Abstract
An endocrine-disrupting chemical (EDC) is an exogenous chemical, or mixture of chemicals, that can interfere with any aspect of hormone action. The potential for deleterious effects of EDC must be considered relative to the regulation of hormone synthesis, secretion, and actions and the variability in regulation of these events across the life cycle. The developmental age at which EDC exposures occur is a critical consideration in understanding their effects. Because endocrine systems exhibit tissue-, cell-, and receptor-specific actions during the life cycle, EDC can produce complex, mosaic effects. This complexity causes difficulty when a static approach to toxicity through endocrine mechanisms driven by rigid guidelines is used to identify EDC and manage risk to human and wildlife populations. We propose that principles taken from fundamental endocrinology be employed to identify EDC and manage their risk to exposed populations. We emphasize the importance of developmental stage and, in particular, the realization that exposure to a presumptive “safe” dose of chemical may impact a life stage when there is normally no endogenous hormone exposure, thereby underscoring the potential for very low-dose EDC exposures to have potent and irreversible effects. Finally, with regard to the current program designed to detect putative EDC, namely, the Endocrine Disruptor Screening Program, we offer recommendations for strengthening this program through the incorporation of basic endocrine principles to promote further understanding of complex EDC effects, especially due to developmental exposures.
Founded in 1916, The Endocrine Society is the world's oldest, largest, and most active organization devoted to research on hormones and the clinical practice of endocrinology. Today, The Endocrine Society's membership consists of more than 15,000 scientists (basic and clinical researchers, physicians, educators, nurses, and students) in more than 100 countries. Society members represent all basic, applied, and clinical interests in endocrinology. Included among The Endocrine Society's members are the world's leading experts on hormones and the endocrine effects of environmental chemicals.
Drawing on the expertise of its members, The Endocrine Society published a Scientific Statement on endocrine-disrupting chemicals (EDC) in June of 2009 (1). The statement resulted from a great deal of scientific interest and research among The Endocrine Society members combined with concern about the consequences of widespread exposure of human and wildlife populations during all life stages to chemicals that can interfere with hormone action. Therefore, The Endocrine Society and its members are keenly interested in applying their collective knowledge and expertise to improve human and wildlife health through the effective chemical safety assessment that is fundamental to successful public health policies. It is important to consider the issue of EDC within the context of normal endocrine function, which is described in a very large body of literature. Fundamental principles of endocrinology must be applied to the design and execution of studies that characterize the ability of chemicals to interfere with hormone action. The Endocrine Society is in a unique position to help inform the ongoing debate about the health effects of endocrine disruptors (ED), and the purpose of this article is to outline (from an endocrine perspective) key issues related to identifying EDC and protecting humans and wildlife from their adverse effects.
The current statement of principles is a commentary that builds upon the groundwork laid in the Scientific Statement by introducing specific guidelines for the application of principles and practices of the discipline of endocrinology to the process of chemical safety assessment. The cornerstone of this process is chemical risk assessment, which is a systematic approach to organizing and analyzing scientific knowledge and information about potentially hazardous activities or substances that might pose risks. Risk assessment is typically divided into four steps: hazard identification, dose-response assessment, exposure assessment, and risk characterization. Endocrine practices and principles should be applied to the design, implementation, and interpretation of screening and testing programs intended to identify EDC (hazard identification) and to the analysis of data to assess the health risks from EDC exposure (hazard identification, dose-response assessment, and risk characterization). Moreover, when attempting to link exposures to outcome in the field of EDC research, it is important to be cognizant of the ways in which hormone action changes over the lifetime of an individual.
Considering these issues, the goal of this document is to provide a concise and cogent justification for the perspective that EDC must be evaluated within the context of fundamental principles of endocrinology. EDC cannot be evaluated as if they are general toxins. Therefore, in this document, we discuss the definition of an EDC and frame the principles of endocrinology that need to be incorporated into studies designed to identify EDC and to characterize their risk to human and wildlife populations. We also discuss the current validated assays employed for the purpose of characterizing chemicals that can disrupt thyroid hormone, estrogen, and androgen action and focus on the principles of endocrinology that would strengthen these assays.
What Is an ED?
The definition of an ED is critical, because it will dictate the evidence required to identify a chemical as an EDC and will inform the subsequent steps of assessing the risk of EDC exposures. Various agencies worldwide have defined an EDC, and we review these definitions with their strengths and limitations here.
The Food Quality Protection Act of 1996 mandated that the United States Environmental Protection Agency (EPA) “develop a screening program, using appropriate validated test systems and other scientifically relevant information, to determine whether certain substances may have an effect in humans that is similar to an effect produced by a naturally occurring estrogen, or other such endocrine effect as the Administrator may designate…. ” (reviewed in Ref. 2). As a result, the ED Screening and Testing Advisory Committee (EDSTAC) was established in 1996 to advise the EPA on methods of screening and testing individual chemicals for endocrine-disrupting activity. To accomplish its goals, the EDSTAC described an ED as “an exogenous chemical substance or mixture that alters the structure or function(s) of the endocrine system and causes adverse effects at the level of the organism, its progeny, populations, or subpopulations of organisms, based on scientific principles, data, weight-of-evidence, and the precautionary principle” (3). Subsequently, other entities have also defined an ED in similar terms as follows.
United States EPA (4). An ED is an exogenous agent that interferes with the production, release, transport, metabolism, binding, action, or elimination of natural hormones in the body responsible for the maintenance of homeostasis and the regulation of developmental processes.
European Union (5). An ED is an exogenous substance that causes adverse health effects in an intact organism, or its progeny, secondary to changes in endocrine function. A potential ED is a substance that possesses properties that might be expected to lead to endocrine disruption in an intact organism.
World Health Organization (6). An ED is an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse effects in an intact organism, or its progeny, or (sub)populations.
When considered in the context of hazard and risk characterization, these definitions are complicated and problematic. Currently, in the regulatory process, chemicals are first evaluated for their potential to cause some overt harmful effect (hazard identification). Subsequent to the identification of a chemical as a hazard, quantitative dose response is developed, and risk characterization (defined as dose response × exposure) (7) is conducted. The concept of risk management is that toxic chemicals can be released into the environment and the human population safely provided releases and exposures are minimized to the extent that adverse effects in humans and wildlife are averted (8). However, estimating the exposure level that will cause no harm requires an accurate measure of the dose response (i.e. how sensitive are populations or subpopulations to the substance in question). Thus, the critical first step of hazard identification must be properly designed and implemented to ensure accurate evaluation of the sensitivity of human and wildlife populations to chemicals that pose potential risks.
All but one of the definitions above define an EDC in terms of both the mode of action (i.e. the ability to interfere with hormone action) and the ability to produce adverse effects (cause harm). This conflates the process by which a chemical is identified as an EDC with the process by which its potency is characterized. We propose that the ability of a chemical to interfere with hormone action is a clear predictor of adverse outcome, much like mutagenicity is a predictor of carcinogenicity. The only uncertainties relate to exposure dose, duration, and whether exposure occurred during critical periods of increased sensitivity during the life cycle so that the risk will not be underestimated. Thus, the definition of an EDC must focus on its ability to interfere with hormone action rather than stipulate adverse outcome, and this is precisely what the EPA definition does. The EPA defines an EDC as an exogenous agent that interferes with some aspect of hormone action and then spells out those aspects known at the time to be affected by environmental chemicals. We therefore simplify the language of the EPA definition to account for current and future information about the range of actions through which chemicals may influence the endocrine system but without changing the definition itself. Therefore, we propose the following version of the EPA definition:
An ED is an exogenous chemical, or mixture of chemicals, that interferes with any aspect of hormone action.
