Abstract
Activity of the hypothalamic-pituitary-adrenocortical (HPA) axis is commonly assessed by measuring glucocorticoids such as cortisol (CORT). For many years, CORT was obtained primarily from blood plasma or urine, whereas later approaches added saliva and feces for noninvasive monitoring of HPA functioning. Despite the value of all these sample matrices for answering many research questions, they remain limited in the temporal range of assessment. Plasma and saliva are point samples that vary as a function of circadian rhythmicity and are susceptible to confounding by environmental disturbances. Even urine and feces generally assess HPA activity over a period of only 24 h or less. We and others have recently developed and validated methods for measuring the concentration of CORT in the body hair of animals (e.g. rhesus monkeys) and scalp hair of humans. CORT is constantly deposited in the growing hair shaft, as a consequence of which such deposition can serve as a biomarker of integrated HPA activity over weeks and months instead of minutes or hours. Since the advent of this methodological advance, hair CORT has already been used as an index of chronic HPA activity and stress in human clinical and nonclinical populations, in a variety of laboratory-housed and wild-living animal species, and in archival specimens that are many decades or even centuries old. Moreover, because human hair is known to grow at an average rate of about 1 cm/month, several studies suggest that CORT levels in hair segments that differ in proximity to the scalp can, under certain conditions, be used as a retrospective calendar of HPA activity during specific time periods preceding sample collection.
The hypothalamic-pituitary-adrenocortical (HPA) system is rapidly activated by stressors. Although thousands of papers have been published relating HPA activity to acutely stressful situations, the field has been hampered by the lack of a simple and reliable index of chronic physiological stress. Investigators previously developed methods for assessing cortisol (CORT) in several different sampling matrices. The most widely used matrices are blood and saliva, both of which yield point estimates of HPA activity that are subject to circadian variation and can be confounded by environmental disturbances. Urinary and fecal samples yield measurements of CORT and/or metabolite excretion that span a number of hours up to a full day in some cases. Collection of multiple samples using any of these matrices may provide a rough composite index of CORT levels over time; however, none of these approaches provides a truly long-term index of HPA activity and the responsiveness of this system to chronic stressors.
Measuring CORT in hair has begun to fill this important need in the stress literature. For many years, forensic scientists have used hair samples to detect banned substances including stimulant drugs and anabolic steroids (1). The first forensic papers to describe methods for detecting various corticosteroids (both natural and synthetic) were published in 2000 by Bévalot et al. (2), Cirimele et al. (3), and Gaillard et al. (4), followed a few years later by quantification of endogenous CORT and cortisone in the hair of normal male and female human subjects (5). Because our laboratory has long been interested in characterizing HPA axis function in rhesus monkeys suffering from spontaneously occurring self-injurious behavior (6), we were prompted by these observations to develop and validate a simple method for measuring hair CORT in monkeys as a biomarker for stress-related HPA activation (7). This initial work demonstrated not only that CORT was readily quantifiable in monkey hair but also that the levels in hair were positively correlated with salivary CORT concentrations. Most importantly, hair CORT was substantially elevated over baseline when the same monkeys were subjected to the major life stressor of an administratively mandated relocation to new housing quarters (7, 8). Subsequent studies by other laboratories have shown that hair CORT levels represent a heritable trait that exhibits substantial intra-individual stability over time in the absence of altered allostatic load (9, 10).
This minireview will examine the putative mechanisms of incorporation and elimination of CORT in hair and consider several methodological issues that are relevant for investigators using this tool in their research. We will assess the extent to which hair CORT is related to levels of CORT in other sample matrices and to estimates of perceived stress, and we will additionally discuss some of the limitations of hair CORT measurement. Given the rapid rise in the number of studies using hair CORT, this review will summarize some of the applications for which hair CORT measurement has been used and provide a brief discussion of future directions in the field.
Putative Mechanisms of CORT Incorporation and Elimination in Hair
CORT incorporation into hair
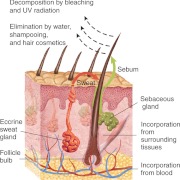
The exact mechanism whereby CORT is incorporated into hair is not yet known. Consequently, because CORT and other steroid hormones are lipophilic substances, scientists must rely on what forensic scientists have discovered about routes of incorporation of lipophilic drugs into the growing hair shaft. Figure 1 depicts the known mechanisms of incorporation of substances into hair. A major route of incorporation is thought to be from the vascular supply to the follicular cells that generate the hair shaft (1, 11). Diffusion from tissues surrounding the follicle is likely to be an additional pathway by which CORT eventually reaches the hair.
Fig. 1.
Mechanisms of incorporation and elimination of lipophilic substances in hair.
Once the growing shaft emerges from the scalp, it is coated with sebum originating from the associated sebaceous gland along with sweat secreted by nearby eccrine glands. Early studies using iv administration of radiolabeled CORT to human subjects demonstrated that this hormone can diffuse from the bloodstream into both sebum and sweat (12, 13). Consequently, there is probably some deposition of CORT from these fluids onto the outside of the hair shaft. However, it remains unclear whether this external CORT is incorporated into the shaft.
