Abstract
Metabolic disease is a significant global health and economic problem. In a phenomenon referred to as fetal programming, offspring of underweight or overweight mothers have an increased incidence of adulthood obesity and metabolic disease. Undernourished individuals have decreased levels of leptin, a regulator of energy balance, whereas obese people develop hyperleptinemia and leptin resistance. We hypothesize that alterations in circulating leptin during pregnancy contribute to programming events caused by maternal nutritional status. To test this hypothesis, pregnant mice were randomly placed in one of three treatment groups: ad libitum feed plus saline injection (control, n = 5), 50% food restriction plus saline injection (restricted, n = 4), or 50% food restriction plus 1 mg/kg·d leptin injection (restricted, leptin treated, n = 4). Mice were treated from 1.5 to 11.5 d after conception and then returned to ad libitum feeding until weaning. At 19 wk after weaning, offspring were placed on a 45% fat diet and then followed up until 26 wk after weaning, at which time they were killed, and samples were collected for further analysis. Our results demonstrate that males are more negatively impacted by high-fat diet than females, regardless of maternal treatment. We provide evidence that differential response to leptin may mediate the sexual dimorphism observed in fetal programming in which male offspring are more affected by maternal undernutrition and female offspring by maternal overnutrition. We show that female offspring born to food-restricted, leptin-supplemented mothers are obese and insulin resistant. This may mimic fetal programming events seen in offspring of overweight women.
Obesity and its associated diseases are a significant problem worldwide, having major effects on morbidity and mortality rates (1). The World Health Organization in 2005 predicted that by 2010 in the United States, 44.2% of adult males and 48.3% of adult females would be obese [body mass index (BMI) ≥ 30 kg/m2] (1). A study in 2010 estimated that 34.3–38.5% of all adults suffer from metabolic syndrome, a condition characterized by central obesity, diabetes, hypertension, and nonalcoholic fatty liver disease (NAFLD), which accounts for 6–7% of all mortalities (2, 3). The developmental origins of health and disease, or fetal programming, hypothesis states that the maternal environment has lasting effects on the fetus into adulthood, which can include increased risk of metabolic syndrome (2, 4). This hypothesis was put forth by epidemiologists Barker and Osmond (5), who discovered correlations between adult cardiovascular disease and low birth weight, infant mortality, and poor maternal health, and nutrition.
Both maternal undernutrition and overnutrition are associated with fetal programming events, although the preponderance of data suggests that male offspring are more influenced by the former and female offspring by the latter (6–11). Studies from the Dutch Hunger Winter indicated that men born to mothers who were undernourished early in pregnancy have a higher incidence of cardiovascular disease and obesity in middle age (7) compared with their counterparts. In these studies, women were largely not followed up due to tracking difficulties. However, when the phenomenon was replicated in animal models, including rats and sheep, maternal undernutrition during early pregnancy often programmed greater adverse outcomes in male offspring (6, 8, 12–15), although a few studies in sheep show a negative effect of early pregnancy food restriction on female offspring (16, 17). On the other hand, global maternal nutrient restriction throughout pregnancy results in multiple aspects of metabolic syndrome in both male and female offspring (18–20). In maternal over nutrition studies, some studies indicate maternal obesity can have a negative impact on both sexes (21–24), whereas other studies show a greater, or exclusive, negative impact on female offspring born to overweight mothers, resulting in obesity, hypertension, and diabetes (9–11). However, no studies that have examined both sexes have shown greater impact of maternal obesity on male offspring. Taken together these studies highlight the differences and similarities between maternal under- and overnutrition as well as the critical role timing and offspring sex have on the outcomes of fetal programming.
One key hormonal regulator of nutritional status and energy homeostasis is leptin (25, 26). Circulating leptin levels are positively correlated to BMI (27), with hyperleptinemia and leptin resistance occurring in obesity (25). In addition to its role in energy homeostasis, leptin affects development in tissues including placenta, hypothalamus, bone (28), and muscle (29). Furthermore, postnatal leptin levels influence the programming of adult disease (see the review in Ref. 30). Thus, we hypothesize that alterations in circulating leptin levels during pregnancy contribute to fetal programming events caused by changes in maternal nutritional status. To test this, we have replicated the model of early pregnancy caloric restriction (7, 12) by feeding mice 50% of ad libitum consumption levels from d 1.5 to 11.5 of pregnancy, a regimen that, as we previously demonstrated, severely reduces serum leptin concentrations (31). A subset of the food-restricted mice were given daily leptin injections. We predicted that offspring programming would be related to maternal hyper- or hypoleptinemia, not to nutrient status per se. In other words, metabolic programming would be evident in male offspring of nutrient-restricted mothers but not when restriction was accompanied by leptin replacement. Similarly, female offspring of undernourished, leptin-treated mothers would exhibit the metabolic programming characteristic of offspring of overnourished mothers.
Materials and Methods
Animals and tissue collection
All animal procedures were approved by University of Missouri Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Swiss-Webster female mice (Harlan, Indianapolis, IN) aged 13–17 wk, were individually housed, and daily food consumption was measured for 2 wk before mating as previously described (31). Mice were mated and then randomly placed in one of three treatment groups: ad libitum feed plus saline injection (control, n = 5), 50% food restriction plus saline injection (restricted, n = 8), or 50% food restricted plus 1 mg/kg · d ip leptin injection (restricted, leptin treated, n = 8). Mice were placed on treatment at 1.5 d after conception (dpc) for 10 d, with injections at 1400 h and food introduced at 1600 h. After treatment, mice were returned to ad libitum feeding. Litter size was recorded and then reduced to eight by culling randomly selected pups to control for litter size during lactation. The sex ratio was determined either by visualization at weaning or by PCR for sex determining region Y on tail clip DNA taken at culling, as previously described (32). Litters were weighed weekly until weaning at 3 wk of age. At weaning, pups were group housed by sex and treatment with up to four males or five females per cage and individually weighed weekly. At 22 wk of age, all offspring were placed on a 45% fat diet (Research Diets, New Brunswick, NJ) until the animals were killed. Ad libitum-fed offspring were killed by carbon dioxide inhalation at 29 wk of age for tissue collection.
Blood samples were taken from all offspring at the time the animals were killed, and serum was stored at −20 C. Liver samples were collected and placed in trireagent (Sigma, St. Louis, MO) or fixed in 4% paraformaldehyde (PFA) overnight and then embedded in optimum cutting temperature compound (Fisher Scientific, Pittsburgh, PA) and stored at −80 C. Subcutaneous fat, visceral fat, and hypothalamus samples were snap frozen in liquid nitrogen. All samples were stored at −80 C. The left kidney was collected, fixed in 4% PFA, bisected midsagitally, embedded in paraffin, and stored at room temperature. A sample of pancreas was also collected, fixed in 4% PFA, embedded in paraffin, and stored at room temperature. Femurs were collected, cleaned, wrapped in gauze, and stored at −20 C in PBS.
Small animal magnetic resonance imaging (MRI)
Body composition was determined in 54 male offspring at 21 wk of age and 54 male and 44 female offspring at 27 wk of age after 5 wk exposure to a high-fat diet (HFD). Body fat percentage was measured using a Micro-MRI high performance 7T MR imaging and spectroscopy system (Varian Inc., Santa Clara, CA) at the Veterans Affairs Biomolecular Imaging Center at the Harry S. Truman Veterans Affairs Hospital and University of Missouri-Columbia, as previously described (33). Animals were anesthetized using isoflurane for the duration of the study. MRI was performed from the diaphragm to tail base. Data analysis was performed using Mnova7software (Mestrelab Research, Santiago de Compostela, Spain).