It is important to recognize that this definition does not imply that all chemicals that interfere with any aspect of hormone action are a significant risk. Risk will depend on the exposure and the potency of the chemical. However, estimating the potency of a chemical in terms of its ability to cause adverse effects is as complicated as studying the role of the endocrine system in development and adult physiology. Therefore, screening and testing for EDC and estimating potency require insight derived from principles of endocrinology that have been developed over decades of research on hormones, their effects, and the consequences of endocrine dysregulation and disease.
Principles of Endocrinology Relevant to EDC
Endocrinology is the study of the mechanisms by which hormones coordinate and control the functions of multiple organ systems and processes throughout life. Because hormones produce different effects at different times during the life cycle, the timing and duration of EDC exposure are important elements of their effects on endocrine systems. The mechanisms by which EDC may interfere with hormone action can be quite complex. Chemicals may bind to hormone receptors and exert direct agonist or antagonist actions, they may exert indirect agonist or antagonist actions, or they may bind to allosteric sites and produce unexpected effects at very low concentrations (1). In addition, chemicals are known to interfere with hormone synthesis or metabolism, transport (in serum or across membranes), or degradation. Therefore, chemicals must be examined for EDC activity in the context of fundamental endocrinology that has arisen from decades of careful research into the mechanisms and consequences of hormone action under normal and pathological circumstances. In addition, our understanding of normal endocrine function is evolving rapidly as researchers apply sophisticated and insightful experimental approaches and state-of-the art technologies to the study of endocrinology. So too, our understanding of EDC actions on endocrine signaling is evolving rapidly. This endocrine literature, largely described in The Endocrine Society Scientific Statement on EDC (1), highlights several important features of the endocrine system that must be considered in the design, execution, and interpretation of studies attempting to identify EDC hazards and to define the risks to human and wildlife health.
Hormone effects are mediated by receptors
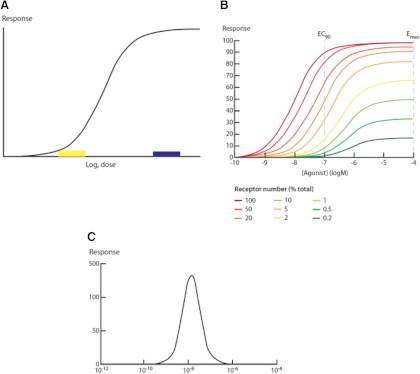
A central tenet of endocrinology is that hormones exert their physiological actions through receptors (9). This simple fact has several implications. First, hormone action is saturable, in terms of both ligand-binding and effect. The magnitude of the effect and the sensitivity of the receptor to ligand (ligand efficacy) depend in part on the affinity of the hormone for its receptor and in part on receptor abundance (e.g. Ref. 10); other, less well understood variables also affect ligand efficacy (11). Moreover, the maximum effect of the hormone typically occurs at ligand concentrations well below those that result in receptor saturation, a phenomenon that has been referred to as “the spare receptor hypothesis” (12 and review in Ref. 13). These observations impose several consequences for the expected shape of dose-response curves induced by hormones and by chemicals that interfere with hormone actions. First, the curves are never linear, although they may contain linear portions. Instead, they tend to be sigmoidal in shape (Fig. 1A) but with important departures from this basic form, as in the case of nonmonotonic dose responses (Fig. 1C). It is the nature of sigmoidal-shaped dose responses that an equivalent change in hormone level (or action) at the low end of the curve will have a proportionally greater effect than at the high end of the curve; in fact, once functional receptor saturation is reached, no further increase in the response will be observed (see Fig. 1). Furthermore, overstimulation of hormone receptors (binding saturation) can down-regulate the receptor, leading to changes in abundance of the receptor and a decrease in sensitivity of the cell to the hormone. This process often results in “high-dose inhibition”; that is, a dose-response curve in which low doses increase the response, and high doses decrease the response. This “inverted U” dose response is an example of a nonmonotonic dose response (Fig. 1C). These observations are universally acknowledged by the endocrine community and have been extensively reviewed (13).
Fig. 1.
A, Typical sigmoidal dose-response curve for hormones. As the dose of hormone increases, the response increases in a logarithmic manner until the point of saturation of the response. Different hormone-receptor interactions will have differences in the dose of hormone or the dynamic range of the log-linear portion of the curve or the maximal response. Some receptors are down-regulated by the hormone, so the dose-response curve will decline at the high dose (this will be a function of both dose and time). Note that a small change in hormone concentration at the low end of the curve (box) will have much greater effects on the response than a similar change in hormone concentration at the high end of the curve (box). It is also important to note that saturation of the response can occur at levels of receptor occupancy in the range of 10%; thus, there are “spare receptors” (e.g. Ref. 73). B, The dose response to the hormone depends on receptor concentration. These data show clearly that as the receptor concentration increases, the hormone becomes “more potent”; that is, it takes significantly less hormone to produce the same response. In fact, at low hormone receptor levels, the maximum response does not achieve the “EC50” response of the high receptor level (from Ref. 10). C, Nonmonotonic dose response curve. The inverted U dose-response curve may have many different mechanisms underlying it. For example, receptor down-regulation at high concentrations of hormone is an important mechanism. However, the addition of separate monotonic dose responses also provides an important mechanism. This issue is reviewed extensively by Vandenberg et al. (13).
Because of the role of receptors in mediating hormone effects, and because hormones exhibit nonlinear dose-response characteristics, EDC will necessarily replicate these characteristics. This has several implications. First, the effect of a high dose of a chemical may not predict the effect of the chemical at a low dose. The most obvious reason for this is the existence of a nonmonotonic dose response. However, at very high doses, chemicals can also produce a number of interacting effects that obscure what would be most important for low-dose exposures. Second, because low doses of endogenous hormones are present and fluctuating, small additions (or subtractions) to their actions will have a significant impact.
These implications are problematic for risk assessments, because it cannot be assumed that high doses always provide information relevant to low-dose exposures, and because it cannot be assumed that there is a threshold. The absence of a threshold for EDC has also been demonstrated experimentally in animals (14, 15), in epidemiological research (16), and theoretically based on mechanisms of hormone action (14). Directly related to this issue is that the human population is chronically exposed to low doses of EDC, which even further necessitates a “no-threshold” approach to risk assessment of these chemicals.
In addition, because some endpoints are more sensitive than others to the actions of endogenous hormones, it is also clear that some endpoints of EDC effects on hormone action will be more sensitive than others. Thus, establishing the potency of a chemical's ability to interfere with hormone action (a key element in the risk assessment process) will require that several of the most sensitive endpoints of hormone action be evaluated.
Endpoints of hormone action
Many hormones exert widespread actions in the body. However, the specific actions of individual hormones often change throughout life, they may be different in males and females, and they may be mediated by different receptors or receptor isoforms expressed in different tissues or at different life stages. To list the extensive examples of these phenomena is beyond the scope of this article, but there are some fundamental patterns that should be highlighted.
