well understood, but the genetic pathways underlying vein/intervein cyto-architectural differences remain largely undescribed. We show here that the Rap1 GTPase plays a critical role in determining cell-type-specific morphologies within the developing wing epithelium. Rap1, together with its effector Canoe, promotes symmetric distribution of the adhesion molecule DE-cadherin about the apicolateral circumference of epithelial cells. We provide evidence that in presumptive vein tissue Rap1/Canoe activity is down-regulated, resulting in adhesive asymmetries and non-hexagonal cell morphologies. In particular Canoe levels are reduced in vein cells as they morphologically differentiate. We also demonstrate that over-expression of Rap1 disrupts vein formation both in the developing epithelium and the adult wing blade. Therefore, vein/intervein morphological differences result, at least in part, from the patterned regulation of Rap1 activity.
Keywords: Rap1, DE-cadherin, Canoe, Thickvein, Ras, wing vein
Introduction
Genetic analysis of the Drosophila wing imaginal disc has revealed many of the mechanisms by which growth and patterning of developing epithelia are controlled. However, at the stage when cell proliferation in the disc is nearly complete (Buttitta et al., 2007), and cell fates along multiple body axes (e.g., anterior/posterior, and dorsal/ventral) have been determined (Bryant, 1975), the epithelium bears little resemblance to an adult appendage. Pupariation (the transition between larval and pre-pupal stages of development) begins the processes of wing disc eversion and subsequent elongation. During this time, dramatic changes in cell shape transform the wing imaginal disc into the appropriate adult structures (Turner and Adler, 1995). The mechanisms underlying this latter stage of disc development, its morphological differentiation, are not well understood. It is important, therefore, to determine how signaling events known to specify cell fates within a developing epithelium are translated into the cyto-architectural changes necessary to achieve the adult form.
The wing blade is an intensely studied portion of the Drosophila wing imaginal disc, and provides an elegant system in which to investigate the morphological differentiation of a particular cell type. Only two cell types predominate in this region of the epithelium: vein and intervein. In the adult structure, veins are linear delaminations of the otherwise opposed dorsal and ventral wing surfaces. These fluid-filled tubes provide wing rigidity that is necessary for flight. Within the blade, veins are positioned in highly stereotypical, species-specific patterns (De Celis and Diaz-Benjumea, 2003). Six longitudinal veins (L1–L6), and two cross-veins (anterior and posterior) characterize the adult Drosophila melanogaster wing (Fig. 1A), and it is well known which developmental signaling pathways distinguish between vein and intervein cell fates in this system (Sotillos and De Celis, 2005). Activation of the Epidermal growth factor receptor (Egfr) is the earliest indication of vein identity (Sturtevant et al., 1993), while subsequent signaling through the Notch and Decapentaplegic (Dpp) pathways refine (de Celis et al., 1997; Huppert et al., 1997) and maintain (de Celis, 1997) the pattern of Egfr activity, respectively. To date, however, most studies have focused on the mechanisms by which vein cells are specified and positioned within the wing epithelium. Egfr/Notch/Dpp target genes that control the morphological changes necessary for vein-cell differentiation have not been thoroughly described.
Fig. 1.
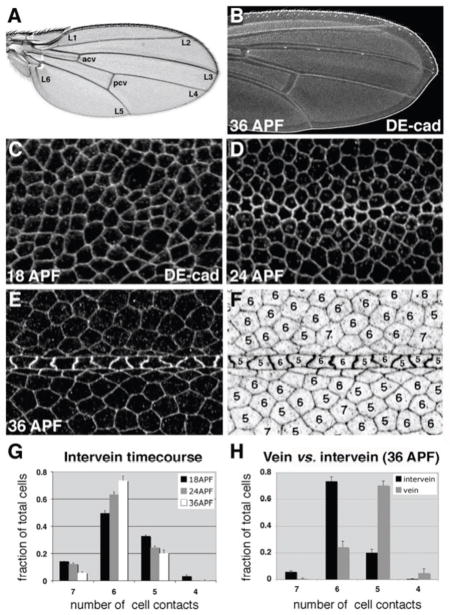
During wing epithelial cell-shape refinement, vein and intervein cells adopt different morphologies.(A) Wild-type adult wing. Longitudinal veins 1–6 (L1–L6) and two crossveins (anterior (acv) and posterior (pcv)) are labeled. (B) Pupal wing (36 h APF) labeled for DE-cadherin. (C–E) Timecourse of cell-shape refinement within the pupal wing. Wings were dissected at 18 h APF (C), 24 h APF (D), or 36 h APF (E), and labeled for DE-cad. Each image is centered on longitudinal vein L3. (F) To quantify cell shape, the number of cell-cell contacts was determined for each cell (36 h APF is shown). (G) Quantification of intervein cell shape refinement. Between 18 and 36 h APF, intervein cell shape variability decreases as hexagons become dominant (n = 4–6 images per time point, with 43–364 cells per image). (H) Quantification of vein and intervein shape differences at 36 h APF. On average, vein cells have one fewer cell-cell contact than surrounding intervein cells (n = 6 images, with 13–64 vein and 27–177 intervein cells per image). Error bars indicate SEM.
An increasing body of evidence suggests that in addition to specifying cell fates, the Egfr, Notch and Dpp pathways are capable of affecting cell morphology within the developing Drosophila wing and elsewhere. For example, Egfr signaling up-regulates the homophilic adhesion molecule DE-cadherin (DE-cad), affecting cell-cell adhesion, epithelial integrity, and cell shape in the Drosophila wing, eye, and trachea (Brown et al., 2006; Cela and Llimargas, 2006; Jeon and Zinn, 2009; Mirkovic and Mlodzik, 2006; O’Keefe et al., 2007). Notch activity in the distal wing regulates actin/myosin levels, creating a population of distinctly shaped cells at the dorsal/ventral boundary that serve an important function during tissue compartmentalization (Major and Irvine, 2005; Major and Irvine, 2006). Finally, loss of Dpp signaling dramatically affects the cytoskeleton of wing cells, resulting in their basal extrusion from the epithelium (Gibson and Perrimon, 2005; Shen and Dahmann, 2005). In contrast, high levels of Dpp signaling correlate with an elongated columnar shape in the wing disc (Widmann and Dahmann, 2009). Roles for Dpp in epithelial morphogenesis have also been described in the pupal retina, the embryonic epidermis during dorsal closure, and the abdominal histoblasts (Cordero et al., 2007; Fernandez et al., 2007; Ninov et al., 2010). It is plausible, therefore, that a single developmental signaling pathway could be used reiteratively to control specification and later the morphological differentiation of a particular cell type.
Here we provide evidence that the Rap1 GTPase plays a critical role in determining the cyto-architectural differences between vein and intervein cells. Rap1 is the GTPase most highly related to Ras (Lundquist, 2006), affecting cell adhesion and migration in numerous developmental and homeostatic contexts. Initial studies focused on the relationship between Rap1 and integrin-based cell adhesion (Bos et al., 2003; Caron, 2003; Su et al., 2003). For example, Rap1 activity is required in lymphocytes for transendothelial migration, an integrin-dependent process (Shimonaka et al., 2003). Subsequently, it was discovered that homophilic cell adhesion through the cadherin family of transmembrane proteins also depends on Rap1. This was first demonstrated in the developing Drosophila wing where Rap1 activity is necessary to maintain an even distribution of DE-cad about the apicolateral circumference of wing epithelial cells (Knox and Brown, 2002). Similarly, vertebrate epithelia lacking Rap1 have reduced levels of E-cadherin (Hogan et al., 2004; Price et al., 2004), and mice with diminished Rap1 activity die during early embryonic stages as a result of cell-adhesion defects (Ohba et al., 2001). We show here that in the Drosophila wing blade, cell-type-specific regulation of Rap1 is necessary for vein differentiation. In particular, Egfr signaling down-regulates Rap1 activity in vein cells, resulting in adhesive asymmetries and non-hexagonal morphologies that characterize the epithelial wing vein structure.
Materials and methods
Genetics
Fly stocks used for this analysis: w1118 (Hazelrigg et al., 1984), hsFLP122; Actin<CD2<Gal4, UAS-GFP (Neufeld et al., 1998; Pignoni and Zipursky, 1997; Struhl and Basler, 1993), UAS-tkvQ235D (Nellen et al., 1996), UAS-RasV12(Karim and Rubin, 1998), UAS-lambda-top (Queenan et al., 1997), UAS-DE-cad (Sanson et al., 1996), hsFLP122; FRT80B, Ub-GFP (Xu and Rubin, 1993), Rap1rB3 (Hariharan et al., 1991), UAS-Rap1V12 (Boettner et al., 2003), UAS-Rap1wt (Boettner et al., 2003), apGal4 (Calleja et al., 1996), Rap1-GFP (Knox and Brown, 2002). To generate labeled clones of cells, larvae were heat shocked at 72 h after egg deposition (AED) for 10 min (flp/Gal4, gain-of-function) or 60 min (flp/FRT, loss-of-function) in a 37 ºC water bath. For two-cell clone analysis, pre-pupae were heat shocked at 0 h after puparium formation (APF) for 3 min.
