Abstract
Protein tyrosine phosphatase 1B (PTP1B) is a ubiquitously expressed tyrosine phosphatase implicated in the negative regulation of leptin and insulin receptor signaling. PTP1B−/− mice possess a lean metabolic phenotype attributed at least partially to improved hypothalamic leptin sensitivity. Interestingly, mice lacking both leptin and PTP1B (ob/ob:PTP1B−/−) have reduced body weight compared with mice lacking leptin only, suggesting that PTP1B may have important leptin-independent metabolic effects. We generated mice with PTP1B deficiency specifically in leptin receptor (LepRb)-expressing neurons (LepRb-PTP1B−/−) and compared them with LepRb-Cre-only wild-type (WT) controls and global PTP1B−/− mice. Consistent with PTP1B's role as a negative regulator of leptin signaling, our results show that LepRb-PTP1B−/− mice are leptin hypersensitive and have significantly reduced body weight when maintained on chow or high-fat diet (HFD) compared with WT controls. LepRb-PTP1B−/− mice have a significant decrease in adiposity on HFD compared with controls. Notably, the extent of attenuated body weight gain on HFD, as well as the extent of leptin hypersensitivity, is similar between LepRb-PTP1B−/− mice and global PTP1B−/− mice. Overall, these results demonstrate that PTP1B deficiency in LepRb-expressing neurons results in reduced body weight and adiposity compared with WT controls and likely underlies the improved metabolic phenotype of global and brain-specific PTP1B-deficient models. Subtle phenotypic differences between LepRb-PTP1B−/− and global PTP1B−/− mice, however, suggest that PTP1B independent of leptin signaling may also contribute to energy balance in mice.
Obesity is a growing health problem in the United States and worldwide (1, 2). Numerous cellular signals play a role in body weight regulation, including leptin. Leptin is a 16-kDa hormone released by adipose tissue into circulation, which plays a major regulatory role in feeding and energy expenditure via action in the brain (3). Circulating leptin levels correlate with total body fat content, signaling to the central nervous system (CNS) current levels of energy stores. Increasing levels of leptin signal a state of energy abundance to the brain, suppressing food intake and increasing energy expenditure. Leptin-deficient ob/ob mice are hyperphagic and develop obesity and insulin resistance (4).
The leptin receptor has multiple isoforms and only the “long form,” LepRb (leptin receptor isoform B), is capable of associating with the intracellular tyrosine kinase Janus kinase 2 (JAK2). Leptin stimulation activates JAK2, resulting in autophosphorylation and phosphorylation of several tyrosine residues on LepRb, allowing for recruitment and activation of downstream signaling molecules. LepRb has broad central expression within the forebrain (hippocampus, hypothalamus, and piriform cortex), midbrain (dorsal raphe nuclei), and hindbrain (cerebellum, reticulotegmental nucleus, and nucleus of the solitary tract) (5–8). The highest concentrations of LepRb-expressing neurons are found in hypothalamic nuclei, including the arcuate nucleus, ventromedial and dorsomedial nuclei of the hypothalamus, and the lateral hypothalamus. Within the arcuate nucleus, two separate neuron populations expressing either agouti-related protein or proopiomelanocortin (POMC) drive opposing effects on energy balance. Leptin decreases food intake and increases energy expenditure by inhibiting orexigenic agouti-related protein neuron activity while stimulating anorexigenic POMC neurons. In the hindbrain, leptin induces phosphorylated-signal transducer and activator of transcription 3 (pSTAT3) immunoreactivity in POMC nucleus of the solitary tract neurons and is also required for energy balance regulation (9, 10).
Leptin signaling is regulated by multiple mechanisms, including tyrosine phosphorylation. Protein tyrosine phosphatase 1B (PTP1B) is an important negative regulator of leptin signaling through the dephosphorylation of JAK2 (11, 12). PTP1B is a ubiquitously expressed enzyme. However, in the brain, it shows enriched expression in areas of high LepRb expression, including in the hypothalamus (11). Consistent with its role as a negative regulator of leptin signaling, global-, whole brain-, or POMC neuron-specific deficiency of PTP1B in mice results in lean and leptin hypersensitive animals (13–15). Muscle-, liver-, or adipocyte-specific PTP1B deletion does not result in a reduction in body weight or adiposity. Therefore, PTP1B's regulation of body mass is likely to be centrally mediated (14, 16–18). Global PTP1B knockout mice also show improvements in insulin sensitivity, consistent with PTP1B being a known negative regulator of insulin receptor (IR) signaling (19, 20). Indeed, deficiency of PTP1B in major insulin-responsive tissues (muscle or liver) results in enhanced IR signaling and improved glucose homeostasis (16, 17). Interestingly, neuron-specific and POMC neuron-specific PTP1B knockout mice also show improvements in peripheral insulin sensitivity even when normalized for body weight and adiposity (14, 15).
The lean metabolic phenotype of PTP1B-deficient mouse models is primarily attributed to improved leptin sensitivity. Thus, compound ob/ob:PTP1B−/− mice would be expected to exhibit a similar metabolic phenotype compared with ob/ob single mutants, due to the complete loss of circulating leptin. Intriguingly, however, ob/ob:PTP1B−/− mice exhibit suppressed body weight gain in comparison with ob/ob single mutants, suggesting that PTP1B's metabolic effects may not be solely due to its regulation of leptin signaling (12). In a separate study, ob/ob mice treated with PTP1B antisense oligonucleotides showed decreased epididymal fat pad weight when compared with saline-treated mice (21). Additionally, leptin receptor-deficient PTP1B double mutants (db/db:PTP1B−/−) exhibit decreased plasma triglyceride and free fatty acids compared with db/db:PTP1B+/− mice (22). Thus, the extent to which PTP1B mediates its metabolic effects via central leptin-independent pathways remains unclear. Here, to begin to address this, we generated mice with PTP1B deficiency in leptin receptor-expressing neurons (LepRb-PTP1B−/−) and examined body weight, adiposity, leptin sensitivity, and glucose homeostasis. LepRb-PTP1B−/− and LepRb-PTP1B+/− mice were compared with LepRb-Cre controls as well as to whole-body PTP1B knockouts to examine whether PTP1B's regulation of energy homeostasis is limited to leptin receptor-expressing neurons.
Materials and Methods
Animal care
All animal care protocols and procedures were approved by the University of Pennsylvania Institutional Care and Use Committee. We maintained mice on a 12-h light, 12-h dark cycle in a temperature-controlled barrier facility, with free access to water and food (standard chow autoclavable Lab Diet 5010 or custom high-fat diet (HFD) Teklad TD93075; Harlan Teklad, Indianapolis, IN). Age-matched littermates were used for all experiments.