Hormones exert very specific effects on development and adult physiology, precisely because they act through receptors that exhibit specific patterns and intensities of distribution. For example, during sexual differentiation, testosterone (T) secreted from the testis acts on the androgen receptor (AR) expressed in fetal tissues to cause the development of the male reproductive tract. It is the location-specific expression of the AR that permits this interaction (17–19). However, the direct action of T in the development of the male reproductive tract (Wolffian duct derivatives) differs considerably from its indirect action in the fetal and adult male brain, which for some areas of the brain is mediated by the estrogen receptor (ER) after T is converted to estradiol by the action of the aromatase enzyme. Therefore, T acts in the male by interacting with either the AR or ER (after aromatization). Furthermore, the AR can be activated by T itself or by its derivative 5α-dihydrotestosterone (DHT) through actions of the enzyme, 5α-reductase. Recent work indicates that T and DHT impose different structural constraints on the AR that may explain the different effects of T and DHT on different tissues (20). These examples also highlight the fact that actions of EDC may be at the level of the steroid biosynthetic or metabolizing enzyme, instead of or in addition to actions on the receptor itself (21). Furthermore, hormone action can be prevented by enzymatic conversion of the hormone from one form to another. For example, cortisol is prevented from acting on the mineralocorticoid receptor in the distal convoluted tubules of the kidney by the action of 11β-hydroxysteroid dehydrogenase, and licorice can block this activity producing hypertension in some people (22).
An important point is that hormone actions during development are often permanent. They affect elements of organ development that have lifetime consequences. Likewise, hormone disruption during development can produce effects that are permanent, some of which do not become manifested until adulthood. These developmental origins of health and disease are exemplified by the effect of the drug diethylstilbestrol (DES) on cancer incidence. Specifically, the female children of women who were prescribed DES during the first trimester of pregnancy have a higher incidence of breast cancer, clear cell adenocarcinoma of the vagina and cervix, and reproductive anomalies (23). These effects of fetal DES exposure occur in adulthood, and there is good experimental evidence that chemical exposures can produce similar actions (24–26). Thus, the adverse effects of EDC exposures during development may require an extended period to be manifested, a period during which a generation of people will have been continuously exposed. This kind of effect is clearly important to incorporate in a screening and testing paradigm.
Taken together, it is clear that hormones have very complex actions simultaneously in different tissues, and it is important to recognize that EDC will exert similarly complex actions but perhaps in patterns that do not exactly replicate the effects of the native hormone. Several characteristics of endocrine systems can explain how EDC can produce selective effects on hormone action. One mechanism is that chemicals can influence hormone metabolism in a tissue-specific manner that can directly interfere with normal hormone actions only in some tissues. In addition, the chemicals themselves may be metabolized (e.g. hydroxylated) in a tissue-specific manner, and the metabolites may directly interfere with hormone action only in those tissues where they are generated. Chemicals, or their metabolites, may also interact with hormone receptors in a tissue-specific manner, either because some tissues exhibit greater receptor density, or because different receptor isoforms are expressed in different tissues (27, 28). Considering these possibilities, it is unrealistic to expect or require completely consistent results of EDC effects on hormone action across all hormone-sensitive endpoints, as EPA's Weight of Evidence document for the ED Screening Program (EDSP) Tier 1 recommends (http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPPT-2010-0877-0021). In addition, EDC are imperfect ligands of hormone receptors and should be expected to interact with them in ways that do not perfectly replicate the actions of the endogenous hormone (29). Thus, it is possible that some EDC can cause a hormone receptor to do something that it would not normally do. Each of these events is a likely explanation for the observation that many EDC influence a subset of a given hormone's effects (e.g. Ref. 30).
In addition, it is known that differences in husbandry, such as phytoestrogen content of different types of animal feed, can impact the outcome of an experiment (31). Unplanned events in an animal colony, such as fire alarms or construction noise, can also influence the physiological status of the animal and thus the outcome of studies on EDC. Although rats and mice are the basis of most basic animal research, differences in species and strains also contribute to differences across studies. Because these environmental contributors introduce inconsistencies, it is important to include positive and negative controls within and between studies so that worthy results will not be ignored simply because they cannot be fully explained (32). The purpose of including animals exposed to an appropriate low dose of control chemical is to demonstrate that: 1) the test system is sensitive to the class of chemical being examined and 2) the sensitivity of the technical approach is sufficient to identify meaningful effects. This latter issue relates to all the sources of introduced biological and technical error that can obscure meaningful results, such as unplanned events in the animal colony as well as the various measurement errors that can occur. Thus, if the experiment is unable to identify effects of the positive control, failure to identify effects of the EDC would not be meaningful. Considering this goal, it is important to employ a dose of the positive control that would challenge the limit of detection for effects.
Characterizing the Endocrine-Disrupting Properties of Environmental Chemicals
The final report of the EDSTAC, published in 1998, recommended a two-tiered system, in which an initial battery of relatively short-term in vitro and in vivo assays was proposed to screen chemicals for potential ED activity, followed by a second tier of “definitive” tests (3). Since then, the United States EPA further developed the Tier 1 assays and engaged in a validation process intended to ensure that the Tier 1 data would be reliable and reproducible across laboratories. The assays that make up the Tier 1 battery (Table 1) were developed on the basis of several criteria: to “a) maximize sensitivity which serves to minimize false negatives, b) include a range of organisms representing differences in metabolism, c) detect all known modes of action by the endocrine endpoints of concern, d) include a sufficient range of taxonomic groups among the test organisms, and e) incorporate sufficient diversity among the endpoints, permitting weight-of-evidence conclusions” (3). Thus, the assays in Tier 1 were designed to identify chemicals that may interfere with estrogen, androgen, or thyroid hormone action, and therefore, would require additional testing (in Tier 2) for the EPA to determine the degree of risk to human and wildlife health. Details of the assays are described by the EPA in a series of test guidelines available on the EPA website at http://www.epa.gov/ocspp/pubs/frs/publications/Test_Guidelines/series890.htm.
Table 1.
Assays included in the EDSP Tier 1
| Screening assay | Test guideline | Receptor binding |
Steroidogenesis |
HPG axis | HTP axis | ||||
|---|---|---|---|---|---|---|---|---|---|
| E | Anti-E | A | Anti-A | E | A | ||||
| In vitro | |||||||||
| ER binding (rat uterine cytosol) | OCSPP 890.1250 | X | X | ||||||
| ERα transcriptional activation | OCSPP 890.1300 OECD 455 | X | |||||||
| AR binding (rat prostate cytosol) | OCSPP 890.1150 | X | X | ||||||
| Steroidogenesis (human cell line H295R) | OCSPP 890.1550 890.1550 |
X | X | ||||||
| Aromatase (human target tissue or cell-line microsomes) | OCSPP 890.1200 | X | |||||||
| In vivo | |||||||||
| Uterotrophic (rat) | OCSPP 890.1600 OECD 440 | X | |||||||
| Hershberger (rat) | OCSPP 890.1400 OECD 441 | X | X | Xa | |||||
| Pubertal male (rat) | OCSPP 890.1500 | X | X | X | X | X | |||
| Pubertal female (rat) | OCSPP 890.1450 | X | X | X | X | X | |||
| Fish short-term reproduction | OCSPP 890.1350 OECD 229 | X | X | X | X | X | X | X | |
| Amphibian metamorphosis (frog) | OCSPP 890.1100 OECD 231 | X | |||||||
Complementary endpoints across assays are indicated (X) within each column.
5α-Reductase inhibition only. OCSPP, Office of Chemical Safety and Pollution Prevention; OECD, Office of Economic and Cooperative Development.
