Abstract
Enterocyte fructose absorption is a tightly regulated process that precedes the deleterious effects of excess dietary fructose in mammals. Glucose transporter (GLUT)8 is a glucose/fructose transporter previously shown to be expressed in murine intestine. The in vivo function of GLUT8, however, remains unclear. Here, we demonstrate enhanced fructose-induced fructose transport in both in vitro and in vivo models of enterocyte GLUT8 deficiency. Fructose exposure stimulated [14C]-fructose uptake and decreased GLUT8 protein abundance in Caco2 colonocytes, whereas direct short hairpin RNA-mediated GLUT8 knockdown also stimulated fructose uptake. To assess GLUT8 function in vivo, we generated GLUT8-deficient (GLUT8KO) mice. GLUT8KO mice exhibited significantly greater jejunal fructose uptake at baseline and after high-fructose diet (HFrD) feeding vs. wild-type mice. Strikingly, long-term HFrD feeding in GLUT8KO mice exacerbated fructose-induced increases in blood pressure, serum insulin, low-density lipoprotein and total cholesterol vs. wild-type controls. Enhanced fructose uptake paralleled with increased abundance of the fructose and glucose transporter, GLUT12, in HFrD-fed GLUT8KO mouse enterocytes and in Caco2 cultures exposed to high-fructose medium. We conclude that GLUT8 regulates enterocyte fructose transport by regulating GLUT12, and that disrupted GLUT8 function has deleterious long-term metabolic sequelae. GLUT8 may thus represent a modifiable target in the prevention and treatment of malnutrition or the metabolic syndrome.
Type 2 diabetes mellitus and metabolic syndrome have reached epidemic proportions spanning both Western and developing societies, yet a full understanding of the underpinnings of these diseases remains elusive. It is apparent, however, that a multifactorial etiology, which includes a net positive energy balance upon a substrate of predisposing genetic factors, underlies these disorders. Positioned at the physical interface between environmental and host factors is the mammalian gastrointestinal tract, the single portal of entry through which environmentally derived nutrition enters the body. Specifically, the intestinal epithelium is the barrier site through which dietary nutrients, including protein, fats, and sugars, are absorbed or rejected. Recent data correlate increased fructose intake with the metabolic syndrome in humans (1, 2) and in rodents (3). Fructose absorption is a prelude to its numerous deleterious effects, thus, much attention has recently been directed toward understanding the mechanisms underlying mammalian fructose uptake and utilization (4–6).
Perhaps the most extensively studied enterocyte hexose transporters include glucose transporters (GLUT)2 and GLUT5 and the sodium-glucose cotransporter-1 (SGLT1). The classical paradigm of intestinal hexose absorption places GLUT5 and SGLT1 at the apical enterocyte membrane, where luminal fructose and glucose, respectively, are transported from the intestinal lumen into the enterocyte intracellular space (reviewed in Ref. 6). GLUT2 resides both in intracellular vesicles that translocate to the apical surface upon luminal nutrient presentation (7) and at the basolateral surface to facilitate glucose and fructose excursion into the portal circulation.
Perturbations in “classical” enterocyte hexose transporter protein abundance, such as GLUT5 and SGLT1, directly affect both hexose flux across the intestinal epithelium and whole-body metabolic homeostasis. Reduced enteric SGLT1 abundance via knockout of the T1R3 regulatory protein decreased the glucose flux rate in brush border membrane vesicles isolated from T1R3−/− mice fed high-glucose diets (8). Strikingly, enhanced enterocyte SGLT1 expression via knockout of the negative SGLT1 regulator, regulatory solute carrier protein family 1 member 1, produced mice that were obese, with 30% greater body weight, 80% increased total fat, and 30% increased serum cholesterol in the context of normal food intake when compared with wild-type (WT) mice (9). Moreover, GLUT5-deficient mice exhibited a malabsorptive phenotype and were resistant to fructose-induced hypertension (10). Together, these studies underscore the idea that gut mucosal hexose transport directly influences mammalian metabolic homeostasis.
In addition to the classical model of intestinal hexose transport, other hexose transporters are now known to be expressed in the intestine, including the facilitative glucose and fructose transporter, GLUT8 (11–14). In colonocytes and in enterocytes, GLUT8 localization was intracellular with a supranuclear distribution near the apical membrane (14). Previous studies, however, failed to characterize the in vivo functions of intestinal GLUT8 in enterocyte hexose handling.
Given that GLUT8 is a poorly understood intracellular GLUT expressed in enterocytes, and given the critical role of intestinal hexose transporters in maintaining global metabolic homeostasis, we tested the hypothesis that GLUT8 regulates intestinal hexose uptake and metabolic homeostasis in vivo. We demonstrate that GLUT8 deficiency enhances fructose uptake in cultured Caco2 human intestinal epithelial cells and in jejunum isolated from mice lacking the gene encoding GLUT8, Slc2a8. Moreover, mice lacking GLUT8 rapidly developed significantly higher serum fructose concentration after oral fructose gavage. We provide evidence that these effects may be mediated by stabilization of the dual-specificity glucose/fructose transporter, GLUT12. Ultimately, altered enterocyte fructose handling in GLUT8-deficient (GLUT8KO) mice correlated with relative hypertension, hyperinsulinemia, and hyperlipidemia after long-term high-fructose diet (HFrD) feeding when compared with high-fructose-fed WT mice. These results implicate GLUT8 as a critical regulator of intestinal fructose transport and of whole-body metabolic homeostasis.
Materials and Methods
Western blot analysis
Western blotting was performed as previously described (15). Primary antibodies were: GLUT5 (Acris Antibodies, San Diego, CA), GLUT8 (11), GLUT9b (16), or GLUT12 (17), each at 1:1000 dilutions in 5% milk and Tris-buffered saline with Tween 20. Secondary antibody was horseradish peroxidase-linked goat antirabbit antibody (1:10,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Secondary antibody was visualized using enhanced chemiluminescence reagent (Amersham, Piscataway, NJ).
Cell cultures and [14C]-fructose uptake assay
Caco2 cell cultures were obtained directly from the American Tissue Culture Center (ATCC) (Manassas, VA) and maintained in standard growth medium per ATCC specifications. Before assay, cells were split into six- or 24-well culture dishes and allowed to attain epithelial morphology with intercellular contacts. Cultures were then incubated in the presence or absence of GLUT8 adenovirus (Applied Biological Materials, Richmond, British Columbia, Canada), GLUT8 lentivirus (Sigma-Aldrich, St. Louis, MO), or fructose (Sigma-Aldrich)-containing medium before analysis. All transfections and hexose incubations before assay were performed in ATCC-recommended standard growth medium containing 5.6 mm d-glucose.
