Abstract
Purpose
Viral infection is the most common aggravating factor for childhood asthma. Asthma may be a risk factor for severe respiratory symptoms in children with lower respiratory tract infections of viral etiology. Influenza A infection enhances Th2-polarization to house dust mites during the acute phase and leads to lung dysfunction in a mouse model. However, there are no data on the relationship between atopic sensitization and H1N1 (Influenza A) infection in humans. To investigate whether atopic sensitization is associated with the severity of H1N1 pneumonia, we compared clinical features and the atopic sensitization rate between children with and without H1N1 infection.
Methods
Using reverse transcription-polymerase chain reactions, we investigated H1N1 virus infection in 214 children who were hospitalized with high fever and respiratory symptoms from September 2009 to February 2010. We also performed immunoassays for total and specific IgEs to six common aeroallergens. Atopy was defined as positivity for more than one specific IgE. The clinical severity of pneumonia was evaluated based on intensive care unit admission, oxygen therapy, steroid therapy, and atelectasis.
Results
There were 70 H1N1-positive children, 42.9% of whom had pneumonia. Children with H1N1 infection were older and had a higher prevalence of atopic sensitization and pneumonia compared with H1N1-negative children. The rate of atelectasis was higher in children with H1N1 pneumonia than in children with non-H1N1 pneumonia. Among children with H1N1 viral infection, those with atopic sensitization had a higher prevalence of intensive care unit admission and oxygen therapy, and a longer duration of hospitalization than non-atopic children. There were no differences between atopic and non-atopic children without H1N1 viral infection.
Conclusions
The prevalence of H1N1-induced severe lower respiratory tract diseases is higher in children with atopic sensitization.
Keywords: H1N1 virus, atopy, pneumonia, severity, children
INTRODUCTION
An outbreak of pandemic influenza A (H1N1) virus infection during 2009-2010 was responsible for severe respiratory disease and deaths worldwide, especially in children and elderly people with chronic diseases.1 In Korea, following identification of the first case of novel influenza A (H1N1) on 2 May 2009, the virus spread at an unprecedented speed, resulting in elevation of the country's flu alert to "red," the highest level of the four-level scale, on 3 November 2009.
Although most influenza A virus infections result in self-limited, uncomplicated disease, higher rates of morbidity and mortality have been noted among children.2 Pneumonia is one of the most common complications in children hospitalized with influenza, and has been reported in 10%-26% of children hospitalized with influenza.3,4 In a recent study, 35% of influenza A (H1N1)-related deaths in children were due to pneumonia.5
Several studies have suggested that asthma may be a risk factor that worsens respiratory symptoms during pandemic H1N1 virus infection. Recent studies have reported that asthma was the most common condition present in both children (29%) and adults (27%) who were hospitalized due to H1N1 viral infection,6 and that asthma was a risk factor that aggravated respiratory conditions in patients with pandemic H1N1 influenza viral infection.7 In another study, asthma was identified as a significant independent risk factor, after controlling for age. Children with pandemic influenza H1N1 were more likely to have asthma than children with seasonal influenza (22% vs. 6%). Furthermore, 42% of the children with pandemic influenza who were admitted to the intensive care unit (ICU) had asthma. Of note, more than 50% of the asthmatic children with pandemic influenza presented with evidence of pneumonia, with or without bronchospasm.8
While asthma is a risk factor for respiratory infection, atopic sensitization may be a risk factor for more severe respiratory symptoms. In a prospective cohort study, atopic sensitization in children was associated with severe respiratory syncytial virus infection requiring hospitalization, compared with the control group (P=0.004).9 Another study suggested that patients with atopic conditions other than asthma were at increased risk for developing severe pneumococcal pneumonia, compared with patients without atopic conditions (adjusted Odd ratio [aOR], 2.13%; Confidence interval [CI], 1.04-4.35; P=0.048).10
Several studies have been conducted regarding the relationship between atopic sensitization and bacterial or viral infection. However, there are few data on the association between atopic sensitization and H1N1 viral infection. In this study, we compared the clinical features and atopic sensitization rate between children with H1N1 viral infection and children with non-H1N1 viral infection during a pandemic H1N1 infection period.
MATERIALS AND METHODS
Study subjects and design
We enrolled 214 children who were hospitalized with influenza-like symptoms in the Department of Pediatrics, Kangbuk Samsung Hospital from September 2009 to February 2010, during an outbreak of pandemic H1N1 virus infection. Influenza-like symptoms were defined as a temperature ≥37.8℃ and at least one of the following symptoms: cough, sore throat, rhinorrhea, myalgia, and headache. All patients were checked for H1N1 viral infection. Detection of the H1N1 virus was performed with nasopharyngeal aspirate samples using a real-time reverse-transcription polymerase chain reaction (RT-PCR) method (LG Life Sciences, Seoul, Korea). We did not perform other viral studies during this period.