Behavioral assays
Behavioral activity was monitored automatically on two male and two female offspring from each mother at 26 wk of age using the Med Associates' Open Field Test Environment (ENV-515) monitors (Georgia, VT) in the Translational Neuroscience Behavior Testing Core at the University of Missouri-Columbia as previously described (33). Sensor break data were used to measure distance traveled. Time spent in the middle of the maze vs. the perimeter was used to estimate behavioral anxiety. Mice were habituated to the testing area for 3–4 h and then placed individually in the chamber for 60 min. Trials were run every other day for a total of three trials per mouse.
Serum assays
Serum leptin and insulin concentrations were measured in offspring using the mouse leptin and rat/mouse insulin ELISA, respectively (Millipore, Billerica, MA) following the manufacturer's guidelines. True serum triglyceride concentrations were measured in each offspring using a serum triglyceride determination kit (Sigma) following the manufacturer's guidelines. Intraperitoneal glucose tolerance tests (IPGTT) were performed on 13 male (five control, four restricted, four leptin) and 25 female offspring (10 control, eight restricted, seven leptin) according to the National Institutes of Health animal models of diabetic complications consortium protocol (http://www.diacomp.org/shared/showFile.aspx?doctypeid=3&docid=11). Briefly, animals were fasted for 6 h, and then a fasting glucose level was obtained by venous tail sample. An ip injection of glucose was then given (1 mg/g), and blood glucose levels were obtained at 30, 60, and 120 min after the injection by venous tail sample using the OneTouch Ultra glucose meter (LifeScan Inc., Milpitas, CA).
Kidney morphology
Kidneys were sectioned and stained with hematoxylin and eosin, and then glomeruli number was counted in the largest kidney cross-section using the counting feature in the ImageJ software (National Institutes of Health, Bethesda, MD).
Bone analysis
Geometric parameters were defined from right femurs of one male and one female offspring from each mother by microcomputed tomography (μCT) scan analysis (Siemens Inveon, Siemens Preclinical Solutions, Knoxville, TN) before ex vivo torsional loading as previously described (34) with some exceptions. The μCT data acquisition scanning protocol was as follows: x-ray settings of 80 kVp voltage and 500 μA current with a 190-msec exposure. The μCT image slices were reconstructed to give a cubic voxel dimension of 0.083 mm and analyzed using Amira 5.3.3 software package (Mercury Computer Systems/TGS, Chelmsford, MA). Marrow cavity diameter (MCD) is reported as the average of the two endosteal diameters. Cortical bone width (CBW) is reported as the average of the four thicknesses on the major and minor axes of the cortical midslice. Geometrical parameter polar moment of area (mm4) was used as an overall measure of the amount of bone as previously described (34). Whole-bone parameters of strength (torsional ultimate strength and strain energy to failure) and stiffness (torsional stiffness) are descriptors of the whole bone, taking into account both the material comprising the bone and the size and shape of the bone. Material properties of strength (tensile strength) and stiffness (shear modulus of elasticity) are descriptors of the material the bone is made of, independent of the size and shape of the bone. After the μCT analyses, the femur was potted in a cylindrical holder and placed in a test fixture designed to apply a pure torsional load on the femur as previously described (34).
Pancreatic morphology
Immunohistochemistry was performed on pancreatic tissue from 42 male and 40 female offspring. Briefly, five 5-μm paraffin sections at intervals of 50 μm or greater were examined per pancreas. After blocking in a 1:10 solution of normal goat serum in PBS for 30 min, each sample was dual stained overnight with an α-cell marker, mouse-antiglucagon (K79bB10; Abcam Inc., Cambridge, MA) and a β-cell marker, rabbit-anti insulin (ab63820; Abcam) at 1:2000 and 1:1000, respectively. Secondary antibodies Alexa Flour 568 goat-antimouse and 488 goat-antirabbit (Invitrogen, Carlsbad, CA) were used at 1:500. A total of 991 islets were examined, 515 female (145 control, 202 restricted, 168 restricted leptin treated) and 476 male (178 control 157 restricted 141 restricted leptin treated), and an average of 12.1 ± 0.4 islets were examined per pup. Islet values were averaged per animal. The α- and β-cell numbers were determined using the manual counting feature of ImageJ (National Institutes of Health). The β-cells were manually circled and area measured, using the Image J region of interest feature, as previously described (35). The average β-cell area per islet was used as an index of β-cell mass.
RNA isolation and real-time RT-PCR analysis
Total cellular RNA was isolated from liver, sc fat, visceral fat, and hypothalamus. Samples were homogenized in trireagent (Sigma), and, after phase separation using phase lock gel heavy tubes (5 Prime Inc., Gaithersburg, MD), the aqueous layer was placed on an RNEasy minicolumn (QIAGEN, Valencia, CA), and total cellular RNA was purified according to manufacturer's instructions.
For liver, visceral and sc fat, and quantitative RT-PCR, 1 μg of RNA was reverse transcribed using the RT2 first strand kit (QIAGEN) following the manufacturer's instructions. Real-time PCR was then performed using a custom RT2 Profiler PCR plate array, containing multiple PCR and RT2 SYBR Green ROX qPCR master mix (QIAGEN) on an Applied Biosystems 7500 real-time PCR system (Applied Biosystems, Foster City, CA) according to the PCR array manufacturer's guidelines. Three housekeeping genes, 18s rRNA (18s ribosomal RNA), Actb (actin-β), and Hprt (hypoxanthine phosphoribosyltransferase 1) were examined; however, due to expression variation of 18s and Hprt, only Actb was used to calculated fold changes. Manufactures external reverse transcription and positive PCR controls were also included.
For hypothalamus quantitative RT-PCR, 500 ng of RNA was reverse transcribed using the SuperScript first-strand synthesis system (Invitrogen) following the manufacturer's instructions. SYBR Green real-time PCR was performed for Agrp (agouti related protein), Cart (cocaine and amphetamine regulated transcript), Npy (neuropeptide Y), Pomc (proopiomelanocortin), Lepra and Leprb (leptin receptor-a and -b). For internal control, Actb was used. Sequences of primers (Integrated DNA Technology, Coralville, IA) can be found in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. Lepra and Leprb primer sequences were developed using Primer Express software (Applied Biosystems), Actb (36), Agrp, Cart, Npy, and Pomc primer sequences were previously published (37).
One male and one female liver sample per mother were analyzed by PCR. Two male and two female sc fat, visceral fat, and hypothalamus samples were examined per mother.
NAFLD analysis
Histological analysis was completed on offspring livers (54 males, 44 females) for NAFLD. Frozen liver samples were sectioned at 8 μm, and three sections at 50-μm intervals were placed on a slide. Samples were stained with Oil red O (Sigma) according to published guidelines (38), counterstained with Mayer's hematoxylin (Sigma), and mounted with glycerin jelly. Liver sections were evaluated for NAFLD based on published guidelines (39, 40). Briefly, common features evaluated by an operator blinded to treatment were hepatocellular ballooning, steatosis, and portal inflammation. Mallory's hyaline was also noted. Each abnormality in each liver section was graded using a 0–3 scale with a value of 0 corresponding to less than 33% of the sample covered with the abnormality. A value of 1 corresponded to 33–50%, 2 to 50–66%, and 3 to presence of the abnormality in 66–100% of the sample. The total score for each section was the sum of the abnormalities, a range of 0–9 (examples, Supplemental Fig. 1). Three section scores per animal were averaged.