Radiolabeled fructose uptake in Caco2 cultures was measured according to established methods (18) with adaptations. Cells were hexose deprived for 10 min in Hank's balanced salt solution in a 37 C, 5% CO2 humidified incubator. The medium was changed to Ringer phosphate buffer containing 2 μm [14C]-d-fructose (American Radiochemicals, Inc., St. Louis, MO) for 2 min at 37 C. The reaction was quenched in ice-cold Hank's balanced salt solution and cultures were washed before lysis in 0.1 n NaOH and 0.1% sodium dodecyl sulfate solution. 70% of each lysate was counted in econosafe liquid scintillation fluid (Fisher Scientific, Waltham, MA). Total protein was quantified in each remaining aliquot by bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL) to normalize uptake for sample protein content. Adenovirus for green fluorescent protein (GFP) and GLUT8 was obtained from Applied Biological Materials. Scrambled and human GLUT8 lentiviruses were obtained from Sigma-Aldrich.
Mice
GLUT8KO mice were generated as described in Adastra (Adastra, K. L., A. I. Frolova, M. M. Chi, D. Cusumano, M. Bade, M. O. Carayannopoulos, and K. H. Moley, unpublished data) on the 129SVE background strain. GLUT12 transgenic (GLUT12TG) mice used for everted jejunal ring assays were generated as described (17). Mice were maintained in a pathogen-free barrier facility throughout all experiments. Female mice were used in the current studies. Mice were fed either a 60% fructose diet (Harlan Teklad, Madison, WI) with water containing 30% fructose or standard rodent chow and sterile water. Mouse weights and food consumed were measured weekly throughout their dietary intervention. Mice were maintained on a HFrD for 5 d before jejunal ring assay, for at least 18 wk before serum collection and insulin tolerance testing and for 24 wk at killing. All animal procedures were approved by the Washington University School of Medicine Animal Studies Committee.
Microvillous length measurements
Fixed, paraffin-embedded sections were stained with hematoxylin and eosin and visualized by standard light microscopy. Villous heights were measured using ImageJ software (version 1.44p).
Everted jejunal ring assay
Jejunal ring assay was performed as described (19, 20). Briefly, WT and GLUT8KO mice were maintained on a HFrD for 5 d before killing and assay. Small bowel was isolated via midline laparotomy, and the jejunum was excised in 2- to 4-mm rings. Adjacent rings were snap frozen or placed in 4% paraformaldehyde for later analysis. Jejunal rings from each animal were rapidly everted and washed in ice-cold Ringer phosphate buffer [115 mm NaCl, 25 mm NaHCO3, 1.2 mm MgCl2, 1.2 mm CaCl2, 2.4 mm K2HPO4, and 0.4 mm KH2PO4 (pH 7.3)]. Rings were transferred to oxygenated Ringer buffer at 37 C containing 37 kBq/ml [14C]-u-fructose or [3H]-2-deoxy-d-glucose (2DG) (American Radiochemicals, Inc.) for 2 min. The experimentally determined linear range of the assay was 2–10 min. The assay was quenched with 300 mm mannitol dissolved in ice-cold Ringer phosphate. Rings were washed in ice-cold Ringer phosphate before 1 h of extraction in 2.5% trichloroacetic acid (Sigma-Aldrich) in a 37 C water bath. The supernatant was counted in Econosafe liquid scintillation fluid (Fisher Scientific). Rings were desiccated overnight and weighed to normalize uptake for dry tissue weight.
Determination of serum fructose concentration
Chow-fed 8- to 12-wk-old WT and GLUT8KO mice were fasted overnight (16 h) before gavage of 2 mg/g body weight d-fructose (Sigma-Aldrich). Serum proteins were extracted in 33 mm HCl, neutralized, and placed in a Teflon well filled with 70% mineral oil, 30% hexadecane mixture, 50 mm Tris HCl (pH 8.1), 0.02% BSA, 1 mm MgCl2, 300 μm ATP, 4 μg/ml hexokinase, and 30 μg/ml fructose-6-phosphate kinase and maintained at room temperature before heat inactivation. Extracts were incubated in solution containing 100 mm Imid HCl (pH 7.0), 0.02% BSA, 2 mm EDTA (pH 7.0), 4 mm beta-mercaptoethanol, 2 mm Na2HAsO4, 600 μm nicotinamide adenine dinucleotide, 10 μg/ml aldolase, 10 μg/ml triose phosphate isomerase, and 100 μg/ml glyceraldehyde-3-phosphate dehydrogenase at room temperature before stopping the secondary reaction with 0.4 n NaOH at 80 C (30 min). An aliquot of this reaction mixture was amplified in nicotinamide adenine dinucleotide reagent (21) for 60 min before boiling and addition of Indicator reagent (21) and spectrophotometric measurement.
Confocal microscopy
Confocal microscopy was performed on 4% paraformaldehyde-fixed, paraffin-embedded microtomed intestinal sections or on methanol-fixed Caco2 cell cultures as previously described (16). GLUT12 and GLUT8 antibodies used for immunofluorescence were used at a dilution of 1:500 at 4 C overnight before incubation with antirabbit Alexa Fluor 488 conjugate (Santa Cruz Biotechnology, Inc.) secondary antibody and with TOPRO-3 iodide (Invitrogen, Carlsbad, CA).
Enterocyte enrichment
Mouse intestinal epithelial fractions were enriched precisely as described previously (22).
Serum insulin measurement
Serum was collected from overnight-fasted WT and GLUT8KO mice, and an aliquot was immediately taken for analysis using the Millipore (Billerica, MA) rat/mouse insulin ELISA kit precisely per manufacturer instructions.
Blood pressure determination
Noninvasive blood pressures on unanesthetized mice were obtained using a Columbus Instruments non-invasive blood pressure (Columbus, OH) tail-cuff and plethysmography device in conjunction with the Mouse Phenotyping Core at Washington University in St. Louis.
Statistical analysis
Unless otherwise indicated, assays described were performed a minimum of three times with identical results. Data are expressed as mean ± sem. Data were analyzed using two-tailed homoscedastic t tests with Bonferroni-Dunn post hoc correction when multiple comparisons were performed on datasets as indicated. P < 0.05 was defined as statistically significant.