We reviewed patient medical charts and collected data including age, sex, presenting symptoms, duration of hospitalization, family history of allergic diseases, and history of ever having been diagnosed with atopic dermatitis, allergic rhinitis, or asthma. We also examined atopic sensitization and chest X-ray findings. We defined severe respiratory illness in patients as being admitted to the ICU; requiring invasive or non-invasive mechanical ventilation; having oxygen saturation below 90% in room air, as measured by pulse oxymetry; and/or receiving systemic steroids for more than 3 days. Patients meeting any of the following criteria were excluded from the study: presence of malignancy, immunodeficiency, or congenital heart disease; presence of an alternative diagnosis during follow-up; and hospitalization during the preceding 72 hours.
A previous history of allergic diseases was defined as any previous diagnosis of asthma, allergic rhinitis, or atopic dermatitis by doctor. Patients were diagnosed with asthma on the basis of wheezing in the chest and the effectiveness of anti-asthmatic medicines such as β2-agonists. Pneumonia was defined as the presence of alveolar consolidation on chest X-ray. Pediatric radiologists interpreted all chest X-rays at the time of admission and classified pneumonia as interstitial, lobar, or bronchopneumonia. They also investigated the presence of parapneumonic effusion and atelectasis. We compared clinical features and atopic sensitization between H1N1-positive and H1N1-negative children and between children with H1N1 viral pneumonia and non-H1N1 pneumonia. We also compared respiratory severity according to atopic sensitization.
This study was approved by the institutional review board of the Kangbuk Samsung Hospital.
Laboratory tests
Blood samples were analyzed to determine white blood cell count, total eosinophil count, C-reactive protein level, total IgE, and allergen-specific IgE antibody. Total IgE concentration and specific IgE levels to six common aeroallergens (Dermatophagoides farina, Dermatophagoides pteronyssinus, cats, dogs, Alternaria, and cockroaches) were assayed using ImmunoCAP (Pharmacia Diagnostics, Uppsala, Sweden). Atopic sensitization was defined as at least one specific IgE level >0.35 IU/mL.
Statistical analysis
Statistical analysis was performed using STATA version 11.1 (StataCorp LP, College Station, TX, USA). Differences between groups were analyzed using Student's t-test and the χ2 test. Quantitative variables are expressed as means±SD. Logistic regression analysis was performed to determine the association between atopic sensitization and respiratory severity. aORs and 95% CIs were derived after adjusting for age, sex, and history of allergic diseases. Values of P<0.05 were considered to indicate statistical significance.
RESULTS
Clinical characteristics of H1N1 infection
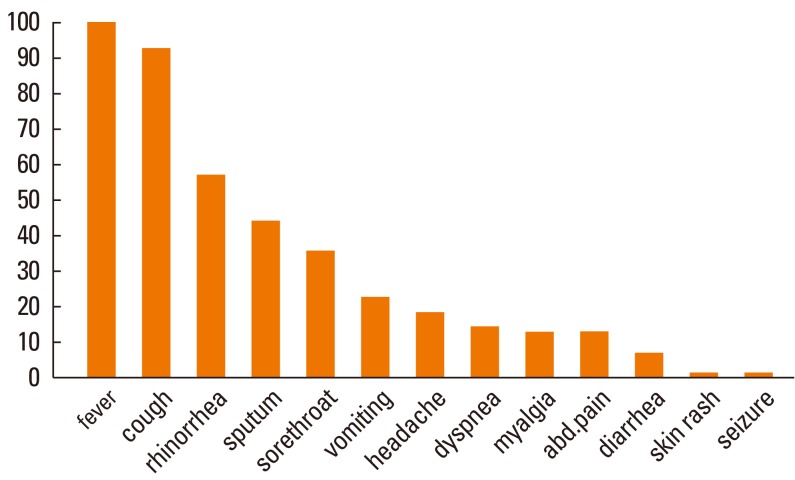
The clinical manifestations in the children with H1N1 viral infection are shown in Fig. 1. The most common presenting symptoms at admission were fever (100%) and cough (93%). Forty-two percent of the children had at least one gastrointestinal symptom such as vomiting, diarrhea, and abdominal pain. Dyspnea was reported in 14.2% of the patients, and less common symptoms included seizure and skin rash (each 1.42%).
Fig. 1.
Clinical features of children with H1N1 viral infection.