Statistical analysis
Data were analyzed using Statistical Analysis System (SAS institute, Cary, NC). Maternal data were analyzed by ANOVA using a general linear model (GLM) of SAS. Offspring weight data were analyzed using repeated-measures ANOVA with pups nested in the mother. Offspring serum insulin, leptin, and triglyceride measurements, heart weight, pancreas morphology, and fat and hypothalamus real-time PCR, data were analyzed using a mixed-model of SAS with offspring nested in the mother. Offspring IPGTT, kidney glomeruli number, and liver real-time PCR data were analyzed by ANOVA using a GLM of SAS. Post hoc analysis was performed using Tukey's test. For NAFLD assessment, median scores were compared individually by offspring and by average per mother using a Mann-Whitney rank sum test with SigmaPlot (Systat Inc., San Jose CA). For comparisons between male and female offspring, regardless of maternal treatment, data were analyzed by ANOVA using a GLM of SAS. For all analyses, P < 0.05 was considered statistically significant.
Results
Pregnancy
Food restriction resulted in 50% pregnancy loss, a rate that was not further modified by leptin injection (Table 1). No pregnancy loss occurred in the control (ad libitum fed) dams. Maternal weight was not different among groups at d 1.5; however, control mothers gained weight and restricted mothers lost it, such that restricted mothers were significantly lighter, independent of leptin treatment (P < 0.05), by d 11.5 (Table 1). In other experiments with the same design, leptin concentrations measured at d 11.5, 2 h after the last injection were significantly increased (P < 0.05) in restricted, leptin-treated mothers over control and restricted mothers (Schlitt, J. M., and L. C. Schulz, unpublished data). There were no differences in leptin concentrations at d 18.5 among treatment groups (unpublished data). Maternal treatment did not alter litter size or sex ratio. Preweaning weights were not different among groups (Table 1).
Table 1.
Pregnancy measurements
| Measurement | Control | Restricted | Leptin |
|---|---|---|---|
| Maternal factors | |||
| Number plug positive | 5 | 8 | 8 |
| Number live litters | 5 | 4 | 4 |
| Starting weight (dpc 1.5) | 26.7 ± 0.7 | 27.0 ± 0.6 | 26.9 ± 0.6 |
| End treatment weight (dpc 11.5) | 29.6 ± 0.6a | 24.3 ± 1.2b | 26.2 ± 0.7b |
| Litter size | 9.3 ± 0.9 | 10.3 ± 0.3 | 9.0 ± 0.5 |
| Measurements at time the animals were killed | |||
| Sex ratio (percent male) | 54.2 ± 0.1 | 49.1 ± 0.1 | 51.9 ± 0.5 |
| Preweaning weights (per pup) | |||
| Day 8 | 6.3 ± 0.3 | 5.6 ± 0.6 | 6.0 ± 0.1 |
| Day 15 | 9.8 ± 0.6 | 9.4 ± 0.4 | 10.3 ± 0.4 |
| Day 22 | 14.0 ± 0.5 | 13.3 ± 0.3 | 14.5 ± 0.3 |
Numbers with different superscripts are significantly different (P < 0.05); n = 5 control, four restricted, and four restricted, leptin treated.
Effect of maternal treatment on offspring obesity
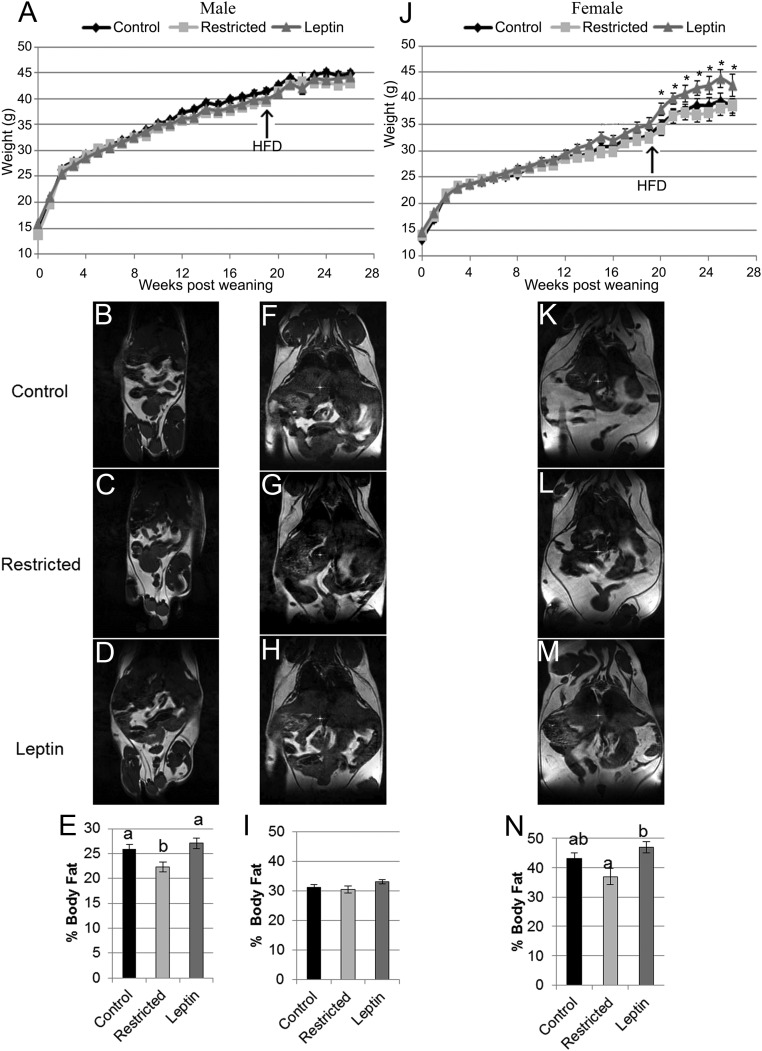
Although there were no differences in body weights among male offspring (Fig. 1A), body fat percentage was significantly reduced (P < 0.05) in male offspring from restricted mothers compared with control male offspring when on a standard diet (Fig. 1, B–E). This effect was attenuated by leptin supplementation to food-restricted mothers. After the HFD, there were no differences in weight or body composition among male offspring from the different maternal treatment groups (Fig. 1, F–I), all of which had significantly (P < 0.0001) higher body fat percentage (31.47 ± 0.01) than on a standard diet (25.41 ± 0.01).
Fig. 1.
Offspring postweaning weights and body composition by treatment. A, Male offspring weights were not different between treatment groups before or after the HFD. J, Female offspring from restricted, leptin-treated mothers tended (#, P < 0.10) to be higher from postweaning wk 13–19 compared with female control and restricted offspring. After the HFD, female restricted, leptin-treated offspring were significantly heavier (*, P < 0.05) compared with control and restricted female offspring. Representative MRI images and graphical representation of male offspring body fat percentage at 18 wk after weaning prior to the HFD (B–E), male offspring body fat percentage at 25 wk after weaning and 5 wk on the HFD (F–I), and female offspring body fat percentage at 25 wk after weaning and 5 wk on the HFD (K–N). E, I, and N, Data are represented as mean ± sem, and columns with different superscripts are significantly different. For comparisons among treatments, n = 5 control, four restricted, and four restricted, leptin-treated for both males and females.
