Abstract
Menopause promotes central obesity, adipose tissue (AT) inflammation, and insulin resistance (IR). Both obesity and the loss of estrogen can activate innate and adaptive immune cells (macrophages, T cells). The respective impacts of weight gain and loss of ovarian hormones on AT inflammation and IR are poorly understood. Here we determined the temporal kinetics of fat accretion, AT inflammation, and IR over a 26-wk time course in ovariectomized (OVX) mice, a model of menopause. OVX and sham-operated (SHM) C57BL6 mice were fed a normal chow diet. Weight, body composition (magnetic resonance imaging), total and regional adiposity, activity, food intake, AT crown-like structures, biohumoral measures, and insulin sensitivity (insulin tolerance testing and homeostatic model assessment) were determined at wk 12, 20, and 26. Macrophages and T cells from perigonadal AT were immunophenotyped by fluorescence-associated cell sorting, and perigonadal adipose tissue (PGAT) gene expression was quantified by quantitative PCR. OVX mice (∼31 g) became fatter than SHM mice (∼26 g) by wk 12, but mice were equally insulin sensitive. PGAT of OVX mice contained more T cells but expressed higher levels of M2-MΦ (arginase-1) and T cell-regulatory (cytotoxic T-lymphocyte antigen 4) genes. At wk 20, both OVX and SHM mice weighed approximately 35 g and were equally insulin sensitive with comparable amounts of PGAT and total body fat. OVX mice became less insulin sensitive than SHM mice by wk 26, coincident with the down-regulation of PGAT arginase-1 (−20-fold) and cytotoxic T-lymphocyte antigen 4 (2-fold) and up-regulation of M1/Th1 genes CD11c (+2-fold), IL12p40 (+2-fold), and interferon-γ (+78-fold). Ovarian hormone loss in mice induces PGAT inflammation and IR by mechanisms that can be uncoupled from OVX-induced obesity.
Compared with premenopausal women, postmenopausal women are at a much greater risk of developing metabolic diseases, such as cardiovascular disease and type 2 diabetes (1). Although the mechanism(s) linking the loss of ovarian hormone production to metabolic disturbances are not fully understood, weight gain and a redistribution of body fat toward a more android (i.e. central) distribution pattern are thought to play major roles (2). However, recent evidence suggests that the loss of ovarian hormone production is associated with symptoms of the metabolic syndrome, even in the absence of body weight changes (3), signifying that the loss of ovarian hormone production per se may disturb metabolic function. The aging process itself is associated with a decline in metabolic functioning; for example, insulin sensitivity declines with age in rodents and humans (4, 5). Although the mechanism(s) driving age-related insulin resistance (IR) are multifactorial and not fully understood, studies on male mice have shown that aging is associated with increased adipose tissue inflammation (6). An emerging body of research suggests that inflammatory processes that occur within the intraabdominal adipose tissue (AAT) of humans and rodents are causatively related to many of the symptoms of the metabolic syndrome (7). Moreover, these inflammatory changes predict metabolic outcomes independent of adiposity (8). Thus, the adipose tissue inflammation that occurs during natural aging may be mechanistically related to the onset of IR. Unfortunately, how the natural aging process affects IR and/or adipose tissue inflammation in female mice has not been investigated. Furthermore, because the aging process itself is associated with increased metabolic risk, how the loss of ovarian hormone production independently contributes to metabolic disturbances is not entirely clear.
Ovariectomy in rodents is a model of human ovarian hormone loss and therefore is used in studies of human menopause. We previously demonstrated that ovariectomy induces rapid weight gain and increases AAT inflammatory gene expression in mice early on (i.e. 12 wk) after surgery (9). Increased adipose tissue inflammatory activation may represent a major mechanism linking ovariectomy to metabolic disease, but unfortunately, the effects of ovariectomy on white adipose tissue (WAT) immune cell infiltration and activation are not known. Evidence that estrogen inhibits excess T cell proliferation (10) and that postmenopausal bone loss is caused at least in part by increased T cell activation in bone marrow (11, 12) suggests a relationship between estrogen loss and inflammatory T cell activation. Determining whether estrogen loss activates T cells in WAT may help explain why postmenopausal women are at greater metabolic risk. In the present study, we followed up ovariectomized (OVX) and sham-operated (SHM) control mice over a 26-wk time course to determine the relationship between AAT inflammation and insulin sensitivity. Because a relationship between AAT inflammation and the metabolic disturbances of ovarian hormone loss has not yet been clearly identified, we characterized the immune cell profile in the AAT of OVX and SHM mice. Inflammatory macrophages (MΦ) (13) and T lymphocytes (i.e. T cells) (14–16) are central orchestrators of the AAT inflammation that contributes to systemic IR; thus, we focused on specific T cell and MΦ populations.
In obese rodents and humans, the gene expression level of CD11c (a marker of M1/inflammatory MΦ) within AAT has been linked to the development of IR (13, 17). Moreover, recent work has pointed to the fact that MΦ and T cell activation may be a more important predictor of metabolic disease than immune cell numbers per se, suggesting that markers such as interferon-γ (IFN-γ), indicative of T cell activation, and IL12p40, indicative of inflammatory MΦ activation, may be most important in predicting IR (18). Building upon our previous findings, we now extend our studies to follow up OVX mice as they age to determine when insulin sensitivity diminishes and how this relates to changes in the immune profile of AAT. Thus, the purpose of this study was to characterize the natural history of specific immune cell changes that occur after ovariectomy by identifying immune cells and their activation states in AAT and determine how the immune cell changes that occur with the progression of ovariectomy contribute to changes in insulin sensitivity, independent of aging and/or dietary changes. Remarkably, our data demonstrate that the early increase in adiposity in OVX mice does not correlate with reduced insulin sensitivity, but rather a reduction in insulin sensitivity develops in these mice compared with their SHM counterparts independent of changes in body composition. Over time, OVX and SHM mice achieve the same level of adiposity, but OVX mice develop increased rates of adipocyte death in association with increased T cell and MΦ activation (IFN-γ and IL12p40 expression) with concomitant reductions in insulin sensitivity compared with the SHM controls. These findings suggest that ovarian hormone loss in mice induces inflammation and IR by mechanisms that are not dependent on obesity and/or the natural aging process.
Materials and Methods
Animals and diets
Female OVX and SHM C57BL6 mice were purchased from Charles River Laboratories (Wilmington, MA); operations were performed at 10 wk of age, and mice were allowed to recover for 1 wk before arriving at the Human Nutrition Research Center on Aging. Animals were housed in an Association for the Assessment and Accreditation of Laboratory Animal Care-approved animal facility with a 12-h light, 12-h dark cycle and given free access to water and food (phytoestrogen free chow, no. 2016S; Harlan Teklad, Indianapolis, IN) unless stated otherwise. Mice were individually housed, and food intake and body weight were monitored weekly. After 12, 20, or 26 wk, mice were fasted overnight (i.e. 8–12 h) and killed by CO2 inhalation followed by cervical dislocation. Blood was collected via cardiac puncture and tissues were immediately harvested and processed for fluorescence-associated cell sorting or flash frozen in liquid nitrogen for later analyses.