Comparison of clinical and laboratory characteristics between children with and without H1N1 infection
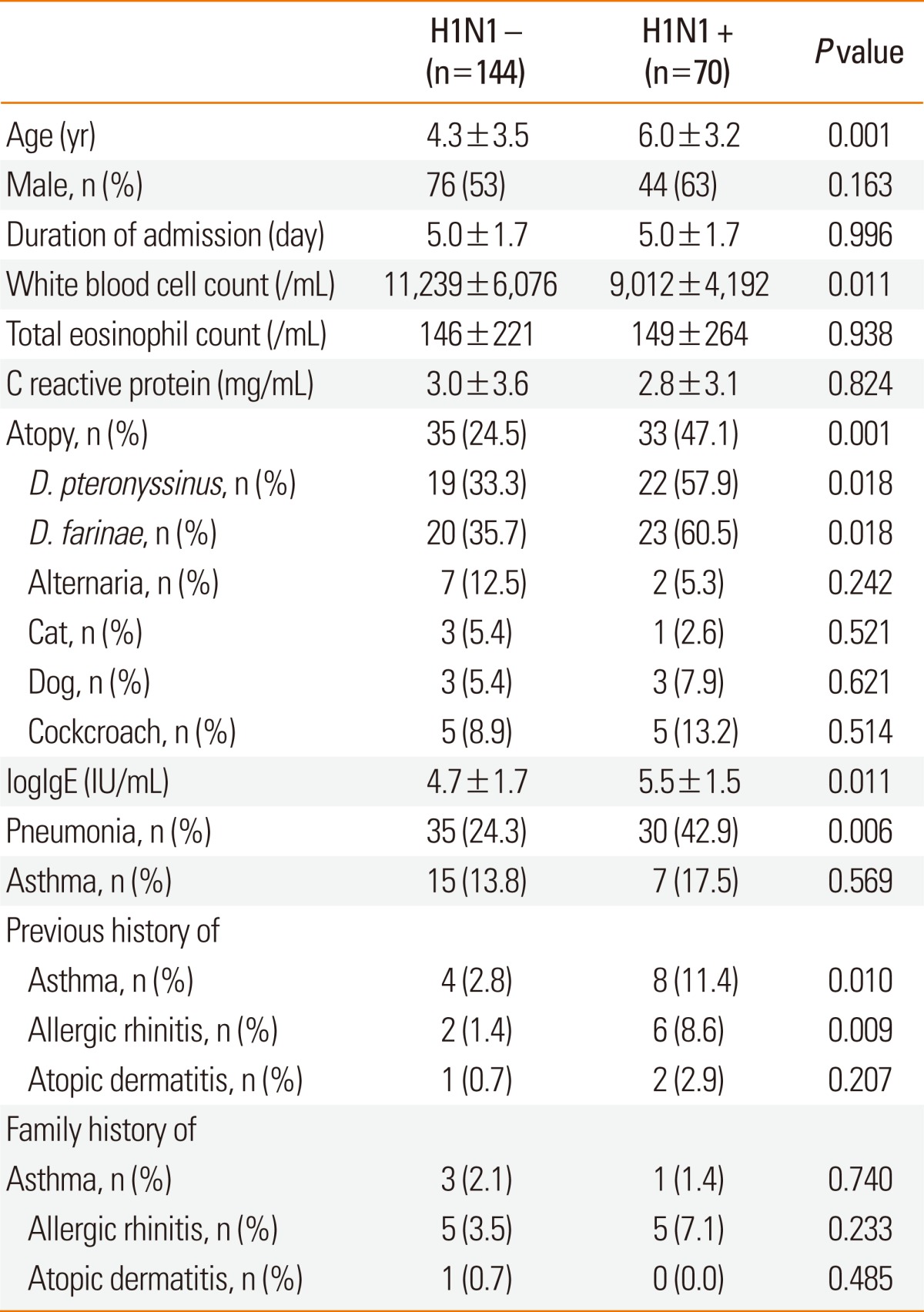
We compared the clinical and laboratory findings of children with and without H1N1 viral infection (Table 1). Among 214 study subjects, 70 children (32.7%) were confirmed to have H1N1 viral infection, and 144 children (67.3%) showed negative results. Patients with H1N1 viral infection were older and had lower white blood cell counts as compared with H1N1-negative children. The mean duration of hospitalization, blood total eosinophil count, and C-reactive protein level did not differ significantly between the two groups. Total IgE concentration, atopic sensitization rate, and positive sensitization rate to D. pteronyssinus and D. farinae were all higher in children with H1N1 infection than in children without H1N1 infection. However, there were no significant differences in the rates of positive sensitization to cats, dogs, Alternaria, and cockroaches. H1N1-positive children were more likely to have a previous history of asthma and allergic rhinitis than those without H1N1 infection, whereas history of atopic dermatitis and family history of asthma, allergic rhinitis, and atopic dermatitis were not significantly different between the groups. The prevalence of pneumonia was significantly higher in H1N1-positive children than in H1N1-negative children (42.9% vs. 24.3%, respectively). There was no significant difference in the prevalence of asthma between the two groups.
Table 1.
Comparison of clinical and laboratory characteristics between children with and without H1N1 infection
Comparison of clinical and laboratory characteristics between children with and without atopy
Sixty-eight children had atopic sensitization (31.8%). Children who had atopic sensitization were older, predominantly male, and had longer hospitalization stays and higher serum total IgE levels than children without atopic sensitization. The prevalence of pneumonia, asthma, and H1N1 infection increased in children with atopic sensitization compared with children without atopic sensitization. The prevalence of a previous history of asthma and atopic dermatitis was significantly higher in children with atopic sensitization than in children without atopic sensitization. However, there was no difference in the prevalence of a family history of allergic diseases (Table 2).
Table 2.