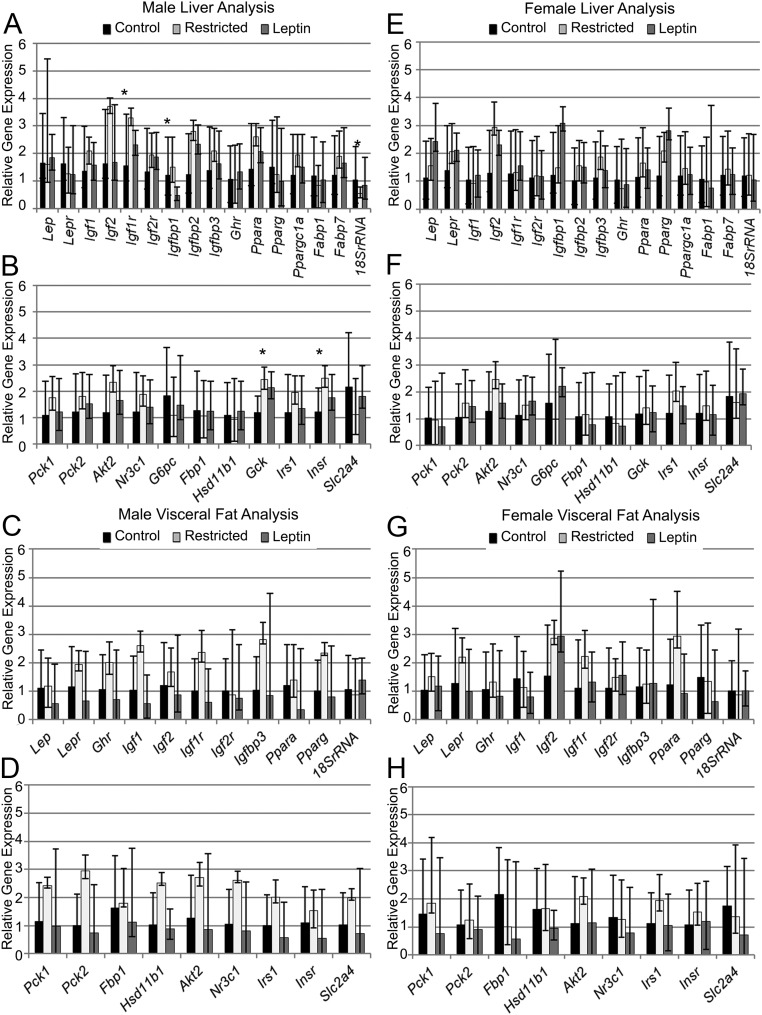
Real-time PCR gene expression analysis of male offspring liver after the HFD revealed that several genes involved in regulating body composition were up-regulated by maternal food restriction, and these effects were attenuated in male offspring from restricted, leptin-treated mothers (Fig. 2A). Notably, several members of the IGF family were altered. Igf1r (IGF-I receptor) was up-regulated (P < 0.05) in male offspring from restricted mothers compared with males from control mothers. Igf1 and Igf2 were not different (P = 0.12) in restricted male offspring compared with restricted, leptin-treated and control male offspring. Igfbp1 (IGF binding protein 1) was significantly decreased (P < 0.05) in male offspring from restricted, leptin-treated mothers compared with male offspring from restricted mothers but not different (P = 0.10) from control male offspring, whereas Igfbp2 and Igfbp3 were not different among treatment groups. There were no differences in expression of Ppara, Pparg, Ppargc1a, leptin, its receptor, or fatty acid binding proteins Fabp1 and Fabp7. Gene expression of 18s rRNA was significantly decreased (P < 0.05) in male offspring from restricted mothers compared with male offspring from control and restricted, leptin-treated mothers. The expression of the same genes was examined in sc (data not shown) and visceral fat from male offspring, and there were no differences among maternal treatment groups (Fig. 2C).
Fig. 2.
Real-time PCR analysis of expression of key metabolic genes in male (left panel, A–D) and female (right panel, E–H) offspring. A, B, E, and F, Liver. C, D, G, and H, Visceral fat. Data are represented as mean fold change relative to control group mean. Error bars represent range of fold changes based on sem of ΔΔCt. *, Significant differences in ΔCt among treatment groups (ANOVA, P < 0.05); n = 5 control, four restricted, and four restricted, leptin treated for both males and females.
Unlike in male offspring, significant weight differences were observed in female offspring, with females born to restricted, leptin-treated mothers being significantly heavier (Fig. 1J). Beginning at 14 wk after weaning, on a standard diet, restricted, leptin-treated female offspring had numerically but not significantly increased (P < 0.1) weights compared with control and restricted female offspring. This was exacerbated by the HFD. Female restricted, leptin-treated offspring body weights were significantly different (P < 0.05) beginning at 20 wk after weaning until the animals were killed (Fig. 1J). The percent body fat of female restricted, leptin-treated offspring was significantly increased (P < 0.05) compared with female restricted offspring, whereas the body fat percentage of female control offspring was intermediate after the HFD (Fig. 1, K–N). No differences were observed in the expression of the genes involved in body composition examined by real-time PCR in liver, sc fat, and visceral fat (Fig. 2, E and G).
At the time the animals were killed, serum leptin levels were not different among treatment groups in male or female offspring but were elevated across all groups (Table 2). No differences were found among groups in heart weight, kidney glomeruli number, or serum triglyceride levels in male or female offspring (Table 2).
Table 2.
Offspring measurements
| Males |
Females |
|||||
|---|---|---|---|---|---|---|
| Control | Restricted | Leptin | Control | Restricted | Leptin | |
| Heart weight (mg) | 228 ± 6 | 227 ± 6 | 220 ± 6 | 166 ± 6 | 166 ± 4 | 174 ± 6 |
| Kidney, n | 195 ± 11 | 166 ± 17 | 178 ± 19 | 154 ± 13 | 150 ± 19 | 152 ± 6 |
| Leptin (ng/ml) | 29.2 ± 2.5 | 32.1 ± 6.5 | 29.1 ± 1.9 | 28.5 ± 3.4 | 37.6 ± 8.2 | 43.9 ± 7.9 |
| Triglycerides (mg/ml) | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.2 | 2.8 ± 0.2 | 2.6 ± 0.2 | 2.6 ± 0.1 |
| Bone biomechanics analysis | ||||||
| Length (mm) | 16.4 ± 0.2 | 16.1 ± 0.1 | 16.2 ± 0.2 | 16.3 ± 0.2 | 16.0 ± 0.2 | 15.9 ± 0.2 |
| Marrow diameter (mm) | 0.67 ± 0.02a | 0.59 ± 0.02b | 0.58 ± 0.03b | 0.50 ± 0.03 | 0.51 ± 0.03 | 0.47 ± 0.03 |
| Cortical bone width (mm) | 0.59 ± 0.01 | 0.63 ± 0.03 | 0.61 ± 0.03 | 0.60 ± 0.03 | 0.59 ± 0.02 | 0.61 ± 0.02 |
| Polar moment of area (mm4) | 1.09 ± 0.04 | 1.09 ± 0.08 | 1.02 ± 0.12 | 0.81 ± 0.07 | 0.78 ± 0.06 | 0.77 ± 0.04 |
| Maximum torque (Nmm) | 46.4 ± 3.0 | 49.5 ± 3.3 | 48.6 ± 3.3 | 39.0 ± 1.8 | 42.5 ± 2.0 | 40.3 ± 2.0 |
| Measured torsional stiffness (Nmm/radian) | 63.4 ± 5.2 | 53.1 ± 5.8 | 63.2 ± 5.8 | 56.6 ± 4.2 | 54.0 ± 4.4 | 55.5 ± 3.9 |
| Material shear modulus (N/mm2) | 405.1 ± 22.5 | 352.1 ± 55.6 | 437.2 ± 15.0 | 496.8 ± 36.5 | 499.3 ± 66.9 | 505.4 ± 38.2 |
| Tensile strength (N/mm2) | 41.9 ± 3.5 | 45.7 ± 3.9 | 47.3 ± 3.9 | 43.7 ± 4.0 | 49.2 ± 4.4 | 46.7 ± 2.3 |
| Energy to failure (U) | 9.5 ± 1.3a | 11.9 ± 1.5b | 11.4 ± 0.5b | 6.9 ± 0.5 | 8.5 ± 0.6 | 8.1 ± 0.6 |
Numbers with different superscripts are significantly different (P < 0.05); n = 5 control, four restricted, and four restricted, leptin treated for both males and females.