Plasma analyses
All blood analytes were quantified using samples collected in the fasted state. Blood glucose was measured with an automated glucometer (One touch Ultra; LifeScan, Inc., Milpitas, CA), and plasma insulin was determined using an ELISA with mouse insulin as a standard (Crystal Chem, Downers Grove, IL). Plasma nonesterified fatty acid (NEFA) and triglyceride (TG) concentrations were quantified using commercial kits (Wako Chemicals, Richmond, VA). Plasma high molecular weight (HMW) adiponectin was measured using commercial kits (ALPCO Diagnostics, Salem, NH).
Measurement of body composition, insulin sensitivity, and spontaneous physical activity
Magnetic resonance imaging (MRI) was used to serially assess body composition in the mice (i.e. fat and lean mass) over time and was performed at the following weeks surgery: 3, 8, 12, 17, 21, and 26 (EchoMRI-700, Houston, TX). Insulin sensitivity was assessed via ip insulin tolerance testing (ITT) and by measurement of fasting glucose and insulin levels and the homeostatic model assessment of insulin sensitivity (HOMA-IR) validated equation (19). For ITT, fasting blood glucose was measured in the 6 h fasted state before insulin injection (0.7 mU/kg); blood was then sampled over a 90-min period using commercially available glucose test strips and a glucometer (One Touch Ultra; LifeScan). The parameter calculated for ITT is area under the curve from T0 to T90. Spontaneous physical activity was measured at 17 and 24 wk, for 4 d at each time point, using metabolic chamber infrared radiation displacement (TSE Calorimetry Systems, Chesterfield, MO) as previously described (9).
Histological analysis of adipose tissue and cell size determination
Intra-AAT was harvested from the perigonadal adipose tissue (PGAT) depot and fixed in zinc formaldehyde overnight at 4 C before being transferred and stored in PBS at 4 C. Tissues were then embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Digital images were captured with an Olympus DX51 light microscope (Tokyo, Japan). Adipocyte volume was calculated from a cross-sectional area obtained from perimeter tracings of adipocytes from digital images obtained from hematoxylin and eosin stains using Image J software (NIH public domain; National Institutes of Health, Bethesda, MD). For each mouse, 100 adipocytes in each of three separate sections were used to determine the mean adipocyte volume.
Immunohistochemistry and crown-like structure (CLS) quantification
Histological sections of AAT were dewaxed in xylenes, rehydrated through an ethanol series, and stained using Mac-2 antibody for the analysis of CLS as previously described (20). Mean CLS quantification was done by counting the number of Mac-2 positive CLS per field in eight fields at 10 times per mouse; four mice per group were analyzed.
Real-time quantitative PCR
RNA was isolated from frozen tissues using commercial extraction kits designed for lipid tissues (QIAGEN, Valencia, CA) and was quantified and assessed for purity using the Nanodrop1000 spectrophotometer (Thermo Scientific, Wilmington, DE). cDNA was generated from 1 μg of RNA using avian myeloma virus reverse transcriptase (Promega, Madison, WI), and real-time quantitative PCR was performed using SYBR Green (Applied Biosystems 7300; Applied Biosystems, Foster City, CA). Fold changes were calculated as 2-ΔΔCT compared with the housekeeping gene, cyclophilin A, using SHM as the reference group at each time point. For time differences, cycle threshold vales relative to cyclophilin A were compared between time points for both groups. All forward and reverse primer sequences are available upon request.
Stromal vascular cell (SVC) isolation
Adipose tissue was placed into Krebs-Henseleit buffer (21) supplemented with 4% fatty acid-free BSA, 5 mmol/liter d-glucose, and 200 nmol/liter phenylisopropyladenosine and then minced; centrifuged (500 × g, 5 min, room temperature); digested (30 min, 37 C) via collagenase containing 50 U of deoxyribonuclease I (Sigma Aldrich, St. Louis, MO); passed through a sterile 100-μm strainer (Fischer Scientific, Franklin, MA); and centrifuged (500 × g, 5 min, room temperature). After incubation with red blood cell lysis buffer, SVC were resuspended (1 × 106 cells per 100 μl) in cold sorting buffer (PBS containing 1 mmol/liter EDTA, 25 mmol/liter HEPES, and 1% fatty acid free BSA) until immunolabeling.
Flow cytometry and SVC phenotyping
SVC were first incubated on ice (10 min) with Fc block (5 μg/ml) (BD PharMingen, San Jose, CA) followed by incubation with fluorophore-conjugated antibodies or isotype controls to obtain extracellular staining. For FOXP3 intracellular staining, cells were incubated in permeabilization buffer for 1–2 h at 4 C and then stained overnight with allophycocyanin-conjugated FOXP3 Ab (or isotype control) using a regulatory T cells (TREG) staining kit (eBioscience, San Diego, CA). Antibodies to detect cell surface antigens included the following: phycoerythrin-Cy5-conjugated CD3 (eBioscience), phycoerythrin-conjugated F4/80 (AbD Serotec, Raleigh, NC), fluorescein isothiocyanate-conjugated CD4 (eBioscience), allophycocyanin-conjugated CD8 (eBioscience), and phycoerythrin-conjugated CD25 (eBioscience). CD-gating strategies included dead cell discrimination and lymphocyte quantification based on forward/side scatter and included unstained cells and appropriate isotype controls. Cells were immunophenotyped using an Accuri cytometer (Ann Arbor, MI) and data analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Liver TG analysis
To assess the degree of hepatic steatosis, liver TG content was measured using commercial kits (Wako L-type TG M kit; Wako Diagnostics, Richmond, VA) and expressed per gram liver tissue.
Statistical analysis
Comparisons were made using Student's t tests or one-way ANOVA with post hoc Tukey's tests where appropriate. Time course analyses (e.g. body weight projection, ITT time points) were assessed using ANOVA with repeated measures analysis. Data are presented as means ± sem. Significance was defined as P < 0.05.
Results
After early rapid weight gain, OVX mice attain similar body weight and body fat as SHM mice
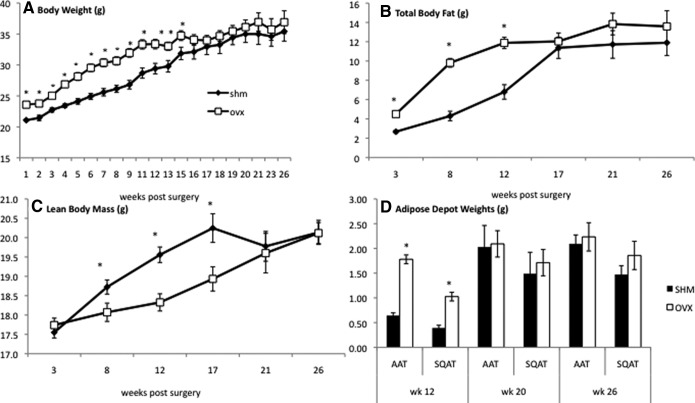
As we have previously demonstrated (9), OVX mice rapidly gain weight after surgery compared with age-matched SHM controls. Initially, OVX mice gained an average of three more grams per week than SHM while consuming a chow diet. We continued to follow both sets of mice as they aged and found that, surprisingly, the mice converged over time and reached similar weights by approximately 17 wk after surgery (at 28 wk of age) (Fig. 1A). In parallel with our studies on weight gain, body composition was determined by MRI, which demonstrated that the early weight gain in OVX mice was due to an increase in fat (Fig. 1B), and not lean, mass (Fig. 1C). Consistent with the body weight changes, there were no significant differences in total body fat or lean mass between OVX and SHM mice by 17 wk after surgery (Fig. 1, B and C). Correspondingly, OVX mice had heavier PGAT and sc adipose tissue (SQAT) pads at 12 wk after surgery, but fat pad differences were no longer evident at 20 or 26 wk (Fig. 1D).