Considering the complexity of hormone action and the known complexity of EDC effects on hormone action, the goal of identifying all EDC in a rapid animal-based screen is impossible to achieve. However, the United States EPA has developed a first step toward accomplishing that goal in the current EDSP and is engaged in a promising new strategy of using high throughput in vitro assays that would be faster and more efficient (33), with the aim of replacing animal testing and evaluating more chemicals to which the human population is already routinely exposed (34). These important steps would be strengthened by the incorporation of specific endocrine principles to support the design of future EDC assays, as well as to support the execution of current assays and interpretation of the current data. Specifically, incorporating endocrine principles can help integrate the large battery of high throughput in vitro assays into risk assessment procedures for EDC. These principles are not targeted to the risk assessment of any particular chemical but are broadly applicable to EDC, nor are they specific to the EDSP itself. Academic studies focused on understanding mechanisms of EDC effects on hormone action that could account for disease trends likewise should incorporate these principles. However, the assays described in Tier 1 of the EDSP (Table 1) provide a useful focal point, around which to illustrate practical application of endocrine principles to improve screening for EDC, thereby enhancing strategies to protect human and wildlife health.
Tier 1 of the EDSP
The assays described in Table 1 represent a combination of in vitro and in vivo assays. They have certain strengths, but they also have identifiable weaknesses. We describe below the relative merits of these assays using specific examples to help focus the ways in which our current knowledge can capitalize on the strengths and minimize the weaknesses. However, two initial points of concern that apply to all of the following examples are that first, the current endpoints being examined in the EPA's EDSP studies to determine the hazard of EDC do not meet the criterion of using the most sensitive outcomes to assess hazard; and second, the in vivo assays will use the traditional approach in regulatory toxicology of starting with a very high dose and examining a few lower doses that are all very high by endocrine standards. The initial (reference) high dose of the chemical is required to be near the maximum tolerated dose (determined to cause some sublethal effect, typically indicated by a decrease in body weight) or a dose of no more than 1 g/kg body weight. This strategy is based on the concept that toxic effects will appear at maximum doses (e.g. LD50, which is the dose that kills 50% of the animals) and that there is a linear relationship between dose and effect. In addition, there are typically only three doses tested that cover about a 50-fold range. Although this approach has been effective in identifying classic toxicants, the EDSP was mandated to identify EDC, which do not behave like toxicants. Two problems with this approach are immediately clear for EDC. First, it is impossible to assess the shape of the dose-response curve with only three doses; and second, the dose-response curve cannot be assumed to be monotonic (or linear), which is the core assumption underlying this “top down” approach to dose selection. The lowest dose tested is assumed to be within 10-fold of the no effect level, even if adverse effects are found at the lowest dose tested, and the calculated no effect dose (which is 10-fold less that the lowest dose tested) is declared a “threshold” dose. This 10-fold assumption is not based on the known differences in hormone potency based on receptor abundance alone, because this can change by 10,000-fold (e.g. Fig. 1B). Therefore, by endocrine standards, the assumed threshold dose is always very high, because it is based on a dose that is sublethal rather than on mechanistic information about the biochemical and molecular actions of the EDC that may be observed at doses more than a million-fold lower than the assumed threshold dose.
Finally, people differ in their baseline exposures to EDC and in their sensitivities to endocrine disruption. In addition, because some endpoints are more sensitive than others to the actions of endogenous hormones, it is also clear that some endpoints of EDC effects on hormone action will be more sensitive than others. Thus, establishing the potency of a chemical's ability to interfere with hormone action (a key element in the risk assessment process) will require that several of the most sensitive endpoints of hormone action are evaluated.
Thus, the data derived from the traditional approach just described will have a high probability of underestimating potency and may miss important effects altogether. As a result, the risk assessment process will come to conclusions that could have negative impacts on public health. We describe specific examples below.
Thyroid
Tier 1 has three in vivo assays that measure chemical effects on the thyroid system, the amphibian metamorphosis assay, and the male and female pubertal assays. For these mammalian assays, according to the EPA protocol, Sprague Dawley rats are treated with test chemical from postnatal day (PND) 22 (female) or 23 (male) to PND 42. The dose of the chemical is dictated to be near the maximum tolerated dose (or no more than 1 g/kg) as indicated by effects on body weight. At PND 42, the animals are euthanized and serum collected. The endpoints for thyroid are serum total T4 and TSH, and thyroid histopathology. Thyroid histopathology is evaluated subjectively using a five-point scale, and commercial kits are used to evaluate hormone levels.
There are several design and guidance features of the pubertal assays that limit their ability to identify chemicals known to interfere with thyroid hormone action. First, the hormone levels are considered highly variable and therefore not reliable. The performance criteria required for hormone measurements according to the protocol would allow for measures of serum total T4 (μg/dl) to range from about 4 to 30 and still be considered normal. This range of serum T4 values in untreated Sprague Dawley rats is not known to exist but appears to be based on the variability reported in the validation studies leading to adoption of these assays for thyroid endpoints. The intraassay variation in the validation studies was reported to be 25–35% (35), but performance criteria for these kit assays routinely conducted by thousands of clinical laboratories require an intraassay coefficient of variation of less than 4%. The intraassay variability may have contributed to the wide range of total T4 values that would be considered normal and undoubtedly contributed to the overall variability of hormone measurements observed in the validation studies.
Another limitation of the EDSP is the EPA's guidance that the primary endpoint for consideration of thyroid hormone action in Tier 1 should be histopathological changes within the thyroid gland itself (36). Chemical-induced changes in thyroid histopathology mostly reflect chemical-induced changes in serum TSH. However, many chemicals reduce serum total and serum-free T4 without eliciting an increase in serum TSH, although it is not clear how this happens (37). Therefore, it remains possible that these chemicals are disrupting thyroid hormone action at sites other than the thyroid gland through mechanisms that do not require changes in TSH. A good example of this scenario is that of polychlorinated biphenyls (PCB).
PCB are a class of industrial chemicals, the production of which was banned by the United States Congress in the 1970s (38). Because of their stability and persistence, PCB remain ubiquitous contaminants in the environment and in the human population even today (38). They are well known to cause a reduction in circulating total and free T4 but do not cause an increase in serum TSH, nor do they change elements of thyroid histopathology (39). However, PCB interfere with thyroid hormone action in the periphery and the brain of experimental animals and are linked to neurobehavioral effects in humans (40–42). Some PCB, or their hydroxylated metabolites, can bind to the thyroid hormone receptor in a competitive binding assay (43) and may exert allosteric effects on the thyroid hormone receptor as well (28, 44). In the Tier 1 EDSP, PCB would cause a decrease in serum T4 but would cause neither an increase in TSH nor a change in thyroid histopathology. The EPA's guidance to more heavily weigh thyroid histopathological changes rather than proper serum T4 measurement would result in the interpretation that, despite their effects on thyroid hormone levels, PCB need not be further examined in Tier 2, because they do not cause the adverse effect of thyroid histopathological changes. In this case, the evaluation of PCB would end at Tier 1 with no measure of the other adverse effects they have been shown to cause. However, the effect of PCB on the thyroid system may be detected in the amphibian metamorphosis assay. In this case, the combination of a positive in the amphibian assay and the reduction in circulating T4 in the pubertal assays, may send PCBs to Tier 2. However, Tier 2 does not have an amphibian assay, nor does it contain endpoints of thyroid hormone action. Therefore, it is quite likely that PCBs would not be identified as antithyroid agents capable of producing population effects at environmental relevant exposures.