Diabetic assessment
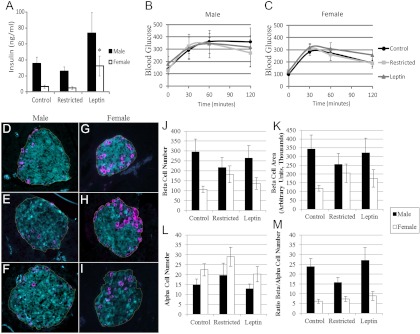
Insulin resistance and type 2 diabetes are consequences of metabolic syndrome. After the HFD, among female offspring, only those born to restricted, leptin-treated mothers were insulin resistant, whereas all male offspring were insulin resistant, based on serum insulin levels and impaired glucose tolerance (Fig. 3). In males, the maternal treatment group had no effect on insulin concentrations, which were uniformly high (Fig. 3A), nor did it affect glucose tolerance, which was impaired in all male offspring (Fig. 3B). Female offspring from restricted, leptin-treated mothers had significantly increased (P < 0.05) serum insulin concentrations compared with control and restricted female offspring (Fig. 3A). Female offspring from restricted, leptin-treated mothers also had numerically higher, but not significantly different, area under the curve after an IPGTT compared with female offspring from control and restricted mothers (Fig. 3C).
Fig. 3.
Diabetic assessment of male and female offspring. A, Serum insulin concentrations in male and female offspring. Glucose tolerance test curves in male (B) and female (C) offspring. D--F, Representative images of pancreas morphology in male control, restricted, and restricted, leptin-treated offspring, respectively. G–I, Representative images of pancreas morphology in female control, restricted, and restricted, leptin-treated offspring, respectively. Line draw around β-cell area represents the selected region of interest for β-cell mass index. Average β-cell number (J), β-cell mass (K), α-cell number (L), and β- to α-cell ratio (M) compared among treatment groups in male and female offspring are shown. Insulin-positive β-cells are stained cyan and glucagon-positive α-cells are magenta. Data are represented as mean ± sem. *, Significant difference from control of same sex (P < 0.05). For comparisons among treatments, n = 5 control, four restricted, and four restricted, leptin treated for both males and females.
Pancreas morphology followed the same patterns as insulin levels and glucose tolerance. The α- and β-cell number, β-cell mass, and β- to α-cell ratio were not different among maternal treatment groups in male offspring (Fig. 3, D–F and J–M). Female offspring from restricted (n = 4) and restricted, leptin-treated (n = 4) mothers had numerically higher but not significantly different β-cell number (P = 0.08) and β-cell mass (P = 0.13) compared with control (n = 5) female offspring (Fig. 3, J—K). The α-cell number and β- to α-cell ratio were not different among female offspring groups (Fig. 3, G–M).
Several adaptations were found in the gene expression of glucose metabolism regulators in the livers of male offspring from restricted mothers (Fig. 2B). Despite the absence of a significant difference in glucose tolerance, male offspring of restricted mothers had gene expression changes indicative of enhanced insulin sensitivity. Gck (glucokinase), a key enzyme in glycogen synthesis and glycolysis, was significantly increased (P < 0.05) in males from restricted mothers compared with controls but not different between male offspring of restricted, leptin-treated mothers and controls (P = 0.08). Insr (insulin receptor) was also significantly increased (P < 0.05) in restricted male offspring compared with controls. Akt2 (thymoma viral protooncogene 2), Nr3c1 (nuclear receptor subfamily 3, group C, member 1, glucocorticoid receptor), and Irs1 (insulin receptor substrate 1) gene expression was not different among the treatment groups. There was no effect of maternal treatment group on the expression of genes related to glucose metabolism in male offspring visceral fat (Fig. 2D). In females, no differences were found in liver or visceral fat gene expression (Figure 2, F and H) among the treatment groups. Overall, after the HFD, male offspring were significantly (P < 0.05) more insulin resistant and glucose intolerant compared with female offspring (Fig. 3, A–C). Male offspring (n = 54) produced significantly (P < 0.05) more insulin (44.8 ± 9.1 ng/ml) than female offspring (n = 44, 13.7 ± 6.6 ng/ml). Males (n = 13) also had significantly (P < 0.05) higher area under the curve (36845 ± 2352) on the IPGTT vs. female offspring (n = 25, 29480 ± 1951). The pancreas morphology assessment revealed that male offspring (n = 42) had significantly decreased (P < 0.0001) α-cell numbers and significantly increased (P < 0.0001) β-cell numbers, β-cell mass, and β- to α-cell ratio compared with female offspring (n = 40) (Fig. 3, D–M).
NAFLD assessment
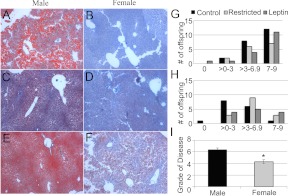
Another common feature of metabolic syndrome is NAFLD. The pathological analysis of offspring livers revealed that the severity of NAFLD did not differ between male offspring (Fig. 4C and Supplemental Figs. 1 and 2). All male offspring showed signs of NAFLD. No significant differences in the severity of NAFLD were observed among treatment groups in female offspring (Fig. 4F). The distributions of total scores for NAFLD were compared between all male (n = 54) and female (n = 44) offspring, regardless of treatment group, and males showed a greater severity of NAFLD overall (P = 0.008, Fig. 4I).
Fig. 4.
NAFLD diagnosis of male and female offspring. Representative images of NAFLD in male control (A), restricted (C), leptin-treated (E), and female control (B), restricted (D), and leptin-treated (F) offspring are shown. Histogram of distribution of NAFLD severity in male (G) and female (H) offspring is also shown. For comparisons among treatments, n = 5 control, four restricted, and four restricted, leptin-treated for both males and females. I, Comparison of mean NAFLD severity ± sem between male and female offspring. *, Significant difference between sexes (P < 0.05), n = 54 males and 44 females.
Brain and behavior
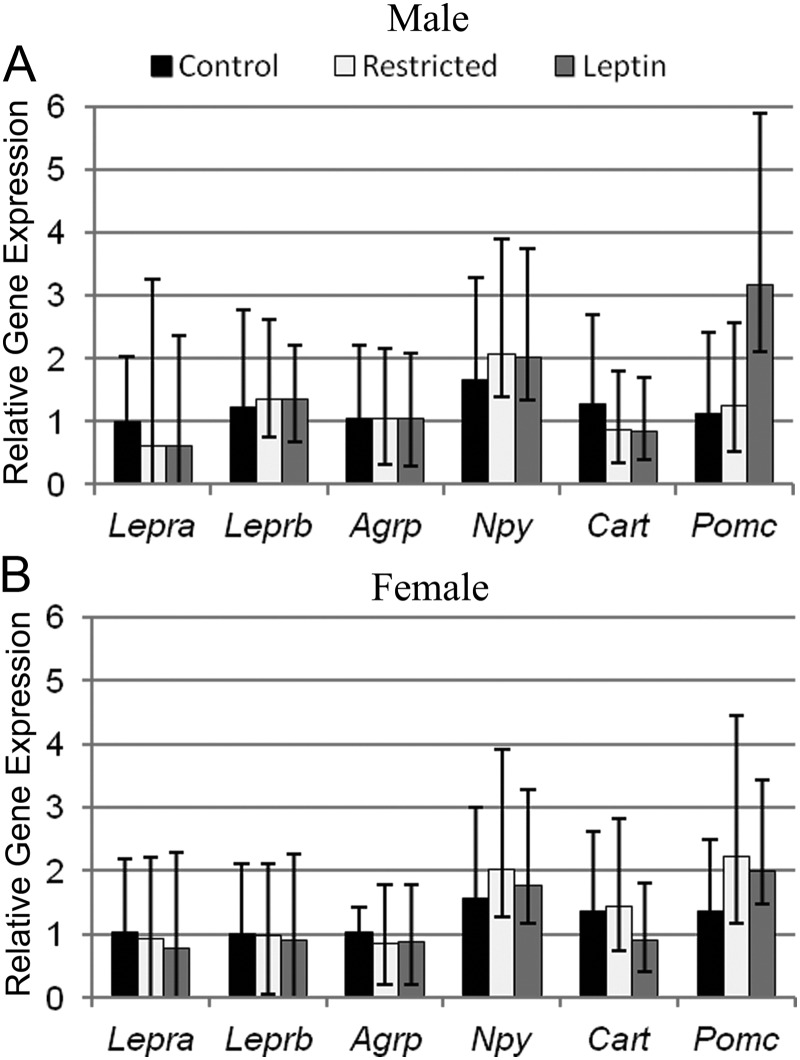
The hypothalamus is a central site in the body that controls feeding behavior and energy homeostasis. Leptin signaling in the hypothalamus via leptin receptors acts on orexigenic and anorexigenic neuropeptides to control satiety (41). Analysis of Lepra and Leprb in the hypothalamus revealed that male offspring from restricted and restricted, leptin-treated mothers tended (P = 0.06) to have decreased Lepra but not Leprb gene expression compared with control male offspring (Fig. 5A). No differences in Lepra or Leprb were found among female offspring (Fig. 5B).