Results
Fructose exposure down-regulates GLUT8 in Caco2 cultures
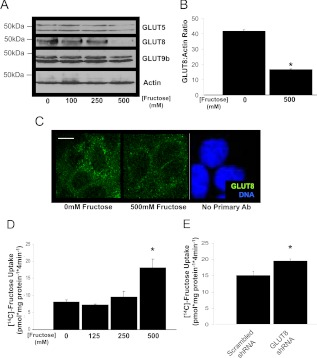
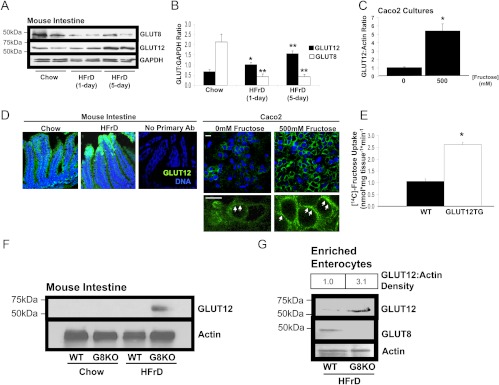
Previous studies demonstrated that luminal hexose exposure regulates enterocyte GLUT2, GLUT5, and SGLT1 protein accumulation. To determine whether fructose exposure regulates GLUT8 protein abundance, Caco2 human colon adenocarcinoma cell cultures were exposed to increasing fructose concentrations, lysed, and immunoblotted for GLUT8. High-fructose medium suppressed GLUT8 in a dose-dependent manner (Fig. 1A) without dramatic changes in GLUT5, GLUT9b, or actin. The average maximal suppression was approximately 70%, observed at 500 mm after 48 h of exposure (Fig. 1B).
Fig. 1.
Caco2 cell fructose exposure suppresses GLUT8 abundance and stimulates fructose uptake. A, Fructose exposure decreases Caco2 GLUT8 protein abundance. Caco2 cultures were incubated with or without d-fructose (48 h), lysed, and immunoblotted for GLUT5 (upper panel), GLUT8 (second panel), GLUT9b (third panel), and actin (bottom panel). B, Quantification of GLUT8:Actin band intensity by ImageJ densitometry. *, P < 0.05 vs. 0 mm group. C, Intracellular localization of GLUT8. Confocal images of Caco2 cultures treated with or without 500 mm d-fructose for 6 h and immunolabeled with GLUT8 primary antibody are shown. Cultures incubated without primary antibody are shown at right. Scale bars, 10 μm. Green represents GLUT8 signal. Blue represents nuclear staining. D, Fructose exposure stimulates Caco2 fructose uptake. Caco2 cells were incubated with or without fructose and radiolabeled fructose uptake was measured. *, P = 0.019 vs. 0 mm group. E, shRNA-mediated GLUT8 knockdown stimulates Caco2 fructose uptake. Caco2 cultures were transfected with lentivirus encoding scrambled shRNA or with virus encoding GLUT8 shRNA before measurement of radiolabeled fructose uptake. *, P < 0.05 vs. scrambled shRNA group. Ab, Antibody.
GLUT8 in intestinal epithelium was previously localized primarily intracellularly under basal conditions (15), but the effect of fructose exposure on GLUT8 localization was not explored. Therefore, we first assessed the effects of fructose exposure on GLUT8 localization by immunofluorescence and confocal microscopy. GLUT8 immunofluorescence was diminished in Caco2 cultures treated with 500 mm fructose. However, fructose exposure did not change cellular localization, and both groups exhibited punctate intracellular staining without appreciable plasma membrane accumulation (Fig. 1C).
To determine whether suppressed GLUT8 correlated with altered fructose transport in Caco2 cells, uptake of radiolabeled fructose was measured in fructose-exposed Caco2 cell cultures (Fig. 1D). Cultures exposed to 500 mm fructose exhibited a 124% (P < 0.02 vs. 0 mm group) increase in fructose uptake. As an index of comparison, a leading commercially available orange-flavored drink contains 140 g/liter or approximately 777 mm added carbohydrate, whereas a popular cola-flavored beverage contains approximately 108 g/liter or 600 mm added carbohydrate (the effective molarity encountered by the intestinal epithelium upon ingesting a solid oral carbohydrate load, such as an apple, is less easily calculated).
GLUT8 knockdown enhances enterocyte fructose uptake in vitro
Fructose exposure increased fructose uptake in correlation with suppressed GLUT8 protein abundance. To test the possibility that GLUT8 protein suppression per se stimulates enterocyte fructose uptake, Caco2 cultures were transfected with lentivirus encoding scrambled or GLUT8 short hairpin RNA (shRNA). Cultures transfected with GLUT8 shRNA exhibited a 43 ± 13% GLUT8 protein knockdown relative to scrambled shRNA cultures (P < 0.05 vs. scrambled shRNA-transfected cultures) (data not shown). Lentiviral transduction of GLUT8 shRNA significantly increased fructose uptake vs. scrambled shRNA-transduced cultures (P < 0.05) (Fig. 1E).
GLUT8KO baseline characteristics
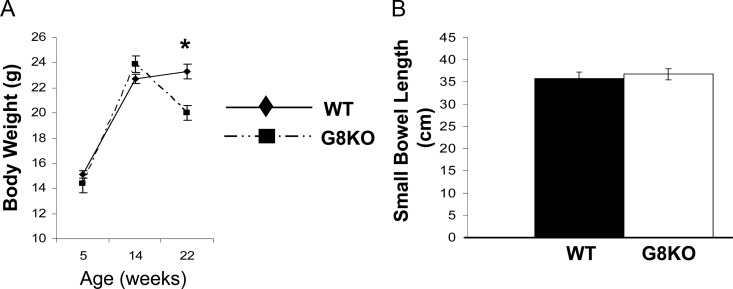
Given that GLUT8 knockdown enhanced enterocyte fructose uptake in vitro, mice globally deficient in Slc2a8, the gene encoding GLUT8 protein, were generated using a homologous gene recombination targeting strategy (herein referenced as GLUT8KO mice). GLUT8KO mice are live born and are fertile. However, crosses involving mice heterozygous or homozygous for the GLUT8KO allele produced slightly reduced litter sizes (Adastra, K. L., A. I. Frolova, M. M. Chi, D. Cusumano, M. Bade, M. O. Carayannopoulos, and K. H. Moley, unpublished data). GLUT8KO mice exhibited similar body weights at weaning (at 4–5 wk postpartum) and at 14 wk of age, but they were 12 ± 5% lighter at 22 wk of age (P < 0.01) (Fig. 2A). Despite decreased body weight, GLUT8KO mice at any age were grossly healthy, exhibiting normal coat color and consistency, normal stool consistency, as well as normal locomotion, social behavior, and feeding behavior in comparison with age-matched WT mice. Biochemically, GLUT8KO mice had normal fasting complete metabolic panels, indicating normal renal, biliary, and hepatic synthetic function testing (data not shown). Gross small bowel length (Fig. 2B) and microvillous height (data not shown) were identical in each group.
Fig. 2.