Mice with LepRb-specific deletion of PTP1B
Ptpn1loxP/loxP mice were generated previously and genotyped by PCR as described (14). Genotyping primer sequences for the Ptpn1 allele were: PTP1B forward 5′-TGCTCACTCACCCTGCTACAA and reverse 5′-GAAATGGCTCACTCCTACTGG; all Ptpn1loxP/loxP mice were originally on a mixed 129Sv/J × C57BL/6 background before mating with LepRb-Ires-Cre mice (gift from M. Meyers; University of Michigan, Ann Arbor, MI), which were on a C57BL/6 background. Genotyping primer sequences for LepRb-Ires-Cre allele were: Cre forward 5′-CCTCTCCACCCAAGCGGCCGGAGAACC and reverse 5′-CCGGCTCCGTTCTTTGGTGGCCCCTTCGCG; wild type (WT) forward 5′-GCCCTCATTAATCTAGTAATGTAGATGG and reverse 5′-ACTAGGGGTCAACTCTC.
Isolating DNA from tissues for detection of recombination of the floxed alleles
Tissues were digested at 55 C overnight in proteinase K digestion buffer [100 mm Tris-HCl (pH 8.5), 5 mm EDTA, 0.2% sodium dodecyl sulfate, 200 mm NaCl, and 300 μg/ml proteinase K]. Saturated NaCl (∼6 m) was added to the digestion, and samples were vortexed vigorously for 1 min. Samples were centrifuged for 20 min at 13,700 × g, and supernatants were transferred to a fresh tube. DNA was precipitated by adding 1 ml of 100% ethanol, and pellets were washed once with 70% ethanol and resuspended in 100 μl of sterile PCR water for analysis. PCR primers for detection of recombined alleles were: Ptpn1Δ/Δ forward 5′-GTGGTGCCTGCAAGAGAACTGAC and reverse 5′-GAAATGGCTCACTCCTACTGG; IL-2 internal control forward 5′-CTAGGCCACAGAATTGAAAGATCT and reverse 5′-GTAGGTGGAAATTCTAGCATCATCC.
Body composition and food intake
At weaning, mice were placed on diets of either standard laboratory chow [Lab Diet 5010; calories provided by protein (28.7%), fat (12.7%), and carbohydrate (58.5%)] or custom HFD [Teklad TD93075; calories provided by protein (21.2%), fat (54.8%), and carbohydrate (24%)]. Body weights were assessed weekly for at least 18 wk, and food intake was measured daily. Body length was measured as nose-rump length at indicated age. Epididymal fat pads were dissected and weighed at indicated age.
Energy expenditure measurements
Adult male mice were transitioned from chow diet to HFD at 9 wk of age. Rectal temperature was measured with a thermistor during light cycle in animals at 11 wk of age (MicroTherma 2T; ThermoWorks, Lindon, UT). Brown adipose tissue (BAT) uncoupling protein 1 (UCP1) gene expression was measured by quantitative real-time PCR (qRT-PCR) as described below.
Leptin sensitivity
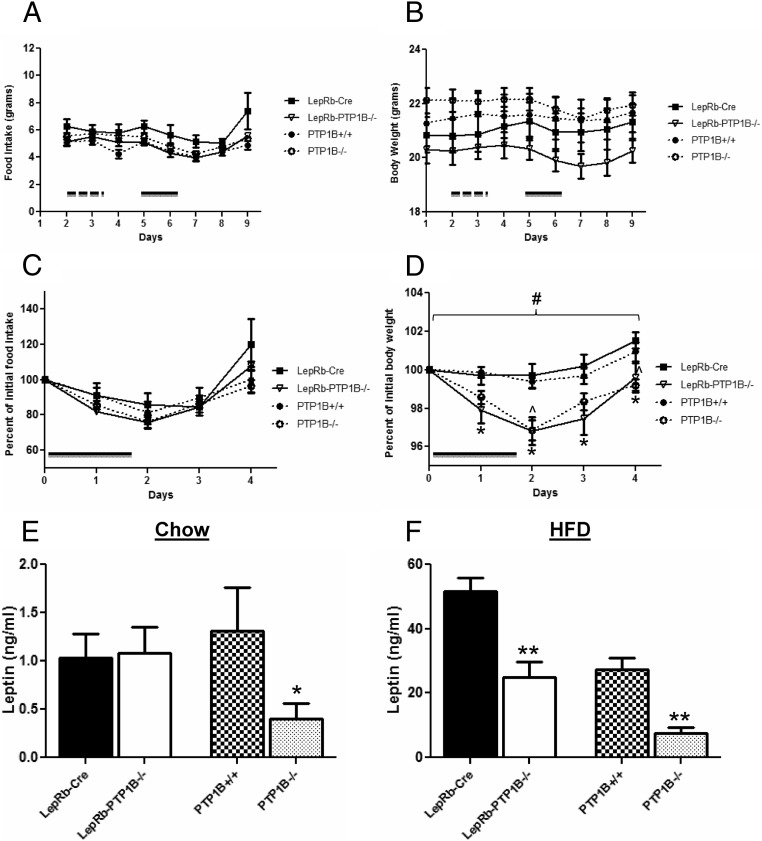
For in vivo leptin sensitivity measurements, recombinant mouse leptin 0.5 μg/g body weight/injection (A. F. Parlow; National Hormone and Peptide Program, Torrance, CA) or an equal volume 0.9% saline was administered ip to male mice on a chow diet (∼7–8 wk of age). Mice were initially injected with saline ip every 12 h over the course of 36 h. After a recovery period of 24 h, leptin was administered morning and evening following the same paradigm. Body weight and food intake were monitored daily for 4 d after the initial leptin injection. Baseline body weight and food intake measurements for the days before the start of leptin injections were averaged and used to calculate percent change. Animals acted as internal controls, because each received both saline and leptin injections using a within subjects design.
Glucose homeostasis
Glucose tolerance tests (GTT) were performed as described previously (13). Glucose dose used for ip injections was 2 mg/g BW (20% solution). Blood glucose was assayed in tail blood using a glucometer (Contour; Bayer, Tarrytown, NY). Fasting insulin levels were determined as described below.