Comparison of clinical and laboratory characteristics between children with and without atopic sensitization
Comparison of respiratory severity and chest X-ray findings between H1N1 and non-H1N1 pneumonia
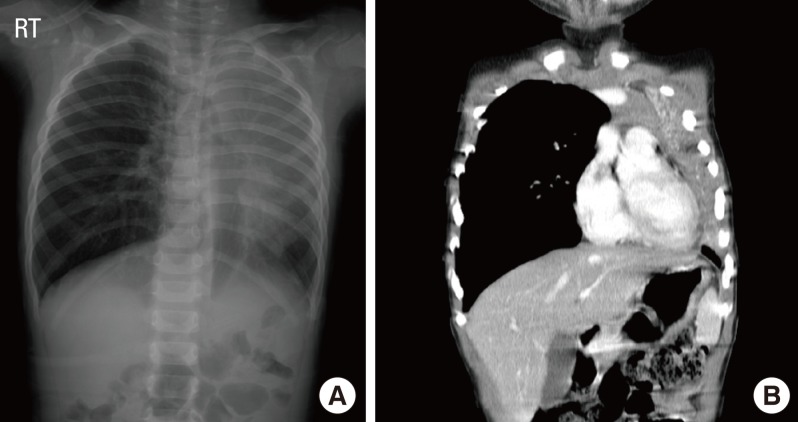
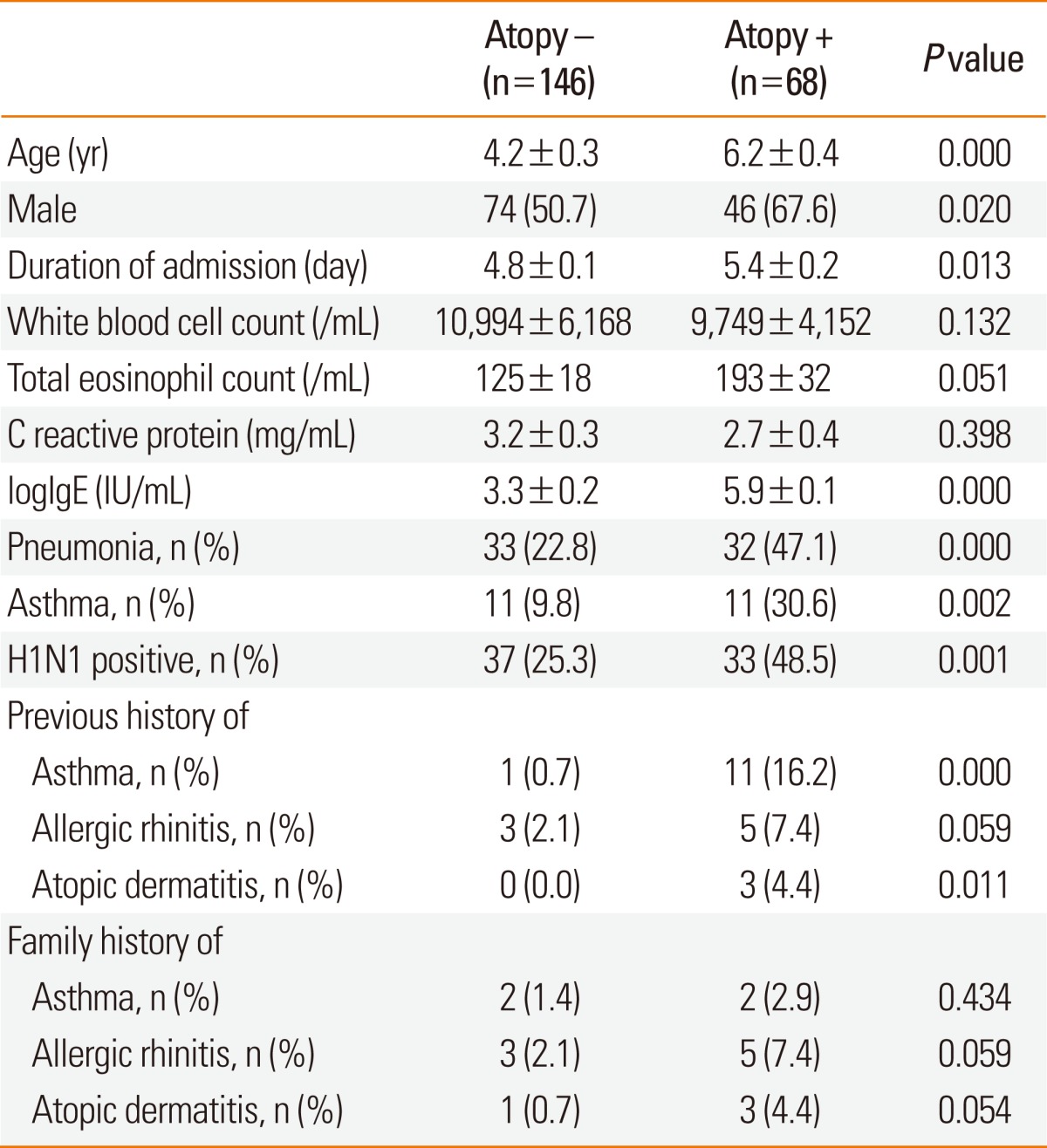
We next investigated respiratory severity and chest X-ray findings in children with pneumonia according to the presence of H1N1 infection (Table 3). The prevalence of pneumonia was 42.9% in children with H1N1 infection and 24.3% in children without H1N1 infection. There were no significant differences in location or types of pneumonia between H1N1-positive and non-H1N1 pneumonia. There was a higher prevalence of atelectasis in H1N1 pneumonia than in non-H1N1 pneumonia. Four children with H1N1 pneumonia demonstrated atelectasis, with one showing massive atelectasis with mediastinal shifting (Fig. 2). Children with H1N1 pneumonia tended to have higher rates of lobar pneumonia, effusion, ICU care, systemic corticosteroid therapy, and oxygen therapy than children with non-H1N1 pneumonia, although the differences in rates were not statistically significant. The rate of atopic sensitization was significantly higher in children with H1N1 pneumonia than in children with non-H1N1 pneumonia (63.3% vs. 37.1%; P=0.035), but the prevalence of a history of allergic diseases did not differ between the groups. None of the patients had organ failure or required mechanical ventilation.