Another potential source of CORT is the hair follicle itself. Research over the past 20 yr has established the skin as an endocrine organ (14). Most importantly, Ito and co-workers (15) demonstrated the existence of a functional HPA-like system in microdissected human hair follicles maintained in organ culture. The cultured follicles were shown to synthesize CRH, ACTH, CORT, and the cognate receptors for these signaling molecules. Furthermore, CORT was released into the culture medium and exerted a negative feedback action on CRH synthesis. More recently, Sharpley and colleagues (16, 17) reported that hair on the arm displayed a rapid increase in immunoreactive CORT after challenge with a cold pressor test. Although these findings suggest the possibility of local stress responses by the skin that could cause increased hair CORT concentrations, such a rapid incorporation of CORT into hair seems inconsistent both with the known structure of hair (containing barriers to diffusion due to its low water and lipid content) (18) and with the segmental analyses discussed in the next section that rely on CORT remaining relatively static within the hair shaft after deposition. Moreover, there are humoral and neuronal pathways whereby centrally mediated stress responses could signal the follicular HPA-like system (19). Additional studies are needed to ascertain the degree of cross talk between these central and peripheral systems.
Evidence for a local HPA-like system raises the important question of how much of the CORT measured in hair has come from the bloodstream. If most of the hair CORT originates from the bloodstream, we would predict that conditions that elevate circulating CORT should likewise be reflected in hair CORT concentrations. Indeed, hair CORT is elevated during the third trimester of pregnancy (20, 21) and in Cushing's syndrome (22, 23), both of which are physiological conditions associated with increased plasma CORT. Furthermore, three ACTH injections over a 14-d period resulted in significant increases in both plasma and hair CORT in dairy cattle (24). It is additionally possible that hair CORT, like CORT in saliva, is derived from the unbound fraction in the plasma. Although this hypothesis has not yet been proven, data from our monkey relocation study support the hypothesis insofar as changes in hair CORT levels over time after the relocation event closely paralleled changes in the free CORT index (the ratio of total plasma CORT to plasma corticosteroid-binding globulin) (8).
CORT elimination from hair
Forensic scientists have shown that washing of hair, cosmetic treatments, and exposure to UV irradiation from the sun are potential sources of drug elimination from hair (Fig. 1). The idea that these processes might also remove CORT from hair first arose from the results of segmental analyses of hair CORT levels in humans. Segmental analysis involves cutting hair samples into segments that vary with respect to their proximity to the scalp with the aim of constructing a retrospective calendar of HPA activity over the preceding several months. The ability to determine such a timeline is based on data showing that human scalp hair taken from the vertex posterior or the occipital region (the two most common areas for human hair sampling) grows at an average rate of about 1 cm/month (25). Therefore, the 1-cm segment closest to the scalp is thought to contain CORT incorporated during the previous month, the next 1-cm segment should contain CORT incorporated 1 month earlier, and so forth. Interestingly, the first reported segmental analysis, which measured hair CORT in 3-cm segments, found a monotonic decrease in CORT concentrations out to 18 cm from the scalp (20). Subsequent studies have found a similar segmental decline in some cases but not others (26). Experimental findings have now demonstrated that the segmental decline in hair CORT is likely due to repeated exposure to shampooing or to water alone, with the possibility of an additional contribution from UV radiation (27, 28). Additionally, exposure both to water and to UV radiation are likely to impact hair CORT levels in wild-living animals or captive animals living in outdoor enclosures.