Mild body weight reduction and normal gross small bowel morphology in GLUT8KO mice. A, Body weights of chow-fed female WT mice and GLUT8KO (denoted G8KO in each figure) mice through 22 wk of age. Data points represent mean body weights at 5, 14, and 22 wk from n = 3, 9, and 8 GLUT8KO mice, respectively, and from n = 11, 9, and 8 WT mice, respectively. *, P < 0.01 vs. WT. B, Normal gross small bowel length in GLUT8KO mice; 4- to 8-wk-old WT and GLUT8KO mice maintained on standard rodent chow were killed, and small bowel was resected from pylorus to terminal ileum and measured. Shown is the mean small intestine length from n = 7 samples per genotype.
Enhanced fructose uptake in GLUT8KO mouse jejunum
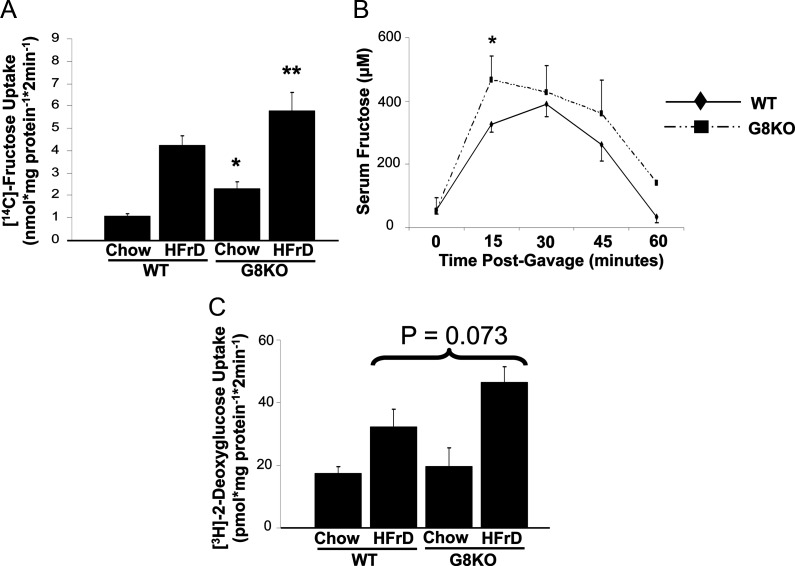
To characterize the role of GLUT8 in small intestinal fructose uptake in vivo, GLUT8KO mice were fed a HFrD for 5 d, after which radiolabeled fructose uptake was measured in isolated jejunal rings. Daily chow consumption did not differ in WT vs. GLUT8KO mice on a long- or short-term HFrD (data not shown), and GLUT8 deletion had no effect on microvillous height after 5-d HFrD exposure (data not shown). However, in the absence of changes in microscopic villous architecture, GLUT8KO mice exhibited elevated basal (119% increase, P < 0.001) and fructose-stimulated (37% increase, P < 0.05) jejunal fructose uptake when compared with WT controls (Fig. 3A). To determine whether enhanced enterocyte fructose uptake yielded increased fructose excursion into the systemic circulation of GLUT8KO mice, 2 g/kg oral fructose gavages were performed in age-matched WT and GLUT8KO mice, followed by serum collection 0, 15, 30, and 60 min after gavage. Serum fructose concentrations were significantly greater (+43.3%, P = 0.01) (Fig. 3B) 15 min after gavage, and this trend toward greater excursion continued throughout the assay time course (Fig. 3B). Although jejunal 2DG uptake was similarly stimulated by HFrD feeding in both genotypes, there was no significant difference in basal or fructose-stimulated 2DG uptake when comparing WT and GLUT8KO mouse jejunum (Fig. 3C). Taken together, these data suggest that GLUT8 is required for normal enterocyte fructose handling, but GLUT8 is dispensible for normal enterocyte glucose handling.
Fig. 3.
Enhanced jejunal fructose uptake in HFrD-fed GLUT8KO mice. A, Enhanced baseline and HFrD-stimulated jejunal fructose uptake in GLUT8KO mice. WT and GLUT8KO mice were fed chow or HFrD for 5 d, and [14C]-fructose uptake was measured. *, P < 0.001 vs. chow-fed WT group; **, P < 0.05 vs. HFrD-fed WT group. B, Increased serum fructose excursion in GLUT8KO mice. WT and GLUT8KO mice were fasted overnight and orally gavaged with unlabeled fructose. Shown is the mean serum fructose concentration at each time point. C, GLUT8 deficiency does not alter baseline or fructose diet-stimulated jejunal 2DG uptake. WT and GLUT8KO mice were fed chow or HFrD for 5 d, killed, and [3H]-2DG uptake in resected jejunal rings was measured. Bars represent pooled mean 2DG uptake from two experiments, n = 10–15 rings per group. P = Not significant for chow-fed GLUT8KO vs. chow-fed WT and for HFrD-fed GLUT8KO vs. HFrD-fed WT groups.
GLUT8 reconstitution in vivo suppresses jejunal fructose uptake
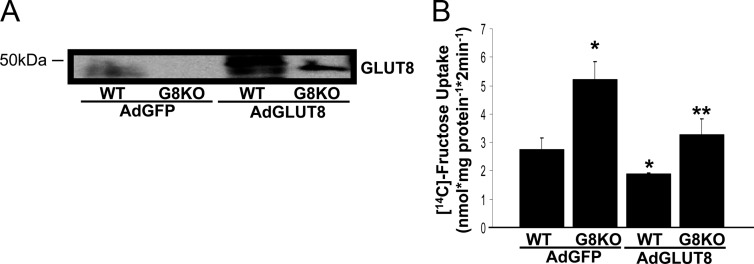
We next measured fructose uptake in WT and GLUT8KO jejunum after reconstituting GLUT8 in the small bowel to test the hypothesis that GLUT8 regulates a rate-limiting step in enterocyte fructose uptake. Orogastric gavage with adenovirus encoding human GLUT8 (AdGLUT8) before HFrD exposure increased jejunal GLUT8 protein abundance (Fig. 4A). Reconstitution of small intestinal GLUT8 significantly suppressed both WT and GLUT8KO jejunal fructose uptake when compared with mice transduced with GFP-encoding adenovirus (Fig. 4B). Jejunal 2DG uptake, however, was unaffected by jejunal GLUT8 reconstitution (data not shown). These data indicate that GLUT8 suppresses enterocyte fructose, but not 2DG, uptake and that dysregulated fructose handling in GLUT8KO small bowel is acutely reversible by GLUT8 reconstitution.
Fig. 4.