Table 3.
Chest X-ray findings and clinical severity of children with H1N1 pneumonia and non-H1N1 pneumonia
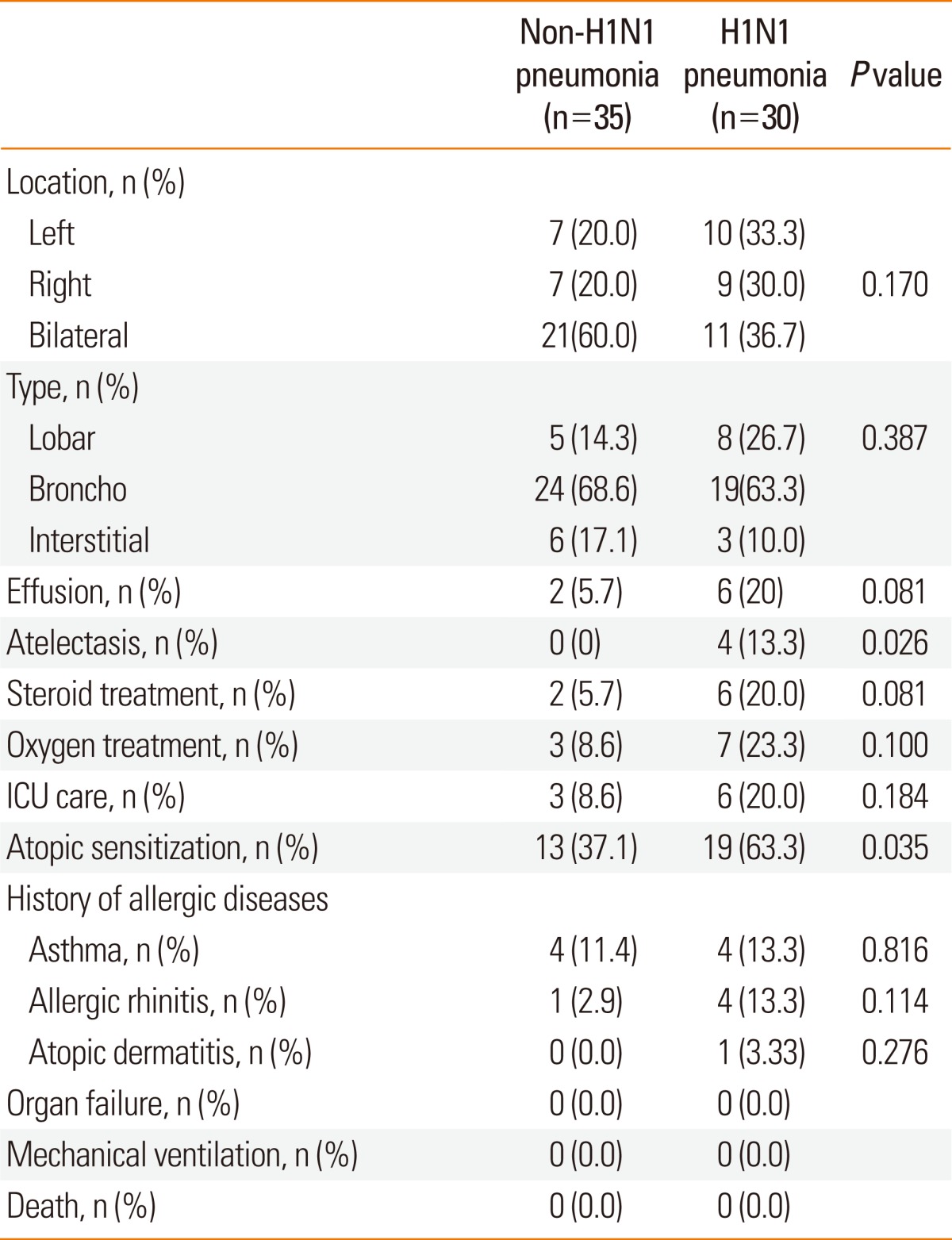
Fig. 2.