Fig. 1.
Body weight, adiposity, and regional fat distribution between OVX and SHM mice different early but not late. Body weight (A) and body fat in grams (B) were higher in OVX mice (white squares) compared with SHM mice (black diamonds) over the first 12 wk but not different by 17 wk, whereas lean body mass (C) was lower in OVX mice over the first 12 wk and not different by 17 wk. Intra-AAT (i.e. PGAT) and SQAT depot weights (D) were also higher in OVX mice (black columns) compared with SHM mice (white columns) only at 12 wk. Error bars indicate sem (n = 6–10/group). *, P < 0.05.
OVX mice maintain lower spontaneous activity levels but reduce food intake over time
We have previously demonstrated that the early rapid weight gain with OVX was secondary to a reduction in physical activity and not increased food intake (9). To determine whether alterations in physical activity over time explained the normalization of body weights, we continued to measure this parameter. To our surprise, despite their weight equivalence by 17 wk, OVX mice continued to be significantly less active than SHM mice throughout the study, as demonstrated by reduced physical activity at 17 and 24 wk (Fig. 2A). However, unlike what was revealed early on, later in the time course, OVX mice consumed less food than SHM mice (Fig. 2B). Almost coincident with the reduction in food intake by OVX mice, body composition became more similar between OVX and SHM mice. From wk 20 onward, the rate of change in lean and fat mass was similar between the two lines of mice. Thus, how food energy was partitioned into lean and fat mass initially was different between SHM and OVX mice, but over time the relative body composition and weights became equivalent.
Fig. 2.
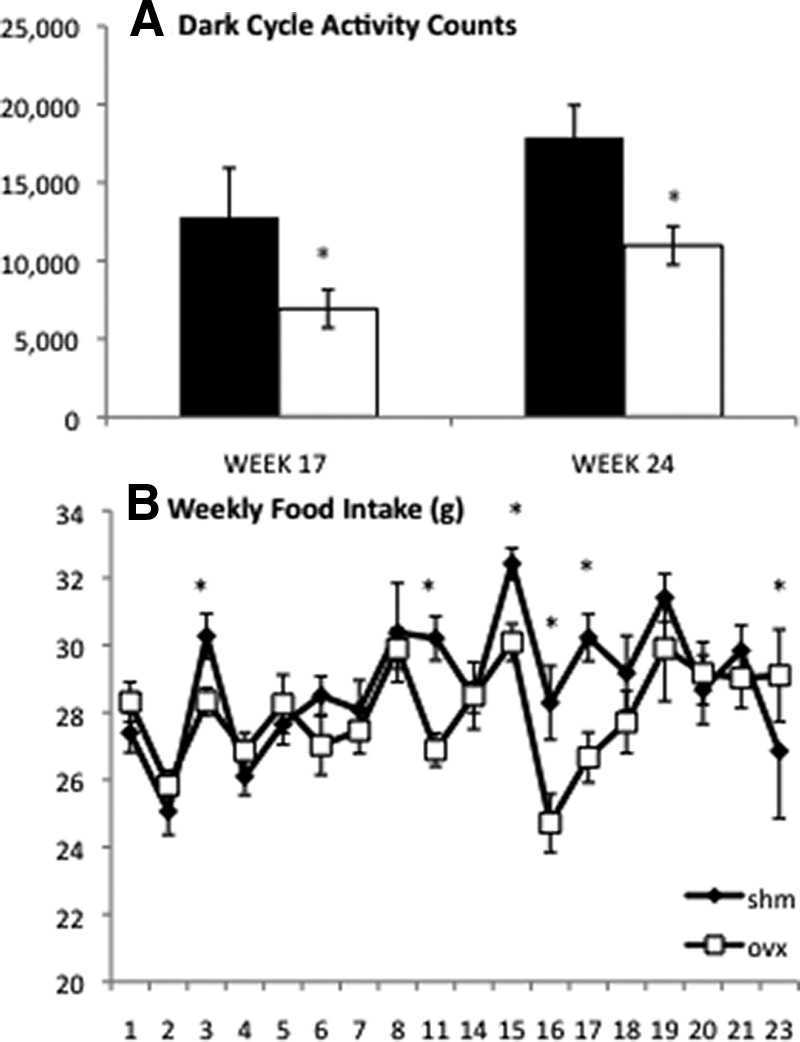
Spontaneous physical activity and food intake are lower in OVX mice. Spontaneous physical activity was lower in OVX mice (white columns) than SHM mice (black columns) at 17 and 24 wk (A). Weekly food intake (B) was significantly lower in OVX mice (white squares) compared with SHM mice (black diamonds) at wk 3, 11, 15, 16, 17, and 23 and not different between groups at the other time points. Error bars indicate sem (n = 4–10/group). *, P < 0.05 between groups and within the time point.
Insulin insensitivity develops late in OVX mice, even at adiposity levels equal to SHM controls
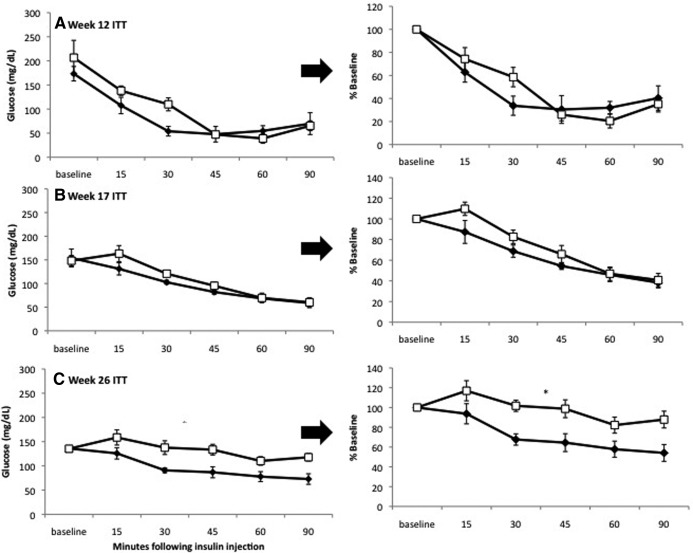
Although OVX mice weighed approximately 5 more grams on average than SHM mice by 12 wk after surgery and had greater total and regional adiposity, they did not exhibit reduced insulin tolerance, as indicated by ITT (Fig. 3A) and HOMA-IR (Table 1). In contrast, although no differences existed between OVX and SHM mice in terms of body weight, adiposity, or mean adipocyte size at 20 or 26 wk, OVX mice were less insulin sensitive compared with SHM by 26 wk (Fig. 3, B and C). The reduced insulin sensitivity was reflected in higher fasting insulin levels and HOMA-IR values (Table 1). Liver weight, hepatic triglyceride content, and plasma levels of TG, NEFA, and HMW adiponectin were comparable between OVX and SHM (Table 1).