Fig. 5.
Real-time PCR analysis of hypothalamic gene expression in male (A) and female (B) offspring. Data are represented as mean fold change relative to control mean ± range of fold change based on sem of ΔΔCt, n = 5 control, four restricted, and four restricted, leptin treated for both males and females.
The hypothalamic gene expression of orexigenic neuropeptides, Agrp and Npy, and anorexigenic neuropeptides, Cart and Pomc, were also examined in male and female offspring (Fig. 5, A and B). Pomc trended (P = 0.06) higher in male offspring from restricted, leptin-treated mothers compared with control and restricted male offspring. Agrp, Npy, and Cart did not differ among maternal treatments in male or female offspring.
Locomotor activity as measured by distance traveled in 1 h was similar among the treatment groups in male and female offspring (Supplemental Table 2). Additionally, anxiety level was not affected by maternal treatment in either male or female offspring (Supplemental Table 2).
Offspring bone biomechanics
Male offspring from food-restricted mothers had stronger bones than controls, and this effect was not attenuated by maternal leptin supplementation. Bone marrow diameter was significantly decreased (P < 0.05) in male offspring from restricted and restricted, leptin-treated mothers compared with control male offspring (Table 2). The bone energy to failure, or total energy the bone can absorb before fracturing, was significantly increased (P < 0.05) in male offspring from restricted and restricted, leptin-treated mothers compared with controls (Table 2). There was a trend (P = 0.06) for energy to failure to be increased in female offspring from restricted mothers compared with control female offspring (Table 2).
Overall, males had stronger bones than females. Males had a significantly larger (P < 0.0001) marrow diameter than females. Males also had significantly increased (P < 0.0001) polar moment of area and (P < 0.001) maximal torque compared with females. Females had a significantly greater (P < 0.005) femur material shear modulus compared with males. All of these differences account for males having a significantly increased (P < 0.005) energy to failure compared with females.
Discussion
Effects of maternal food restriction on male offspring
Based on previous studies in humans, rats, and sheep (7, 8, 12), we predicted that maternal food restriction during early pregnancy would result in higher incidence of metabolic syndrome features (elevated body weight, body fat percentage, glucose intolerance and NAFLD) in male offspring and that this would be exacerbated by a HFD (6). If anything, maternal food restriction had the opposite effect. On a standard diet, male offspring from restricted mothers had a body weight similar to controls, with a lower body fat percentage, and thus a higher lean mass. After exposure to the HFD, all male offspring, regardless of maternal treatment, exhibited features of the metabolic syndrome: increase in weight, high body fat percentage, glucose intolerance, and NAFLD. However, even after the HFD, male offspring of restricted mothers appeared to be more resistant to metabolic syndrome than controls. They had increased expression of IGF pathway components, indicative of their potential for increased lean mass, particularly muscle (42), and had significantly increased bone strength compared with control offspring. The IGF signaling axis can be programmed by maternal undernutrition (43–45). Skeletal growth is also regulated by the IGF signaling axis (46). There was an up-regulation of expression of multiple insulin signaling components including Gck and Insr in male offspring from restricted mothers compared with control male offspring.
There are a few potential explanations for the unexpected effect of maternal food restriction on male offspring. One may be a species difference, particularly with regard to the precise timing of food restriction. Although it is the period with the strongest evidence across multiple species, there are no data specifically in mice on food restriction during early pregnancy. Food restriction throughout pregnancy in mice and rats can program obesity after a HFD (19, 47). Although restriction throughout pregnancy has shown similar effects in rats and mice, there is a lack of effect seen in humans (48), suggesting different timing sensitivities. It may also be of note that food restriction in our study began on d 1.5, thus missing the periconceptual period, which may be crucial.
Another possibility is that leptin levels were not actually reduced in the food-restricted mothers. Previous work from our laboratory demonstrated in detail that 50% food restriction from d 1.5–11.5 severely decreases maternal leptin concentrations, (31). In this study, both control and restricted mothers were given a saline injection to control for handling stress in the leptin-treated mothers. When we measured leptin levels under these conditions, 2 h after the leptin injection, there was no difference in leptin concentrations in control and restricted mothers (Schlitt, J. M., and L. C. Schulz, unpublished data). A direct comparison of leptin concentrations in a small number of restricted mothers who were or were not given saline injections suggests that it may have increased serum leptin levels (unpublished data). We do not know whether the injection effect lasted beyond 2 h. There is one important piece of evidence suggesting that the food-restricted mothers were truly leptin deficient: leptin injection reversed most of the effects of food restriction on offspring.
Bone is also known to be affected by fetal programming (49). In one study, whole pregnancy protein restriction caused a decrease in offspring bone mass (50). This is an opposite outcome compared with our observations, with early-pregnancy nutrient restriction resulting in male offspring having stronger bones. One possibility for these differences is the timing of treatment; a shorter period of nutrient deprivation may allow offspring to compensate and thus develop stronger bones. Furthermore, although leptin affects bone mass in adulthood (for review, see Ref. 51), we found no effect of leptin during early pregnancy on adult bone strength.
Our second prediction was that effects of maternal food restriction on male offspring would be reversed by leptin treatment, and this did occur. Before the HFD, body composition of male offspring of restricted, leptin-treated mothers was more similar to controls than restricted offspring. Similarly, effects of maternal food restriction on the IGF system and glucose metabolism of male offspring after the HFD were partially or completely attenuated by the leptin treatment. The only exception was increased bone strength of restricted offspring, which was not attenuated by leptin treatment. Thus, we conclude that d 1.5-d11.5 maternal food restriction programs reduced susceptibility of male offspring to metabolic syndrome and that this is due, in part, to reduced maternal leptin.
HFD more severely impacted male offspring than female offspring
The data presented here clearly indicate that males, regardless of maternal treatment group, are more susceptible to metabolic syndrome than females when on a HFD. Overall, male offspring were diabetic at the time the animals were killed and developed more severe NAFLD compared with female offspring. This is consistent with statistics indicating that men have a higher incidence of metabolic syndrome compared with women (3) and with previous data in mice (52). Males also had stronger bones compared with females, a phenomenon that has been previously described (53, 54).