Radiographic findings of children with H1N1 pneumonia. (A) This chest X-ray shows atelectasis of the left upper lobe and consolidation in the left lower lobe. The mediastinal structure was markedly shifted toward the left side. (B) A chest CT scan demonstrates massive atelectasis of the left upper lobe, mediastinal shift to the left side, and pneumonic consolidation and pleural effusion in the left lower lobe.
Respiratory severity and laboratory findings in children with and without H1N1 viral infection according to atopic sensitization
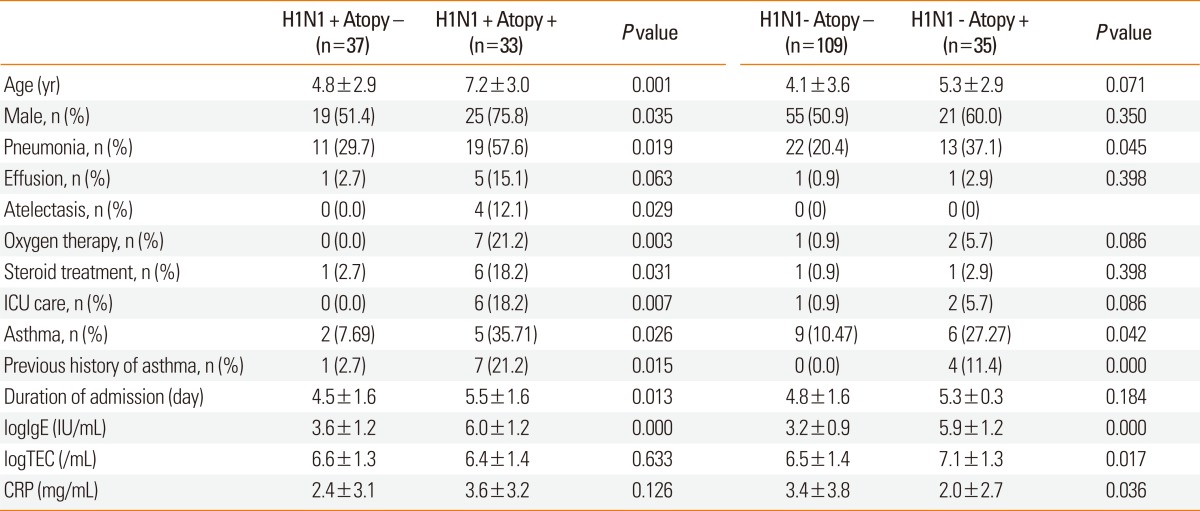
We compared respiratory severity according to atopic sensitization in children with and without H1N1 viral infection (Table 4). Atopic sensitization was present in 24.5% and 47.1% of the children without and with H1N1 viral infection, respectively. In the H1N1 group, children with atopic sensitization were older and had a longer duration of hospitalization than non-atopic children, whereas these parameters did not differ according to atopic sensitization in children in the non-H1N1 group. The prevalence of pneumonia was higher with atopic sensitization than without atopic sensitization in both the H1N1 (57.6% vs. 29.7%, respectively; P=0.019) and non-H1N1 groups (37.1% vs. 20.4%, respectively; P=0.045). However, compared with non-atopic children, ICU care, atelectasis, and oxygen therapy were more common in atopic children with H1N1 viral infection, but not in atopic children without H1N1 viral infection. Compared with children without atopy, more atopic children with H1N1 infection received systemic corticosteroid treatment (18.2% vs. 2.7%, respectively) and had parapneumonic effusion on chest radiography (15.1% vs. 2.7%, respectively). The mean serum total IgE level, prevalence of asthma, and previous history of asthma were also higher in children with atopic sensitization than in those without atopy. Atopic children without H1N1 viral infection showed higher blood eosinophil counts and lower C-reactive protein levels, whereas these parameters showed no significant differences in children with H1N1 viral infection.
Table 4.
Respiratory severity in children with or without H1N1 virus-infection according to atopic sensitization
CRP, C-reactive protein; ICU, intensive care unit; TEC, total eosinophil count.
DISCUSSION
In this study, pneumonia was more prevalent in hospitalized children with H1N1 viral infection than in children without H1N1 infection, showing similar results to those from another study that demonstrated the percentage of children with pneumonia or atelectasis was significantly higher in the pandemic H1N1 influenza infection group than in the seasonal influenza infection group.7 In a recent animal study using a ferret model, the presence of virus-infected cells was limited to the nasal turbinates in the seasonal influenza virus-inoculated model, whereas many infected cells were found in the trachea, bronchus, and bronchioles as well as the nasal cavity in ferrets inoculated with H1N1 influenza virus.11 This finding suggests that pandemic H1N1 influenza virus replicates more extensively in the lower respiratory tract, causing more severe lower respiratory tract diseases than those caused by seasonal influenza.