Fig. 3.
ITT reveals IR in OVX at 26 wk only. At 12 wk (A) and 20 wk (B), no significant differences in insulin responsiveness were found between OVX mice (white squares) compared with SHM mice (black diamonds). By 26 wk, OVX mice were significantly more IR than SHM mice (C). Error bars indicate sem (n = 4–6/group). *, P < 0.05 based on repeated-measures ANOVA.
Table 1.
Metabolic characteristics of OVX and SHM mice at 12, 20, and 26 wk after surgery
| Week | 12 |
20 |
26 |
|||
|---|---|---|---|---|---|---|
| Group (n) | SHM (10) | OVX (10) | SHM (6) | OVX (6) | SHM (10) | OVX (9) |
| Body weight (g) | 25.7±0.6 | 30.8±0.9a | 35.8±1.7 | 35.0±1.6 | 34.3±1.6 | 36.2±2.0 |
| Liver weight (g) | 1.2±0.1 | 1.0±0.1 | 1.2±0.1 | 1.2±0.1 | 1.2±0.1 | 1.2±0.1 |
| Liver TG (mg/g) | 94.4±15.4 | 87.30±21.9 | 47.1±5.3 | 40.4±7.0 | ||
| FPG (mg/dl) | 130.8±4.2 | 152.5±5.4 | 155.7±17.6 | 157.2±8.8 | 151.2±7.8 | 179.5±8.7 |
| FPI (ng/ml) | 0.8±0.1 | 0.9±0.1 | 0.6±0.1 | 0.83±0.2 | 0.9±0.1 | 1.3±0.2a |
| Glucose ITT AUC (mmol/liter) | 429.7±49.0 | 511.0±45.3 | 445.3±18.7 | 514.9±30.4 | 467.6±36.8 | 650.5±46.8a |
| HOMA-IR | 6.1±0.1 | 8.1±1.0 | 6.3±1.4 | 8.1±2.1 | 8.5±1.2 | 15.2±2.4a |
| NEFA (mmol/liter) | 0.9±0.2 | 1.4±0.2 | 0.8±0.1 | 0.7±0.1 | ||
| FPTG (mg/dl) | 67.0±5.1 | 63.0±4.1 | 64.4±2.9 | 59.4±5.1 | 63.2±4.2 | 57.7±4.4 |
| HMW adiponectin (μg/ml) | 14.2±4.6 | 22.6±2.8 | 23.2±3.8 | 30.8±3.3 | 26.3±2.9 | 29.0±2.9 |
Data are represented as mean ± sem. FPG, Fasting plasma glucose; FPI, fasting plasma insulin; AUC, area under the curve; FPTG, fasting plasma triglyceride level.
P < 0.05, OVX vs. SHM within time point.
OVX mice have greater rates of AAT adipocyte death
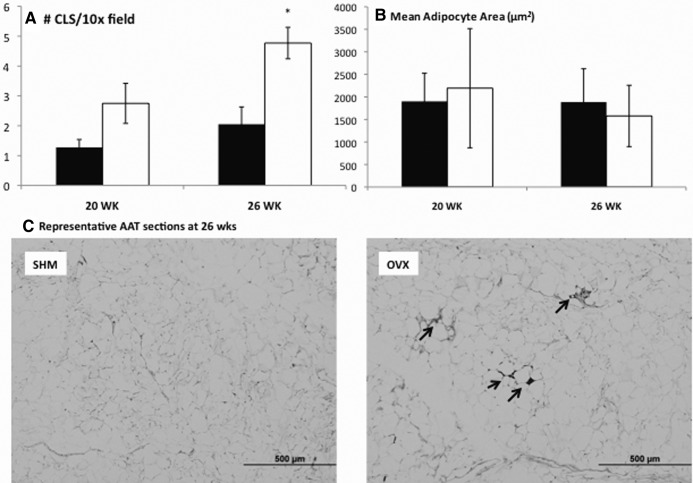
Previously our laboratory had demonstrated that increased rates of adipocyte death and associated MΦ surrounding the remnant lipid droplet of dead adipocytes termed CLS correlated with the development of IR in diet-induced obesity (20, 22, 23). To determine whether the reduction in insulin sensitivity in OVX at 26 wk was coincident with increased adipocyte death, CLS were quantified in OVX and SHM mice matched for adiposity and AAT adipocyte size using histological analysis (Fig. 4A). Although OVX tended to have more CLS at 20 wk (P = 0.087), similar to the case with IR, the difference did not reach statistical significance until wk 26. Although adipocyte size differences between OVX and SHM mice were readily observed early (i.e. 12 wk) as reported previously (9), by 20 wk, no significant differences in adipocyte size existed between groups, and this remained true at 26 wk (Fig. 4B). Previous studies had demonstrated that increasing adipocyte size was associated with increased formation of CLS; however, here the higher CLS formation observed in OVX compared with SHM mice occurred in the absence of greater mean adipocyte size.
Fig. 4.
More adipocyte death indicated by CLS quantification in OVX at 26 wk. CLS quantified in AAT using MAC-2 staining revealed more MΦ in OVX mice (white columns) compared with SHM mice (black columns) (A). No differences in mean adipocyte size were found at either 20 or 26 wk (B). Representative histological staining (×10 field) from OVX mice (right panel) and SHM mice (left panel) at 26 wk (C). CLS are indicated by arrows. Error bars indicate sem (n = 4/group). *, P < 0.05.