Methodological Considerations for Hair Collection and Processing
Investigators are faced with a number of important issues in using hair as a sampling matrix, several of which are briefly discussed in this section. These issues include 1) where in the head (or body) to sample from, 2) how to obtain the hair, 3) how much hair to obtain, 4) whether or not to wash the hair sample, 5) how to process the hair before extraction, and 6) how to measure the extracted CORT. 1) Regardless of the site of hair sampling, it is obviously important to keep the site constant across subjects. For human studies, hair is typically obtained from the posterior vertex of the head, an area that has been shown to produce less intra-individual variation in CORT levels than other head regions (29). For rhesus monkeys, hair is collected from the nape of the neck because this area is relatively uncontaminated from self-grooming. 2) Hair should always be cut or shaved close to the skin (scalp) rather than plucked so as to avoid including follicles in the sample and to prevent possible blood contamination. In animals such as nonhuman primates, each hair shaft grows to a certain length after which growth stops until the hair is shed. Consequently, hair samples obtained without previous shaving will contain a mixture of hairs that have incorporated CORT over different time periods. This problem can be overcome by using a shave-reshave approach that establishes a known timeline of CORT incorporation (see for example Ref. 8). 3) Amounts of hair obtained vary widely across studies, from 250 mg in an earlier rhesus monkey study (7) to as little as 5 or 10 mg in more recent human studies (10, 30). Although it is theoretically possible to measure the CORT content of a single hair shaft if one has a sufficiently sensitive assay, such a measure may not be representative of overall hair CORT levels because hair growth occurs in cycles composed of three different phases that could differ in their CORT incorporation properties (18). 4) Some investigators do not wash hair samples before processing and extraction because washing removes a certain percentage of CORT from the sample and their goal is to measure total hair CORT content (see for example Ref. 31). In contrast, two brief washes with isopropanol removed only 5–10% of total CORT content of monkey hair (7). Subsequent studies were conducted with polar bear hair in which additional washes were performed to clean up samples visibly contaminated with blood and/or adipose tissue (32). The results of this work support the existence of two fractions of hair CORT: a fraction that presumably contains external CORT deposited via sebum, sweat, or other contaminants and that can be removed by mild washing procedures and a more tightly bound fraction that is believed to consist of CORT incorporated into the interior of the hair matrix and that can only be extracted by stronger treatment of the sample. 5) Hair samples are typically processed after washing and drying (if those steps are included) by mincing into small fragments (∼1 mm long) or by grinding into a powder using a ball mill (Retsch, Newtown, PA) or a mini-beadbeater (BioSpec Products, Bartlesville, OK). Preliminary studies with monkey hair showed significantly greater CORT recovery after grinding compared with mincing (7). 6) CORT is usually extracted from the minced fragments or powdered hair with methanol, a solvent that has the dual advantage of causing swelling of the hair (thus facilitating analyte liberation by diffusion) (11) and readily solubilizing steroid hormones such as CORT. After evaporation of the organic solvent, the extract is reconstituted in an appropriate medium and analyzed for its CORT content. Hair CORT has been analyzed by means of enzyme immunoassay, RIA, chemiluminescent immunoassay, HPLC-mass spectrometry, and HPLC with fluorescence detection. The choice of assay is not particularly important as long as it has the requisite specificity for CORT and a degree of sensitivity appropriate for the size of the samples being analyzed.
Relationship between Hair CORT Levels and CORT in Other Sample Matrices
Recent research has examined potential relationships between hair CORT and CORT levels in other sample matrices such as saliva, urine, and feces. In comparing hair and salivary CORT in the same subjects, the results have ranged from no significant correlation in two human studies (29, 33) to a correlation of approximately 0.8 in our initial work with adult male rhesus monkeys maintained in a highly stable environment (8). Two recent studies of healthy humans found moderate but statistically significant correlations of 0.3–0.4 between hair and salivary CORT (34, 35). In addition, correlations between hair CORT levels and area under the curve of salivary CORT across trimesters in pregnant women ranged from 0.29 (nonsignificant) in the first trimester to 0.57 (P = 0.01) in the third trimester (21). Taken together, these findings suggest that long-term HPA activity as assessed by hair CORT concentrations is related to short-term activity assessed by salivary CORT levels under some conditions but not others. The nature of the conditions that afford a significant correlation between these two variables remains to be determined. Alternatively, hair CORT might be more closely related to urinary or fecal CORT excretion because of the longer time frames encompassed by the latter sample matrices. Indeed, significant correlations ranging from 0.33 for 24-h urinary CORT excretion in humans (29) to 0.67 and 0.90 for fecal CORT excretion in dogs and cats, respectively, have been reported (36).
Relationship between Hair CORT Levels and Degree of Perceived Stress
A number of other studies have been directed toward determining whether hair CORT levels are related to perceived stress in humans. Two such studies found significant correlations of 0.47 in healthy pregnant women (37) and 0.59 in male patients being treated for adrenal insufficiency (38); however, many other studies found either no relationship between hair CORT and perceived stress (34, 39–43) or a slight negative correlation between the two variables (10, 30). Failure to obtain a significant relationship between perceived stress and other measures of HPA activity (i.e. salivary or plasma CORT) has been reported before and has been termed lack of psychoendocrine covariance (39, 44). This phenomenon could be due to a differential responsiveness of the neural circuitry controlling the HPA axis compared with the circuitry mediating the subjective experience of stress. In the case of hair, however, it is important to note that perceived stress is typically measured at just one or a few time points, whereas hair CORT is thought to reflect an integral of adrenocortical activity over many weeks or months. Perhaps if measures of perceived stress were obtained repeatedly over the same time period during which CORT is being deposited in the hair shaft, a positive psychoendocrine covariance would be observed.
Applications of Hair CORT
In just a few years since its inception as a new biomarker of HPA axis activity, hair CORT has been used in a wide variety of applications. Most such applications fall into one of the categories listed in Table 1 and are briefly discussed below.
Table 1.