Regulatory processes in the 1970s were insufficient to identify PCB as harmful, thus necessitating an act of Congress to ban them (38). Considering the discussion above, the Tier 1 battery of screens within the EDSP does not differ enough from regulatory standards of the 1970s and would be significantly enhanced by incorporating developmental exposures and additional endpoints of thyroid hormone action. A number of standard assays have been proposed that could ameliorate these weaknesses (e.g. cerebellar histogenesis among many possible endpoints) (45). Many chemicals to which the human population is exposed will exhibit a similar profile of effects in the current Tier 1 of the EDSP.
Androgens
The Tier 1 assays (Table 1) evaluate chemicals for their ability to interfere with androgen action primarily in three assays, the AR binding assay (rat prostate), the Hershberger assay (rat), and the male rat pubertal assay. The binding assay uses the cytosol fraction from rat ventral prostate as a source of the AR in an in vitro displacement assay using the synthetic androgen [3H]-methyltrienolone as the tracer. The Hershberger bioassay is intended to serve as a mechanistic in vivo screening assay for androgen agonists, antagonists, and 5α-reductase inhibitors. Developed in the 1930s and 1940s, it is a short-term screening assay using changes in weight of five androgen-dependent tissues of castrated peripubertal male rats: the ventral prostate, seminal vesicle, levator ani-bulbocavernosus muscle, Cowper's glands, and glans penis. Similarly, the male rat pubertal assay incorporates measures of androgen-dependent organ weights, age and body weight at time of preputial separation, testis histology, and serum T levels. Thus, the concept of using these three assays is that if a chemical directly interacts with the AR, it will be identified in the binding study, and if it has functional consequences on the AR or T biosynthesis, it will affect male reproductive organ weight.
The design of this approach is based on the premise that binding assays as described will provide a comprehensive view of chemical interactions with the AR and that reproductive organ weight is a sensitive proxy measure of androgen disruption at all endpoints. However, these assays are not sensitive to some types of antiandrogens, such as phthalates. Phthalates (phthalic acid esters) are a family of chemicals commonly used as plasticizers, and their presence in a large number of consumer products makes their distribution in the environment ubiquitous (46, 47).
Although two commonly used phthalates, diethylhexyl phthalate (DEHP) and dibutyl phthalate, and their in vivo, bioactive metabolites monoethylhexylphthalate and monobutylphtalate, disrupt male reproductive development in an antiandrogenic fashion, the activities of these compounds are not manifest via the classic antiandrogenic mechanism of an antagonist with high affinity for the AR (48, 49) and would not be detected in in vitro AR binding assays. Rather, endocrine-disrupting activity of phthalates is directed at early development of the fetal testis. Exposure of rats to DEHP (or monoethylhexylphthalate) and dibutyl phthalate (or monobutylphthalate) during gestation causes a significant reduction in fetal T levels during the critical masculinization window between embryonic d 15 and 19. In utero exposure of rats to phthalates causes histological evidence of testicular dysgenesis consisting of reduced numbers of Sertoli and germ cells, malformed seminiferous cords/tubules with intracordal/intratubular Leydig cells, and immature Sertoli cells. These effects can only be ascertained by histologic examination during testicular development and are not predicted by AR binding assays with rat prostate cytosol or steroidogenic assays with human H295R cells.
Malformation of reproductive tissues in male rats is most dramatic after fetal and lactational exposure to phthalates with significant postnatal developmental anomalies, including reduced anogenital distance, nipple retention, presence of a vaginal pouch, cleft phallus, hypospadias, epididymal agenesis, undescended testes, and reduced accessory sex gland (prostate, seminal vesicles) weights. Pubertal administration of DEHP can result in delayed onset of puberty assessed by age of preputial separation, alterations in testis histopathology, reduced serum T and elevated LH levels, and decreased accessory sex gland weights. Although these effects would be observable in the pubertal male and Hershberger assays, they occur at doses higher than those that elicit effects during the fetal period, an observation that could lead to the inaccurate interpretation that lower exposure levels are safe because the most sensitive period for exposure is not being examined.
Distinct differences have been shown in the sensitivity of Long-Evans and Sprague Dawley rat strains to the pubertal administration of DEHP, with Long-Evans rats being more sensitive to DEHP effects on some endpoints and less sensitive on other endpoints (50). This demonstrates that selection of the model system is an important consideration when testing for endocrine-disrupting properties. Moreover, various reports indicate that one primate model, the marmoset, may be resistant to the deleterious testicular actions of phthalates, in that reduced T biosynthesis, abnormal testicular histology, and altered accessory sex gland development were not observed in this species. However, epidemiological studies suggest that humans are sensitive to the antiandrogenic actions of phthalates. Differences in the pharmacokinetics and pharmacodynamics of phthalates in this monkey model likely explains the lack of sensitivity of the marmoset to phthalate exposure (51). The apparent dichotomy between the human and nonhuman primate data has yet to be resolved. Despite these outstanding questions, it is clear that DEHP has the potential to act as an androgen disruptor and that it, in fact, does so under a number of conditions.
Estrogens
Estrogenicity (and antiestrogenicity) is more intensively tested in the battery of Tier 1 EDSP than effects on either androgen or thyroid hormone actions. The in vitro assays include an ER binding assay using rat uterine cytosol, human ERα transcriptional activation using a human cell line (HeLa-9903) stably transfected with human ERα and an assay for the enzyme aromatase (estrogen synthetase) activity using human recombinant microsomes. In addition, there is a fish short-term reproduction assay, female rat pubertal assay, and rat uterotrophic assay. As described above for the androgen assays, these assays are not optimally sensitive to chemicals that interfere in some ways with estrogen action. We provide two examples below.
Bisphenol A (BPA)
Nuclear ER binding assays as well as transcriptional activation assays indicate that BPA has at least a 10,000-fold lower affinity for the two estrogen nuclear receptors than 17β-estradiol. In isolation, these results would suggest that BPA at environmentally relevant levels of exposure would not pose a public health problem. However, experiments published by the Kortenkamp group indicate that, in conditions resembling the living condition, BPA and other xenoestrogens act additively with ovarian estrogens, and this phenomenon was observed at very low xenoestrogen levels within the range of environmental exposure [Kortenkamp and co-workers (52)]. Similarly, in vitro experiments addressing the role of ER embedded in the plasma membrane indicate that for some endpoints, BPA is equipotent to ovarian estradiol, and significant effects of BPA at a dose of 0.01 pm have been reported (53, 54).
Depending on the rodent species and strain, uterotropic effects of BPA have been observed at the relatively high doses between 100 and 800 mg/kg body weight, suggesting that environmental BPA exposure poses no problems as the environmental exposure levels are orders of magnitude lower than those needed to induce a significant increase of the wet weight of the uterus. However, long-term adverse effects on the female mouse reproductive system, including the uterus, due to exposure to very low doses of BPA during early development have been reported by the United States National Toxicology Program, and the lowest dose tested (0.1 μg/kg · d) showed the highest percent of uterine tumors (55). These effects of exposure to BPA for only 7 d during fetal life would not be identified or accounted for in the EDSP due to the absence of assays that involve exposure during fetal and neonatal life, when the animals are most sensitive to BPA (56). One of a number of reasons for the high sensitivity of fetuses and neonates to EDC such as BPA is the maxim in pediatric medicine that “babies are not little adults,” and they have a limited capacity to metabolize xenobiotics. BPA also produces significant effects in the adult, with some of the effects being mediated by the ERα (57) and others by ERβ (58). However, the effects in adults occur at higher doses than those that impact the fetus (59), and there is evidence that the dose-response curve is not monotonic (55–58).