OVX mice have more T cells in AAT
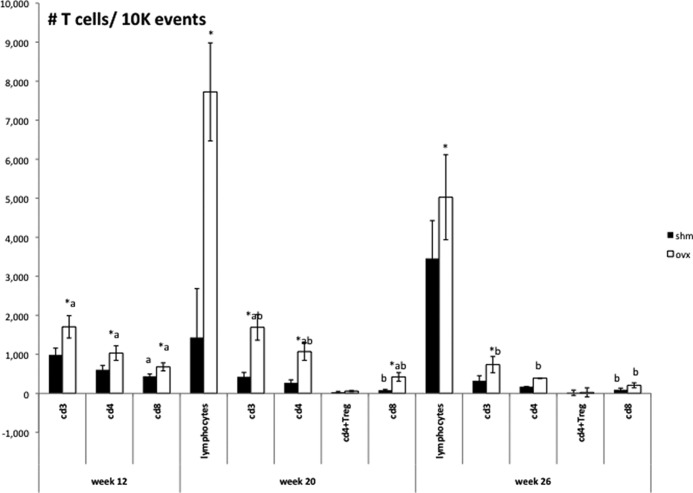
In obesity, the formation of CLS around dying adipocytes is part of the inflammatory process in adipose tissue (24). Moreover, there are alterations in T cells and T cell subsets in AAT. To determine whether adipocyte death was associated with increased immune cell infiltration into the AAT depot, T cells were measured in OVX compared with SHM at 12, 20, and 26 wk after surgery. At 12 wk after surgery (when OVX were considerably fatter than SHM mice), OVX AAT contained more CD3+ cells and CD4+ and CD8+ T cell subsets (P < 0.05) (Fig. 5). Surprisingly, by 20 wk (when OVX and SHM mice had equivalent body weight, body fatness, and fat distribution, OVX mice had approximately 3-fold more CD3+, CD4+, and CD8+ T cells than SHM mice (P < 0.05). Notably, the greater numbers of T cells in the AAT of OVX mice did not coincide with reduced insulin sensitivity. Rather, the loss of insulin sensitivity in OVX mice was observed after 26 wk, at which time the numbers of CD3+, CD4+, and CD8+ T cell populations were significantly reduced as compared with wk 20 (Fig. 5). OVX mice still tended to have more T cells in AAT than SHM mice (P ≤ 0.1 for CD3+, CD4+, and CD8+ T cells. The number of CD4+T regulatory cells (i.e. TREG) was not different between SHM and OVX mice at any time point (Fig. 5). Considered together, these results suggest that, in contrast to diet-induced obesity, quantitative changes in T cell populations in the AAT of OVX mice are not positively (CD4+, CD8+) or negatively (TREG) associated with adiposity or impaired insulin sensitivity.
Fig. 5.
AAT T lymphocyte content is greater in OVX mice but changes over time. AAT SVC T cells were quantified using flow cytometry at 12, 20, and 26 wk. At 12 wk, OVX mice (white columns) had more CD3+, CD4+, and CD8+ T cells than SHM mice (black columns). At 20 wk, OVX mice had significantly more CD3+, CD4+, and CD8+ T cells and were not different from SHM mice in the number of CD4+TREG. The number of CD8+ T cells decreased in SHM mice from 12 to 20 wk. By 26 wk, CD3+, CD4+, and CD8+ T cell populations were significantly reduced in OVX mice compared with 12 wk, whereas only CD8+ T cells were reduced in SHM mice compared with 12 wk. No differences in CD4+TREG were observed at 26 wk, nor were significant time differences observed for this subpopulation. Error bars indicate sem (n = 6–9/group). *, P < 0.05 between groups within time point for each T cell classification; different letters represent differences (P < 0.05) between time points within groups and within T cell classifications.
Alterations in adipose tissue inflammatory mediators and cells of OVX mice are coincident with reduced insulin tolerance
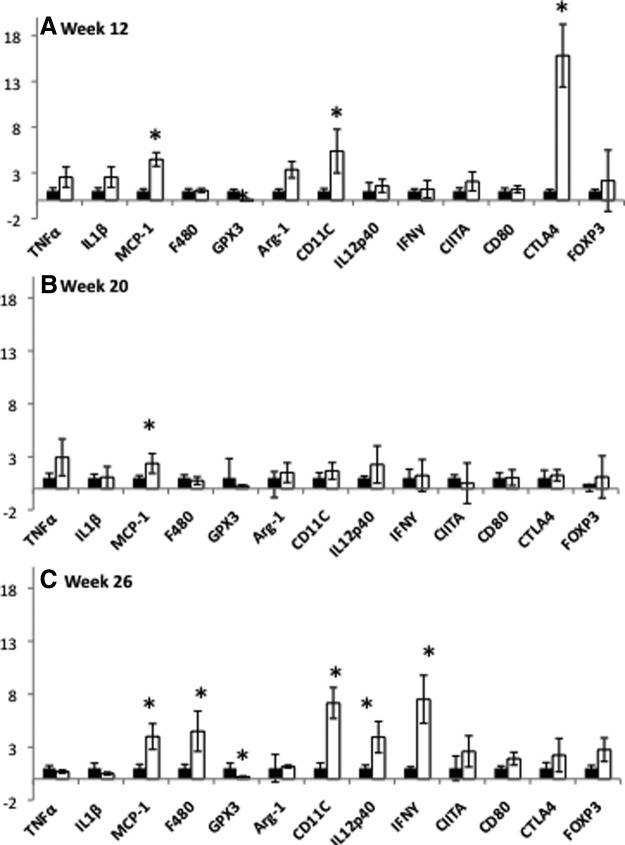
Because significant differences in insulin tolerance were observed at 26 wk in the absence of robust differences in AAT T cell numbers, we next investigated whether the immune cells present in OVX AAT were more activated compared with controls. Real-time quantitative PCR was used to determine the relative levels of specific immune cell activation markers in OVX and SHM adipose tissues. At 12 wk, transcript levels of the MΦ chemoattractant, monocyte chemoattractant protein-1 and the M1 mΦ marker, CD11c were significantly greater in the AAT of OVX compared with SHM mice (Fig. 6A). This modest increase in AAT CD11c expression at 12 wk was not associated with IR in OVX mice, nor was the decrease in glutathione peroxidase 3 (GPX3), an antioxidant enzyme that is negatively correlated with the development of obesity-associated IR (25). Interestingly, the T cell marker, cytotoxic T lymphocyte antigen 4 (CTLA4), which is expressed on CD4+ helper T cells and transmits an inhibitory signal to T cells, was elevated in OVX mice relative to SHM by approximately 15-fold at this time. (Fig. 7At 20 wk (when body fatness was comparable in OVX and SHM mice), macrophage chemoattractant protein-1 was the only inflammation-related gene that remained significantly up-regulated in OVX mice compared with SHM mice (Fig. 6B). By 26 wk, however, the gene expression pattern in the AAT of OVX mice indicated increased infiltration of CD11c+ macrophages (F4/80+ cells), the up-regulation of the T cell costimulatory cytokine IL-12, and corresponding stimulation of Th1 gene expression (IFN-γ). To determine how expression levels changed over time, additional analyses were done comparing gene expression levels relative to the 12-wk time point. These analyses revealed that IFN-γ transcript levels increased 78-fold in AAT of OVX mice between wk 12 and 26 (P < 0.05), indicating a robust Th1 transition. Consistent with T cell activation at wk 26, expression of CTLA4 was no longer greater in OVX mice compared with SHM mice (Fig. 6C). Overall, these results reveal a progressive M1 mΦ polarization (increased CD11c+ and IL-12 expression) and Th1 T cell activation (IFN-γ expression) in the AAT of OVX mice between wk 12 and 26. Supporting this notion of an M1/Th1 transition is the observation that transcript levels of the hallmark M2 marker, arginase-1 (Arg-1), decreased in OVX mice by approximately 20-fold between wk 12 and 26, Importantly, this M1/Th1 transition in AAT coincided with the reduction in insulin sensitivity in OVX from 12 to 26 wk.