Categories of applications for which hair CORT has been used
Chronic stress
Not surprisingly hair CORT has been used to investigate the influence of major life stressors on chronic HPA activity. Several studies have now demonstrated that hair CORT concentrations are indeed elevated in subjects undergoing significant stress compared either with matched controls or with the same subjects before stress imposition. Nonhuman primate examples include rhesus macaques, bonnet macaques, and vervet monkeys subjected to relocation stress (7–9, 45). Human studies of hair CORT and stress include individuals suffering from chronic pain (46), individuals who were unemployed for at least 12 months compared with those who had jobs (39), individuals consigned to shift work compared with day workers (47), endurance athletes who undergo severe physical stress (42), patients hospitalized with acute myocardial infarction compared with control patients (48), alcohol-dependent individuals undergoing withdrawal compared with abstinent alcoholics or control subjects (41), and newborn infants who required hospitalization in the neonatal intensive care unit compared with healthy newborns (49).
Endocrine disorders
Among the clinical applications of hair CORT is the determination of long-term changes in CORT levels in patients with Cushing's syndrome or adrenal insufficiency. Initial studies showed that hair CORT can play a useful role in monitoring disease progression and treatment efficacy in such patients (22, 23). This approach has also revealed that some patients suffering from adrenal insufficiency may have received inappropriately high doses of hydrocortisone replacement therapy (38).
Neuropsychiatric disorders
Several neuropsychiatric disorders are characterized by dysregulation of the HPA axis, notably major depression and posttraumatic stress disorder (PTSD). Recent work by Dettenborn and colleagues (50) found increased CORT concentrations in the first and second 3-cm hair segments (from the scalp) of depressed patients compared with matched healthy controls. Two PTSD studies have also been published thus far. The first reported increased hair CORT in Ugandan PTSD patients who had been severely traumatized compared with severely traumatized Ugandans who had not developed PTSD (51). The second study examined female adolescents who experienced a major earthquake in China in 2008 (52). Subjects exposed to the earthquake had higher hair CORT levels than controls who were not affected by the earthquake, but within the earthquake-exposed group, the adolescents who had developed PTSD had lower levels than those who had not developed the disorder. Finally, Steudte et al. (33) found decreased hair CORT concentrations in patients with generalized anxiety disorder compared with healthy controls. These studies illustrate that hair CORT can provide an important addition to the existing literature on salivary, plasma, or urinary CORT concentrations in patients with neuropsychiatric disorders.
Development
Early postnatal development is a period of both normative changes in HPA activity and sensitivity of the system to different rearing environments. Like plasma CORT, hair CORT levels decline during the first several years of life in a number of species. Such changes have been observed in rhesus monkeys [6–24 months of age (53); 2 yr vs. 3.5 yr of age (54)], vervet monkeys [<0.5–2.5 yr of age (55); 1–12 yr of age (56)], Guinea baboons [12–72 months (55)], and cattle [15 d old vs. 2 yr old (24)]. The two rhesus monkey studies also reported differences in developmental hair CORT profiles as a function of rearing environment (53, 54). Thus, at least in many nonhuman primates and in cattle, CORT levels are high during early postnatal development and gradually decrease over time. Hair CORT is also a sensitive indicator of altered HPA axis activity produced by different rearing environments.
Behavioral temperament
Behavioral temperament (defined as “the phenomenon that individual behavioral differences are consistent over time and/or across situations” in Ref. 57, p. 294) has previously been associated with HPA axis activity using traditional measures such as salivary CORT (58). More recently, hair CORT levels were found to be lower in vervet monkeys with a high novelty-seeking phenotype compared with low novelty-seeking animals (59), hair CORT during infancy predicted later anxiety-like behaviors as well as performance on an object permanence task in rhesus monkeys (53, 60), hair CORT in adult dogs was positively related to behavioral reactivity after exposure to the sounds of a thunderstorm (61), and hair CORT was positively correlated with agonistic behaviors in free-roaming female cats in Tel Aviv (62) but also positively correlated with a docility index in eastern chipmunks in a Quebec forest visited by tourists (63). Further research is needed to ascertain the genetic and environmental factors that contribute jointly to temperament and HPA activity.
Wildlife/conservation
Because hair collection is less invasive than blood drawing and measuring CORT in hair provides an integrated rather than a point measure of HPA activity, hair CORT is now used in wildlife and environmental conservation studies. One such study demonstrated the feasibility of measuring hair CORT in polar bears in East Greenland (32), which was followed by additional research showing that CORT levels were related to the concentrations of several persistent organohalogen pollutants in the same samples (64). Macbeth and co-workers (65) have also measured hair CORT in free-ranging grizzly bears in Alberta. Additional applications of hair CORT to the assessment of stress in wild-living animal populations are expected in the coming years.
Historical/archival samples
CORT is stable in intact hair for at least a year, even when maintained at ambient temperature (24). Measurable levels of this hormone persist for even longer periods of time, which permitted the assessment of CORT in museum polar bear fur specimens collected in the late 19th and early 20th centuries (66). In fact, the mean CORT level from the museum samples was approximately twice as high as the mean level in samples collected from free-ranging bears over the past 24 yr. This outcome seems highly unlikely if there had been significant CORT degradation within the hair over time. Even more remarkably, hair CORT has been measured in Peruvian human archaeological samples that were as much as 1500 yr old (67). The stability of CORT in hair compared with other biological compartments makes it ideal for using archival samples to obtain an index of HPA activity in long-deceased subjects.