RNA extraction and real-time PCR
Mice were killed at the onset of the light cycle (0800–1000 h) after overnight food withdrawal (fasted). Tissues were rapidly dissected and flash frozen in liquid nitrogen. Total RNA was extracted from tissues using TRIzol (Invitrogen, Carlsbad, CA) and the RNeasy kit (QIAGEN, Valencia, CA). cDNA was synthesized from 1 μg of total RNA using the Advantage RT-for-PCR kit (CLONTECH, Mountain View, CA). The relative mRNA levels of Ucp1, and Cidea, were assessed and quantified by qRT-PCR. Hprt1 (SABiosciences, Valencia, CA) was used as an internal control. The qRT-PCR reactions were carried out using RT2 SYBR Green qPCR Master Mix (SABiosciences), and samples were run using the Eppendorf Mastercycler ep realplex. Primer sequences for Ucp1 and Cidea were reported previously (23). Relative mRNA expression was calculated using the comparative Ct method as described previously (14).
Serum analysis
Trunk blood was collected from mice after overnight fast at indicated age. Serum was separated by centrifugation at 6000 × g. Serum insulin and leptin were measured by ELISA (Crystal Chem, Inc., Downers Grove, IL).
Statistics
Results are expressed as mean ± sem. Comparisons between groups were made by unpaired two-tailed Student's t test or two-way ANOVA with repeated measures in one factor followed by Fisher's protected least significant difference test, as appropriate. A P value less than 0.05 was considered to be statistically significant.
Results
Generation of LepRb-specific PTP1B−/− mice
To generate LepRb-specific PTP1B-deficient mice, we crossed Ptpn1loxP/loxP mice to LepRbcre/cre knockin mice in which Cre recombinase is specifically expressed in LepRb-expressing cells (LepRb-Ires-Cre) (5, 24–26). The resulting Ptpn1+/loxP LepRb+/cre mice were subsequently crossed to LepRbcre/cre mice to yield Ptpn1+/loxP LepRbcre/cre mice. Ptpn1+/loxP LepRbcre/cre mice were crossed together to yield Ptpn1loxP/loxP LepRbcre/cre (hereafter termed LepRb-PTP1B−/−), Ptpn1+/loxP LepRbcre/cre (hereafter termed LepRb-PTP1B+/−), and Ptpn1+/+ LepRbcre/cre (LepRb-Cre) “WT,” Cre-only littermate controls. To increase numbers of Ptpn1loxP/loxP LepRbcre/cre and to generate all appropriate controls, we also crossed Ptpn1+/loxP LepRb+/cre mice with Ptpn1loxP/loxP mice to yield Ptpn1loxP/loxP LepRb+/cre, which were then subsequently crossed together. This generated additional Ptpn1loxP/loxP LepRbcre/cre (LepRb-PTP1B−/−) and Ptpn1loxP/loxP LepRb+/+ (PTP1B fl/fl) controls. Finally, in a parallel cross, we crossed PTP1B+/− mice together to generate whole-body PTP1B−/− and their respective PTP1B+/+ WT littermate controls.
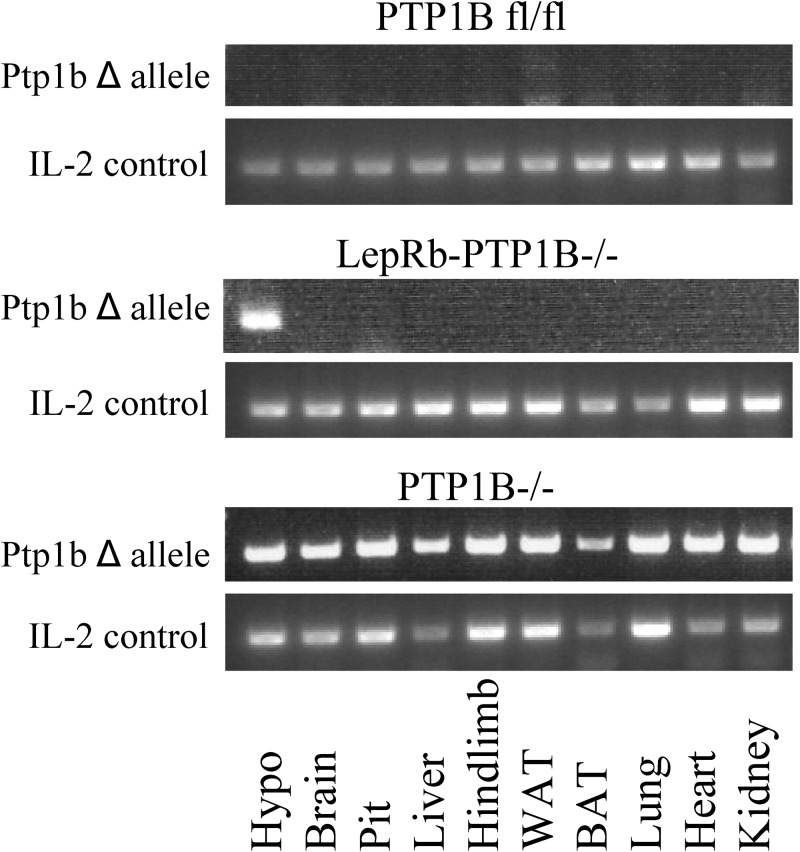
To verify deletion of the PTP1B gene, we extracted DNA from a variety of tissues (hypothalamus, brain without hypothalamus, pituitary, liver, hindlimb muscle, epididymal white adipose, interscapular brown adipose, lung, heart, and kidney) and assessed for deletion of the floxed allele via PCR. Deletion was only detected in hypothalamus tissue from LepRb-PTP1B−/− (Fig. 1). Although LepRb is also expressed outside of the hypothalamus (5, 6, 27), no deletion was detected in whole brain without hypothalamus or peripheral tissues of LepRb-PTP1B−/− mice, likely due to dilution of signal by non-LepRb expressing cells and generally limited LepRb expression. No deletion was detected in any tissues isolated from WT control animals, whereas deletion of the PTP1B gene was detected in all tissues from whole-body PTP1B−/− mice acting as positive controls.
Fig. 1.
Detection of deletion of PTP1B floxed allele in LepRb-PTP1B−/− mice. DNA was isolated from different tissues [hypothalamus (Hypo), brain without hypothalamus, pituitary (Pit), liver, hindlimb, white adipose tissue (WAT), BAT, lung, heart, and kidney], and deletion of floxed allele was detected by PCR.