Chronic oral exposure of the mouse mammary tumor virus-erbB2/neu mouse model to BPA during adult life (from PND 56 to killing) increased breast tumor multiplicity, decreased the latency period, and increased the number of metastases only at the lowest doses (2.5 and 25 μg/kg · d), whereas higher doses did not affect these endpoints (60). There is also now considerable evidence from studies with rats and mice, as well as human breast cells, indicating that BPA increases the risk of breast cancer and interferes with breast cancer therapy. In addition, there is evidence for nonmonotonic dose-response curves for effects on the mammary gland (61, 62).
Specifically, in several rat models, perinatal administration of BPA (2.5–500 μg/kg · d) resulted in the development of preneoplastic mammary lesions (63, 64) and increased neoplastic outcomes when animals were treated with a chemical carcinogen during adult life (65, 66). As stated previously, the EDSP is not structured to examine low-dose effects elicited in a nonlinear or nonmonotonic fashion.
Additional Considerations of Tier 1
Dioxin-like activities
An example of EDC that might not be easily identified in the EDSP toxicological testing is dioxin-like compounds, such as PCB, which have pleotropic effects on multiple endocrine systems. Discussed in the section on thyroid hormone, PCB are a family of structurally stable, synthetic compounds that were widely used in industry beginning in the 1930s. The United States Congress banned manufacture of PCB in the United States in 1977 due to the dioxin-like activity of congeners used in commercial mixtures of PCB. However, significant quantities of persistent PCB are still detectable in both the environment and in the food chain due to bioaccumulation and biomagnification (67, 68). A recent study estimating PCB concentrations in humans shows that people born as recently as 2010 have PCB body burdens (69).
PCB are classified as dioxin-like coplanar, or noncoplanar, based upon the arrangement of chlorine atoms around the biphenyl core. Structural differences between the classes of PCB influence the binding affinity to hormone and neurotransmitter receptors and their ability to act as an agonist or antagonist. This means that the ability to predict effects of PCB requires knowledge of the effects of the individual PCB in a mixture, because exposure inevitably involves mixtures (70).
Because of mixed properties of PCB and the weak binding of some PCB congeners to ER (71), there is high probability that PCB would be missed in Tier 1 screens for estrogenic activity. Furthermore, assays that involve transfection of ER (such as the transcriptional activation assay with transfected HeLa cells) have relatively low sensitivity, making it unlikely this assay would detect PCB or other estrogenic compounds that in mixtures can result in additive effects (52). Assays involving uterotrophic activity and the timing of puberty in females also have low sensitivity, making them poor proxies for estrogenic activity that may be exerted at the cellular/molecular level, something that would not be discerned from just measuring uterine size or timing of the onset of secondary sexual characteristics in rodents.
Given the specific assays in Tier 1 and EPA's guidance document, it is likely that PCB would not be considered an EDC in the estrogen assays, as just described, or in the thyroid assays, as described earlier in this document. This is concerning, because there is an abundance of experimental evidence showing that PCB interfere in complex ways with various hormone systems, and there is evidence in humans of the impacts of PCB exposures to reproduction, cognitive function, immune function, and other health outcomes (73). It may be relevant to remember that PCB production was banned by an act of Congress, not through the regulatory processes in place in the 1970s. It is troubling that, despite the advances in science over the past 40 yr, the current assays and guidelines in the regulatory domain would continue to underestimate PCB toxicity. Moreover, the absence of any assay that would detect a compound with dioxin-like activity in a sensitive manner in the EDSP Tier 1 assay protocols is a significant omission.
Conclusions
The Endocrine Society's Scientific Statement published in 2009 (1) provided an exhaustive summary of the scientific background that justifies concern for the effects of EDC exposures to human and wildlife population health. In that document, a number of recommendations are proposed for research and practice guiding the understanding of EDC in four categories: clinical research, basic science, epidemiology, and clinical practice. These recommendations are still germane today, and the current document outlines specific ways in which science can be employed for public health and wildlife protection.
Importantly, we propose a simplified definition of EDC that separates the fundamental characteristic of interfering with, or disrupting, hormone action from the extraneous criteria of potential downstream adverse effects. This definition is important as we consider the types of evidence required to identify chemicals with endocrine-disrupting properties and that require further consideration for public health protection. We further propose a number of endocrine principles that will strengthen the ability of current screening programs to identify EDC as well as improve new generations of assays used for this purpose. These principles, enumerated in Table 2, are based on the fundamentals of endocrinology but also on our current knowledge of EDC effects on endocrine systems. Vandenberg et al. (13) exhaustively review the evidence for the key principles of low-dose toxicity and nonmonotonic dose-response curves.
Table 2.
Endocrine Principles Applied to EDC Research
Recommendations for the future
|
Endocrine Principles Applied to EDC Research
|
Principles of the biology of endocrine systems
|
Principles of hormone action
|
Tier 1 EDSP
|
The route of exposure and dosing strategy are not optimized to identify EDC. Moreover, the timing of exposure does not include development.
|
Because hormone actions are pleiotropic, a single experimental design will not be optimal for testing the ability of an EDC to interfere with all hormone actions. Therefore, screens and tests for EDC need to be optimized for sensitive endpoints of hormone action, many of which are developmental. In addition, because academic research on EDC has often been both optimized to critically evaluate EDC actions on important hormone actions and intensively scrutinized in peer review, including grant, journal, and institutional panels, this research needs to be incorporated into the processes agencies engage to protect public and wildlife health. In turn, this requires that experts in the basic biology of the system under investigation (i.e. proposed to be affected by an EDC) must be active participants in the review process.
Acknowledgments
This work was funded in part by grants from the NIEHS (ES10026 to R.T.Z., ES01839 to F.S.v.S., and ES08314 to A.S.), the NICHD (HD05574 to T.R.B.), The New York Community Trust (P12-000024) and the Forsythia Foundation (T.J.W.), and the Passport Foundation (R.T.Z.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AR
- Androgen receptor
- BPA
- bisphenol A
- DEHP
- diethylhexyl phthalate
- DES
- diethylstilbestrol
- DHT
- 5α-dihydrotestosterone
- ED
- endocrine disruptor
- EDC
- endocrine-disrupting chemical
- EDSP
- ED Screening Program
- EDSTAC
- ED Screening and Testing Advisory Committee
- EPA
- Environmental Protection Agency
- ER
- estrogen receptor
- PCB
- polychlorinated biphenyl
- PND
- postnatal day
- T
- testosterone.