GLUT8 reconstitution in mouse jejunum normalizes HFrD-fed GLUT8KO jejunal fructose uptake. A, Adenoviral reconstitution of GLUT8 protein in mouse jejunum. WT and GLUT8KO mice orally gavaged with adenovirus (Ad) encoding GFP or GLUT8 per 20 g of body weight and fed HFrD for 24 h before jejunal ring excision and immunoblot analysis for GLUT8. Shown is a representative immunoblot of three experiments with similar results. B, Normalized fructose uptake after adenoviral reconstitution of GLUT8 in mouse jejunum. Shown is the mean fructose uptake. *, P < 0.01 vs. WT AdGFP group by two-tailed t test with Bonferroni-Dunn post hoc correction. **, P < 0.01 vs. GLUT8KO AdGFP group.
GLUT8 regulates fructose-induced accumulation of the GLUT12 glucose/fructose transporter
GLUT8 is an intracellular transporter that suppresses enterocyte fructose uptake. We therefore hypothesized that fructose regulates the abundance and cell-surface localization of another enterocyte fructose transporter in a GLUT8-regulated manner. Among the transmembrane hexose transporters with fructose transport capacity, GLUT12 is indeed expressed in the mammalian intestine (23). Moreover, GLUT12 is plasma membrane localized in multiple cell types (17); the abundance and localization of enterocyte GLUT12 in response to HFrD, however, was not yet known. We thus fed age-matched WT mice chow or HFrD for up to 5 d before GLUT12 immunoblot analysis (Fig. 5A). HFrD stimulated an approximately 3-fold increase in GLUT12 abundance in mouse intestinal homogenates after 5 d of HFrD (P < 0.01 vs. chow-fed mice), and this was associated with significant suppression of GLUT8 abundance under identical conditions (P < 0.01 vs. chow-fed mice) (Fig. 5B). We then attempted to model fructose-stimulated GLUT12 accumulation in vitro, and indeed, incubation in fructose-containing culture medium stimulated a 5-fold increase in Caco2 GLUT12 abundance (Fig. 5C). This phenomenon did not occur in the context of increased osmolarity, however, because incubation with 500 mm l-glucose did not alter Caco2 GLUT12 protein levels (data not shown).
Fig. 5.
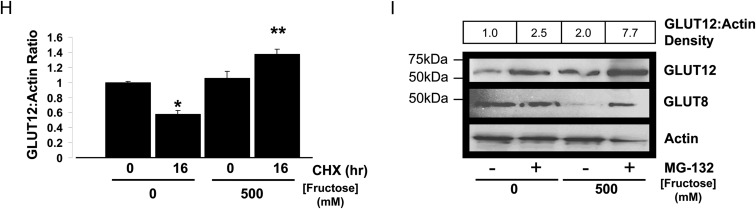
GLUT8 regulates fructose-induced GLUT12 accumulation. A, Inverse fructose-induced regulation of GLUT8 and GLUT12 abundance. Crude intestinal lysates from WT mice fed chow or HFrD (1–5 d) were immunoblotted for GLUT8 (upper panel) GLUT12 (middle panel), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (lower panel). Immunoblots represent at least three similar experiments with identical results. B, Pooled densitometric quantification of GLUT12 (black bars) and GLUT8 bands (white bars) from chow- and HFrD-fed WT mice. *, P < 0.05 vs. chow-fed group; **, P < 0.01 vs. chow-fed group. C, Caco2 cultures were incubated with or without fructose, lysed, and immunoblotted for GLUT12 and actin. Data are mean GLUT12:Actin density ratios n = 3 per group. *, P < 0.05 vs. 0 mm fructose group. D, GLUT12 is localized in jejunal epithelium and at the cell surface of Caco2 cultures. Left, Mice fed chow or HFrD were killed, and jejunal sections were subjected to GLUT12 immunofluorescence and confocal microscopy. Right, Caco2 cultures incubated with or without 500 mm fructose were subjected to GLUT12 immunofluorescence and confocal microscopy. Green represents GLUT12 signal. Blue represents nuclear staining. Scale bars, 10 μm. E, GLUT12 overexpression increases mouse jejunal fructose uptake. Bars represent the mean radiolabeled fructose uptake in WT and GLUT12TG mouse jejunum. *, P < 0.001 by two-tailed t testing. F, GLUT8 suppresses HFrD-induced GLUT12 up-regulation. WT and GLUT8KO mice were fed chow or HFrD diets for 5 d and killed before lysis of crude jejunum and immunoblotting against GLUT12 (upper panel) and against actin (lower panel). G, GLUT8 suppresses HFrD-induced GLUT12 in enterocyte-enriched fractions. Enterocyte fractions from WT and GLUT8KO mice fed chow or HFrD were enriched by differential centrifugation of collagenase-dispase jejunal digests. Enterocyte fraction lysates were immunoblotted for GLUT12 (upper panel), stripped, and reprobed for GLUT8 (middle panel) and actin (lower panel). H, De novo protein synthesis is not required for fructose-induced GLUT12 accumulation. Caco2 cultures were incubated with or without 10 μg/ml cycloheximide (CHX) in the presence or absence of 500 mm fructose. Lysates were immunoblotted for GLUT12 and for actin. Shown are mean GLUT12:Actin density ratios. *, P < 0.05 vs. control cultures; **, P < 0.05 vs. fructose-treated alone. I, 26S proteasomal blockade enhances fructose-induced GLUT12 accumulation. Caco2 cultures were incubated with or without 20 μm MG-132 for 6 h in the presence or absence of 500 mm fructose, lysed, and immnoblotted for GLUT8 (upper panel) and GLUT12 (middle panel). Blots were stripped and reprobed for actin (lower panel) as a loading control. Ab, Antibody.
To determine whether fructose feeding altered intestinal epithelial GLUT12 localization or abundance, jejuna from WT mice fed chow or HFrD for 5 d were sectioned, immunolabeled for endogenous GLUT12, and subjected to confocal microscopy. GLUT12 exhibited enterocyte-specific immunofluorescence, and in concordance with immunoblotting data, GLUT12 immunofluorescence increased after HFrD feeding (Fig. 5D, left panels). Moreover, GLUT12 in unstimulated Caco2 cultures exhibited an immunofluorescence pattern consistent with intracellular and cell-surface localization (Fig. 5D, right panels, arrows), whereas 500 mm fructose incubation enhanced the immunofluorescence signal at the cell periphery (Fig. 5D, right panels, arrows).
We next assessed the hexose transport capabilities of GLUT12 in vivo. Previous studies showed GLUT12-transported glucose in oocytes, and this transport is inhibited by d-fructose, d-glucose, and 2DG (24). To directly test whether GLUT12 is an in vivo enterocyte fructose transporter, we compared radiolabeled fructose and 2DG uptake in jejunum isolated from chow-fed WT mice or from mice globally overexpressing murine GLUT12 (GLUT12TG mice) (17). No significant changes in 2DG uptake were observed (data not shown). However, GLUT12TG mice exhibited a 2.5-fold increase in jejunal fructose uptake vs. WT mice (P < 0.001) (Fig. 5E). In vitro adenovirus-mediated GLUT12 overexpression in isolated Caco2 cultures also significantly increased fructose uptake vs. GFP-transfected Caco2 cultures (data not shown), suggesting that GLUT12 is a rate-limiting enterocyte fructose transporter both in vitro and in vivo.