Fetal programming of metabolic syndrome occurred in female offspring from restricted, leptin-treated mothers
Female offspring from restricted, leptin-treated mothers developed multiple signs and symptoms of metabolic syndrome, including obesity and insulin resistance, compared with control and restricted female offspring. Significant weight differences were detected only after HFD, but it is not clear whether the diet alone, increasing age, or a combination, caused the weights to diverge significantly, and we did not conduct other measures in the absence of HFD. Some maternal nutritional programming studies have shown that effects of fetal programming may become apparent with age alone (17, 55), whereas others have found that feeding a HFD is necessary to exacerbate programming effects (19, 56). Our findings indicate that in utero exposure to increased maternal leptin levels (hyperleptinemia), even during food restriction, can lead to fetal programming events in female offspring. These findings mimic fetal programming studies of maternal obesity in which female offspring are obese and diabetic (9, 11). In contrast, maternal food restriction during early pregnancy generally does not affect female offspring (6, 12) because it did not in this study. Leptin levels are positively correlated with BMI in nonpregnant women and in first-trimester pregnant women (27, 57). By giving leptin to restricted mothers, we have isolated the effects of hyperleptinemia from other consequences of maternal obesity. Thus, exposure to hyperleptinemia during gestation is one mechanism by which obesity, especially in female offspring, is perpetuated.
Leptin may play a role in the sexual dimorphism in nutritional fetal programming
The effects of maternal nutritional status on offspring are known to be sexually dimorphic. Previous studies in animal models and humans indicate that male offspring are more susceptible to programming by maternal food restriction (7, 8, 12), whereas female offspring are more susceptible to fetal programming by maternal obesity (9–11). Our data indicate that male offspring are more affected by maternal hypoleptinemia and female offspring by maternal hyperleptinemia. Only female mice born to restricted mothers with hyperleptinemia during the first half of gestation were more likely to become obese and insulin resistant. Only male offspring born to food restricted mothers were different from controls. The ability of leptin to reverse this effect of maternal food restriction shows that it is due at least in part to hypoleptinemia. Based on these observations, we conclude that differential responses to leptin contribute to the sexual dimorphism seen in fetal programming events caused by under- and overnutrition. Further studies are needed to examine the mechanism through which leptin may act to affect fetal programming events in a sex-specific manner.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support provided by Lixin Ma and Ming Yang and the Veterans Affairs Biomolecular Imaging Center at the Harry S. Truman Veterans Affairs Hospital University of Missouri-Columbia, Dennis Miller and the University of Missouri Center for Translational Neuroscience Behavior Testing Core. We also thank Tina Wang for her assistance in evaluating kidney morphology.
This work was supported by National Institutes of Health Grant HD055231.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- μCT
- microcomputed tomography
- dpc
- d after conception
- GLM
- general linear model
- HFD
- high-fat diet
- IPGTT
- ip glucose tolerance test
- MRI
- magnetic resonance imaging
- NAFLD
- nonalcoholic fatty liver disease
- PFA
- paraformaldehyde.
References
- 1. Ono T, Guthold R, Strong K. 2005. World Health Organization global comparable estimates, http://infobase.who.int
- 2. Rinaudo P, Wang E. 2012. Fetal Programming and metabolic syndrome. Annu Rev Physiol 74:107–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ford ES, Li C, Zhao G. 2010. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes 2:180–193 [DOI] [PubMed] [Google Scholar]
- 4. McMillen IC, Robinson JS. 2005. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85:571–633 [DOI] [PubMed] [Google Scholar]
- 5. Barker DJ, Osmond C. 1986. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1:1077–1081 [DOI] [PubMed] [Google Scholar]
- 6. Jones AP, Assimon SA, Friedman MI. 1986. The effect of diet on food intake and adiposity in rats made obese by gestational undernutrition. Physiol Behav 37:381–386 [DOI] [PubMed] [Google Scholar]
- 7. Ravelli GP, Stein ZA, Susser MW. 1976. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 295:349–353 [DOI] [PubMed] [Google Scholar]
- 8. Ford SP, Hess BW, Schwope MM, Nijland MJ, Gilbert JS, Vonnahme KA, Means WJ, Han H, Nathanielsz PW. 2007. Maternal undernutrition during early to mid-gestation in the ewe results in altered growth, adiposity, and glucose tolerance in male offspring. J Anim Sci 85:1285–1294 [DOI] [PubMed] [Google Scholar]
- 9. Han J, Xu J, Epstein PN, Liu YQ. 2005. Long-term effect of maternal obesity on pancreatic β cells of offspring: reduced β cell adaptation to high glucose and high-fat diet challenges in adult female mouse offspring. Diabetologia 48:1810–1818 [DOI] [PubMed] [Google Scholar]
- 10. Bayol SA, Simbi BH, Bertrand JA, Stickland NC. 2008. Offspring from mothers fed a ‘junk food’ diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol 586:3219–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. 2003. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41:168–175 [DOI] [PubMed] [Google Scholar]
- 12. Jones AP, Friedman MI. 1982. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science 215:1518–1519 [DOI] [PubMed] [Google Scholar]
- 13. Hyatt MA, Gopalakrishnan GS, Bispham J, Gentili S, McMillen IC, Rhind SM, Rae MT, Kyle CE, Brooks AN, Jones C, Budge H, Walker D, Stephenson T, Symonds ME. 2007. Maternal nutrient restriction in early pregnancy programs hepatic mRNA expression of growth-related genes and liver size in adult male sheep. J Endocrinol 192:87–97 [DOI] [PubMed] [Google Scholar]
- 14. Anguita RM, Sigulem DM, Sawaya AL. 1993. Intrauterine food restriction is associated with obesity in young rats. J Nutr 123:1421–1428 [DOI] [PubMed] [Google Scholar]
- 15. Jones AP, Pothos EN, Rada P, Olster DH, Hoebel BG. 1995. Maternal hormonal manipulations in rats cause obesity and increase medial hypothalamic norepinephrine release in male offspring. Brain Res Dev Brain Res 88:127–131 [DOI] [PubMed] [Google Scholar]
- 16. Burt BE, Hess BW, Nathanielsz PW, Ford SP. 2007. Flock differences in the impact of maternal dietary restriction on offspring growth and glucose tolerance in female offspring. Soc Reprod Fertil Suppl 64:411–424 [DOI] [PubMed] [Google Scholar]
- 17. George LA, Zhang L, Tuersunjiang N, Ma Y, Long NM, Uthlaut AB, Smith DT, Nathanielsz PW, Ford SP. 2012. Early maternal undernutrition programs increased feed intake, altered glucose metabolism and insulin secretion, and liver function in aged female offspring. Am J Physiol Regul Integr Comp Physiol 302:R795–R804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howie GJ, Sloboda DM, Vickers MH. 2011. Maternal undernutrition during critical windows of development results in differential and sex-specific effects on postnatal adiposity and related metabolic profiles in adult rat offspring. Br J Nutr:1–10 [DOI] [PubMed] [Google Scholar]
- 19. Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. 2000. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab 279:E83–E87 [DOI] [PubMed] [Google Scholar]
- 20. Vickers MH, Breier BH, McCarthy D, Gluckman PD. 2003. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol Regul Integr Comp Physiol 285:R271–R273 [DOI] [PubMed] [Google Scholar]