Fig. 6.
Comparison of adipose tissue gene expression between OVX and SHM mice at 12, 20, and 26 wk after surgery reveals profound immune cell activation in OVX mice at 26 wk. AAT mRNA expression relative to SHM mice is shown at 12 wk (A); AAT mRNA expression relative to SHM is shown at 20 wk (B); AAT mRNA expression relative to SHM is shown at 26 wk (C). Black columns, SHM mice; white columns, OVX mice; error bars indicate sem (n = 6–10/group). *, P < 0.05 between groups within the time point.
Fig. 7.
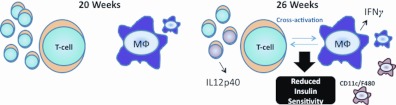
Schematic representing AAT immune cell differences at 20 and 26 wk. At 20 wk, although more T cells are present in the AAT of OVX mice, no differences in insulin sensitivity exist. By 26 wk, although OVX and SHM mice remain equivalent in terms of obesity and adipose tissue mass/distribution, OVX mice are less insulin sensitive than SHM mice, which coincides with T cell and MΦ cross-activation as illustrated by greater gene expression of IL12p40, which is secreted by activated T cells, and IFN-γ, which is secreted by activated MΦ bearing the marker CD11c.
Discussion
We demonstrate for the first time that ovariectomy in mice fed a normal chow diet leads to a reduction in insulin sensitivity well after the initial body weight increase, at a time when OVX and SHM mice are equally obese. The insulin insensitivity manifests at approximately 26 wk after surgery when mice are about 9 months of age, equivalent to late-middle age in humans. Reduced insulin sensitivity in OVX mice is concomitant with increased adipocyte death and inflammatory activation in the AAT depot.
Premenopausal women have greater relative adiposity, lower visceral adiposity, and improved whole-body insulin sensitivity compared with age-matched men (26). The mechanisms underlying such sex differences are not completely understood, but the fact that women tend to lose this metabolic protection after menopause suggests that ovarian sex hormones play a role (27). Indeed, postmenopausal women tend to adopt a more male-like adipose tissue distribution pattern with increased visceral (i.e. intraabdominal) adiposity, a major determinant of metabolic dysfunction (28). It is unclear whether sex hormones play an independent role in metabolic health or whether the effects are mediated indirectly, through changes in body composition and/or aging. In this extended time-course study, we found that the previously reported body composition difference between OVX and SHM C57BL6 mice diminishes over time such that it is nonexistent by 20 wk after surgery. This allowed us to investigate the independent role played by loss of ovarian hormones, in the absence of alterations in adiposity, on WAT inflammation, and systemic insulin sensitivity. Our findings suggest that female sex hormones may directly affect WAT inflammatory cell activation and may even predict systemic reductions in insulin tolerance.
Recent evidence suggests that there are profound sex differences in metabolic parameters in rodents similar to what has been shown in humans. Using microarray data, Grove et al. (29) identified sex differences in the high-fat diet (HFD)-induced obesity inflammation profile, with greater AAT inflammatory gene expression observed in male compared with female mice. Those authors compared their groups of mice with OVX females and found that the OVX mice adopted a more male-like phenotype of higher inflammatory gene expression, suggesting that ovarian hormones are responsible for the metabolic protection seen in female mice. The findings presented here, that OVX mice have greater AAT inflammation compared with SHM in the absence of adiposity differences, support this notion. Medrikova et al. (30) found that female mice exhibit less WAT MΦ infiltration with long-term HFD feeding than do males even at comparable adiposity levels. In that study, fewer CLS were present in the females' AAT at a time point when no differences in adipocyte size existed compared with male mice. Here we show a similar pattern between SHM and OVX mice at 26 wk: more CLS were detected in the AAT of OVX compared with SHM mice despite equal mean adipoctye size and total adiposity. It is unclear how ovarian hormones might protect adipocytes from inflammation. A hypothesis that could be addressed in the future is that ovarian hormones allow for increased adipocyte expandability, which protects the tissue against adipocyte cell death and inflammation.
Surprisingly, the body weight gain observed in OVX compared with SHM mice on chow diet was an early phenomenon: the OVX and SHM mice in this study converged over time and became similar in body weight and body composition. It has been established that OVX mice are less physically active than SHM (9, 31), and it is thought that it is the loss of estrogen that results in the reduced activity in OVX mice because studies have confirmed that estrogen directly acts on the preoptic area of the brain to increase physical activity (32). It appears that the OVX mice in our study compensated over time for their reduced physical activity by reducing their food intake (Fig. 2), thus resulting in the mice having similar body weights later in the time course. During the first 12 wk, when OVX mice had significantly more fat mass compared with their age-matched SHM counterparts, they also had heavier PGAT (i.e. AAT) and SQAT fat pads (Fig. 1). These early differences between groups diminished by the 20-wk time point and remained nonexistent until the end of the study, providing a useful model with which metabolic functioning in OVX and SHM mice could be compared without the confounding factors of increased adiposity, body composition differences, and/or aging.
In obesity, WAT inflammation, including increased numbers of MΦ, inflammatory mediators, and, more recently, T cells, predict systemic IR in male mice (16, 33, 34). It is not well understood how loss of ovarian hormones alters immune cell populations in AAT. Because ovariectomy-mediated bone loss has been attributed at least partially to increased T cell proliferation and activation (12), we measured T cell numbers and the activation state in the AAT of OVX and SHM mice. We demonstrate that early on, ovariectomy robustly increases T cell populations in the AAT, consistent with the increased adiposity. However, although OVX mice tend to have more CD3+, CD4+, and CD8+ T cells in their AAT at all time points, it is not until 26 wk after surgery that diminished insulin sensitivity develops in OVX mice compared with equally obese SHM mice, suggesting that it is not T cell numbers per se but immune cell activity that affects insulin responsiveness. Surprisingly, an overall reduction in T cell populations took place from 20 to 26 wk in OVX mice, resulting in the OVX and SHM mice having virtually equal numbers of T cells by the 26-wk time point. This included the CD8+ cytotoxic T cell subset, which has been associated with IR in HFD-induced obesity studies (35).
Histological analysis revealed more cell death (i.e. more CLS) in the OVX compared with SHM mice at 26 wk (Fig. 4A). Adipocyte death has been implicated as a possible cause of adipose tissue inflammation, in which MΦ surround dead adipocytes and secrete inflammatory mediators (22–24). The increased MΦ accumulation in the AAT in OVX mice was confirmed by increased F4/80 and CD11c gene expression (Fig. 6C). The fact that no differences existed in mean adipocyte size between groups suggests that AAT of OVX mice becomes more inflamed with greater numbers of MΦ, independent of differences in adipose size. Others have reported an increase in adipocyte lipolysis between OVX and SHM mice at 10 wk after surgery concomitant with larger adipocytes in OVX (36). Here the lack of difference in plasma NEFA suggests that lipolysis rates were not different between OVX and SHM and may imply that the increased lipolysis exhibited by OVX mice as reported by Stubbins et al. (36) was attributable to the adipocyte size differences observed and not loss of ovarian hormones per se. We and others have previously shown that increased adipocyte death is correlated with adipocyte size (20), but the mechanism behind this relationship is not clear. The present data suggest that cell size is not the only factor that contributes to adipocyte death and emphasize the important role that female hormones may play in protecting against adipocyte death.