References
- 1. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30:293–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eldridge JC, Laws SC. 2010. The U.S. EPA's Tier 1 screening battery for endocrine disruptor compounds. New York: Informa Healthcare USA, Inc [Google Scholar]
- 3. (EDSTAC)USEPA EDSaTAC 1998. Endocrine disrupter screening and testing advisory committee final report. Washington, DC: United States Government; http://www.epa.gov/scipoly/oscpendo/history/finalrpt.htm [Google Scholar]
- 4. Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S, Lucier G, Luster M, Mac MJ, Maczka C, Miller R, Moore J, Rolland R, Scott G, Sheehan DM, Sinks T, Tilson HA. 1996. Research needs for the risk assessment of health and environmental effect of endocrine disruptors: a report of the USEPA-sponsored workshop. Environ Health Perspect 104(Suppl 4):715–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Commission E. 1996. European workshop on the impact of endocrine disruptors on human health and wildlife. In: Environment and climate research programme DX. Brussels: European Commission [Google Scholar]
- 6. Damstra T, Barlow S, Bergman A, Kavlock RJ, van der Kraak G. eds. 2002. Global assessment of the state-of-the-science of endocrine disruptors. Geneva: World Health Organization [Google Scholar]
- 7. Putzrath RM, Wilson JD. 1999. Fundamentals of health risk assessment. Use, derivation, validity and limitations of safety indices. Risk Anal 19:231–247 [DOI] [PubMed] [Google Scholar]
- 8. Borgert CJ, Mihaich EM, Quill TF, Marty MS, Levine SL, Becker RA. 2011. Evaluation of EPA's Tier 1 endocrine screening battery and recommendations for improving the interpretation of screening results. Regul Toxicol Pharmacol 59:397–411 [DOI] [PubMed] [Google Scholar]
- 9. Kovacs WJ, Ojeda SR. 2012. Textbook of endocrine physiology. 6th ed Oxford: Oxford University Press [Google Scholar]
- 10. Charlton SJ. 2009. Agonist efficacy and receptor desensitization: from partial truths to a fuller picture. Br J Pharmacol 158:165–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeyakumar M, Carlson KE, Gunther JR, Katzenellenbogen JA. 2011. Exploration of dimensions of estrogen potency: parsing ligand binding and coactivator binding affinities. J Biol Chem 286:12971–12982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. 2003. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 111:994–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, Vom Saal FS, Welshons WV, Zoeller RT, Myers JP. 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheehan DM. 2006. No-threshold dose-response curves for nongenotoxic chemicals: findings and applications for risk assessment. Environ Res 100:93–99 [DOI] [PubMed] [Google Scholar]
- 15. Sheehan DM, Willingham E, Gaylor D, Bergeron JM, Crews D. 1999. No threshold dose for estradiol-induced sex reversal of turtle embryos: how little is too much? Environ Health Perspect 107:155–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, McCormack P, Luben R, Khaw KT, Wareham NJ, Galloway TS. 2012. Urinary bisphenol: a concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation [DOI] [PubMed] [Google Scholar]
- 17. Murashima A, Miyagawa S, Ogino Y, Nishida-Fukuda H, Araki K, Matsumoto T, Kaneko T, Yoshinaga K, Yamamura K, Kurita T, Kato S, Moon AM, Yamada G. 2011. Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation. Endocrinology 152:1640–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Renfree MB, Fenelon J, Wijiyanti G, Wilson JD, Shaw G. 2009. Wolffian duct differentiation by physiological concentrations of androgen delivered systemically. Dev Biol 334:429–436 [DOI] [PubMed] [Google Scholar]
- 19. Welsh M, Saunders PT, Sharpe RM. 2007. The critical time window for androgen-dependent development of the Wolffian duct in the rat. Endocrinology 148:3185–3195 [DOI] [PubMed] [Google Scholar]
- 20. Jasuja R, Ulloor J, Yengo CM, Choong K, Istomin AY, Livesay DR, Jacobs DJ, Swerdloff RS, Miksovská J, Larsen RW, Bhasin S. 2009. Kinetic and thermodynamic characterization of dihydrotestosterone-induced conformational perturbations in androgen receptor ligand-binding domain. Mol Endocrinol 23:1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arase S, Ishii K, Igarashi K, Aisaki K, Yoshio Y, Matsushima A, Shimohigashi Y, Arima K, Kanno J, Sugimura Y. 2011. Endocrine disrupter bisphenol A increases in situ estrogen production in the mouse urogenital sinus. Biol Reprod 84:734–742 [DOI] [PubMed] [Google Scholar]
- 22. Whorwood CB, Sheppard MC, Stewart PM. 1993. Licorice inhibits 11β-hydroxysteroid dehydrogenase messenger ribonucleic acid levels and potentiates glucocorticoid hormone action. Endocrinology 132:2287–2292 [DOI] [PubMed] [Google Scholar]
- 23. Veurink M, Koster M, Berg LT. 2005. The history of DES, lessons to be learned. Pharm World Sci 27:139–143 [DOI] [PubMed] [Google Scholar]
- 24. Walker DM, Gore AC. 2011. Transgenerational neuroendocrine disruption of reproduction. Nat Rev Endocrinol 7:197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gore AC, Patisaul HB. 2010. Neuroendocrine disruption: historical roots, current progress, questions for the future. Front Neuroendocrinol 31:395–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. 2008. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One 3:e3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ibhazehiebo K, Iwasaki T, Kimura-Kuroda J, Miyazaki W, Shimokawa N, Koibuchi N. 2011. Disruption of thyroid hormone receptor-mediated transcription and thyroid hormone-induced Purkinje cell dendrite arborization by polybrominated diphenyl ethers. Environ Health Perspect 119:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miyazaki W, Iwasaki T, Takeshita A, Tohyama C, Koibuchi N. 2008. Identification of the functional domain of thyroid hormone receptor responsible for polychlorinated biphenyl-mediated suppression of its action in vitro. Environ Health Perspect 116:1231–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McKinney JD, Waller CL. 1998. Molecular determinants of hormone mimicry: halogenated aromatic hydrocarbon environmental agents. J Toxicol Environ Health B Crit Rev 1:27–58 [DOI] [PubMed] [Google Scholar]
- 30. Shioda T, Chesnes J, Coser KR, Zou L, Hur J, Dean KL, Sonnenschein C, Soto AM, Isselbacher KJ. 2006. Importance of dosage standardization for interpreting transcriptomal signature profiles: evidence from studies of xenoestrogens. Proc Natl Acad Sci USA 103:12033–12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruhlen RL, Howdeshell KL, Mao J, Taylor JA, Bronson FH, Newbold RR, Welshons WV, vom Saal FS. 2008. Low phytoestrogen levels in feed increase fetal serum estradiol resulting in the “fetal estrogenization syndrome” and obesity in CD-1 mice. Environ Health Perspect 116:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. vom Saal FS, Welshons WV. 2006. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ Res 100:50–76 [DOI] [PubMed] [Google Scholar]
- 33. Knudsen TB, Kavlock RJ, Daston GP, Stedman D, Hixon M, Kim JH. 2011. Developmental toxicity testing for safety assessment: new approaches and technologies. Birth Defects Res B Dev Reprod Toxicol 92:413–420 [DOI] [PubMed] [Google Scholar]
- 34. De Wever B, Fuchs HW, Gaca M, Krul C, Mikulowski S, Poth A, Roggen EL, Vila MR. 2012. Implementation challenges for designing integrated in vitro testing strategies (ITS) aiming at reducing and replacing animal experimentation. Toxicol In Vitro [DOI] [PubMed] [Google Scholar]
- 35. Borrell B. 2010. Toxicology: the big test for bisphenol A. Nature 464:1122–1124 [DOI] [PubMed] [Google Scholar]