Limitations of Hair CORT Measurement
Several limitations should be kept in mind when using the hair CORT approach. First, because hair CORT is thought to represent an integrated measure of HPA activity over long time periods, this approach is not sensitive to changes in the circadian rhythmicity of HPA activity or the awakening CORT response. Hair CORT levels also might not detect the impact of relatively brief stressors that occurred during the period of hormone deposition. Hence, this approach should be thought of as complementary to measurements of salivary and/or plasma CORT, not as a replacement for such measurements. Second, whereas the use of hair CORT will likely be of particular value to researchers interested in psychosocial and environmental stressors like those mentioned earlier, it is important to keep in mind that elevated HPA activity can occur under a variety of conditions, including physical exercise, metabolic abnormalities, and infectious disease. Indeed, one recent study found increased hair CORT in amateur endurance athletes (42), and another reported a significant positive correlation between hair CORT and body mass index (43). Third, the degree to which hair CORT is derived from the circulation has not yet been determined, nor is it certain that CORT in hair comes from the free fraction in the plasma.
The hypothesis that hair CORT is derived primarily from the bloodstream is supported by previously cited studies showing increased hair CORT levels during the third trimester of pregnancy, in Cushing's syndrome patients, and in cattle given repeated ACTH injections; however, such evidence is not definitive, and therefore additional research is needed. Finally, studies of plasma, salivary, and urinary CORT have led to a growing recognition that chronic stress can lead to HPA hypoactivity rather than hyperactivity under certain conditions (68). Hair CORT in stressed populations may similarly exhibit bidirectional changes depending on experimental variables such as the time of sampling in relation to stress onset.
Summary and Future Directions
Hair CORT provides a new approach to assessing long-term HPA activity over periods of weeks to months. Use of this novel measure should help resolve controversies in the current neuroendocrine literature (e.g. the status of HPA activity in PTSD patients) as well as open up new avenues for research. For example, it should be possible to assess in utero CORT exposure from about 28 wk of gestation to the time of birth by measuring the hormone in scalp hair from human newborns (69). Although limited by an apparent washout effect, segmental analysis of hair CORT content still has the potential to provide a retrospective calendar of HPA activity over a period of several months, particularly when combined with an appropriate cross-sectional comparison (see, for example, Refs. 20 and 52). Lastly, other steroid hormones are also deposited in growing hair and can be measured by appropriate analytical methods. There are published reports of hair testosterone levels in men and women (70, 71), and esterified steroids such as nortestosterone decanoate and estradiol benzoate have been analyzed in cattle hair after treatment with these growth-promoting compounds (72). In addition, our laboratory is currently working to develop and validate procedures for measuring corticosterone in rat and mouse hair so that this technique becomes available to investigators using rodent models.
Acknowledgments
We thank the many students, postdocs, and research assistants who contributed to the development, characterization, and application of our hair CORT methodology.
This work was supported by National Institutes of Health Grants RR11122 and RR00168.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CORT
- Cortisol
- HPA
- hypothalamic-pituitary-adrenocortical
- PTSD
- posttraumatic stress disorder.
References
- 1. Barroso M, Gallardo E, Vieira DN, López-Rivadulla M, Queiroz JA. 2011. Hair: a complementary source of bioanalytical information in forensic toxicology. Bioanalysis 3:67–79 [DOI] [PubMed] [Google Scholar]
- 2. Bévalot F, Gaillard Y, Lhermitte MA, Pépin G. 2000. Analysis of corticosteroids in hair by liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Biomed Sci Appl 740:227–236 [DOI] [PubMed] [Google Scholar]
- 3. Cirimele V, Kintz P, Dumestre V, Goullé JP, Ludes B. 2000. Identification of ten corticosteroids in human hair by liquid chromatography-ionspray mass spectrometry. Forensic Sci Int 107:381–388 [DOI] [PubMed] [Google Scholar]
- 4. Gaillard Y, Vayssette F, Pépin G. 2000. Compared interest between hair analysis and urinalysis in doping control. Results for amphetamines, corticosteroids and anabolic steroids in racing cyclists. Forensic Sci Int 107:361–379 [DOI] [PubMed] [Google Scholar]
- 5. Raul JS, Cirimele V, Ludes B, Kintz P. 2004. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem 37:1105–1111 [DOI] [PubMed] [Google Scholar]