LepRb-PTP1B−/− mice have decreased body weight on chow and HFD
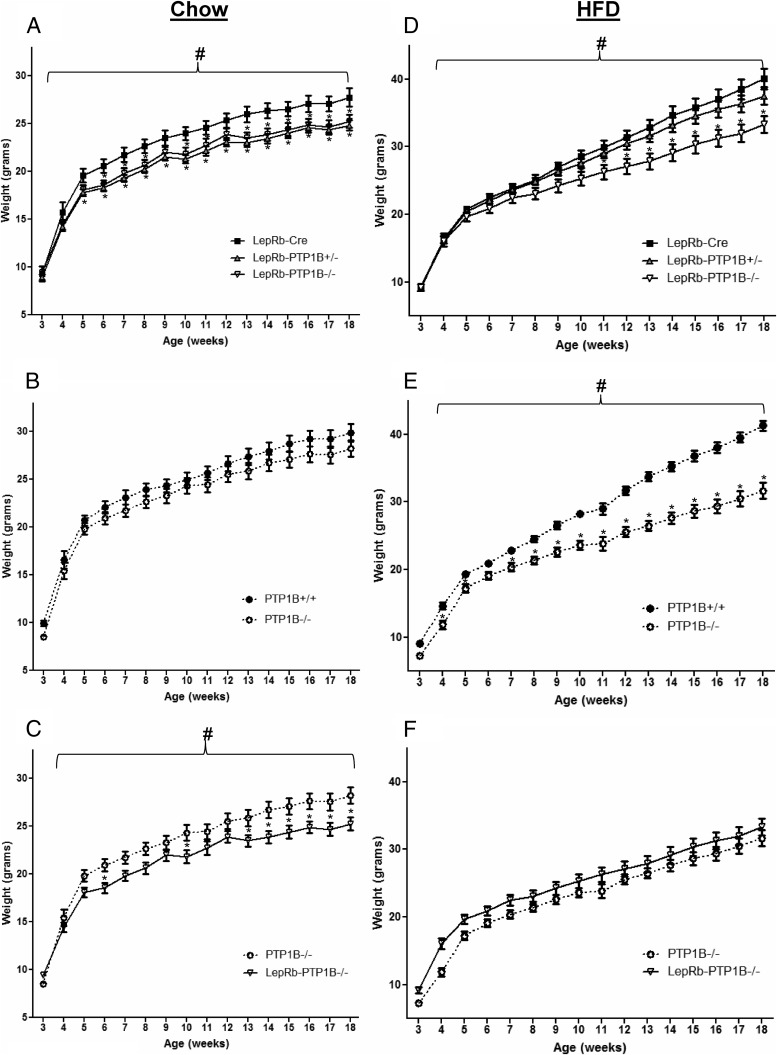
To assess whether energy balance is affected by PTP1B deficiency in LepRb-expressing neurons, we examined body weight in animals placed on normal chow or HFD at weaning. Both LepRb-PTP1B−/− and LepRb-PTP1B+/− mice show significantly decreased body weight on chow compared with Cre-only WT controls (Fig. 2A). No body weight differences were found between PTP1B fl/fl “floxed” and LepRb-Cre-only controls (data not shown). In contrast to LepRb-PTP1B−/− mice, global PTP1B−/− mice are similar in body weight compared with their WT littermate controls on chow diet as previously shown (Fig. 2B) (11). When compared with whole-body PTP1B−/− mice, LepRb-specific PTP1B−/− mice weigh less on chow (Fig. 2C). On HFD, LepRb-PTP1B−/− mice display a significant reduction in body weight compared with WT LepRb-Cre controls (Fig. 2D). Consistent with a previous report for PTP1B+/− mice on HFD (13), LepRb-PTP1B+/− mice on HFD do not display decreased body weight compared with WT controls. Global PTP1B−/− mice show reduced body weight on HFD to a comparable extent as that of LepRb-PTP1B−/− mice, demonstrating for the first time that the protection against diet-induced obesity seen in PTP1B-deficient models is largely (if not exclusively) due to PTP1B deficiency within LepRb-expressing neurons (Fig. 2, E and F).
Fig. 2.
LepRb-PTP1B−/− mice have reduced body weight on chow and HFD. A, Body weights of male LepRb-PTP1B−/− (n = 11), LepRb-PTP1B+/− (n = 16), and LepRb-Cre mice (n = 9) on chow. B, Body weights of male whole-body PTP1B−/− (n = 14) and WT controls (n = 9) on chow. C, Body weights of male LepRb-PTP1B−/− and whole-body PTP1B−/− mice on chow. D, Body weights of male LepRb-PTP1B−/− (n = 13), LepRb-PTP1B+/− (n = 23), and LepRb-Cre mice (n = 16) on HFD. E, Body weights of male whole-body PTP1B−/− (n = 14) and WT controls (n = 13) on HFD. F, Body weights of male LepRb-PTP1B−/− and whole-body PTP1B−/− mice on HFD. All values are mean ± sem. Weight curves analyzed by two-way ANOVA with repeated measures followed by pairwise comparisons: #, P < 0.05 by two-way ANOVA with repeated measures; *, P < 0.05 by Fisher's LSD post hoc test for indicated group compared with Cre (A and D) or WT control (E); *, P < 0.05 by Fisher's LSD post hoc test for LepRb-PTP1B−/− compared with PTP1B−/− mice (C).