Immunohistochemistry and image analysis
Pupal wings were dissected at 18, 24, or 36 h APF in phosphate-buffered saline (PBS) and fixed for 20 min in 4% paraformaldehyde in PBS. After washes in PBS-Triton (0.1%) (PBT), wings were placed in blocking solution (PBT plus 4% normal goat serum) for 2 h at room temperature, or overnight at 4 ºC. Wings were subsequently incubated in primary antibody overnight at 4 ºC. Washes and secondary antibody incubation followed standard protocols. Antibodies used for these studies were directed against DE-cad (1:100; Developmental Studies Hybridoma Bank), Canoe (1:100; gift from D. Yamamoto), or DSRF (1:500; Active Motif, Carlsbad, CA). Alexa Fluor conjugated secondary antibodies were used (1:1500; Invitrogen, Carlsbad, CA). Adult wings were placed in EtOH for 1 h, transferred to methylsalicylate for 1 h, and mounted in Canada balsam/methyl salicylate (1:1).
Fluorescent images were acquired using a Zeiss LSM 510 or a Leica TCS SP5 confocal microscope. When analyzing GFP-expressing clones of cells, the GFP signal was detected throughout the cytoplasm and nucleus. As adherens junctions and nuclei are found at different planes of focus, the GFP signal did not accurately delimit clone boundaries in stacks of confocal images. Thin confocal sections at the apical surface were therefore used to precisely determine clone boundaries (images not shown). Adobe Photoshop was used to compile images. Pixel intensities along linear vectors were determined using ImageJ. Graphs were generated and statistical analyses (Student’s t-test) performed using Microsoft Excel.
Results
Epithelial cell-shape differences between vein and intervein precursors in the pupal wing
In the developing Drosophila wing, cell proliferation is complete at approximately 20 h APF (Buttitta et al., 2007; Milan et al., 1996; Schubiger and Palka, 1987). At this time, remodeling of cell-cell adhesive contacts creates a highly uniform lattice of hexagonally shaped epithelial cells; a process termed hexagonal packing (Classen et al., 2005). We confirmed this result by dissecting wing epithelia from 18-, 24-, and 36-h APF animals (genotype: w1118) and labeling them with antibodies directed against DE-cad (Fig. 1B–E). DE-cad is a key component of the apicolateral adherens junction complex, and delineates the apical shape of each wing epithelial cell when visualized (Oda et al., 1994; Woods et al., 1997). To quantify changes in epithelial cell shape, the number of cell-cell contacts (sidedness) was determined manually for each cell within a given image (Fig. 1F). As reported previously (Classen et al., 2005), epithelial cell-shape variability for cells within intervein territories was greatly reduced at 36 h APF compared to earlier time points. By 36 h APF, hexagons predominated, while seven-, five-, and four-sided cells had become far less common (Fig. 1G).
Cells fated to form wing veins, however, did not adopt hexagonal morphologies. Specified during larval stages of development (Sturtevant et al., 1993), vein cells remain morphologically indistinct for a number of days. By 36 h APF, however, each vein consists of many, linearly arrayed, trapezoidal-shaped cells (in contrast to the surrounding hexagonal intervein cells) (Fig. 1E,F). When the shape of 36-h APF vein cells was quantified, five-sided cells predominated (Fig. 1H). While the signals that direct vein-cell fate have been well described, the downstream effectors that determine vein cyto-architecture are less clear. We have investigated, therefore, the mechanism by which five-sided vein cells are created within the hexagonal wing epithelium.
Asymmetric cell adhesion in developing wing veins
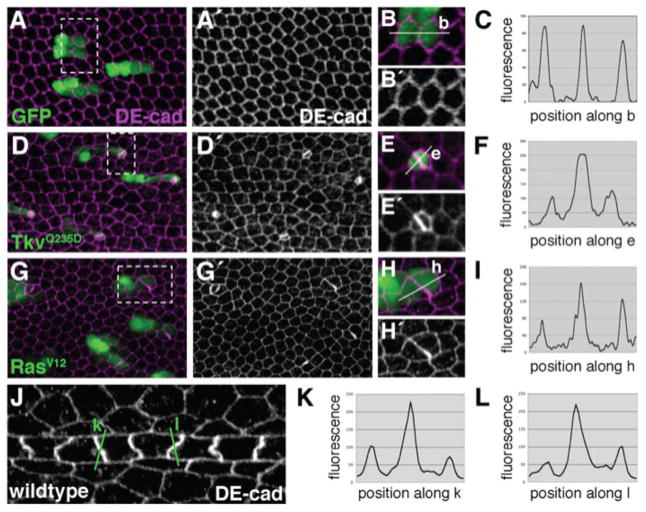
As a first step toward understanding how vein-cell shape is controlled, we characterized cell-cell contacts within small ectopic patches of vein tissue. Using the flp/Gal4 system, animals were heat shocked at pupariation (0 h APF) to generate two-cell clones that expressed an activated version of the Thickvein (Tkv) receptor (TkvQ235D) (genotype: hsFLP122; Act<CD2<Gal4, UAS-GFP/UAS-tkvQ235D). Wings were dissected at 36 h APF and labeled for DE-cad. Tkv is the Drosophila BMP4 receptor homologue and is principally activated by the secreted ligand Dpp. Extensive experimentation has shown it to be necessary and sufficient for the specification of wing vein cell fate (de Celis, 1997; Sotillos and De Celis, 2005). TkvQ235D was able to induce ectopic vein tissue during this 36-h period, indicated by down-regulation of the intervein marker DSRF (Montagne et al., 1996) (data not shown). Compared to control clones that expressed only GFP (Fig. 2A,B), two-cell clones that expressed TkvQ235D were apically constricted (Fig. 2D,E), and the number of cell-cell contacts was reduced (Supp. Fig. S1). Most striking, however, was the pattern of DE-cad localization. While DE-cad levels were generally elevated in TkvQ235D-expressing cells, extremely high levels were observed at the vein-vein interface (the border between the two cells of the clone) (Fig. 2F). This was in contrast to wild-type intervein cells that had an even distribution of DE-cad about their apical circumference (Fig. 2C). Statistical analyses of adherens junction localization are presented in Supplemental Figure S2A,B.
Fig. 2.
Vein cells asymmetrically distribute DE-cad.(A–I) Using the flp/Gal4 system, animals were heat-shocked at 0 h APF to generate two-cell clones (GFP-positive). Wings were dissected at 36 h APF and labeled for DE-cad. (B,E,H) Magnified views of individual clones are shown (boxed regions). White lines indicate vectors along which fluorescence intensity was measured and graphed (C,F,I). (A–C) Cells expressing GFP are generally hexagonal and have an even distribution of DE-cad about their apicolateral circumference. (D–F) Ectopic vein cells expressing TkvQ235D are apically constricted, no longer hexagonal, and asymmetrically concentrate DE-cad at the vein-vein interface. (G–I) Ectopic vein cells expressing RasV12 also asymmetrically concentrate DE-cad at the vein-vein interface. (J) Wild-type wing (36 h APF) labeled for DE-cad. Magnified view of vein L3 is shown. Green lines indicate vectors along which fluorescence intensity was measured and graphed (K,L). DE-cad levels are higher at vein-vein points of contact than vein-intervein. Note that y-axis scales are not identical between panels.
To determine whether this asymmetric distribution of DE-cad is a general feature of vein cells, or TkvQ235D-specific, we expressed an activated version of the Ras GTPase (RasV12) using the same experimental protocol. In the developing wing, Ras is a critical component of the Egfr signaling cascade (Diaz-Benjumea and Hafen, 1994), and ras lies genetically upstream of tkv in differentiating vein cells (de Celis, 1997; Sotillos and De Celis, 2005). As with Tkv, Egfr/Ras activity is both necessary and sufficient for wing vein formation (Diaz-Benjumea and Hafen, 1994; Karim and Rubin, 1998; Prober and Edgar, 2000; Sturtevant and Bier, 1995; Sturtevant et al., 1993). When the UAS-RasV12 transgene was expressed from 0–36 h APF in two-cell clones, similar effects on cell sidedness (Supp. Fig. S1) and DE-cad distribution were observed (Fig. 2G–I). In particular, DE-cad levels were highest at vein-vein interfaces (Fig. 2I; Supp. Fig. S2C,D), as was the case with TkvQ235D. A similar phenotype was also seen using an activated version of Egfr (lambda-top), indicating that this is not a Ras-specific effect (Supp. Fig. S3). The phenotypes associated with Egfr/Ras and Dpp signaling pathways were not identical, however, as TkvQ235D had a more dramatic effect on apical constriction.
Whereas we initially noticed adherens junction asymmetries in two-cell clones of ectopic vein cells, this property is also clearly visible in the wild-type wing. When levels of DEcad were quantified in images of 36-h APF wild-type wings, higher levels of DE-cad were found at vein-vein cell interfaces than at vein-intervein interfaces (Fig. 2J–L; Supp. Fig. S2I,J). These experiments reveal two basic properties of vein cyto-architecture. First, vein cells asymmetrically distribute DE-cad about their apicolateral circumference (in contrast to intervein cells that are characterized by a symmetrical distribution of DE-cad). DE-cad levels were highest at vein-vein interfaces, indicating that vein cells preferentially adhere to one another. Second, vein cells generally have fewer cell-cell contacts than intervein cells: approximately five, rather than six. As adherens junctions play such a critical role in determining epithelial cell shape (Lecuit and Lenne, 2007), the five-sided characteristic of vein cells is likely a secondary consequence of altered cell adhesion.