Several studies have proposed that asthma is a risk factor that worsens respiratory conditions in patients infected with pandemic H1N1 influenza.8,12 In this study, we also found that the prevalence of H1N1 infection was higher in children with a history of asthma and allergic rhinitis. However, in terms of pneumonia prevalence, there was no difference between children with and without asthma, whereas the atopic sensitization rate was higher in children with H1N1 pneumonia than in those with non-H1N1 pneumonia. In addition, among nine children who received ICU care, 16.7% with H1N1 pneumonia had a history of asthma compared with 66.7% with non-H1N1 pneumonia. Although the sample size was not sufficient to draw a firm conclusion, this observation may imply that a history of asthma may not be a risk factor for severe respiratory infection in children with H1N1 pneumonia.
Interestingly, the most striking finding in our study was that among children with H1N1 infection, children who had atopic sensitization showed more severe respiratory symptoms than children without atopic sensitization. Children with atopic sensitization had a higher prevalence of pneumonia and atelectasis, a longer duration of hospitalization, and an increased need for ICU care and oxygen therapy. In children with non-H1N1 infection, the prevalence of severe respiratory symptoms was not different between children with and without atopic sensitization. Therefore, we suggest severe pneumonia requiring ICU care and oxygen and steroid treatment is more likely to be related to atopic sensitization than to asthma in children with H1N1 virus infection. The pathogenesis may be explained by a different host cytokine expression profile and immune response to H1N1 virus. In a mouse study, the inhalation of ovalbumin after inoculation with H1N1 influenza virus produced a high level of specific IgE and increased airway responsiveness.13 However, neither ovalbumin sensitization alone nor virus infection alone affected IgE production and airway responsiveness. In another mouse study, bovine serum albumin sensitization during the acute phase of influenza A infection recruited eosinophils into the lung and enhanced IL-10 production from CD4+ T cells, thereby provoking Th2 responses.14 Thus, both virus factors and host immune factors appear to be important in IgE-mediated viral lower respiratory tract diseases, and atopic sensitization may be a prerequisite condition for virus-induced severe respiratory disease.
In this study, a higher proportion of atopic children with H1N1 viral infection had atelectasis and required ICU care, and oxygen and steroid therapy. This observation highlights the importance of influenza prevention through vaccination, especially in children with atopic sensitization, even when they have no obvious history of allergic diseases. A Japanese study also suggested that pandemic H1N1 viral infection could induce pneumonia and atelectasis in atopic children without a previous history of asthma.7 In addition, patients with atopy have been shown to exhibit low natural killer cell activity.15 We speculate that both an abnormality of innate immunity in atopic individuals and an affinity of the pandemic H1N1 virus for the respiratory tract may lead to increased susceptibility to severe lower respiratory tract diseases induced by H1N1 infection.
In our study, fever and cough were the most common symptoms of H1N1 infection, as in other studies.16,17 We found a high frequency (42.7%) of gastrointestinal symptoms such as vomiting and diarrhea, which are known to be more frequent among children than adults with influenza.17,18 Fortunately, no children with H1N1 pneumonia in our study required mechanical ventilation, had organ failure, or died during the pandemic H1N1 infection, because they received prompt treatment, including antiviral therapy, intravenous steroid treatment, and aggressive physiotherapy.
This study is limited by its retrospective design and small sample size. Due to a small sample size, we could not determine whether atopic sensitization itself, apart from asthma, was associated with H1N1 virus-induced severe lower respiratory tract diseases. Only a few association studies on atopic sensitization and clinical severity of pneumonia in H1N1-infected children have been performed, and further studies are needed to investigate the association and pathophysiological mechanism between viral infections and Th2 responses. Our study was also limited by an inability to differentiate primary pandemic influenza pneumonia cases from cases of secondary infections caused by other viruses or bacteria such as mycoplasma. This study only investigated H1N1 viral infection, and there is a need for studies examining other viral or mycoplasma infections that can cause pneumonia.