GLUT12 is an enterocyte fructose transporter that is up-regulated in concert with fructose-induced GLUT8 suppression. We tested whether GLUT8 is required for normal enterocyte GLUT12 regulation. GLUT2 and GLUT5 protein levels were not statistically different when comparing immunoblots from HFrD-fed WT and GLUT8KO-enriched enterocyte lysates (data not shown). In addition, GLUT2, GLUT5, and GLUT12 mRNA abundances were identical as determined by quantitative RT-PCR analysis in HFrD-fed WT vs. GLUT8KO mice (data not shown). In contrast, immunoblot analysis revealed increased GLUT12 protein in GLUT8KO mouse crude jejunal lysates (Fig. 5F) and in GLUT8KO-enriched enterocyte fractions (Fig. 5G) after HFrD feeding vs. HFrD-fed WT mice. Taken together, fructose exposure induces GLUT12 in an enterocyte-autonomous manner, and this up-regulation is attenuated by GLUT8.
We next sought to determine whether de novo protein synthesis was required for fructose-induced GLUT12 up-regulation in Caco2 cultures. In fructose-unexposed cultures, the protein synthesis inhibitor cycloheximide decreased GLUT12 abundance over 16 h of incubation (Fig. 5H). In contrast, GLUT12 protein increased significantly in the presence of cycloheximide and 500 mm fructose medium (Fig. 5H), suggesting that de novo protein synthesis did not fully account for GLUT12 accumulation. Moreover, GLUT12 protein abundance increased when Caco2 cultures were incubated with the proteasomal inhibitor MG-132 alone (Fig. 5I, upper panel), and this increase was enhanced in the presence of 500 mm fructose. GLUT8 was conversely down-regulated when cultures were incubated with 500 mm fructose alone, but the proteasomal inhibitor MG-132 attenuated fructose-induced GLUT8 degradation (Fig. 5I, middle panel). Together, these data suggest that GLUT12 exists in a dynamic equilibrium of synthesis and proteasomal degradation that is regulated by fructose.
Worsened metabolic syndrome-like state in long-term HFrD-fed GLUT8KO mice
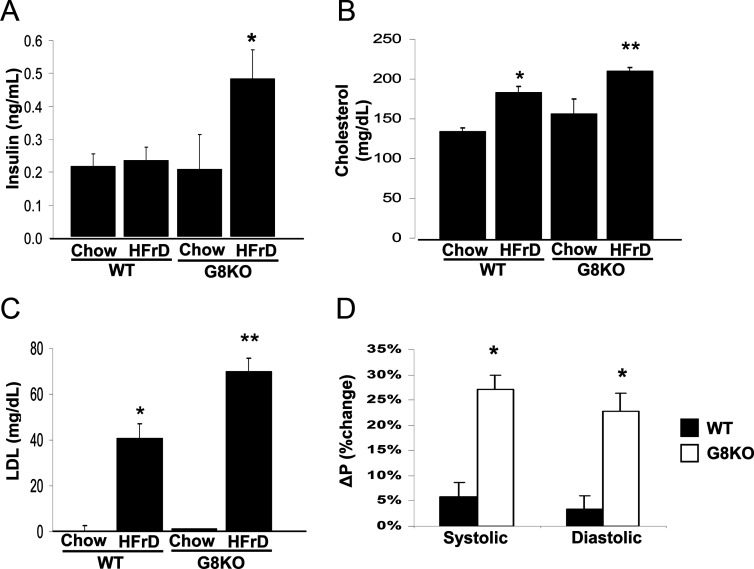
Mice fed a chronic HFrD develop a metabolic syndrome-like state, featuring obesity, hyperlipidemia, and insulin resistance (3). Our in vitro and in vivo models demonstrated that GLUT8 deficiency resulted in enhanced fructose uptake across the enterocyte membrane and excursion of fructose into the serum. To elucidate the long-term metabolic sequelae of HFrD feeding in GLUT8KO mice, 12-wk-old WT and GLUT8KO mice were thus initiated on a 24-wk chow or HFrD intervention. All groups consumed similar food and water quantities (data not shown) and gained significant weight vs. congenic, chow-fed mice. However, no significant differences in final weight or in weight change from baseline were observed between HFrD-fed WT and GLUT8KO groups throughout the intervention period (data not shown). Fasting insulin was more than 2-fold greater in GLUT8KO mice (+108%, P < 0.05) on HFrD vs. similarly treated WT mice (Fig. 6A). In accordance with these data, a modest trend toward insulin resistance by insulin tolerance testing in HFrD-fed GLUT8KO mice was observed in comparison with HFrD-fed WT mice, but total area under the blood glucose-vs.-time curve did not reach statistical significance (P = 0.08; data not shown). Fasting serum lipid panels in WT mice fed HFrD each yielded significant increases in total cholesterol and low-density lipoprotein (LDL) when compared with chow-fed WT mice (Fig. 6, B and C, respectively). GLUT8KO mice, however, had a more pronounced response to chronic HFrD when compared with HFrD-fed WT mice, featuring higher total cholesterol and LDL vs. WT HFrD-fed mice (Fig. 6, B and C). Additionally, HFrD induced a significantly greater elevation in systolic blood pressure (SBP) and diastolic blood pressure (DBP) in GLUT8KO mice vs. WT mice (Fig. 6D). In WT mice, fructose feeding increased mean SBP and DBP by 5.8 and 3.3%, respectively. SBP and DBP in GLUT8KO mice increased by 27.2 and 22.7%, respectively. No significant differences in baseline SBP or DBP were observed between WT and GLUT8KO mice (data not shown). Serum triglycerides, alanine aminiotransferase and aspartate aminotransferase (markers of hepatocyte damage), and C-reactive protein (a general inflammatory marker) were unchanged in WT and GLUT8KO mice fed HFrD (data not shown). Together, these results demonstrate a relative hypertension, hyperinsulinemia, and dyslipidemia in HFrD-fed GLUT8KO mice. GLUT8 is therefore required to attenuate HFrD-induced metabolic syndrome.
Fig. 6.