Rap1 regulates vein differentiation
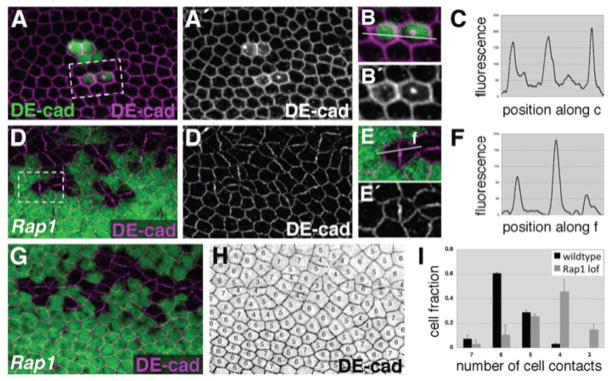
Taking a candidate-gene approach, we began to explore the mechanisms that control vein-cell adhesion and morphology. We have previously demonstrated that from early stages of wing development presumptive vein cells express higher levels of DE-cad than adjacent intervein cells. Furthermore, Egfr/Ras signaling is critical for this adhesive difference (O’Keefe et al., 2007). We asked, therefore, whether the effects on adhesion and shape that we observed in ectopic clones of vein cells resulted from increased levels of DE-cad. To test this hypothesis, we over-expressed DE-cad in two-cell clones, but did not observe a dramatic effect on shape. Intervein cells expressing DE-cad were hexagonal (Fig. 3A,B; Supp. Fig. S1), and maintained an even distribution of DE-cad (Fig. 3C). Up-regulation of DE-cad, therefore, is not sufficient to confer vein-cell shape within an intervein territory.
Fig. 3.
Cells that lack Rap1 activity phenotypically resemble vein cells.(A–C) Using the flp/Gal4 system, animals were heat shocked at 0 h APF to generate two-cell clones (GFP-positive). (D–I) Using the flp/FRT system, Rap1 loss-of-function clones (GFP-negative) were generated during larval stages. (A–I) Wings were dissected at 36 h APF and labeled for DE-cad. (B,E) Magnified views of boxed regions are shown. White lines indicate vectors along which fluorescence intensity was measured and graphed (C,F). DE-cad asymmetries in vein cells do not result simply from increased levels of DE-cad, as two-cell clones over-expressing DE-cad are hexagonal (A,B) and have an even apicolateral distribution of DE-cad (C). Pairs of Rap1 mutant cells frequently scatter within the epithelium, displaying cell-shape (D,E) and DE-cad-distribution phenotypes (F) similar to ectopic vein cells. (G–I) When sidedness of Rap1 mutant cells was measured and quantified they were found to have dramatically fewer cell-cell contacts than surrounding wild-type cells (n = 4 images, with 20–34 Rap1 and 47–145 wild-type cells per image). Error bars indicate SEM. Note that y-axis scales are not identical between panels.
Alternatively, vein-cell shape could depend on the asymmetric distribution of adherens junctions, rather than the absolute level of DE-cad. The Rap1 GTPase has been shown to regulate cell adhesion and migration in many developmental and homeostatic contexts. In particular, epithelial cells require Rap1 activity to maintain an even distribution of adherens junctions about their apicolateral circumference (Knox and Brown, 2002). Using the flp/FRT system, Rap1 mutant clones of cells were generated during larval stages and wings were dissected and analyzed at 36 h APF (genotype: hsFLP122; FRT80b, Rap1/FRT80b, Ubi-GFP). As has been described previously, Rap1 loss-of-function clones scattered within the plane of the wing epithelium as a result of ineffective cell-cell adhesion (Knox and Brown, 2002) (Fig. 3D,G). Frequently, pairs of cells separated from the main body of the clone, generating, in effect, two-cell clones. These Rap1 mutant pairs of cells had a rounded shape and an asymmetric distribution of DE-cad (even when located within intervein regions) (Knox and Brown, 2002) (Fig. 3E,F), which appeared remarkably similar to two-cell clones of ectopic vein cells (RasV12-expressing cells in particular) (compare Fig. 2H and Fig. 3E). When the number of cell-cell contacts was measured in Rap1 mutant clones (Fig. 3H) a dramatic effect was seen. Most Rap1 mutant cells had fewer than six cell-cell contacts, as four cell-cell contacts were most common (Fig. 3I). Importantly, Rap1 mutant cells within intervein regions maintain DSRF expression, and therefore intervein-cell fate. Based on these observations, we hypothesized that in vein-cell precursors the non-hexagonal morphology and the asymmetric distribution of adherens junctions result, at least in part, from the down-regulation of Rap1 activity.
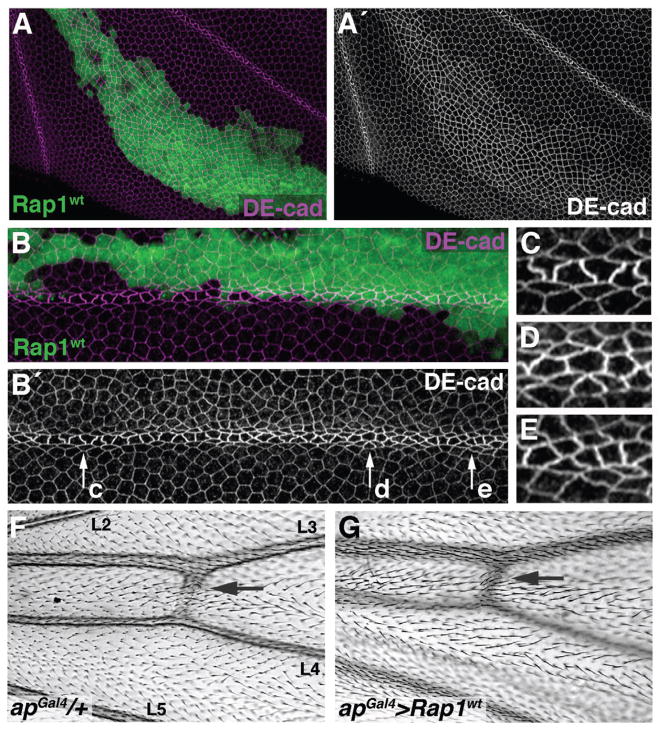
If less Rap1 activity is necessary for the morphological differentiation of vein cells, then over-expression of Rap1 should disrupt this process. To test this idea, we expressed an activated version of the Rap1 protein (Rap1V12) in the developing wing. Cells expressing Rap1V12down-regulated the intervein marker DSRF, however, indicating a switch to vein identity, and rendering this experiment inconclusive (data not shown). As an alternative means of increasing Rap1 activity, we over-expressed a wild-type version of Rap1 in clones of cells. Vein/intervein cell fate was not affected in Rap1 gain-of-function clones (as measured by DSRF) (Supp. Fig. S4), but DE-cad was elevated (Fig. 4A). This, in combination with the loss-of-function data, clearly indicates that Rap1 functions to stabilize adhesive contacts between wing epithelial cells. The most dramatic effect of Rap1 over-expression, however, was seen in vein cells, where vein-shape refinement was inhibited (Fig. 4B). Vein cells over-expressing Rap1 maintained symmetric distributions of apicolateral adherens junctions, and therefore did not adopt their characteristic trapezoidal conformation (compare Fig. 4C to Fig. 4D,E). Accordingly, when adult wings containing Rap1 over-expressing clones were examined, vein discontinuities (a phenotype never seen in wild-type controls) were often observed (data not shown).
Fig. 4.
Increased levels of Rap1 stabilize DE-cad and disrupt vein differentiation.(A–E) Clones of cells over-expressing wild-type Rap1 (GFP-positive) were generated during larval stages. Wings were dissected at 36 h APF and labeled for DE-cad. (A) DE-cad levels are elevated within clones over-expressing Rap1. (B) Rap1 over-expression disrupts vein-cell morphology. Arrows labeled c–e indicate vein regions that are magnified in C–E. (C) Wild-type portion of the vein showing cells with asymmetric distributions of DE-cad. (D,E) Vein cells over-expressing Rap1 are shown. DE-cad is symmetrically distributed and vein cyto-architecture is abnormal. (F) Veins within wild-type wings consist of distinct tubular structures. Veins L2–L5 are labeled. (G) The apGal4 driver was used to over-express Rap1 throughout the dorsal wing surface. Pigmented cuticle associated with vein tissue is found in appropriate regions, but vein lumens are often disrupted (indistinct edges). To demonstrate a consistent plane of focus, a sensory structure (campaniform sensilla) on the dorsal surface of each wing is indicated (arrows, F,G).
To more definitively characterize the adult-wing phenotype associated with Rap1 over-expression, apterous-Gal4 (apGal4) was used to express Rap1 throughout the dorsal surface of the wing from mid-larval stages of development (Calleja et al., 1996). In wild-type wings, vein cells form distinct tubular structures (Fig. 4F). Rap1 over-expression did not affect wing-vein patterning (i.e., stripes of pigmented cuticle appeared in appropriate locations), but the tubular lumens were often less distinct or absent altogether (Fig. 4G). Although it is unclear how the apical shape of vein epithelial precursors (which are assembled together like a lattice beam used in construction) functionally contributes to the adult vein, Rap1-mediated disruption of this cyto-architecture affected vein morphology within the adult structure.