- 36. EPA U 2011. Endocrine disruptor screening program weight-of-evidence: evaluating results of EDSP Tier 1 screening to identify the need for Tier 2 testing. Washington, DC: Office of Chemical Safety and Pollution Prevention [Google Scholar]
- 37. Hood A, Hashmi R, Klaassen CD. 1999. Effects of microsomal enzyme inducers on thyroid-follicular cell proliferation, hyperplasia, and hypertrophy. Toxicol Appl Pharmacol 160:163–170 [DOI] [PubMed] [Google Scholar]
- 38. Erickson MD. 2001. PCB properties, uses, occurrence, and regulatory history. In: Robertson LW, Hansen LG, eds. PCBs: recent advances in environmental toxicology and health effects. Lexington, KY: The University Press of Kentucky; xii–xxx [Google Scholar]
- 39. Klaassen CD, Hood AM. 2001. Effects of microsomal enzyme inducers on thyroid follicular cell proliferation and thyroid hormone metabolism. Toxicol Pathol 29:34–40 [DOI] [PubMed] [Google Scholar]
- 40. Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT. 2011. Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development. Endocrinology 152:2909–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Amano I, Miyazaki W, Iwasaki T, Shimokawa N, Koibuchi N. 2010. The effect of hydroxylated polychlorinated biphenyl (OH-PCB) on thyroid hormone receptor (TR)-mediated transcription through native-thyroid hormone response element (TRE). Ind Health 48:115–118 [DOI] [PubMed] [Google Scholar]
- 42. Langer P, Kocan A, Tajtáková M, Susienková K, Rádiková Z, Koska J, Ksinantová L, Imrich R, Hucková M, Drobná B, Gasperíková D, Trnovec T, Klimes I. 2009. Multiple adverse thyroid and metabolic health signs in the population from the area heavily polluted by organochlorine cocktail (PCB, DDE, HCB, dioxin). Thyroid Res 2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. You SH, Gauger KJ, Bansal R, Zoeller RT. 2006. 4-Hydroxy-PCB106 acts as a direct thyroid hormone receptor agonist in rat GH3 cells. Mol Cell Endocrinol 257–258:26–34 [DOI] [PubMed] [Google Scholar]
- 44. Gilbert ME, Rovet J, Chen ZP, Koibuchi N. 2011. Developmental thyroid hormone disruption: prevalence, environmental contaminants and neurodevelopmental consequences. Neurotoxicology [DOI] [PubMed] [Google Scholar]
- 45. Zoeller RT, Tyl RW, Tan SW. 2007. Current and potential rodent screens and tests for thyroid toxicants. Crit Rev Toxicol 37:55–95 [DOI] [PubMed] [Google Scholar]
- 46. Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, Saunders M, Skaare JU. 2009. Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health B Crit Rev 12:225–249 [DOI] [PubMed] [Google Scholar]
- 47. Kamrin MA. 2009. Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health B Crit Rev 12:157–174 [DOI] [PubMed] [Google Scholar]
- 48. Gray LE, Jr, Barlow NJ, Howdeshell KL, Ostby JS, Furr JR, Gray CL. 2009. Transgenerational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicol Sci 110:411–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Foster PM. 2006. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl 29:140–147; discussion 181–145 [DOI] [PubMed] [Google Scholar]
- 50. Noriega NC, Howdeshell KL, Furr J, Lambright CR, Wilson VS, Gray LE., Jr 2009. Pubertal administration of DEHP delays puberty, suppresses testosterone production, and inhibits reproductive tract development in male Sprague-Dawley and Long-Evans rats. Toxicol Sci 111:163–178 [DOI] [PubMed] [Google Scholar]
- 51. Kurata Y, Makinodan F, Shimamura N, Katoh M. 2012. Metabolism of di (2-ethylhexyl) phthalate (DEHP): comparative study in juvenile and fetal marmosets and rats. J Toxicol Sci 37:33–49 [DOI] [PubMed] [Google Scholar]
- 52. Rajapakse N, Silva E, Kortenkamp A. 2002. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect 110:917–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alonso-Magdalena P, Quesada I, Nadal A. 2011. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol 7:346–353 [DOI] [PubMed] [Google Scholar]
- 54. Watson CS, Jeng YJ, Kochukov MY. 2010. Nongenomic signaling pathways of estrogen toxicity. Toxicol Sci 115:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Newbold RR, Jefferson WN, Padilla-Banks E. 2009. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect 117:879–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Welshons WV, Nagel SC, vom Saal FS. 2006. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 147:S56–S69 [DOI] [PubMed] [Google Scholar]
- 57. Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A. 2008. A pancreatic insulin content regulation by the estrogen receptor ERα. PLoS One 3:e2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Soriano S, Alonso-Magdalena P, García-Arévalo M, Novials A, Muhammed SJ, Salehi A, Gustafsson JA, Quesada I, Nadal A. 2012. A rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor β. PLoS One 7:e31109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A. 2010. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect 118:1243–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jenkins S, Wang J, Eltoum I, Desmond R, Lamartiniere CA. 2011. Chronic oral exposure to bisphenol A results in a nonmonotonic dose response in mammary carcinogenesis and metastasis in MMTV-erbB2 mice. Environ Health Perspect 119:1604–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. LaPensee EW, Ben-Jonathan N. 2010. Novel roles of prolactin and estrogens in breast cancer: resistance to chemotherapy. Endocr Relat Cancer 17:R91–R107 [DOI] [PubMed] [Google Scholar]
- 62. Soto AM, Vandenberg LN, Maffini MV, Sonnenschein C. 2008. Does breast cancer start in the womb? Basic Clin Pharmacol 102:125–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. 2007. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol 23:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, Muñoz-de-Toro M. 2007. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect 115:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Betancourt AM, Eltoum IA, Desmond RA, Russo J, Lamartiniere CA. 2010. In utero exposure to bisphenol A shifts the window of susceptibility for mammary carcinogenesis in the rat. Environ Health Perspect 118:1614–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jenkins S, Raghuraman N, Eltoum I, Carpenter M, Russo J, Lamartiniere CA. 2009. Oral exposure to bisphenol a increases dimethylbenzanthracene-induced mammary cancer in rats. Environ Health Perspect 117:910–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lake JL, McKinney R, Lake CA, Osterman FA, Heltshe J. 1995. Comparisons of patterns of polychlorinated biphenyl congeners in water, sediment, and indigenous organisms from New Bedford Harbor, Massachusetts. Arch Environ Contam Toxicol 29:207–220 [Google Scholar]
- 68. McFarland VA, Clarke JU. 1989. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: considerations for a congener-specific analysis. Environ Health Perspect 81:225–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Quinn CL, Wania F, Czub G, Breivik K. 2011. Investigating intergenerational differences in human PCB exposure due to variable emissions and reproductive behaviors. Environ Health Perspect 119:641–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hansen LG. 1998. Stepping backward to improve assessment of PCB congener toxicities. Environ Health Perspect 106(Suppl 1):171–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ma R, Sassoon DA. 2006. PCBs exert an estrogenic effect through repression of the Wnt7a signaling pathway in the female reproductive tract. Environ Health Perspect 114:898–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Carpenter DO. 2006. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health 21:1–23 [DOI] [PubMed] [Google Scholar]
- 73. Welshons WV, Jordan VC. 1987. Adaptation of estrogen-dependent MCF-7 cells to low estrogen (phenol red-free) culture. Eur J Cancer Clin Oncol 23:1935–1939 [DOI] [PubMed] [Google Scholar]