LepRb-PTP1B−/− mice have decreased adiposity and body length on HFD
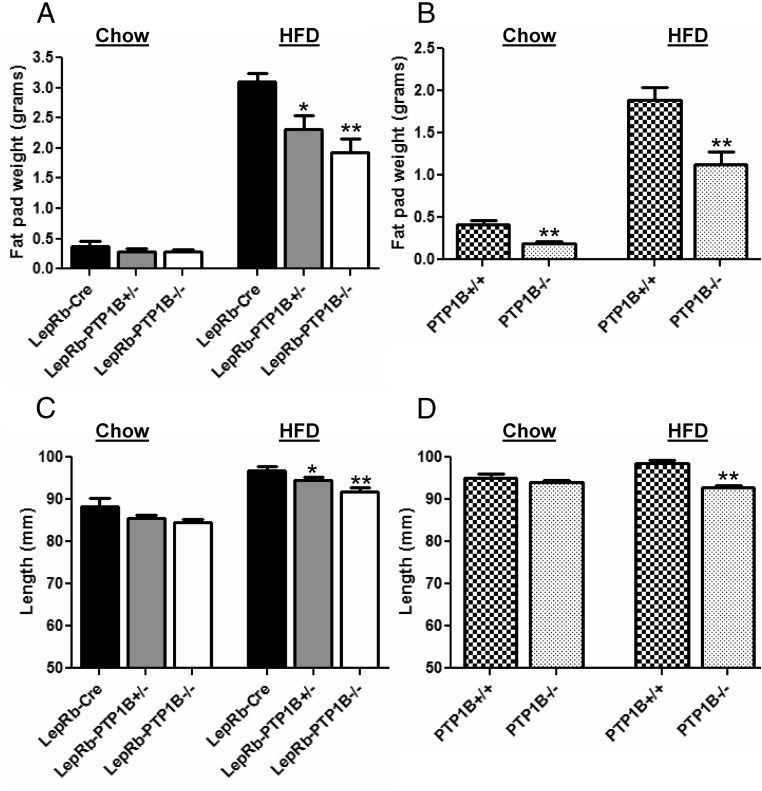
To determine whether the decreased body weight of LepRb-PTP1B−/− mice reflects decreases in fat mass or linear growth, we measured epididymal fat pad weights and body length. LepRb-PTP1B−/− mice on chow diet show a nonsignificant trend toward decreased adiposity as measured by epididymal fat pad weight when compared with Cre-only WT mice (Cre vs. LepRb-PTP1B−/−, 0.368 ± 0.083 vs. 0.278 ± 0.036 g; P = 0.341) (Fig. 3A). In contrast, global PTP1B−/− mice have decreased adiposity compared with controls despite similar body weight (PTP1B+/+ vs. PTP1B−/−, 0.410 ± 0.059 vs. 0.193 ± 0.020 g; P < 0.01) (Fig. 3B). On HFD, adiposity is significantly decreased in LepRb-PTP1B−/−, LepRb-PTP1B+/−, and global PTP1B−/− mice at 18 wk of age compared with their respective controls (Fig. 3, A and B).
Fig. 3.
LepRb-PTP1B−/− mice have decreased adiposity and body length on HFD. A, Epididymal fat pad weight of male LepRb-PTP1B−/− (n = 10 chow, n = 10 HFD), LepRb-PTP1B+/− (n = 16 chow, n = 9 HFD), and LepRb-Cre mice (n = 9 chow, n = 8 HFD). B, Epididymal fat pad weight of male whole-body PTP1B−/− mice (n = 14 chow, n = 14 HFD) and WT controls (n = 9 chow, n = 7 HFD). C, Nose-rump length of male LepRb-PTP1B−/− (n = 6 chow, n = 10 HFD), LepRb-PTP1B+/− (n = 12 chow, n = 9 HFD), and LepRb-Cre mice (n = 6 chow, n = 8 HFD). D, Nose-rump length of male whole-body PTP1B−/− mice (n = 8 chow, n = 14 HFD) and WT controls (n = 13 chow, n = 7 HFD). All values are mean ± sem; *, P < 0.05 for indicated group compared with Cre or WT control; **, P < 0.01 for indicated group compared with Cre or WT control.
Body length of LepRb-PTP1B−/− mice on chow is unchanged when compared with Cre-only WT controls (Fig. 3C). Likewise, whole-body PTP1B−/− mice show no difference in body length compared with their respective controls on chow (Fig. 3D). On HFD, LepRb-PTP1B−/− and LepRb-PTP1B+/− mice display significantly decreased body length compared with Cre-only controls (Fig. 3C). Similarly, whole-body PTP1B−/− on HFD also show decreased linear growth compared with controls (Fig. 3D).
Food intake and core temperature in LepRb-PTP1B−/− mice
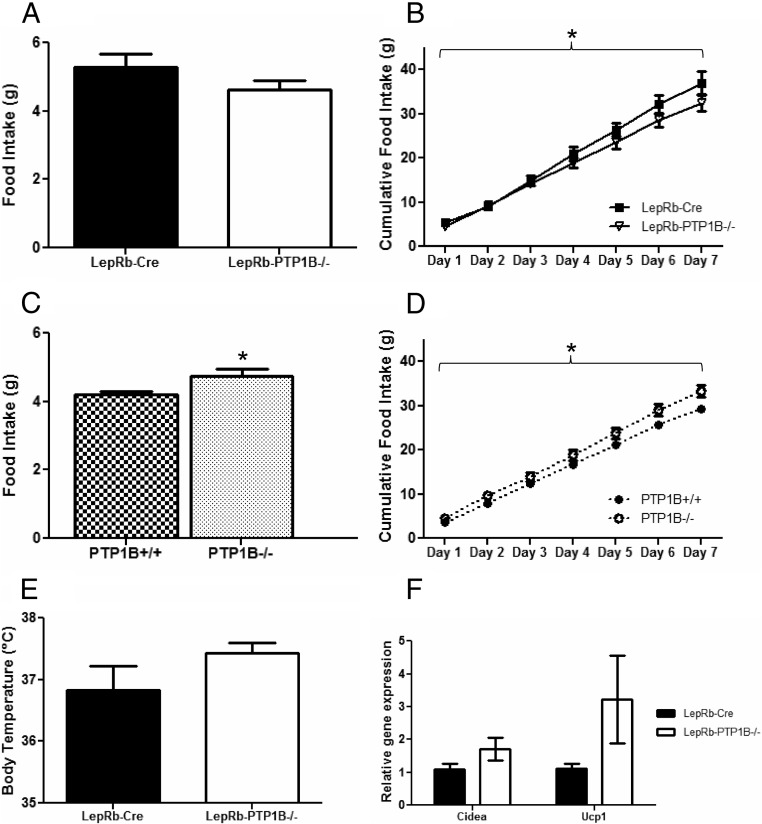
To determine the cause of the reduced body weight and adiposity in LepRb-PTP1B−/− mice, we measured daily food intake. Average daily food intake on both chow (Fig. 4A) and HFD (data not shown) is slightly decreased in LepRb-PTP1B−/− mice compared with Cre-only WT, although these differences do not reach statistical significance. To more carefully assess food intake over time, cumulative chow food intake over the course of 1 wk was measured. Cumulative food intake is decreased in LepRb-PTP1B−/− mice compared with controls on chow (Fig. 4B). In contrast, global PTP1B−/− mice have increased average daily and cumulative food intake on chow, consistent with past reports (Fig. 4, C and D) (13).
Fig. 4.