Thus, either loss of Rap1 or Rap1 over-expression creates a disconnect between cell fate and morphology. Rap1 loss-of-function clones of cells within intervein territories maintain high levels of DSRF expression (i.e., maintain an intervein cell fate), but adopt the morphological properties of vein cells. In contrast, vein precursors that over-express Rap1 maintain low levels of DSRF expression (i.e., maintain a vein cell fate) but adopt the morphological properties of intervein cells. Taken together, these results are consistent with the hypothesis that Rap1 activity is down-regulated in vein cells compared to intervein cells, which leads to an asymmetric apicolateral distribution of DE-cad. In turn, these adhesive asymmetries result in fewer cell-cell contacts, and a non-hexagonal cell shape.
Rap1 activity in the pupal-wing epithelium
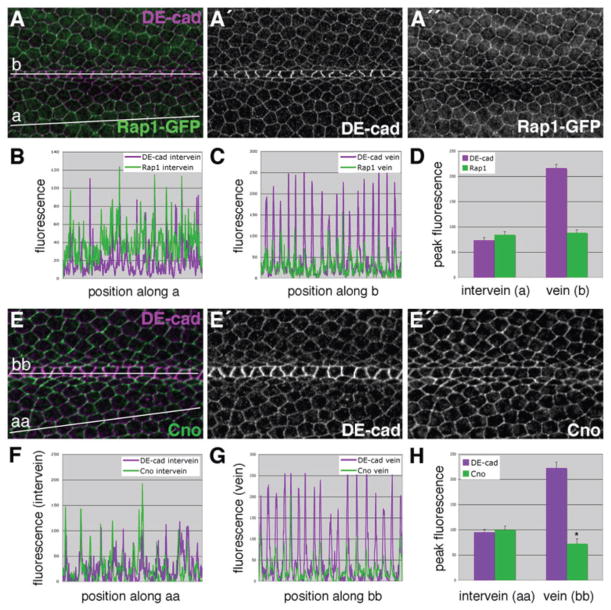
If Rap1 indeed contributes to the morphological differentiation of vein cells, the level of Rap1 protein and/or Rap1 activity should be lower in vein cells of the pupal wing (when compared to intervein cells). We therefore examined levels of Rap1 protein and the Rap1 effector Canoe in vein and intervein cells. Wings expressing a Rap1-GFP fusion protein (controlled by the endogenous Rap1 promoter (Knox and Brown, 2002)) were dissected at 36 h APF and stained for DE-cad (Fig. 5A). As seen previously, DE-cad levels were elevated at vein-vein interfaces, and quantification of pixel intensities confirmed this observation (Fig. 5B,C). Compared to the average intervein cell-cell junction, there was approximately two-fold more DE-cad present at vein-vein junctions per unit area (Fig. 5D) (See Supp. Fig. S5 for details concerning this quantitative analysis). In contrast, we found no differences in Rap1-GFP levels between vein and intervein cell junctions (Fig. 5A,D). These data reveal DE-cad/Rap1 stoichiometric differences between vein and intervein cells. If we assume a 1:1 ratio between DE-cad and Rap1 in intervein cells, then this ratio is ~2.5:1 in vein cells (i.e., vein cells have fewer molecules of Rap1 for every adherens junction complex).
Fig. 5.
Relative to DE-cad, levels of both Rap1 and Cno are reduced at vein-vein cell junctions.(A) Wings from Rap1-GFP animals were dissected at 36 h APF and labeled for DE-cad. For both DE-cad and Rap1-GFP, fluorescence intensity was measured along two vectors: intervein (a), and vein (b). Raw data is plotted for intervein (B) and vein (C) cells. (D) Fluorescence values specifically from cell-cell junctions were extracted to more quantitatively compare fluorescence (See Supp. Fig. S5). Significantly more DE-cad localizes to vein-vein cell junctions than intervein-intervein cell junctions. In contrast, vein and intervein cell junctions contain similar levels of Rap1-GFP. (E) A 36-h APF wild-type wing double-labeled for DE-cad and Cno. For both DE-cad and Cno, fluorescence was measured along two vectors, intervein (aa) and vein (bb). Raw data (F,G) and peak values (H) are plotted. Analysis indicates that at vein-vein cell junctions DE-cad levels are higher, while Cno levels are slightly lower (compared to intervein values). Error bars indicate SEM. *p • 0.05 when compared to the corresponding intervein Cno value via the Student’s t-test. Note that y-axis scales are not identical between panels.
As an indicator of Rap1 activity, localization of the scaffolding protein Cno was examined in the developing wing. Cno binds to active Rap1 (Boettner et al., 2000; Boettner et al., 2003; Linnemann et al., 1999; Su et al., 2003) and in several developmental contexts (including the Drosophila wing) has been shown to act as a critical downstream effector of Rap1 activity (Boettner et al., 2003; Su et al., 2003; Xie et al., 2005). In particular, cno is necessary to maintain an even distribution of DE-cad about the apical circumference of epithelial cells, phenocopying Rap1 in this developmental context (O’Keefe et al., 2009). Rap1 activity is necessary for the localization of Cno to adherens junctions, where it forms a link to the actin-myosin cytoskeleton. This link is critical for apical constriction of mesodermal cells during gastrulation, for example (Mandai et al., 1997; Sawyer et al., 2009).
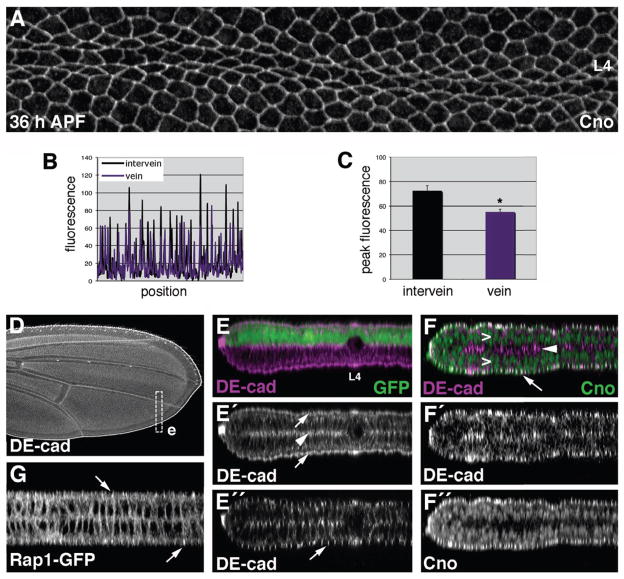
Wings from 36-h APF animals were dissected and double-labeled for DE-cad and Cno (Fig. 5E). When pixel intensities from these images were analyzed, a small but statistically significant drop in Cno was detected at vein-vein interfaces (compared to intervein-intervein interfaces) (Fig. 5F–H). To confirm that the anti-DE-cad antibody was not affecting the Cno localization pattern in this experiment, we labeled 36-h APF wings for Cno alone. A similar pattern was seen (Fig. 6A) and quantification confirmed that Cno levels were reduced in vein cells (Fig. 6B,C; Supp. Fig. S2K,L; Supp. Fig. S6). As was seen with Rap1, therefore, vein cells have far fewer molecules of Cno for every adherens junction complex (compared to intervein cells). This is consistent with the hypothesis that vein cells lack sufficient Rap1 activity (i.e., effector capacity) to maintain a symmetric apicolateral distribution of adherens junctions, and that the patterned regulation of Rap1 activity plays an important role in determining vein-cell morphology.
Fig. 6.
Localization patterns of Cno, DE-cad, and Rap1 in the pupal-wing epithelium.(A) Wild-type36-h APF wing labeled for Cno. Vein L4 is indicated. (B) Using the image from panel A, vectors were drawn through either intervein or vein territories (see Supp. Fig. S6). (B) Fluorescence values along these two vectors are shown. (C) Peak values of fluorescence (from regions of cell-cell contact) were averaged, and analysis indicates that significantly less Cno is present at vein-vein cell junctions than intervein-intervein cell junctions. (D) Pupal wing (36 h APF) labeled for DE-cadherin. (E–G) Optical cross-sections through the bi-layered pupal-wing epithelium are shown (36 h APF). (E) To highlight the apical/basal architecture of the wing, a projection of multiple adjacent sections through vein L4 and the posterior wing margin is shown. Approximate location of the section is indicated by the boxed region (e) in panel (D). The apGal4 driver was used to express GFP in the dorsal half of the wing epithelium, and the wing was labeled for DE-cad. Vein cells on both wing surfaces contract along the apical/basal axis to form a lumen (vein L4 is labeled). (E′) Arrows indicate the apical surfaces of the dorsal and ventral wing epithelia, whereas an arrowhead indicates the apposed basal surfaces. (E″) A single optical section of the same wing is shown to highlight the apicolateral adherens junctions (arrow). (F) Pupal wing (36 h APF) double-labeled for DE-cad and Cno. These proteins co-localize at adherens junctions (arrow), but Cno is absent from the basal domain (arrowhead). Cno also localizes to nuclei (>), which do not contain DE-cad. (G) Highest levels of Rap1-GFP are found at apicolateral adherens junctions (arrows), whereas lower levels are found throughout the cell cortex (36 h APF). Error bars indicate SEM. *p • 0.05 when compared to intervein Cno levels via the Student’s t-test. Note that y-axis scales are not identical between panels.