Exacerbated fasting hyperinsulinemia, hyperlipidemia, and relative hypertension in GLUT8KO mice (G8KO). A, WT and GLUT8KO mice were maintained on chow or on HFrD for 18 wk and fasted overnight. Serum was collected, and insulin was measured as in experimental procedures. Shown is the mean insulin concentration ± se for n = 5–8 mice in each group. *, P < 0.05 vs. WT HFrD group. B and C, Exacerbated hyperlipidemia in HFrD-fed GLUT8KO mice. Graphed in B and C are the mean total cholesterol and calculated LDL concentrations, respectively, for chow- and HFrD-fed WT and GLUT8KO mice. n = 9–11 mice per group. *, P < 0.05 vs. WT chow-fed group; **, P < 0.05 vs. HFrD-fed WT group. D, Enhanced blood pressure elevation in response to chronic high-fructose feeding in GLUT8KO mice. Mice were fed chow or HFrD for 24 wk and subjected to tail-cuff plethysmography. Graphed is the mean percent change in systolic (left) or diastolic (right) pressure vs. chow-fed control mice ± se; n = from 9–11 mice, each with more than or equal to three readings per mouse per group.
Discussion
From a teleological standpoint, an organism's ability to efficiently absorb and handle fructose was critical to survival in nutrient-poor environments in which glucose, fructose, and sucrose predominated as the available dietary sugars. Accordingly, multiple transporters with fructose transport capacity exist in the gut to fulfill this critical task, including GLUT2 (6, 7), GLUT5 (6), GLUT7 (25), GLUT8 (11–14), and GLUT12 (23). Although the specific functions of these transporters might overlap to some extent, the functions of GLUT family members in the gut are clearly not redundant: GLUT5 is almost exclusively a high-capacity fructose transporter (5), GLUT7 is a high-affinity, low-capacity fructose transporter (4), whereas GLUT2 (6) and GLUT12 (24) transport both fructose and glucose. Although GLUT5 and GLUT2 are classically thought to be the primary enterocyte fructose transporters, we demonstrate that GLUT8 attenuates enterocyte fructose transport by regulating GLUT12 protein abundance in vitro and in vivo. We further provide evidence that disrupted GLUT8 function has deleterious long-term metabolic sequelae under HFrD-fed conditions.
The in vivo function of GLUT8 was previously incompletely characterized. Functionally, GLUT8 was demonstrated as the transporter responsible for insulin-stimulated uptake in the blastocyst (11). Moreover, Gorovits et al. (26) postulated a role for GLUT8 in global metabolic homeostasis, showing dynamic hepatic GLUT8 regulation throughout development and in type 1 and type 2 diabetic models. However, Membrez et al. (27) reported mild overall phenotypes in mice lacking the C terminus encoded by exon 10 of the Slc2a8 gene. Mice lacking the GLUT8 C terminus were born live and developed normally but in slightly sub-Mendelian ratios. These mice had normal body weight, but they exhibited mild heart and brain abnormalities, including p-wave elongation and enhanced hippocampal neuronal proliferation, respectively. Importantly, this model exhibited normal glucose homeostasis in the absence of environmental stressors.
Our findings recapitulate the findings of the GLUT8 C terminus knockout model by Membrez et al. (27) with regard to grossly normal glucose homeostasis observed in unperturbed GLUT8KO mice. However, we found that enterocyte fructose transport and excursion into the systemic circulation was markedly perturbed at baseline and after acute HFrD feeding. Dysregulated fructose handling in GLUT8KO mice also correlated with relative hypertension, dyslipidemia, and hyperinsulinemia that developed after chronic high-fructose feeding. One might speculate that the absence of GLUT8 in GLUT8KO mice shifts enterocyte substrate preference toward fructose. Given that most standard rodent chows consist of significantly less than 1% carbohydrate derived from fructose, this substrate predilection might explain the lower body weight and normal metabolic profiles in GLUT8KO mice maintained on standard rodent chow.
Our data suggest that GLUT12 is in dynamic equilibrium between de novo protein synthesis and regulated degradation that is regulated by GLUT8, providing a plausible mechanism underlying, at least in part, GLUT8 regulation of enterocyte fructose transport. Intestinal hexose-sensing paradigms involving transmembrane transporters have been postulated, such as the glucose-sensing pathway involving SGLT1 and apical GLUT2 (reviewed in Ref. 28). Given the Michaelis-Menten constant for half-maximal fructose transport (Kt) of 100–150 mm in rat brush border membranes (29), and given that many subjects can consume large oral fructose loads (e.g. 600–800 mm carbohydrate in many popular sugar-sweetened beverages, with restaurant maximum beverage sizes ranging from 960 to 1260 ml) without inducing an osmotic diarrhea, one might postulate that GLUT8 and GLUT12 participate in an analogous fructose-sensing pathway that matches dietary fructose load with absorptive capacity. Future studies aimed at the precise trafficking and signaling mechanisms underlying GLUT8 and GLUT12 regulation are warranted.
Our in vivo model of fructose feeding without significant amounts of other dietary hexoses should be considered in extrapolating the results to the more physiological setting of a meal, which can be a carbohydrate milieu containing more than one hexose (e.g. glucose, sucrose, and/or fructose). However, the 0 mm fructose and 500 mm fructose incubation media used for our in vitro modeling each contained 5.6 mm glucose. This glucose content is likely to be of consequence, given the Michaelis-Menten constant for mouse intestinal brush border membrane glucose transport is 1.92 mm (30). We therefore expect that the in vitro model in which we observed GLUT8-GLUT12 regulation most closely approximates the hexose milieu of a meal, whereas the strength of the GLUT8KO model in vivo is a more reductionist, relatively pure fructose-stimulated approach to GLUT12 regulation in the absence of GLUT8.
In summary, we show that GLUT8 regulates enterocyte fructose transport and whole-body metabolic homeostasis in response to fructose feeding. Novel members of the GLUT family, therefore, have critical functions in the enterocyte that have yet to be fully explored. Given the deleterious long-term metabolic consequences of disrupted GLUT8 function, this mouse model may represent a new paradigm for conceptualizing the pathogenesis and treatment of under- and overnutrition and development of the metabolic syndrome.
Acknowledgments
This work was supported by the Pediatric Scientist Development Program (PSDP) National Institutes of Health Grant 5K12HD000850-27 (to B.J.D.) and R01HD040390-07 (to K.H.M.). The Washington University Mouse Phenotyping Core is supported by Diabetes Research and Training Center Grant P60 DK020579.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATCC
- American Tissue Culture Center
- DBP
- diastolic blood pressure
- 2DG
- 2-deoxy-d-glucose
- GFP
- green fluorescent protein
- GLUT
- glucose transporter
- GLUT8KO
- GLUT8 deficient
- GLUT12TG
- GLUT12 transgenic
- HFrD
- high-fructose diet
- LDL
- low-density lipoprotein
- SBP
- systolic blood pressure
- SGLT1
- sodium-glucose cotransporter-1
- shRNA
- short hairpin RNA
- WT
- wild type.