In conclusion, the results of the present study showed that the prevalence of H1N1 virus-induced severe lower respiratory tract diseases is higher in atopic children than in non-atopic children. From these results, it is highly recommended that children with atopic sensitization receive vaccination against influenza, especially during pandemic influenza viral infections.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Iskander M, Booy R, Lambert S. The burden of influenza in children. Curr Opin Infect Dis. 2007;20:259–263. doi: 10.1097/QCO.0b013e3280ad4687. [DOI] [PubMed] [Google Scholar]
- 3.Dawood FS, Fiore A, Kamimoto L, Nowell M, Reingold A, Gershman K, Meek J, Hadler J, Arnold KE, Ryan P, Lynfield R, Morin C, Baumbach J, Zansky S, Bennett NM, Thomas A, Schaffner W, Kirschke D, Finelli L Emerging Infections Program (EIP) Network. Influenza-associated pneumonia in children hospitalized with laboratory-confirmed influenza, 2003-2008. Pediatr Infect Dis J. 2010;29:585–590. doi: 10.1097/inf.0b013e3181d411c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrag SJ, Shay DK, Gershman K, Thomas A, Craig AS, Schaffner W, Harrison LH, Vugia D, Clogher P, Lynfield R, Farley M, Zansky S, Uyeki T Emerging Infections Program Respiratory Diseases Activity. Multistate surveillance for laboratory-confirmed, influenza-associated hospitalizations in children: 2003-2004. Pediatr Infect Dis J. 2006;25:395–400. doi: 10.1097/01.inf.0000214988.81379.71. [DOI] [PubMed] [Google Scholar]
- 5.Finelli L, Fiore A, Dhara R, Brammer L, Shay DK, Kamimoto L, Fry A, Hageman J, Gorwitz R, Bresee J, Uyeki T. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics. 2008;122:805–811. doi: 10.1542/peds.2008-1336. [DOI] [PubMed] [Google Scholar]
- 6.Mahut B, Refabert L, Marchac V, Iniguez JL, Aubertin G, Tamalet A, Lebras MN, Troadec C, Chatellier G, Delclaux C. Influenza-like illness responsible for severe exacerbations in asthmatic children during H1N1 pandemic: a survey before vaccination. J Asthma. 2011;48:224–227. doi: 10.3109/02770903.2011.555032. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa S, Hirano R, Hashimoto K, Haneda Y, Shirabe K, Ichiyama T. Characteristics of atopic children with pandemic H1N1 influenza viral infection: pandemic H1N1 influenza reveals 'occult' asthma of childhood. Pediatr Allergy Immunol. 2011;22:e119–e123. doi: 10.1111/j.1399-3038.2010.01090.x. [DOI] [PubMed] [Google Scholar]
- 8.O'Riordan S, Barton M, Yau Y, Read SE, Allen U, Tran D. Risk factors and outcomes among children admitted to hospital with pandemic H1N1 influenza. CMAJ. 2010;182:39–44. doi: 10.1503/cmaj.091724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stensballe LG, Kristensen K, Simoes EA, Jensen H, Nielsen J, Benn CS, Aaby P Danish RSV Data Network. Atopic disposition, wheezing, and subsequent respiratory syncytial virus hospitalization in Danish children younger than 18 months: a nested case-control study. Pediatrics. 2006;118:e1360–e1368. doi: 10.1542/peds.2006-0907. [DOI] [PubMed] [Google Scholar]
- 10.Jung JA, Kita H, Yawn BP, Boyce TG, Yoo KH, McGree ME, Weaver AL, Wollan P, Jacobson RM, Juhn YJ. Increased risk of serious pneumococcal disease in patients with atopic conditions other than asthma. J Allergy Clin Immunol. 2010;125:217–221. doi: 10.1016/j.jaci.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munster VJ, de Wit E, van den Brand JM, Herfst S, Schrauwen EJ, Bestebroer TM, van de Vijver D, Boucher CA, Koopmans M, Rimmelzwaan GF, Kuiken T, Osterhaus AD, Fouchier RA. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science. 2009;325:481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki S, Suzuki Y, Yamamoto N, Matsumoto Y, Shirai A, Okubo T. Influenza A virus infection increases IgE production and airway responsiveness in aerosolized antigen-exposed mice. J Allergy Clin Immunol. 1998;102:732–740. doi: 10.1016/S0091-6749(98)70012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato F, Nakazawa M, Yamamiya S, Tamura C, Hongo N, Hotta C, Minami M. Effect of BSA antigen sensitization during the acute phase of influenza A viral infection on CD11c+ pulmonary antigen presenting cells. Allergol Int. 2009;58:445–454. doi: 10.2332/allergolint.08-OA-0081. [DOI] [PubMed] [Google Scholar]
- 15.Jensen JR, Sand TT, Jørgensen AS, Thestrup-Pedersen K. Modulation of natural killer cell activity in patients with atopic dermatitis. J Invest Dermatol. 1984;82:30–34. doi: 10.1111/1523-1747.ep12259055. [DOI] [PubMed] [Google Scholar]
- 16.Jeon MH, Chung JW, Choi SH, Kim TH, Lee EJ, Choo EJ. Pneumonia risk factors and clinical features of hospitalized patients older than 15 years with pandemic influenza A (H1N1) in South Korea: a multicenter study. Diagn Microbiol Infect Dis. 2011;70:230–235. doi: 10.1016/j.diagmicrobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Launay E, Ovetchkine P, Saint-Jean M, Coïc L, Ducruet T, Charest H, Desmarais N, Lamarre V, Tapiéro B. Novel influenza A (H1N1): clinical features of pediatric hospitalizations in two successive waves. Int J Infect Dis. 2011;15:e122–e130. doi: 10.1016/j.ijid.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Friedman MJ, Attia MW. Clinical predictors of influenza in children. Arch Pediatr Adolesc Med. 2004;158:391–394. doi: 10.1001/archpedi.158.4.391. [DOI] [PubMed] [Google Scholar]