To generate a more complete picture of protein localization patterns within the pupal-wing epithelium, we examined optical cross-sections. At 36 h APF, the wing consists of a dorsal and a ventral epithelium that are opposed at their basal surfaces. Vein cells, however, constrict along the apical/basal axis to form a lumen (Fig. 6E). As we have previously demonstrated (O’Keefe et al., 2007), high levels of DE-cad localize to both apico- and baso-lateral regions within the cell cortex of intervein cells (Fig. 6E). Cno co-localized with DE-cad at apicolateral adherens junctions, but was not found basally (Fig. 6F). Cno also localized to the nucleus, which has been previously reported in the embryo (Sawyer et al., 2009). Finally, high levels of Rap1-GFP were detected apico-laterally (Fig. 6G), although lower levels were found throughout the cell cortex. This Rap1 pattern is consistent with previous findings in the wing disc (Knox and Brown, 2002). This analysis revealed substantial differences between the DE-cad, Cno, and Rap1 patterns of localization, suggesting that the precise regulation of their localization is important during epithelial differentiation.
Ras signaling down-regulates Cno
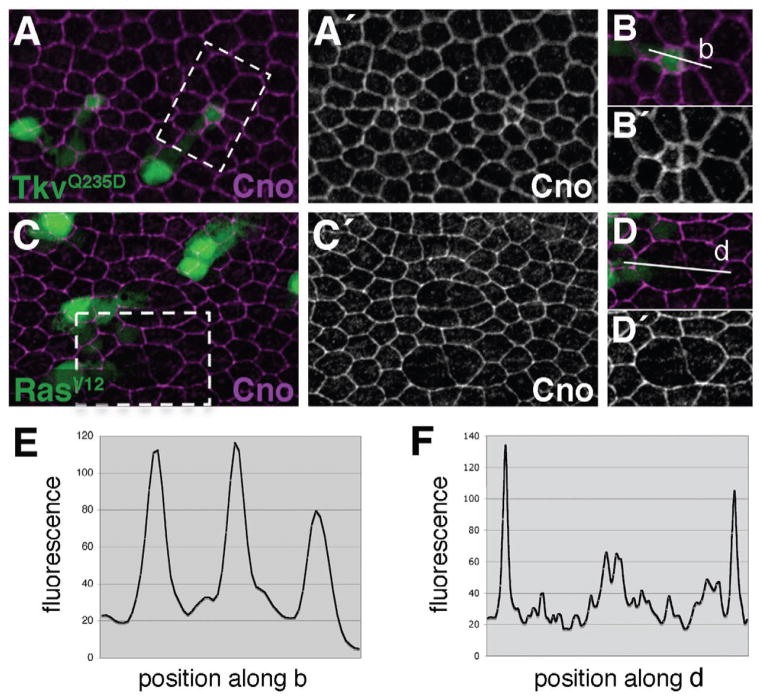
We next examined Cno distribution in two-cell clones of ectopic vein cells. Clones of cells expressing either TkvQ235D or RasV12were generated at 0 h APF. Wings were dissected at 36 h APF and labeled for Cno. In TkvQ235D-expressing cells, Cno levels were generally unaffected (compared to surrounding wild-type tissue) (Fig. 7A,B), and an even apicolateral distribution of Cno was maintained (Fig. 7E; Supp. Fig. S2E,F). In RasV12-expressing cells, however, Cno levels were down-regulated at vein-vein cell contacts (Fig. 7C,D,F; Supp. Fig. S2G,H; Supp. Fig. S7). This indicates that the Egfr/Ras and Dpp/Tkv signaling pathways play non-equivalent roles in vein cell differentiation. Whereas these two pathways act in concert to specify vein cell fate, Ras plays the dominant role in down-regulating Rap1 activity to affect vein morphology (see model, Fig. 8G). Furthermore, these measurements support our hypothesis that cells within differentiating wing-vein territories lack sufficient Rap1 activity to maintain an even distribution of DE-cad about their apicolateral circumferences. This compromise in Rap1 function leads to asymmetric adhesive contacts and, at least in part, to the non-hexagonal shape that characterizes vein cells.
Fig. 7.
Ras signaling regulates Cno levels. Clones expressing either TkvQ235D (A,B), or RasV12 (C,D) were generated at 0 h APF. Wings were dissected at 36 h APF and labeled for Cno. (B) and (D) represent magnified views of boxed regions in A and C, respectively. (A) TkvQ235D-expressing cells are apically constricted, but Cno levels are not dramatically affected compared to surrounding wild-type tissue. (C) In contrast, Cno levels are down-regulated in RasV12-expressing cells. (E) and (F) indicate fluorescence intensity values measured along vectors (b) and (d), respectively. (E) Cno remains essentially evenly distributed about the apicolateral circumference of TkvQ235D-expressing cells. (F) In contrast, Cno levels are dramatically lower at junctions between RasV12-expressing cells. Note that y-axis scales are not identical between panels.
Fig. 8.
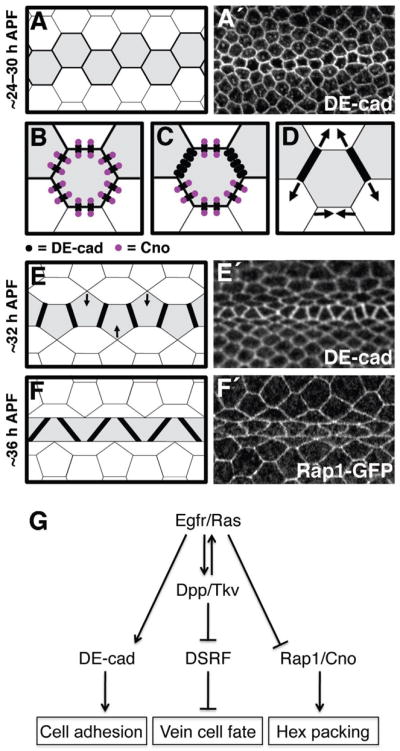
Models of adhesive changes and signaling events that determine vein–cell morphology in the wing epithelium. For schematics (A–F) shapes colored grey indicate vein-cell precursors, whereas black lines indicate DE-cad levels (thicker lines indicate higher levels of DE-cad). For representative images (A′,E′,F′), visualized proteins are indicated. (A) By ~24–30 h APF, vein precursors have narrowed to a single row of cells, which express high levels of DE-cad. (B–D) After ~24–30 h APF adhesive asymmetries arise in vein cells as Cno levels are down-regulated. (B) In hexagonal vein precursors (~24–30 h APF) there is a ~1:1 stoichiometry between DE-cad and Cno (i.e., each adherens junction is associated with a molecule of Cno). (C) Ras signaling leads to reduced levels of Cno in vein precursors, resulting in Cno-free adherens junctions that accumulate at vein-vein cell contacts. (D) Adhesive asymmetries cause vein-vein cell contacts to expand at the expense of vein-intervein regions of contact (arrows), resulting in pentagonal vein precursors (E). The row of vein precursors then straightens (arrows in E), and the 36-h APF conformation is attained (F). (G) The Egfr/Ras and Dpp/Tkv signaling pathways act in concert to specify vein-cell fate within the Drosophila wing epithelium. Egfr/Ras plays the critical role in determining the epithelial morphology of these cells, however, by regulating DE-cad and Cno in a Dpp-independent fashion.
Discussion
During the early, proliferative phase of epithelial development each cell strives to maintain adhesive contacts with its neighbors (Garcia-Bellido et al., 1973), generating, on average, a field of hexagonal-shaped cells (Gibson et al., 2006). This uniformity is transient, however, as multiple cell types are frequently specified within a single epithelium, each with a unique function and cyto-architecture. Mechanisms must exist, therefore, for cell-type-specific shapes to emerge as these heterogeneous epithelia begin to morphologically differentiate. We show here that in the Drosophila wing the regulation of Rap1 activity is one means by which non-hexagonal epithelial cell shapes are generated.
Our studies have focused on the Drosophila wing vein. Within the wing blade, veins comprise a small subset of cells, and during pupal stages of development we have shown that vein-precursor cells adopt a unique shape (trapezoidal), compared to surrounding intervein cells (hexagonal). Presumptive vein cells are first identified by high levels of Egfr activity (Sturtevant et al., 1993), and we have shown previously that Egfr signaling up-regulates the homophilic adhesion molecule DE-cad in these cells (both transcriptionally and post-translationally) (O’Keefe et al., 2007). High levels of cadherin generally result in apical constriction (Schlichting and Dahmann, 2008), a prominent characteristic of the adult vein. DE-cad is only one component of this morphogenetic process, however, as increased levels of DE-cad did not result in a vein-like trapezoidal shape (Fig. 4A). We asked, therefore, what other mechanisms might determine the non-hexagonal morphology of vein precursors.