Food intake, core temperature, and expression of BAT genes in epididymal white adipose. A, Average daily food intake of male LepRb-PTP1B−/− and LepRb-Cre mice on chow (n = 6/group). B, Cumulative food intake of male LepRb-PTP1B−/− and LepRb-Cre mice on chow. C, Average daily food intake of male whole-body PTP1B−/− and WT controls on chow (n = 6/group). D, Cumulative food intake of male whole-body PTP1B−/− and WT controls on chow. E, Core temperature of male LepRb-PTP1B−/− and LepRb-Cre mice after 2 wk of HFD (n = 6/group). F, Expression of Cidea and Ucp1 in epididymal white adipose of male LepRb-PTP1B−/− and LepRb-Cre mice on chow. All values are mean ± sem; *, P < 0.05 for indicated group compared with Cre or WT control. For cumulative food intake: *, P < 0.05 by two-way ANOVA with repeated measures.
Global, brain-specific, and POMC neuron-specific PTP1B−/− models exhibit a lean metabolic phenotype and increased energy expenditure (13–15, 28). To assess whether LepRb-PTP1B−/− mice showed enhanced diet-induced adaptive thermogenesis, we measured core temperature after placing adult animals (8 wk of age) on a HFD for 2 wk. Core temperature of LepRb-PTP1B−/− mice is elevated by more than 0.5 C, although this does not reach statistical significance (Cre vs. LepRb-PTP1B−/−, 36.83 ± 0.39 vs. 37.43 ± 0.16 C; P = 0.183) (Fig. 4E). Because BAT oxidizes chemical energy and is the central tissue promoting thermogenesis, we examined expression of Ucp1 in BAT. Interscapular BAT Ucp1 expression is unchanged in LepRb-PTP1B−/− mice compared with Cre-only controls (data not shown). Although no differences were seen in BAT Ucp1 expression, we measured expression of BAT gene markers in epididymal white adipose to see whether LepRb-PTP1B−/− mice showed increased “browning” of white fat, which would indicate enhanced energy expenditure (23, 29–31). Interestingly, in chow-fed animals, the brown fat markers, Cidea and Ucp1, show a trend toward increased expression in the epididymal white fat of LepRb-PTP1B−/− mice compared with Cre-only controls (all values Cre vs. LepRb-PTP1B−/−: Cidea, 1.098 ± 0.170 vs. 1.711 ± 0.339, P = 0.162; Ucp1, 1.108 ± 0.167 vs. 3.227 ± 1.335, P = 0.159) (Fig. 4F).
LepRb-PTP1B−/− mice show enhanced leptin sensitivity
Mice with whole-body, brain-specific, or POMC neuron-specific PTP1B deficiency demonstrate leptin hypersensitivity (11, 12, 14, 15). Given the leptin receptor expressing cell-specific deletion of PTP1B in this model, we expected LepRb-PTP1B−/− mice to recapitulate this improvement in leptin sensitivity. No changes in food intake or body weight were noted in any genotype in response to saline injection (Fig. 5, A and B). In response to leptin treatment (0.5 μg/g per injection, ip), food intake is suppressed to a similar extent in LepRb-PTP1B−/−, PTP1B−/−, and WT controls (Fig. 5, A and C). However, LepRb-PTP1B−/− and PTP1B−/− mice show a significantly greater suppression of body weight compared with their respective WT controls (Fig. 5, B and D), consistent with our hypothesis that LepRb-PTP1B−/− mice display a similar level of leptin hypersensitivity as whole-body PTP1B−/− mice. Additionally, we measured serum leptin levels from overnight-fasted mice on chow or HFD. On chow diet, serum leptin levels are unchanged in LepRb-PTP1B−/− mice compared with Cre-only WT, whereas global PTP1B−/− mice show decreased serum leptin compared with PTP1B WT, reflecting their reduced adiposity on chow (Fig. 5C). On HFD, serum leptin levels are reduced in LepRb-PTP1B−/− mice compared with Cre-only WT and in global-PTP1B−/− compared with their respective controls (Cre vs. LepRb-PTP1B−/−: 51.55 ± 4.33 vs. 24.87 ± 4.96 ng/ml, P < 0.01; PTP1B+/+ vs. PTP1B−/−: 27.36 ± 3.56 vs. 7.602 ± 1.71, P < 0.01) (Fig. 5D).
Fig. 5.
LepRb-PTP1B−/− mice have increased leptin sensitivity. A, Male mice (7 wk of age on a chow diet) were initially injected with saline ip every 12 h over the course of 36 h indicated by the dashed bar. After 24 h of recovery, mice were injected with leptin (0.5 μg/g body weight every 12 h for 48 h) ip indicated by the solid bar (n = 6/genotype). LepRb-PTP1B−/−, whole-body PTP1B−/− mice, Cre, and WT controls show suppression of food intake in response to leptin. B, Male LepRb-PTP1B−/− and whole-body PTP1B−/− mice show suppression of body weight, whereas Cre and WT controls do not respond to low dose leptin. C, Percent change in food intake in response to leptin injection compared with saline baseline. D, Percent change in body weight in response to leptin injection compared with saline baseline. E, Fasting serum leptin levels in male LepRb-PTP1B−/− (n = 17) and LepRb-Cre mice (n = 16), and whole-body PTP1B−/− mice (n = 14) and WT controls (n = 9) on chow (18 wk old). F, Fasting serum leptin levels in male LepRb-PTP1B−/− (n = 7) and LepRb-Cre mice (n = 8), and whole-body PTP1B−/− mice (n = 13) and WT controls (n = 7) on HFD (18 wk old). All values are mean ± sem. Leptin sensitivity analyzed by two-way ANOVA with repeated measures followed by pairwise comparisons: #, P < 0.05 by two-way ANOVA with repeated measures; *, P < 0.05 by Fisher's LSD post hoc test for LepRb-PTP1B−/− compared with Cre control; ^, P < 0.05 by Fisher's LSD post hoc test for PTP1B−/− vs. WT control. For serum leptin comparisons: *, P < 0.05 for indicated group compared with Cre or WT control; **, P < 0.01 for indicated group compared with Cre or WT control.