References
- 1. Brown IJ, Stamler J, Van Horn L, Robertson CE, Chan Q, Dyer AR, Huang CC, Rodriguez BL, Zhao L, Daviglus ML, Ueshima H, Elliott P. 2011. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: international study of macro/micronutrients and blood pressure. Hypertension 57:697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferder L, Ferder MD, Inserra F. 2010. The role of high-fructose corn syrup in metabolic syndrome and hypertension. Curr Hypertens Rep 12:105–112 [DOI] [PubMed] [Google Scholar]
- 3. Abdullah MM, Riediger NN, Chen Q, Zhao Z, Azordegan N, Xu Z, Fischer G, Othman RA, Pierce GN, Tappia PS, Zou J, Moghadasian MH. 2009. Effects of long-term consumption of a high-fructose diet on conventional cardiovascular risk factors in Sprague-Dawley rats. Mol Cell Biochem 327:247–256 [DOI] [PubMed] [Google Scholar]
- 4. Thorens B, Mueckler M. 2010. Glucose transporters in the 21st century. Am J Physiol Endocrinol Metab 298:E141–E145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferraris RP. 2001. Dietary and developmental regulation of intestinal sugar transport. Biochem J 360:265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drozdowski LA, Thomson ABR. 2006. Intestinal sugar transport. World J Gastroenterol 12:1657–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leturque A, Brot-Laroche E, Le Gall M, Stolarczyk E, Tobin V. 2005. The role of GLUT2 in dietary sugar handling. J Physiol Biochem 61:529–537 [DOI] [PubMed] [Google Scholar]
- 8. Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP. 2007. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104:15075–15080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Osswald C, Baumgarten K, Stümpel F, Gorboulev V, Akimjanova M, Knobeloch KP, Horak I, Kluge R, Joost HG, Koepsell H. 2005. Mice without the regulator gene Rsc1A1 exhibit increased Na+-D-glucose cotransport in small intestine and develop obesity. Mol Cell Biol 25:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barone S, Fussell SL, Singh AK, Lucas F, Xu J, Kim C, Wu X, Yu Y, Amlal H, Seidler U, Zuo J, Soleimani M. 2009. Slc2a5 (Glut5) is essential for the absorption of fructose in the intestine and generation of fructose-induced hypertension. J Biol Chem 284:5056–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, Devaskar SU, Moley KH. 2000. GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst. Proc Natl Acad Sci USA 97:7313–7318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doege H, Schürmann A, Bahrenberg G, Brauers A, Joost HG. 2000. GLUT8, a novel member of the sugar transport facilitator family with glucose transport activity. J Biol Chem 275:16275–16280 [DOI] [PubMed] [Google Scholar]
- 13. Ibberson M, Riederer BM, Uldry M, Guhl B, Roth J, Thorens B. 2002. Immunolocalization of GLUTX1 in the testis and to specific brain areas and vasopressin-containing neurons. Endocrinology 143:276–284 [DOI] [PubMed] [Google Scholar]
- 14. Romero A, Gomez O, Terrado J, Mesonero JE. 2009. Expression of GLUT8 in mouse intestine: identification of alternative spliced variants. J Cell Biochem 106:1068–1078 [DOI] [PubMed] [Google Scholar]
- 15. DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. 2006. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem 281:32841–32851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carayannopoulos MO, Schlein A, Wyman A, Chi M, Keembiyehetty C, Moley KH. 2004. GLUT9 is differentially expressed and targeted in the preimplantation embryo. Endocrinology 145:1435–1443 [DOI] [PubMed] [Google Scholar]
- 17. Purcell SH, Aerni-Flessner LB, Willcockson AR, Diggs-Andrews KA, Fisher SJ, Moley KH. 2011. Improved insulin sensitivity by GLUT12 overxpression in mice. Diabetes 60:1478–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rooj AK, Kimura Y, Buddington RK. 2010. Metabolites produced by probiotic Lactobacilli rapidly increase glucose uptake by Caco-2 cells. BMC Microbiol 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krimi RB, Letteron P, Chedid P, Nazaret C, Ducroc R, Marie JC. 2009. Resistin-like molecule-β inhibits SGLT-1 activity and enhances GLUT2-dependent jejunal glucose transport. Diabetes 58:2032–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gouyon F, Caillaud L, Carriere V, Klein C, Dalet V, Citadelle D, Kellett GL, Thorens B, Leturque A, Brot-Laroche E. 2003. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J Physiol 552:823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kato T, Berger SJ, Carter JA, Lowry OH. 1973. An enzymatic cycling method for nicotinamide-adenine dinucleotide with malic and alcohol dehydrogenases. Anal Biochem 53:86–97 [DOI] [PubMed] [Google Scholar]
- 22. Campbell FC. 2010. Isolation and culture of mouse intestinal cells. In: Ward A, Tosh D, eds. Mouse cell culture, methods in molecular biology. 1st ed New York: Humana Press; 197–206 [DOI] [PubMed] [Google Scholar]
- 23. Miller PJ, Finucane KA, Hughes M, Zhao FQ. 2005. Cloning and expression of bovine glucose transporter GLUT12. Mamm Genome 16:873–883 [DOI] [PubMed] [Google Scholar]
- 24. Rogers S, Chandler JD, Clarke AL, Petrou S, Best JD. 2003. Glucose transporter GLUT12—functional characterization in Xenopus laevis oocytes. Biochem Biophys Res Comm 308:422–426 [DOI] [PubMed] [Google Scholar]
- 25. Cheeseman CI. 2008. GLUT7: a new intestinal facilitated hexose transporter. Am J Physiol Endocrinol Metab 295:E238–E241 [DOI] [PubMed] [Google Scholar]
- 26. Gorovits N, Cui L, Busik JV, Ranalletta M, Hauguel de-Mouzon S, Charron MJ. 2003. Regulation of hepatic GLUT8 expression in normal and diabetic models. Endocrinology 144:1703–1711 [DOI] [PubMed] [Google Scholar]
- 27. Membrez M, Hummler E, Beermann F, Haefliger JA, Savioz R, Pedrazzini T, Thorens B. 2006. GLUT8 is dispensable for embryonic development but influences hippocampal neurogenesis and heart function. Mol Cell Biol 26:4268–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kellett GL, Brot-Laroche E. 2005. Apical GLUT2 a major pathway of intestinal sugar absorption. Diabetes 54:3056–3062 [DOI] [PubMed] [Google Scholar]
- 29. Corpe CP, Burant CF, Hoekstra JH. 1999. Intestinal fructose absorption: clinical and molecular aspects. J Pediatr Gastr Nutr 28:364–374 [DOI] [PubMed] [Google Scholar]
- 30. Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. 2012. Na(+)-d-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]