In addition to elevated levels of DE-cad, another distinguishing feature of pupal vein cells is an asymmetric distribution of DE-cad about their apicolateral circumference, a phenotype most apparent when two-cell clones of ectopic veins were examined. As loss of Rap1 leads to asymmetric DE-cad (Knox and Brown, 2002; O’Keefe et al., 2009), we hypothesized that Rap1 activity is down-regulated in vein precursor cells compared to surrounding intervein precursors. Consistent with this hypothesis, Rap1 over-expression dramatically disrupted pupal vein cell shape without affecting cell fate (i.e., DSRF levels). Rap1 over-expressing vein cells had more symmetric DE-Cad distributions, and did not adopt a trapezoidal morphology (Fig 4B–E). This often led to morphological vein defects in the adult wing (Fig. 4G). In addition, the localization patterns of Rap1-GFP and Canoe suggested lower levels of Rap1 activity in pupal-vein precursors (compared with surrounding intervein cells) (Figs 5, 6). We have previously demonstrated that the generation of Rap1 loss-of-function clones during larval stages results in vein loss (O’Keefe et al., 2009). Rap1 activity, therefore, plays a dual role in wing-vein formation. First, during larval and early pupal stages, Rap1 stabilizes adhesive contacts between adjacent epithelial cells, thereby facilitating Egfr signaling and maintaining vein-cell fate. Hours later, as the wing begins to differentiate, down-regulation of Rap1 activity drives the morphological changes necessary for vein formation.
How does the down-regulation of Rap1 activity specifically increase DE-cad levels at vein-vein cell contacts? Rap1 recruits Cno to adherens junctions, where Cno forms a physical link between adherens junctions and the actin cytoskeleton (Sawyer et al., 2009). As such, Cno primarily acts as a non-enzymatic scaffolding protein, which suggests that stoichiometry between DE-cad and Cno is important. Based on our immunofluorescence analysis of apicolateral cell junctions in the wing, there is a large disparity between Cno and DE-cad levels in vein cells, as Egfr/Ras signaling both up-regulates DE-cad, and down-regulates Cno. We infer from these data that vein cells contain far fewer adherens junction complexes that are associated with a molecule(s) of Cno (compared to intervein cells). As Cno represents the critical Rap1 effector in this context, these Cno-free adherens junction complexes would be functionally dissociated from Rap1 signaling, and free to localize in an asymmetric fashion. Relieved from spatial constraints concerning symmetry, adherens junction complexes would accumulate at vein-vein interfaces, where chances of encountering an intercellular binding partner are highest for two reasons: 1) adjacent vein cells express higher levels of DE-cad than adjacent intervein cells, and 2) adjacent vein cells contain Cno-free adherens junction complexes, which are similarly relieved from symmetry constraints (Fig. 8C).
The formation of asymmetrical adhesive contacts in presumptive vein cells is coincident with changes in apical cell shape. We asked, therefore, how changes in DE-cad localization might affect vein-cell shape, and have proposed a simple model based on examinations of a timecourse of vein differentiation (Fig. 8). The balance between intercellular adhesion and cortical tension is a critical determinant of cell shape. Increased adhesion expands cell contacts, and cortical tension opposes this effect (Kafer et al., 2007; Lecuit and Lenne, 2007). Our data suggest that after ~24 h APF, vein-vein cell contacts are characterized by high levels of adhesion (i.e., DE-cad) and decreased levels of cortical tension (i.e., Cno, which links adherens junctions to the actin cytoskeleton). We hypothesize that these factors drive the expansion of vein-vein contacts at the expense of one vein-intervein cell contact (Fig. 8D), resulting in the formation of a pentagon (Fig. 8E). Real-time imaging of vein differentiation will be used in the future to test this model of morphogenesis.
The Egfr/Ras and Dpp signaling pathways act in concert to specify vein-cell fate. At 12–16 h APF, Egfr/Ras activity turns on dpp expression in presumptive vein cells (de Celis, 1997). After this stage of development, Dpp is required to maintain vein identity and high levels of Egfr/Ras signaling in presumptive vein cells (creating a positive feed-back loop) (Sotillos and De Celis, 2005). In contrast, these developmental signaling pathways have very different effects on cell adhesion and epithelial cell morphology. We have shown previously that Egfr/Ras activity up-regulates DE-cad levels in vein precursors, and that it does so in a Dpp-independent fashion (O’Keefe et al., 2007). Results presented here indicate that Egfr/Ras signaling also plays the dominant role in regulating Rap1/Cno. Two-cell clones that express RasV12 phenotypically resembled Rap1 loss-of-function cells (more so than TkvQ235D clones). In addition, RasV12 down-regulated the critical Rap1 effector Cno, whereas this effect was not evident in TkvQ235D-expressing cells. As loss of Cno disassociates actin-myosin contractility from cell shape (Sawyer et al., 2009), RasV12 two-cell clones were less apically constricted than TkvQ235D-expressing cells. Egfr/Ras signaling is also associated with asymmetric adhesive contacts in other developmental contexts. In the Drosophila eye, for example, Egfr/Ras signaling is required in photoreceptors. Much like vein cells, photoreceptors adhere more tightly to one another than to surrounding cells (Hayashi and Carthew, 2004; Mirkovic and Mlodzik, 2006). This raises the possibility that Egfr down-regulates Rap1 activity in multiple cell types following their specification, enabling them to differentiate appropriate cell shapes. Finally, it will be interesting to determine how the Egfr/Ras and Dpp signaling pathways regulate other aspects of vein-cell morphology (e.g., constriction along the apical/basal axis to generate a lumen).
In the wing, Egfr/Ras signaling does not affect Rap1/Cno activity at every developmental stage. High levels of Egfr/Ras signaling are detected in vein cells at the beginning of the third larval instar (Sturtevant et al., 1993), but vein/intervein cell-shape differences are not observed before ~24 h APF. As such, the Rap1/Cno complex likely represents a pupal-specific target of Egfr signaling. We have shown, therefore, that a single developmental signaling pathway can first determine a cell’s fate, and later contribute towards its morphological differentiation. Critical to this process, therefore, are genetic and/or epigenetic factors that temporally regulate the output of Egfr/Ras signaling. In the future it will be important to identify such factors not only for the Egfr/Ras pathway, but other developmental signaling pathways as well.
Finally, it is becoming increasingly clear that Rap1 affects cancer progression, often by promoting metastasis. In cancer cells, levels of Rap1 activity are typically high, which stimulates migration and metastasis by up-regulating integrin-based cell adhesion. Such is the case in pancreatic, prostate, and breast cancers (Bailey et al., 2009; McSherry et al., 2011; Ricono et al., 2009). However, loss of Rap1 can also cause metastasis by down-regulating cadherin and disrupting the epithelial integrity of the tumor (e.g, ovarian and prostate cancer) (Yajnik et al., 2003). Within this disease context, the Egfr/Ras and Rap1 signaling networks often interact. Most recently, Egfr activation of Rap1 has been shown to promote metastasis of human pancreatic carcinoma cells (Huang et al., 2011). The precise mechanisms by which Egfr/Ras signaling affects Rap1 activity (both during normal development and disease) must be deciphered, therefore, if we are to understand and/or mitigate these metastatic processes.
Supplementary Material
Two-cell clones of ectopic veins have fewer cell-cell contacts. Clones of cells were generated at 0 h APF. Wings were dissected at 36 h APF and labeled for DE-cad. Whereas clones over-expressing DE-cad resemble GFP controls, activated versions of both Tkv and Ras reduce cell-cell contacts (n = 19–140 cells per genotype).
Statistical analysis of adherens junction localization. Two-cell clones were generated at 0 h APF. Wings were dissected at 36 h APF and labeled for either DE-cad (A,C,I) or Cno (E,G,K). Fluorescence was quantified along green and purple vectors, which represent vein-intervein and vein-vein cell junctions, respectively. Both TkvQ235D (B) and RasV12 (D) significantly increase levels of DE-cad at vein-vein cell junctions (compared to vein-intervein junctions within the same cells). In contrast, TkvQ235D does not affect levels of Cno (F), whereas RasV12 down-regulates Cno at vein-vein cell junctions (H). Distributions of DE-cad (J) and Cno (L) within wild-type vein cells are shown. Graphs represent data from 5–10 images. Error bars indicate SEM. *p • 0.05, via the paired Student’s t-test.
Egfr signaling regulates adhesive asymmetries in vein cells. Two-cell clones expressing an activated version of Egfr (lambda-top) were generated at 0 h APF (GFP-positive). Wings were dissected at 36 h APF and labeled for DE-cad. High levels of DE-cad accumulate at boundaries between vein cells. Arrows indicate two clones that clearly demonstrate this phenotype.
Rap1 over-expression does not affect vein/intervein cell fate. Clones of cells were generated during larval stages. Wings were dissected at 36 h APF and labeled for DSRF. Over-expression of wild-type Rap1 does not affect DSRF.
Fluorescence intensity measurements along linear vectors, and at cell-cell junctions. (A,B) Pupal wing (36 h APF) labeled for DE-cad. Image is centered on vein L3. (A) Fluorescence intensity was measured along vectors drawn through vein (purple) and intervein (green) territories. (A′) Graph of fluorescence intensity is shown. (B) Fluorescence intensity was determined at cell junctions (*) within vein and intervein territories. (B′) Graph indicates average values of fluorescence intensity at cell junctions. Error bars indicate SEM. *p • 0.05, via the Student’s t-test.
Pupal wing (36 h APF) labeled for Cno. White lines indicate vectors (vein and intervein) along which fluorescence was measured. Graphs of data are shown in Fig. 6B,C.