Gene expression data indicated increased MΦ (IL12p40) and T cell (IFN-γ) activation in the AAT of OVX mice by 26 wk after surgery. This inflammatory profile, combined with decreased Arg-1 gene expression, is suggestive of an M1/inflammatory MΦ phenotype. Importantly, this shift in inflammatory state occurred in parallel with reduced insulin responsiveness, supporting a recent report that inflammatory activation, and not quantitative differences in immune cells per se, predict IR (37). Although AAT inflammatory gene expression also increased (albeit to a much lower extent) in SHM mice, the inflammatory profile was critically different in that MΦ and T cell activation occurred primarily in OVX as illustrated by the robust increases in IL12p40 and IFN-γ combined with the reduction in Arg-1. These differences, in conjunction with reduced CTLA4 and GPX3 expression in OVX mice, likely contributed to the impaired insulin sensitivity in OVX as compared with SHM mice at 26 wk. In this regard, diminished (∼14-fold) GPX3 expression in OVX between wk 12 and 26 is particularly noteworthy because overexpression of this antioxidant enzyme in WAT has been shown to reduce inflammatory gene expression and IR in obese mice (25). Although this is the first report that ovariectomy reduces WAT mRNA levels of GPX3, it has been reported that estrogen significantly up-regulates GPX3 gene expression in WAT (38).
Studies with mice and humans have also shown that obesity is associated with a reduction in CD4+ TREG, a T cell population that has been associated with protection against IR (39). Although we saw no differences in the total number of CD4+TREG between OVX and SHM mice, OVX tended to have a lower percentage of CD4+ T cells that were TREG than did SHM at 20 wk (P = 0.08). Of particular importance, the T cell inhibitory factor, CTLA4, was elevated in OVX at the early time point (before mice became insulin insensitive) but not at the later time point when OVX mice were significantly less sensitive to insulin than SHM mice, suggesting an inhibition of T cell activation early in the time course may have protected the mice. This study is the first to report the changes in T cell populations within the WAT of female mice in the absence of HFD feeding and suggests that HFD and ovariectomy have different effects on T cell populations in WAT. The mechanisms responsible for the time-course changes in AAT T cell populations need to be addressed in future studies.
Although this study links the loss of ovarian hormone production in mice to systemic insulin insensitivity and WAT immune cell infiltration and activation, the findings cannot be specifically attributed to loss of estrogen because other ovarian hormones or possibly a combination cannot be ruled out. It is also important to mention that, although ovariectomy is the most common animal model of human menopause, it is not without limitations. For example, postmenopausal women still produce some ovarian testosterone and the adrenals synthesize C19 steroids, which are converted to testosterone in the periphery; rodents do not make C19 steroids and therefore lack estrogen as well as testosterone. In addition, tissue level insulin sensitivity was not investigated in this study, nor was the euglycemic hyperinsulinemic clamp used; thus, whether it was improvements in skeletal muscle, adipocyte, or liver insulin sensitivity that contributed to the observed enhancement of systemic insulin tolerance cannot be stated.
This model of long-term OVX allowed us to make comparisons of more subtle inflammatory conditions between OVX and SHM AAT because their body weight, body fatness, fat distribution pattern, mean adipocyte volume, circulating HMW adiponectin, and circulating NEFA and TG were comparable at the 26-wk time point. The reduction in insulin sensitivity observed in OVX mice compared with their SHM counterparts was correlated with increased infiltration of M1 mΦ and T cells as well as increased MΦ and T cell activation in the AAT depot despite the OVX mice no longer being fatter than their SHM counterparts. The cytokine, IL12p40 has been demonstrated to activate T cells to produce and release IFN-γ (18); both were increased in OVX by 26 wk. Intriguingly, it has recently been reported that T cells from obese mice produce more IFN-γ than lean controls and that IFN-γ knockout animals are protected from IR, even when fed a HFD (18). We hypothesize that T cell MΦ cross-activation, including increased IFN-γ production from T cells, led to an inflammatory profile in the AAT of OVX that contributed to the reduced insulin tolerance that was observed (Fig. 7). This inflammatory profile was supported by histological measurements, demonstrating increased incidence of adipocyte death indicated by elevated CLS. Whether the increased cell death contributed to the increased inflammatory profile in AAT or vice versa cannot be determined from the present study. Nonetheless, our data strongly suggest the idea that it is the physiology of the WAT, including the balance of inflammatory mediators, that helps promote IR and not obesity per se. These findings also suggest that IR, and perhaps other metabolic disturbances, may occur during menopause, even when obesity is not present. Such metabolic disturbances may relate, at least in part, to an increase in WAT inflammation, thus implicating loss of ovarian hormones to alterations in adipose tissue immune cell activities.
Acknowledgments
We thank Dr. Brooke Stephens Hassan for her helpful insight in interpretation of the metabolic chamber data and to Dr. Christine Graham for help with histology. Author contributions included the following: V.J.V.P. helped design the study, conducted the experiments, analyzed the data, and wrote the manuscript; K.J.S. helped direct the study, helped with fluorescence-associated cell sorting, and revised the manuscript; C.X. helped with several of the experiments; E.C., G.B., and J.D. helped harvest tissues and offered critical insight; and M.S.O. and A.S.G. helped direct the study.
This work was supported by grant R24 DK087669, DK082574, and R24 DK0867669 from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases to (A.S.G.), grant RO1DK074979-01A1 (to A.S.G., K.J.S., and M.S.O.), grant 58-1950-7-707 from USDA Agricultural Service (to A.S.G. and M.S.O.), and grants T32- DK062032-20 and GB-T32-HL069772-09 (to J.D.).
Disclosure Summary: There is no conflict of interest to report.
Footnotes
- AAT
- Abdominal adipose tissue
- Arg-1
- arginase-1
- CLS
- crown-like structure
- CTLA4
- cytotoxic T lymphocyte antigen 4
- GPX3
- glutathione peroxidase 3
- HFD
- high-fat diet
- HMW
- high molecular weight
- HOMA-IR
- homeostatic model assessment of insulin sensitivity
- IFN-γ
- interferon-γ
- IR
- insulin resistance
- ITT
- insulin tolerance testing
- MΦ
- macrophage
- MRI
- magnetic resonance imaging
- NEFA
- nonesterified fatty acid
- OVX
- ovariectomized
- PGAT
- perigonadal adipose tissue
- SHM
- sham operated
- SQAT
- sc adipose tissue
- SVC
- stromal vascular cell
- TG
- triglyceride
- TREG
- regulatory T cells
- WAT
- white adipose tissue.