LepRb-PTP1B−/− mice show improvements in glucose homeostasis
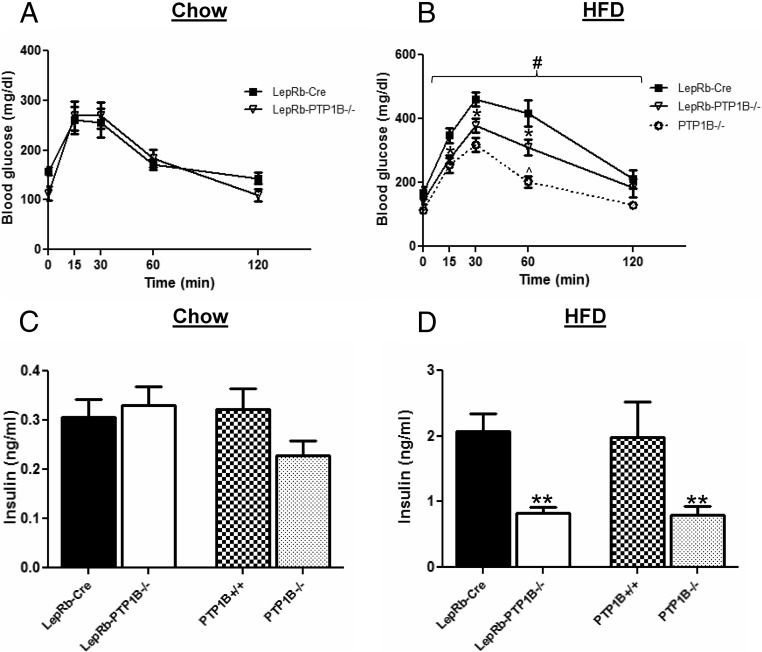
Because we previously found that neuronal deficiency of PTP1B can improve peripheral glucose homeostasis (14, 15), we examined glucose tolerance in LepRb-PTP1B−/− mice compared with controls. On chow, LepRb-PTP1B−/− mice display similar glucose clearance compared with Cre-only controls as measured by a GTT and have similar fasting serum insulin levels compared with controls (Fig. 6, A and C). On HFD, however, LepRb-PTP1B−/− show significantly improved glucose tolerance and fasting serum insulin compared with Cre-only controls (Cre vs. LepRb-PTP1B−/−, 2.076 ± 0.264 vs. 0.826 ± 0.087 ng/ml; P < 0.01) (Fig. 6, B and D). As expected, global PTP1B−/− mice also show significantly enhanced glucose clearance and lower fasting serum insulin levels compared with controls (PTP1B+/+ vs. PTP1B−/−, 1.987 ± 0.535 vs. 0.792 ± 0.135 ng/ml; P < 0.01) and displayed an additional enhancement in glucose clearance compared with LepRb-PTP1B−/− mice most likely due to the additional deletion of PTP1B in muscle and liver (13, 16, 17, 28).
Fig. 6.
LepRb-PTP1B−/− mice on HFD have improved glucose tolerance. A, GTT for male LepRb-PTP1B−/− (n = 10) and LepRb-Cre mice (n = 7) on chow (8–10 wk old). B, GTT for male LepRb-PTP1B−/− (n = 8), LepRb-Cre (n = 8), and whole-body PTP1B−/− mice (n = 6) on HFD (8–10 wk old). C, Fasting serum insulin levels in male LepRb-PTP1B−/− (n = 17) and LepRb-Cre mice (n = 16), and whole-body PTP1B−/− mice (n = 14) and WT controls (n = 9) on chow (18 wk old). D, Fasting serum insulin levels in male LepRb-PTP1B−/− (n = 10) and LepRb-Cre mice (n = 8), and whole-body PTP1B−/− mice (n = 14) and WT controls (n = 7) on HFD (18 wk old). All values are mean ± sem. GTT analyzed by two-way ANOVA with repeated measures followed by pairwise comparisons: #, P < 0.05 by two-way ANOVA with repeated measures; *, P < 0.05 by Fisher's LSD post hoc test for LepRb-PTP1B−/− compared with Cre control; ^, P < 0.05 by Fisher's LSD post hoc test for PTP1B−/− vs. LepRb-PTP1B−/− mice. For serum insulin comparisons: **, P < 0.01 for indicated group compared with Cre or WT control.
Discussion
The extent to which PTP1B's metabolic effects are mediated exclusively via LepRb-expressing neurons was previously unknown. Our data clearly demonstrate an important role for PTP1B in LepRb-expressing neurons in the control of body weight and leptin sensitivity in mice. Additionally, our findings highlight subtle differences between PTP1B's metabolic role in the whole body compared with its restricted effects within leptin receptor-expressing neurons, suggesting an additional leptin-independent metabolic role for PTP1B.
Global PTP1B deficiency, as well as neuronal PTP1B deficiency, results in a lean metabolic phenotype with decreased HFD-induced adiposity in mice (13, 14). We demonstrate that LepRb-PTP1B−/− mice display a significant decrease in body weight and adiposity when fed a HFD, which overall is indistinguishable from global PTP1B−/− mice. Thus, the lean metabolic phenotype of global PTP1B−/− mice fed a HFD likely results from PTP1B deficiency within LepRb-expressing neurons. Similar to mice with neuronal PTP1B deficiency, LepRb-PTP1B−/− mice fed a chow diet weighed less than WT controls and global PTP1B−/− mice. However, global PTP1B−/− mice do not show decreased body weight on chow diet. Previous studies have found that muscle-, liver-, or adipocyte-specific deletion of PTP1B does not result in decreased body weight or adiposity, whereas deletion of PTP1B in adipocytes and macrophages using the adipocyte protein 2-driven Cre results in weight gain (14, 16–18). The lack of any body weight difference in chow-fed global PTP1B−/− mice compared with controls may result from the combined effects of PTP1B deficiency in both brain and macrophages; this interesting possibility remains to be explored. Interestingly, global PTP1B−/− mice have reduced adiposity on chow diet, despite similar body weight as WT, whereas LepRb-PTP1B−/− mice show a trend toward reduced adiposity on chow, despite lower body weight.
The decreased body weight and adiposity of LepRb-PTP1B−/− mice are potentially a combined result of decreased food intake over time and increased energy expenditure due to enhanced leptin sensitivity. Indeed, cumulative food intake is lower in LepRb-PTP1B−/− mice, and core temperature and markers of white adipose browning tend to be higher. Overall these differences are subtle in magnitude. However, this phenotype is consistent within the context of other PTP1B-deficient models, which show enhanced energy expenditure measured by indirect calorimetry (13–15), and is also consistent with the fact that the reduction in body weight occurs slowly over time.
Previous models of PTP1B deficiency have all demonstrated clear elevations in energy expenditure (13–15), whereas only brain-specific PTP1B−/− mice showed a decrease in food intake. Other neuronal populations that express PTP1B (separate from LepRb- expressing neurons) may also play a role in food intake regulation, explaining the more pronounced suppression in food intake seen in complete brain-specific PTP1B knockouts. For example, insulin has been shown to have food intake-reducing effects (32–37) and may act upon cell populations both overlapping and distinct from LepRb-expressing populations. The IR is broadly expressed within the CNS, including the hypothalamus. Moreover, PTP1B is a known regulator of insulin signaling via dephosphorylation of IR and insulin receptor substrate (38–42). Although PTP1B deletion in LepRb neurons may in turn sensitize insulin signaling pathways, the extent to which LepRb and IR are coexpressed within the CNS is unclear, and in the case of POMC neurons, leptin and insulin appear to signal in separate, distinct subpopulations (43).