RasV12 has opposite effects on DE-cad and Cno. (A–D) Clones expressing RasV12 were generated at 0 h APF. Wings were dissected at 36 h APF and labeled for DE-cad and Cno. Indicated channels from a single field of view are shown. At junctions between RasV12-expressing cells, DE-cad levels are elevated (C), while Cno levels are lower (D). Arrows indicate a pair of cells in which these effects are particularly dramatic.
Highlights.
In the Drosophila wing epithelium, vein cell precursors are not hexagonal.
Adherens junctions are asymmetrically distributed within presumptive vein cells.
Two-cell patches of ectopic vein tissue phenocopy Rap1 loss-of-function.
Rap1 over-expression disrupts cell-shape refinement of vein precursors.
The Egfr/Ras signaling pathway down regulates the Rap1 effector Cno in vein cells.
Acknowledgments
We would like to thank Benjamin Boettner, Iswar Hariharan, Daisuke Yamamoto, the Developmental Studies Hybridoma Bank, and the Bloomington Drosophila Stock Center for fly stocks and reagents. In addition, overdue thanks to Jeffrey Rasmussen for assistance with image analysis, and Savraj Grewal for pointing us in the right direction. Thanks to Kathy Hanley for help with statistics, Peter Cooke and Julio Vasquez for help with microscopy, and the entire Edgar and Curtiss labs for advice and entertainment throughout the process. Finally, thanks to Dino, Mr. O’s, and the Sanur night market campur for constant life-sustaining/enjoying nourishment. This work was supported by NCI 1 U54 CA132381 to FHCRC, NCI 1 U54 CA132383 to NMSU, and the National Science Foundation MRI-DBI-095817.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey CL, Kelly P, Casey PJ. Activation of Rap1 promotes prostate cancer metastasis. Cancer Res. 2009;69:4962–4968. doi: 10.1158/0008-5472.CAN-08-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner B, Govek EE, Cross J, Van Aelst L. The junctional multidomain protein AF-6 is a binding partner of the Rap1A GTPase and associates with the actin cytoskeletal regulator profilin. Proc Natl Acad Sci U S A. 2000;97:9064–9069. doi: 10.1073/pnas.97.16.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner B, Harjes P, Ishimaru S, Heke M, Fan HQ, Qin Y, Van Aelst L, Gaul U. The AF-6 homolog canoe acts as a Rap1 effector during dorsal closure of the Drosophila embryo. Genetics. 2003;165:159–169. doi: 10.1093/genetics/165.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL, de Bruyn K, Enserink J, Kuiperij B, Rangarajan S, Rehmann H, Riedl J, de Rooij J, van Mansfeld F, Zwartkruis F. The role of Rap1 in integrin-mediated cell adhesion. Biochem Soc Trans. 2003;31:83–86. doi: 10.1042/bst0310083. [DOI] [PubMed] [Google Scholar]
- Brown KE, Baonza A, Freeman M. Epithelial cell adhesion in the developing Drosophila retina is regulated by Atonal and the EGF receptor pathway. Dev Biol. 2006 doi: 10.1016/j.ydbio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Bryant PJ. Pattern formation in the imaginal wing disc of Drosophila melanogaster: fate map, regeneration and duplication. J Exp Zool. 1975;193:49–77. doi: 10.1002/jez.1401930106. [DOI] [PubMed] [Google Scholar]
- Buttitta LA, Katzaroff AJ, Perez CL, de la Cruz A, Edgar BA. A double-assurance mechanism controls cell cycle exit upon terminal differentiation in Drosophila. Dev Cell. 2007;12:631–643. doi: 10.1016/j.devcel.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Calleja M, Moreno E, Pelaz S, Morata G. Visualization of gene expression in living adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- Caron E. Cellular functions of the Rap1 GTP-binding protein: a pattern emerges. J Cell Sci. 2003;116:435–440. doi: 10.1242/jcs.00238. [DOI] [PubMed] [Google Scholar]
- Cela C, Llimargas M. Egfr is essential for maintaining epithelial integrity during tracheal remodelling in Drosophila. Development. 2006;133:3115–3125. doi: 10.1242/dev.02482. [DOI] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Cordero JB, Larson DE, Craig CR, Hays R, Cagan R. Dynamic decapentaplegic signaling regulates patterning and adhesion in the Drosophila pupal retina. Development. 2007;134:1861–1871. doi: 10.1242/dev.002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Celis JF. Expression and function of decapentaplegic and thick veins during the differentiation of the veins in the Drosophila wing. Development. 1997;124:1007–1018. doi: 10.1242/dev.124.5.1007. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Bray S, Garcia-Bellido A. Notch signalling regulates veinlet expression and establishes boundaries between veins and interveins in the Drosophila wing. Development. 1997;124:1919–1928. doi: 10.1242/dev.124.10.1919. [DOI] [PubMed] [Google Scholar]
- De Celis JF, Diaz-Benjumea FJ. Developmental basis for vein pattern variations in insect wings. Int J Dev Biol. 2003;47:653–663. [PubMed] [Google Scholar]
- Diaz-Benjumea FJ, Hafen E. The sevenless signalling cassette mediates Drosophila EGF receptor function during epidermal development. Development. 1994;120:569–578. doi: 10.1242/dev.120.3.569. [DOI] [PubMed] [Google Scholar]
- Fernandez BG, Arias AM, Jacinto A. Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech Dev. 2007;124:884–897. doi: 10.1016/j.mod.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A, Ripoll P, Morata G. Developmental compartmentalisation of the wing disk of Drosophila. Nat New Biol. 1973;245:251–253. doi: 10.1038/newbio245251a0. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Patel AB, Nagpal R, Perrimon N. The emergence of geometric order in proliferating metazoan epithelia. Nature. 2006;442:1038–1041. doi: 10.1038/nature05014. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–1789. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- Hariharan IK, Carthew RW, Rubin GM. The Drosophila roughened mutation: activation of a rap homolog disrupts eye development and interferes with cell determination. Cell. 1991;67:717–722. doi: 10.1016/0092-8674(91)90066-8. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- Hazelrigg T, Levis R, Rubin GM. Transformation of white locus DNA in drosophila: dosage compensation, zeste interaction, and position effects. Cell. 1984;36:469–481. doi: 10.1016/0092-8674(84)90240-x. [DOI] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;24:6690–6700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Anand S, Murphy EA, Desgrosellier JS, Stupack DG, Shattil SJ, Schlaepfer DD, Cheresh DA. EGFR-dependent pancreatic carcinoma cell metastasis through Rap1 activation. Oncogene. 2011 doi: 10.1038/onc.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert SS, Jacobsen TL, Muskavitch MA. Feedback regulation is central to Delta-Notch signalling required for Drosophila wing vein morphogenesis. Development. 1997;124:3283–3291. doi: 10.1242/dev.124.17.3283. [DOI] [PubMed] [Google Scholar]
- Jeon M, Zinn K. Receptor tyrosine phosphatases control tracheal tube geometries through negative regulation of Egfr signaling. Development. 2009;136:3121–3129. doi: 10.1242/dev.033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafer J, Hayashi T, Maree AF, Carthew RW, Graner F. Cell adhesion and cortex contractility determine cell patterning in the Drosophila retina. Proc Natl Acad Sci USA. 2007;104:18549–18554. doi: 10.1073/pnas.0704235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim FD, Rubin GM. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development. 1998;125:1–9. doi: 10.1242/dev.125.1.1. [DOI] [PubMed] [Google Scholar]
- Knox AL, Brown NH. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science. 2002;295:1285–1288. doi: 10.1126/science.1067549. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- Linnemann T, Geyer M, Jaitner BK, Block C, Kalbitzer HR, Wittinghofer A, Herrmann C. Thermodynamic and kinetic characterization of the interaction between the Ras binding domain of AF6 and members of the Ras subfamily. J Biol Chem. 1999;274:13556–13562. doi: 10.1074/jbc.274.19.13556. [DOI] [PubMed] [Google Scholar]
- Lundquist EA. Small GTPases. WormBook; 2006. pp. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major RJ, Irvine KD. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development. 2005;132:3823–3833. doi: 10.1242/dev.01957. [DOI] [PubMed] [Google Scholar]
- Major RJ, Irvine KD. Localization and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev Dyn. 2006;235:3051–3058. doi: 10.1002/dvdy.20966. [DOI] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSherry EA, Brennan K, Hudson L, Hill AD, Hopkins AM. Breast cancer cell migration is regulated through junctional adhesion molecule-A-mediated activation of Rap1 GTPase. Breast Cancer Res. 2011;13:R31. doi: 10.1186/bcr2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan M, Campuzano S, Garcia-Bellido A. Cell cycling and patterned cell proliferation in the Drosophila wing during metamorphosis. Proc Natl Acad Sci U S A. 1996;93:11687–11692. doi: 10.1073/pnas.93.21.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkovic I, Mlodzik M. Cooperative activities of drosophila DE-cadherin and DN-cadherin regulate the cell motility process of ommatidial rotation. Development. 2006;133:3283–3293. doi: 10.1242/dev.02468. [DOI] [PubMed] [Google Scholar]