- 6. Tiefenbacher S, Novak MA, Lutz CK, Meyer JS. 2005. The physiology and neurochemistry of self-injurious behavior: A nonhuman primate model. Front Biosci 10:1–11 [DOI] [PubMed] [Google Scholar]
- 7. Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, Meyer JS. 2006. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen Comp Endocrinol 147:255–261 [DOI] [PubMed] [Google Scholar]
- 8. Davenport MD, Lutz CK, Tiefenbacher S, Novak MA, Meyer JS. 2008. A rhesus monkey model of self injury: Effects of relocation stress on behavior and neuroendocrine function. Biol Psychiatry 63:990–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fairbanks LA, Jorgensen MJ, Bailey JN, Breidenthal SE, Grzywa R, Laudenslager ML. 2011. Heritability and genetic correlation of hair cortisol in vervet monkeys in low and higher stress environments. Psychoneuroendocrinology 36:1201–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stalder T, Steudte S, Miller R, Skoluda N, Dettenborn L, Kirschbaum C. 2012. Intraindividual stability of hair cortisol concentrations. Psychoneuroendocrinology 37:602–610 [DOI] [PubMed] [Google Scholar]
- 11. Pragst F, Balikova MA. 2006. State of the art in hair analysis for detection of drug and alcohol abuse. Clin Chim Acta 370:17–49 [DOI] [PubMed] [Google Scholar]
- 12. Cook TJ, Spector AR. 1964. Excretion of intravenously administered radioactive hydrocortisone in skin surface lipids. J Invest Dermatol 43:413–414 [DOI] [PubMed] [Google Scholar]
- 13. Jenkins ME, Rivarola MA, Brusilow SW, Migeon CJ. 1969. Excretion of 4-14C-cortisol and 1,2-3H-d-aldosterone in human thermal sweat. J Clin Endocrinol Metab 29:1102–1106 [DOI] [PubMed] [Google Scholar]
- 14. Slominski A. 2005. Neuroendocrine system of the skin. Dermatology 211:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. 2005. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal (HPA) axis and synthesize cortisol. FASEB J 19:1332–1334 [DOI] [PubMed] [Google Scholar]
- 16. Sharpley CF, Kauter KG, McFarlane JR. 2009. An initial exploration of in vivo hair cortisol responses to a brief pain stressor: latency, localization and independence effects. Physiol Res 58:757–761 [DOI] [PubMed] [Google Scholar]
- 17. Sharpley CF, Kauter KG, McFarlane JR. 2010. Hair cortisol concentration differs across site and person: Localisation and consistency of responses to a brief pain stressor. Physiol Res 59:979–983 [DOI] [PubMed] [Google Scholar]
- 18. Harkey MR. 1993. Anatomy and physiology of hair. Forensic Sci Int 63:9–18 [DOI] [PubMed] [Google Scholar]
- 19. Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. 2006. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol 126:1697–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. 2009. Hair as a retrospective calendar of cortisol production: increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology 34:32–37 [DOI] [PubMed] [Google Scholar]
- 21. D'Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. 2011. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav 104:348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomson S, Koren G, Fraser LA, Rieder M, Friedman TC, Van Uum SH. 2010. Hair analysis provides a historical record of cortisol levels in Cushing's syndrome. Exp Clin Endocrinol Diabetes 118:133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manenschijn L, Koper JW, Lamberts SW, van Rossum EF. 2011. Evaluation of a method to measure long term cortisol levels. Steroids 76:1032–1036 [DOI] [PubMed] [Google Scholar]
- 24. González-de-la-Vara Mdel R, Valdez RA, Lemus-Ramirez V, Vázquez-Chagoyán JC, Villa-Godoy A, Romano MC. 2011. Effects of adrenocorticotropic hormone challenge and age on hair cortisol concentrations in dairy cattle. Can J Vet Res 75:216–221 [PMC free article] [PubMed] [Google Scholar]
- 25. LeBeau MA, Montgomery MA, Brewer JD. 2011. The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Sci Int 210:110–116 [DOI] [PubMed] [Google Scholar]
- 26. Russell E, Koren G, Rieder M, Van Uum S. 2012. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 37:589–601 [DOI] [PubMed] [Google Scholar]
- 27. Hamel AF, Meyer JS, Henchey E, Dettmer AM, Suomi SJ, Novak MA. 2011. Effects of shampoo and water washing on hair cortisol concentrations. Clin Chim Acta 412:382–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li J, Xie Q, Gao W, Xu Y, Wang S, Deng H, Lu Z. 2012. Time course of cortisol loss in hair segments under immersion in hot water. Clin Chim Acta 413:434–440 [DOI] [PubMed] [Google Scholar]
- 29. Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SHM. 2007. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med 30:E183–E191 [DOI] [PubMed] [Google Scholar]