References
- 1. Polotsky HN, Polotsky AJ. 2010. Metabolic implications of menopause. Semin Reprod Med 28:426–434 [DOI] [PubMed] [Google Scholar]
- 2. Perry A, Wang X, Goldberg R, Ross R, Jackson L. 2008. The relationship between cardiometabolic and hemostatic variables: influence of race. Metabolism 57:200–206 [DOI] [PubMed] [Google Scholar]
- 3. Heidari R, Sadeghi M, Talaei M, Rabiei K, Mohammadifard N, Sarrafzadegan N. 2010. Metabolic syndrome in menopausal transition: Isfahan Healthy Heart Program, a population based study. Diabetol Metab Syndr 2:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Escriva F, Agote M, Rubio E, Molero JC, Pascual-Leone AM, Andres A, Satrustegui J, Carrascosa JM. 1997. In vivo insulin-dependent glucose uptake of specific tissues is decreased during aging of mature Wistar rats. Endocrinology 138:49–54 [DOI] [PubMed] [Google Scholar]
- 5. Manetta J, Brun JF, Callis A, Mercier J, Prefaut C. 2001. Insulin and non-insulin-dependent glucose disposal in middle-aged and young athletes versus sedentary men. Metabolism 50:349–354 [DOI] [PubMed] [Google Scholar]
- 6. Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN. 2007. Aging up-regulates expression of inflammatory mediators in mouse adipose tissue. J Immunol 179:4829–4839 [DOI] [PubMed] [Google Scholar]
- 7. Nishimura S, Manabe I, Nagai R. 2009. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med 8:55–60 [PubMed] [Google Scholar]
- 8. Klöting N, Fasshauer M, Dietrich A, Kovacs P, Schön MR, Kern M, Stumvoll M, Blüher M. 2010. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab 299:E506–E515 [DOI] [PubMed] [Google Scholar]
- 9. Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. 2009. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 150:2161–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, Sierra O, Pacifici R. 2003. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-γ-induced class II transactivator. Proc Natl Acad Sci USA 100:10405–10410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grassi F, Tell G, Robbie-Ryan M, Gao Y, Terauchi M, Yang X, Romanello M, Jones DP, Weitzmann MN, Pacifici R. 2007. Oxidative stress causes bone loss in estrogen-deficient mice through enhanced bone marrow dendritic cell activation. Proc Natl Acad Sci USA 104:15087–15092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pacifici R. 2007. T cells and postmenopausal osteoporosis in murine models. Arthritis Res Ther 9:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu H, Perrard XD, Wang Q, Perrard JL, Polsani VR, Jones PH, Smith CW, Ballantyne CM. 2010. CD11c expression in adipose tissue and blood and its role in diet-induced obesity. Arterioscler Thromb Vasc Biol 30:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. 2008. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32:451–463 [DOI] [PubMed] [Google Scholar]
- 15. Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M, Fischer-Posovszky P, Barth TF, Dragun D, Skurk T, Hauner H, Blüher M, Unger T, Wolf AM, Knippschild U, Hombach V, Marx N. 2008. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 28:1304–1310 [DOI] [PubMed] [Google Scholar]
- 16. Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, Andoh Y, Fujii S, Iwabuchi K, Onoé K, Tsutsui H. 2010. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Arterioscler Thromb Vasc Biol 30:193–199 [DOI] [PubMed] [Google Scholar]
- 17. Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. 2008. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab 8:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. 2008. Interferon-γ, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res 103:467–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. 1985. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- 20. Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. 2007. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56:2910–2918 [DOI] [PubMed] [Google Scholar]
- 21. Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. 1991. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 266:11341–11346 [PubMed] [Google Scholar]
- 22. Murano I, Barbatelli G, Parisani V, Latini C, Muzzonigro G, Castellucci M, Cinti S. 2008. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res 49:1562–1568 [DOI] [PubMed] [Google Scholar]
- 23. DeFuria J, Bennett G, Strissel KJ, Perfield JW, 2nd, Milbury PE, Greenberg AS, Obin MS. 2009. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr 139:1510–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. 2005. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 25. Lee YS, Kim AY, Choi JW, Kim M, Yasue S, Son HJ, Masuzaki H, Park KS, Kim JB. 2008. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol Endocrinol 22:2176–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geer EB, Shen W. 2009. Gender differences in insulin resistance, body composition, and energy balance. Gend Med 6(Suppl 1):60–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ren J, Kelley RO. 2009. Cardiac health in women with metabolic syndrome: clinical aspects and pathophysiology. Obesity (Silver Spring) 17:1114–1123 [DOI] [PubMed] [Google Scholar]
- 28. Tousignant B, Faraj M, Conus F, Garrel D, Brochu M, Rabasa-Lhoret R, Coderre L. 2008. Body fat distribution modulates insulin sensitivity in post-menopausal overweight and obese women: a MONET study. Int J Obes (Lond) 32:1626–1632 [DOI] [PubMed] [Google Scholar]
- 29. Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. 2010. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 34:989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Medrikova D, Jilkova ZM, Bardova K, Janovska P, Rossmeisl M, Kopecky J. 2012. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int J Obes (Lond) 36(2):262–72 [DOI] [PubMed] [Google Scholar]
- 31. Gorzek JF, Hendrickson KC, Forstner JP, Rixen JL, Moran AL, Lowe DA. 2007. Estradiol and tamoxifen reverse ovariectomy-induced physical inactivity in mice. Med Sci Sports Exerc 39:248–256 [DOI] [PubMed] [Google Scholar]
- 32. Takeo T, Sakuma Y. 1995. Diametrically opposite effects of estrogen on the excitability of female rat medial and lateral preoptic neurons with axons to the midbrain locomotor region. Neurosci Res 22:73–80 [DOI] [PubMed] [Google Scholar]
- 33. Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, Stephens JM, Mynatt RL, Dixit VD. 2010. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol 185:1836–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. 2010. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 18:1918–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. 2009. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 15:914–920 [DOI] [PubMed] [Google Scholar]
- 36. Stubbins RE, Najjar K, Holcomb VB, Hong J, Núñez NP. 2012. Estrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes Metab 14:58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. 2011. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17:179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lundholm L, Putnik M, Otsuki M, Andersson S, Ohlsson C, Gustafsson JA, Dahlman-Wright K. 2008. Effects of estrogen on gene expression profiles in mouse hypothalamus and white adipose tissue: target genes include glutathione peroxidase 3 and cell death-inducing DNA fragmentation factor, alpha-subunit-like effector A. J Endocrinol 196:547–557 [DOI] [PubMed] [Google Scholar]
- 39. Deiuliis J, Shah Z, Shah N, Needleman B, Mikami D, Narula V, Perry K, Hazey J, Kampfrath T, Kollengode M, Sun Q, Satoskar AR, Lumeng C, Moffatt-Bruce S, Rajagopalan S. 2011. Visceral adipose inflammation in obesity is associated with critical alterations in T regulatory cell numbers. PLoS One 6:e16376. [DOI] [PMC free article] [PubMed] [Google Scholar]