- Montagne J, Groppe J, Guillemin K, Krasnow MA, Gehring WJ, Affolter M. The Drosophila Serum Response Factor gene is required for the formation of intervein tissue of the wing and is allelic to blistered. Development. 1996;122:2589–2597. doi: 10.1242/dev.122.9.2589. [DOI] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, de la Cruz AF, Johnston LA, Edgar BA. Coordination of growth and cell division in the Drosophila wing. Cell. 1998;93:1183–1193. doi: 10.1016/s0092-8674(00)81462-2. [DOI] [PubMed] [Google Scholar]
- Ninov N, Menezes-Cabral S, Prat-Rojo C, Manjon C, Weiss A, Pyrowolakis G, Affolter M, Martin-Blanco E. Dpp signaling directs cell motility and invasiveness during epithelial morphogenesis. Curr Biol. 2010;20:513–520. doi: 10.1016/j.cub.2010.01.063. [DOI] [PubMed] [Google Scholar]
- O’Keefe DD, Gonzalez-Nino E, Burnett M, Dylla L, Lambeth SM, Licon E, Amesoli C, Edgar BA, Curtiss J. Rap1 maintains adhesion between cells to affect Egfr signaling and planar cell polarity in Drosophila. Dev Biol. 2009;333:143–160. doi: 10.1016/j.ydbio.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe DD, Prober DA, Moyle PS, Rickoll WL, Edgar BA. Egfr/Ras signaling regulates DE-cadherin/Shotgun localization to control vein morphogenesis in the Drosophila wing. Dev Biol. 2007;311:25–39. doi: 10.1016/j.ydbio.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H, Uemura T, Harada Y, Iwai Y, Takeichi M. A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev Biol. 1994;165:716–726. doi: 10.1006/dbio.1994.1287. [DOI] [PubMed] [Google Scholar]
- Ohba Y, Ikuta K, Ogura A, Matsuda J, Mochizuki N, Nagashima K, Kurokawa K, Mayer BJ, Maki K, Miyazaki J, Matsuda M. Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. Embo J. 2001;20:3333–3341. doi: 10.1093/emboj/20.13.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignoni F, Zipursky SL. Induction of Drosophila eye development by decapentaplegic. Development. 1997;124:271–278. doi: 10.1242/dev.124.2.271. [DOI] [PubMed] [Google Scholar]
- Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279:35127–35132. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- Prober DA, Edgar BA. Ras1 promotes cellular growth in the Drosophila wing. Cell. 2000;100:435–446. doi: 10.1016/s0092-8674(00)80679-0. [DOI] [PubMed] [Google Scholar]
- Queenan AM, Ghabrial A, Schupbach T. Ectopic activation of torpedo/Egfr, a Drosophila receptor tyrosine kinase, dorsalizes both the eggshell and the embryo. Development. 1997;124:3871–3880. doi: 10.1242/dev.124.19.3871. [DOI] [PubMed] [Google Scholar]
- Ricono JM, Huang M, Barnes LA, Lau SK, Weis SM, Schlaepfer DD, Hanks SK, Cheresh DA. Specific cross-talk between epidermal growth factor receptor and integrin alphavbeta5 promotes carcinoma cell invasion and metastasis. Cancer Res. 2009;69:1383–1391. doi: 10.1158/0008-5472.CAN-08-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson B, White P, Vincent JP. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature. 1996;383:627–630. doi: 10.1038/383627a0. [DOI] [PubMed] [Google Scholar]
- Sawyer JK, Harris NJ, Slep KC, Gaul U, Peifer M. The Drosophila afadin homologue Canoe regulates linkage of the actin cytoskeleton to adherens junctions during apical constriction. J Cell Biol. 2009;186:57–73. doi: 10.1083/jcb.200904001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting K, Dahmann C. Hedgehog and Dpp signaling induce cadherin Cad86C expression in the morphogenetic furrow during Drosophila eye development. Mech Dev. 2008;125:712–728. doi: 10.1016/j.mod.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Schubiger M, Palka J. Changing spatial patterns of DNA replication in the developing wing of Drosophila. Dev Biol. 1987;123:145–153. doi: 10.1016/0012-1606(87)90436-2. [DOI] [PubMed] [Google Scholar]
- Shen J, Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–1790. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J Cell Biol. 2003;161:417–427. doi: 10.1083/jcb.200301133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotillos S, De Celis JF. Interactions between the Notch, EGFR, and decapentaplegic signaling pathways regulate vein differentiation during Drosophila pupal wing development. Dev Dyn. 2005;232:738–752. doi: 10.1002/dvdy.20270. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Sturtevant MA, Bier E. Analysis of the genetic hierarchy guiding wing vein development in Drosophila. Development. 1995;121:785–801. doi: 10.1242/dev.121.3.785. [DOI] [PubMed] [Google Scholar]
- Sturtevant MA, Roark M, Bier E. The Drosophilarhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes Dev. 1993;7:961–973. doi: 10.1101/gad.7.6.961. [DOI] [PubMed] [Google Scholar]
- Su L, Hattori M, Moriyama M, Murata N, Harazaki M, Kaibuchi K, Minato N. AF-6 controls integrin-mediated cell adhesion by regulating Rap1 activation through the specific recruitment of Rap1GTP and SPA-1. J Biol Chem. 2003;278:15232–15238. doi: 10.1074/jbc.M211888200. [DOI] [PubMed] [Google Scholar]
- Turner CM, Adler PN. Morphogenesis of Drosophila pupal wings in vitro. Mech Dev. 1995;52:247–255. doi: 10.1016/0925-4773(95)00405-p. [DOI] [PubMed] [Google Scholar]
- Widmann TJ, Dahmann C. Dpp signaling promotes the cuboidal-to-columnar shape transition of Drosophila wing disc epithelia by regulating Rho1. J Cell Sci. 2009;122:1362–1373. doi: 10.1242/jcs.044271. [DOI] [PubMed] [Google Scholar]
- Woods DF, Wu JW, Bryant PJ. Localization of proteins to the apico-lateral junctions of Drosophila epithelia. Dev Genet. 1997;20:111–118. doi: 10.1002/(SICI)1520-6408(1997)20:2<111::AID-DVG4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Xie Z, Huganir RL, Penzes P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron. 2005;48:605–618. doi: 10.1016/j.neuron.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yajnik V, Paulding C, Sordella R, McClatchey AI, Saito M, Wahrer DC, Reynolds P, Bell DW, Lake R, van den Heuvel S, Settleman J, Haber DA. DOCK4, a GTPase activator, is disrupted during tumorigenesis. Cell. 2003;112:673–684. doi: 10.1016/s0092-8674(03)00155-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-cell clones of ectopic veins have fewer cell-cell contacts. Clones of cells were generated at 0 h APF. Wings were dissected at 36 h APF and labeled for DE-cad. Whereas clones over-expressing DE-cad resemble GFP controls, activated versions of both Tkv and Ras reduce cell-cell contacts (n = 19–140 cells per genotype).
Statistical analysis of adherens junction localization. Two-cell clones were generated at 0 h APF. Wings were dissected at 36 h APF and labeled for either DE-cad (A,C,I) or Cno (E,G,K). Fluorescence was quantified along green and purple vectors, which represent vein-intervein and vein-vein cell junctions, respectively. Both TkvQ235D (B) and RasV12 (D) significantly increase levels of DE-cad at vein-vein cell junctions (compared to vein-intervein junctions within the same cells). In contrast, TkvQ235D does not affect levels of Cno (F), whereas RasV12 down-regulates Cno at vein-vein cell junctions (H). Distributions of DE-cad (J) and Cno (L) within wild-type vein cells are shown. Graphs represent data from 5–10 images. Error bars indicate SEM. *p • 0.05, via the paired Student’s t-test.
Egfr signaling regulates adhesive asymmetries in vein cells. Two-cell clones expressing an activated version of Egfr (lambda-top) were generated at 0 h APF (GFP-positive). Wings were dissected at 36 h APF and labeled for DE-cad. High levels of DE-cad accumulate at boundaries between vein cells. Arrows indicate two clones that clearly demonstrate this phenotype.
Rap1 over-expression does not affect vein/intervein cell fate. Clones of cells were generated during larval stages. Wings were dissected at 36 h APF and labeled for DSRF. Over-expression of wild-type Rap1 does not affect DSRF.
Fluorescence intensity measurements along linear vectors, and at cell-cell junctions. (A,B) Pupal wing (36 h APF) labeled for DE-cad. Image is centered on vein L3. (A) Fluorescence intensity was measured along vectors drawn through vein (purple) and intervein (green) territories. (A′) Graph of fluorescence intensity is shown. (B) Fluorescence intensity was determined at cell junctions (*) within vein and intervein territories. (B′) Graph indicates average values of fluorescence intensity at cell junctions. Error bars indicate SEM. *p • 0.05, via the Student’s t-test.
Pupal wing (36 h APF) labeled for Cno. White lines indicate vectors (vein and intervein) along which fluorescence was measured. Graphs of data are shown in Fig. 6B,C.
RasV12 has opposite effects on DE-cad and Cno. (A–D) Clones expressing RasV12 were generated at 0 h APF. Wings were dissected at 36 h APF and labeled for DE-cad and Cno. Indicated channels from a single field of view are shown. At junctions between RasV12-expressing cells, DE-cad levels are elevated (C), while Cno levels are lower (D). Arrows indicate a pair of cells in which these effects are particularly dramatic.