- 30. Karlén J, Ludvigsson J, Frostell A, Theodorsson E, Faresjö T. 2011. Cortisol in hair measured in young adults: a biomarker of major life stressors? BMC Clin Pathol 11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gow R, Thomson S, Rieder M, Van Uum S, Koren G. 2010. An assessment of cortisol analysis in hair and its clinical applications. Forensic Sci Int 196:32–37 [DOI] [PubMed] [Google Scholar]
- 32. Bechshøft TØ, Sonne C, Dietz R, Born EW, Novak MA, Henchey E, Meyer JS. 2011. Cortisol levels in hair of East Greenland polar bears. Sci Total Environ 409:831–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steudte S, Stalder T, Dettenborn L, Klumbies E, Foley P, Beesdo-Baum K, Kirschbaum C. 2011. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatry Res 186:310–314 [DOI] [PubMed] [Google Scholar]
- 34. van Holland BJ, Frings-Dresen MHW, Sluiter JK. 20 December 2011. Measuring short-term and long-term physiological stress effects by cortisol reactivity in saliva and hair. Int Arch Occup Environ Health 10.1007/s00420-011-0727-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xie Q, Gao W, Li J, Qiao T, Jin J, Deng H, Lu Z. 2011. Correlation of cortisol in 1-cm hair segment with salivary cortisol in human: hair cortisol as an endogenous biomarker. Clin Chem Lab Med 49:2013–2019 [DOI] [PubMed] [Google Scholar]
- 36. Accorsi PA, Carloni E, Valsecchi P, Viggiani R, Gamberoni M, Tamanini C, Seren E. 2008. Cortisol determination in hair and faeces from domestic cats and dogs. Gen Comp Endocrinol 155:398–402 [DOI] [PubMed] [Google Scholar]
- 37. Kalra S, Einarson A, Karaskov T, Van Uum S, Koren G. 2007. The relationship between stress and hair cortisol in healthy pregnant women. Clin Invest Med 30:E103–E107 [DOI] [PubMed] [Google Scholar]
- 38. Gow R, Koren G, Rieder M, Van Uum S. 2011. Hair cortisol content in patients with adrenal insufficiency on hydrocortisone replacement therapy. Clin Endocrinol (Oxf) 74:687–693 [DOI] [PubMed] [Google Scholar]
- 39. Dettenborn L, Tietze A, Bruckner F, Kirschbaum C. 2010. Higher cortisol content in hair among long-term unemployed individuals compared to controls. Psychoneuroendocrinology 35:1404–1409 [DOI] [PubMed] [Google Scholar]
- 40. Dowlati Y, Herrmann N, Swardfager W, Thomson S, Oh PI, Van Uum S, Koren G, Lanctôt KL. 2010. Relationship between hair cortisol concentrations and depressive symptoms in patients with coronary artery disease. Neuropsychiatr Dis Treat 6:393–400 [PMC free article] [PubMed] [Google Scholar]
- 41. Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, Dettenborn L. 2010. Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biol Psychol 85:357–360 [DOI] [PubMed] [Google Scholar]
- 42. Skoluda N, Dettenborn L, Stalder T, Kirschbaum C. 2012. Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology 37:611–617 [DOI] [PubMed] [Google Scholar]
- 43. Stalder T, Steudte S, Alexander N, Miller R, Gao W, Dettenborn L, Kirschbaum C. 2012. Cortisol in hair, body mass index and stress-related measures. Biol Psychol 90:218–223 [DOI] [PubMed] [Google Scholar]
- 44. Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, Wüst S. 2008. Covariance between psychological and endocrine responses to pharmacological challenge and psychosocial stress: A question of timing. Psychosom Med 70:787–796 [DOI] [PubMed] [Google Scholar]
- 45. Laudenslager ML, Natvig C, Corcoran CA, Blevins MW, Pierre PJ, Bennett AJ. 4 April 2012. The influences of perinatal challenge persist into the adolescent period in socially housed bonnet macaques (Macaca radiata). Dev Psychobiol 10.1002/dev.21030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Van Uum SH, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G. 2008. Elevated content of cortisol in hair of patients with severe chronic pain: A novel biomarker for stress. Stress 11:483–488 [DOI] [PubMed] [Google Scholar]
- 47. Manenschijn L, van Kruysbergen RG, de Jong FH, Koper JW, van Rossum EF. 2011. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J Clin Endocrinol Metab 96:E1862–E1865 [DOI] [PubMed] [Google Scholar]
- 48. Pereg D, Gow R, Mosseri M, Lishner M, Rieder M, Van Uum S, Koren G. 2011. Hair cortisol and the risk for acute myocardial infarction in adult men. Stress 14:73–81 [DOI] [PubMed] [Google Scholar]
- 49. Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, Koren G. 2007. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology 92:42–49 [DOI] [PubMed] [Google Scholar]
- 50. Dettenborn L, Muhtz C, Skoluda N, Stalder T, Steudte S, Hinkelmann K, Kirschbaum C, Otte C. 2012. Introducing a novel method to assess cumulative steroid concentrations: Increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress 15:348–353 [DOI] [PubMed] [Google Scholar]
- 51. Steudte S, Kolassa IT, Stalder T, Pfeiffer A, Kirschbaum C, Elbert T. 2011. Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology 36:1193–1200 [DOI] [PubMed] [Google Scholar]
- 52. Luo H, Hu X, Liu X, Ma X, Guo W, Qiu C, Wang Y, Wang Q, Zhang X, Zhang W, Hannum G, Zhang K, Liu X, Li T. 2012. Hair cortisol level as a biomarker for altered hypothalamic-pituitary-adrenal activity in female adolescents with posttraumatic stress disorder after the 2008 Wenchuan earthquake. Biol Psychiatry 72:65–69 [DOI] [PubMed] [Google Scholar]