- 21. Yan X, Huang Y, Zhao JX, Long NM, Uthlaut AB, Zhu MJ, Ford SP, Nathanielsz PW, Du M. 2011. Maternal obesity-impaired insulin signaling in sheep and induced lipid accumulation and fibrosis in skeletal muscle of offspring. Biol Reprod 85:172–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Strakovsky RS, Zhang X, Zhou D, Pan YX. 2011. Gestational high fat diet programs hepatic phosphoenolpyruvate carboxykinase gene expression and histone modification in neonatal offspring rats. J Physiol 589:2707–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ong ZY, Muhlhausler BS. 2011. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB J 25:2167–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Samuelsson AM, Matthews PA, Argenton M, Christie MR, McConnell JM, Jansen EH, Piersma AH, Ozanne SE, Twinn DF, Remacle C, Rowlerson A, Poston L, Taylor PD. 2008. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51:383–392 [DOI] [PubMed] [Google Scholar]
- 25. Djiane J, Attig L. 2008. Role of leptin during perinatal metabolic programming and obesity. J Physiol Pharmacol 59(Suppl 1):55–63 [PubMed] [Google Scholar]
- 26. Hill JW, Elmquist JK, Elias CF. 2008. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab 294:E827–E832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. 1996. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab 81:4406–4413 [DOI] [PubMed] [Google Scholar]
- 28. Iwaniec UT, Boghossian S, Lapke PD, Turner RT, Kalra SP. 2007. Central leptin gene therapy corrects skeletal abnormalities in leptin-deficient ob/ob mice. Peptides 28:1012–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sáinz N, Rodríguez A, Catalán V, Becerril S, Ramírez B, Gómez-Ambrosi J, Frühbeck G. 2009. Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1α in ob/ob mice. PLoS One 4:e6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pico C, Jilkova ZM, Kus V, Palou A, Kopecky J. 2011. Perinatal programming of body weight control by leptin: putative roles of AMP kinase and muscle thermogenesis. Am J Clin Nutr 94:1830S–1837S [DOI] [PubMed] [Google Scholar]
- 31. Schlitt JM, Schulz LC. 2012. The source of leptin, but not leptin depletion in response to food restriction, changes during early pregnancy in mice. Endocrine 41:227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zwingman T, Erickson RP, Boyer T, Ao A. 1993. Transcription of the sex-determining region genes Sry and Zfy in the mouse preimplantation embryo. Proc Natl Acad Sci USA 90:814–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calderan L, Marzola P, Nicolato E, Fabene PF, Milanese C, Bernardi P, Giordano A, Cinti S, Sbarbati A. 2006. In vivo phenotyping of the ob/ob mouse by magnetic resonance imaging and 1H-magnetic resonance spectroscopy. Obesity (Silver Spring) 14:405–414 [DOI] [PubMed] [Google Scholar]
- 34. Carleton SM, McBride DJ, Carson WL, Huntington CE, Twenter KL, Rolwes KM, Winkelmann CT, Morris JS, Taylor JF, Phillips CL. 2008. Role of genetic background in determining phenotypic severity throughout postnatal development and at peak bone mass in Col1a2 deficient mice (oim). Bone 42:681–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim JH, Stewart TP, Soltani-Bejnood M, Wang L, Fortuna JM, Mostafa OA, Moustaid-Moussa N, Shoieb AM, McEntee MF, Wang Y, Bechtel L, Naggert JK. 2006. Phenotypic characterization of polygenic type 2 diabetes in TALLYHO/JngJ mice. J Endocrinol 191:437–446 [DOI] [PubMed] [Google Scholar]
- 36. Schroder AL, Pelch KE, Nagel SC. 2009. Estrogen modulates expression of putative housekeeping genes in the mouse uterus. Endocrine 35:211–219 [DOI] [PubMed] [Google Scholar]
- 37. Heijboer AC, Voshol PJ, Donga E, van Eden CG, Havekes LM, Romijn JA, Pijl H, Corssmit EP. 2005. High fat diet induced hepatic insulin resistance is not related to changes in hypothalamic mRNA expression of NPY, AgRP, POMC and CART in mice. Peptides 26:2554–2558 [DOI] [PubMed] [Google Scholar]
- 38. Penney DP, Powers JM, Frank M, Willis C, Churukian C. 2002. Analysis and testing of biological stains—the Biological Stain Commission procedures. Biotech Histochem 77:237–275 [DOI] [PubMed] [Google Scholar]
- 39. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. 1999. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94:2467–2474 [DOI] [PubMed] [Google Scholar]
- 40. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. 2005. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321 [DOI] [PubMed] [Google Scholar]
- 41. Wauman J, Tavernier J. 2012. Leptin receptor signaling: pathways to leptin resistance. Front Biosci 17:2771–2793 [DOI] [PubMed] [Google Scholar]
- 42. Duan C, Ren H, Gao S. 2010. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol 167:344–351 [DOI] [PubMed] [Google Scholar]
- 43. Hyatt MA, Budge H, Walker D, Stephenson T, Symonds ME. 2007. Ontogeny and nutritional programming of the hepatic growth hormone-insulin-like growth factor-prolactin axis in the sheep. Endocrinology 148:4754–4760 [DOI] [PubMed] [Google Scholar]
- 44. El-Khattabi I, Grégoire F, Remacle C, Reusens B. 2003. Isocaloric maternal low-protein diet alters IGF-I, IGFBPs, and hepatocyte proliferation in the fetal rat. Am J Physiol Endocrinol Metab 285:E991–E1000 [DOI] [PubMed] [Google Scholar]
- 45. Li C, Schlabritz-Loutsevitch NE, Hubbard GB, Han V, Nygard K, Cox LA, McDonald TJ, Nathanielsz PW. 2009. Effects of maternal global nutrient restriction on fetal baboon hepatic insulin-like growth factor system genes and gene products. Endocrinology 150:4634–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yakar S, Courtland HW, Clemmons D. 2010. IGF-1 and bone: new discoveries from mouse models. J Bone Miner Res 25:2543–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, Kawamura M, Takemura M, Kakui K, Ogawa Y, Fujii S. 2005. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab 1:371–378 [DOI] [PubMed] [Google Scholar]
- 48. Stanner SA, Bulmer K, Andrès C, Lantseva OE, Borodina V, Poteen VV, Yudkin JS. 1997. Does malnutrition in utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad Siege Study, a cross sectional study. BMJ 315:1342–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cooper C, Westlake S, Harvey N, Javaid K, Dennison E, Hanson M. 2006. Review: developmental origins of osteoporotic fracture. Osteoporos Int 17:337–347 [DOI] [PubMed] [Google Scholar]
- 50. Mehta G, Roach HI, Langley-Evans S, Taylor P, Reading I, Oreffo RO, Aihie-Sayer A, Clarke NM, Cooper C. 2002. Intrauterine exposure to a maternal low protein diet reduces adult bone mass and alters growth plate morphology in rats. Calcif Tissue Int 71:493–498 [DOI] [PubMed] [Google Scholar]
- 51. Karsenty G, Yadav VK. 2011. Regulation of bone mass by serotonin: molecular biology and therapeutic implications. Annu Rev Med 62:323–331 [DOI] [PubMed] [Google Scholar]
- 52. Hong J, Stubbins RE, Smith RR, Harvey AE, Núñez NP. 2009. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J 8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seeman E. 2001. Clinical review 137: sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab 86:4576–4584 [DOI] [PubMed] [Google Scholar]
- 54. Iuliano-Burns S, Hopper J, Seeman E. 2009. The age of puberty determines sexual dimorphism in bone structure: a male/female co-twin control study. J Clin Endocrinol Metab 94:1638–1643 [DOI] [PubMed] [Google Scholar]
- 55. Petry CJ, Dorling MW, Pawlak DB, Ozanne SE, Hales CN. 2001. Diabetes in old male offspring of rat dams fed a reduced protein diet. Int J Exp Diabetes Res 2:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bellinger L, Sculley DV, Langley-Evans SC. 2006. Exposure to undernutrition in fetal life determines fat distribution, locomotor activity and food intake in ageing rats. Int J Obes (Lond) 30:729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hedley P, Pihl K, Krebs L, Larsen T, Christiansen M. 2009. Leptin in first trimester pregnancy serum: no reduction associated with small-for-gestational-age infants. Reprod Biomed Online 18:832–837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.