- 53. Dettmer AM, Novak MA, Suomi SJ, Meyer JS. 2012. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: Hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology 37:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Feng X, Wang L, Yang S, Qin D, Wang J, Li C, Lv L, Ma Y, Hu X. 2011. Maternal separation produces lasting changes in cortisol and behavior in rhesus monkeys. Proc Natl Acad Sci USA 108:14312–14317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fourie NH, Bernstein RM. 2011. Hair cortisol levels track phylogenetic and age related differences in hypothalamic-pituitary-adrenal (HPA) axis activity in non-human primates. Gen Comp Endocrinol 174:150–155 [DOI] [PubMed] [Google Scholar]
- 56. Laudenslager ML, Jorgensen MJ, Fairbanks LA. 10 April 2012. Developmental patterns of hair cortisol in male and female nonhuman primates: Lower hair cortisol levels in vervet males emerge at puberty. Psychoneuroendocrinology 10.1016/j.psyneuen.2012.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Réale D, Reader SM, Sol D, McDougall PT, Dingemanse NJ. 2007. Integrating animal temperament within ecology and evolution. Biol Rev 82:291–318 [DOI] [PubMed] [Google Scholar]
- 58. Dettling AC, Parker SW, Lane S, Sebanc A, Gunnar MR. 2000. Quality of care and temperament determine changes in cortisol concentrations over the day for young children in childcare. Psychoneuroendocrinology 25:819–836 [DOI] [PubMed] [Google Scholar]
- 59. Laudenslager ML, Jorgensen MJ, Grzywa R, Fairbanks LA. 2011. A novelty seeking phenotype is related to chronic hypothalamic-pituitary-adrenal activity reflected by hair cortisol. Physiol Behav 104:291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dettmer AM, Novak MF, Novak MA, Meyer JS, Suomi SJ. 2009. Hair cortisol predicts object permanence performance in infant rhesus macaques (Macaca mulatta). Dev Psychobiol 51:706–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Siniscalchi M, McFarlane JR, Kauter KG, Quaranta A, Rogers LJ. 24 March 2012. Cortisol levels in hair reflect behavioural reactivity of dogs to acoustic stimuli. Res Vet Sci 10.1016/j.rvsc.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 62. Finkler H, Terkel J. 2010. Cortisol levels and aggression in neutered and intact free-roaming female cats living in urban social groups. Physiol Behav 99:343–347 [DOI] [PubMed] [Google Scholar]
- 63. Martin JG, Réale D. 2008. Animal temperament and human disturbance: Implications for the response of wildlife to tourism. Behav Processes 77:66–72 [DOI] [PubMed] [Google Scholar]
- 64. Bechshøft TØ, Sonne C, Dietz R, Born EW, Muir DC, Letcher RJ, Novak MA, Henchey E, Meyer JS, Jenssen BM, Villanger GD. 2012. Associations between complex OHC mixtures and thyroid and cortisol hormone levels in East Greenland polar bears. Environ Res 116:26–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Macbeth BJ, Cattet MRL, Stenhouse GB, Gibeau ML, Janz DM. 2010. Hair cortisol concentration as a noninvasive measure of long-term stress in free-ranging grizzly bears (Ursus arctos): considerations with implications for other wildlife. Can J Zool 88:935–949 [Google Scholar]
- 66. Bechshøft TØ, Rigét FF, Sonne C, Letcher RJ, Muir DC, Novak MA, Henchey E, Meyer JS, Eulaers I, Jaspers VL, Eens M, Covaci A, Dietz R. 2012. Measuring environmental stress in East Greenland polar bears, 1892–1927 and 1988–2009: what does hair cortisol tell us? Environ Int 45:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Webb E, Thomson S, Nelson A, White C, Koren G, Rieder M, Van Uum S. 2010. Assessing individual systemic stress through cortisol analysis of archaeological hair. J Archaeol Sci 37:807–812 [Google Scholar]
- 68. Miller GE, Chen E, Zhou ES. 2007. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull 133:25–45 [DOI] [PubMed] [Google Scholar]
- 69. Gareri J, Koren G. 2010. Prenatal hair development: implications for drug exposure determination. Forensic Sci Int 196:27–31 [DOI] [PubMed] [Google Scholar]
- 70. Thomson S, Koren G, Van Steen V, Rieder M, Van Uum SH. 2009. Testosterone concentrations in hair of hypogonadal men with and without testosterone replacement therapy. Ther Drug Monit 31:779–782 [DOI] [PubMed] [Google Scholar]
- 71. Deshmukh NIK, Barker J, Petroczi A, Naughton DP. 2012. Detection of testosterone and epitestosterone in human hair using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 67–68:154–158 [DOI] [PubMed] [Google Scholar]
- 72. Duffy E, Mooney MH, Elliott CT, O'Keeffe M. 2009. Studies on the persistence of estradiol benzoate and nortestosterone decanoate in hair of cattle following treatment with growth promoters, determined by ultra-high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1216:8090–8095 [DOI] [PubMed] [Google Scholar]