Like global-, brain-, and POMC-PTP1B−/− mice, LepRb-PTP1B−/− also display improved glucose tolerance and decreased circulating insulin levels on HFD. Whether these indications of improved insulin sensitivity are independent of differences in body weight and adiposity is unclear because LepRb-PTP1B−/− mice exhibit decreased body weight on chow and HFD. There is precedent for central PTP1B regulation of peripheral insulin sensitivity; POMC-PTP1B−/− mice display improved insulin sensitivity even when controlled for body weight and adiposity. Given the degree of overlap (at the neuronal level) in expression of POMC and LepRb, regulation of peripheral insulin sensitivity by neuronal PTP1B may, at least partially, account for the improvements seen in LepRb-PTP1B−/− mice. Central leptin signaling has been closely tied to the control of peripheral glucose homeostasis (44, 45), and enhancing central leptin sensitivity can improve insulin sensitivity at the periphery. Like PTP1B deficiency, deletion of suppressor of cytokine signaling-3, a separate known negative regulator of leptin signaling, results in improved peripheral glucose homeostasis (46, 47). Thus, enhanced leptin sensitivity of LepRb-PTP1B−/− mice may underlie the improvements seen in glucose tolerance and fasting insulin levels.
The subtle metabolic differences between LepRb-PTP1B−/− and global PTP1B−/− mice point toward a distinct, metabolically significant role for PTP1B outside of leptin receptor-expressing neurons. The significant reduction in adiposity seen in chow-fed global PTP1B−/− compared with LepRb-PTP1B−/− mice may be explained by PTP1B deficiency in non-LepRb-expressing neurons and/or in other tissues of global PTP1B−/− mice. For example, PTP1B has been shown to regulate BAT adipogenesis; preadipocyte cell lines derived from PTP1B−/− mice show enhanced BAT differentiation when compared with WT-derived cell lines (48, 49). PTP1B deletion in whole body or brain results in increased activity of the fuel sensing enzyme AMP-activated protein kinase as well as increased UCP1 expression in BAT; increased AMP-activated protein kinase activity in BAT promotes expression of fatty acid oxidation and mitochondrial markers (50). Additionally, nonleptin signaling pathways that play a role in the central control of energy homeostasis may be sensitized in non-LepRb-expressing neurons, potentially contributing to the reduced adiposity phenotype limited to global PTP1B−/− mice on chow. It should be noted that although both LepRb-PTP1B−/− and global PTP1B−/− mice are on a mixed 129Sv/J × C57BL/6 background, the two lines were generated in parallel, and subtle differences in genetic strain may also contribute to the metabolic differences observed.
In models of diet-induced obesity, whether leptin resistance is a cause or consequence of the obese state is incompletely understood. Current evidence support leptin resistance as a consequence and subsequent facilitator of the obese state: HFD-fed mice exhibit baseline increases in arcuate pSTAT3 levels in absence of exogenous leptin (51). Diet-induced obese animals tend to reduce food intake and body weight after removal of the palatable diet, thus cellular leptin resistance is not enough to maintain the obese phenotype. In a model of obesity proposed by Myers et al. (52), increased caloric intake promotes weight gain, increased adiposity, and cellular leptin resistance; the development of leptin resistance in turn facilitates further weight gain. This model suggests that cellular leptin resistance develops over time. Therefore, the benefits of increased leptin sensitivity from LepRb-specific PTP1B deficiency would not be immediately noticeable in young animals. In spite of this, global PTP1B−/− mice on HFD show body weight differences early on, near weaning age (13). Although these differences are small, our data were consistent with Klaman et al. (13), with global PTP1B−/− mice displaying significant differences in body weight appearing as early as 1–2 wk after weaning (Fig. 2E). On the other hand, LepRb-PTP1B−/− mice display decreased body weight on HFD at 7 wk after weaning (10 wk of age). This early weight difference between global PTP1B−/− and LepRb-PTP1B−/− mice may be explained by PTP1B outside of LepRb-expressing neurons sensitizing nonleptin signaling pathways, which may affect early growth and development. Alternatively, the temporal expression pattern of PTP1B within LepRb-expressing neurons may be affecting early growth. For example, LepRb-PTP1B−/− mice are capable of expressing PTP1B in LepRb-expressing progenitors until LepRb expression occurs during development. LepRb expression in the brain occurs as early as embryonic d 14 (53). However, global PTP1B−/− mice lack PTP1B in all neurons throughout development. Thus, the early loss of PTP1B in LepRb progenitors and/or non-LepRb neurons may account for the small, early weight differences seen between global PTP1B−/− and LepRb-PTP1B−/− mice.
Taken together, the data here suggest that PTP1B has both leptin-dependent and leptin-independent metabolic roles. Deletion of PTP1B in LepRb-expressing neurons clearly results in increased leptin sensitivity and a lean metabolic phenotype, consistent with previous models of targeted PTP1B deficiency in vivo. Although PTP1B deficiency in LepRb-expressing neurons encompasses a majority of the metabolic phenotype in PTP1B-deficient models, differences between LepRb-PTP1B−/− and global PTP1B−/− mice suggest that PTP1B's contribution to energy homeostasis regulation also extends outside of leptin signaling.
Acknowledgments
We thank Kimberly Rak, Ceren Ozek, and Ian Penkala for technical assistance with experiments; Dr. Patrick Seale for BAT gene qPCR primers; Dr. Benjamin Neel (University of Toronto, Toronto, Canada) and Dr. Barbara Kahn (Beth Israel Deaconess Medical Center, Boston, MA) for PTP1Bfl/fl mice; and Dr. Martin Myers for LepRb-Cre mice.
This work was supported by National Institutes of Health (NIH) Grant R01DK082417 (to K.K.B.), the University of Pennsylvania Diabetes Endocrinology Research Center and the Mouse Phenotyping, Physiology, and Metabolism Core NIH Grant 5P30DK019525, and NIH Fellowship F31NS074684 (to R.C.T.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- Brown adipose tissue
- CNS
- central nervous system
- GTT
- glucose tolerance test
- HFD
- high-fat diet
- IR
- insulin receptor
- JAK2
- Janus kinase 2
- LepRb
- leptin receptor isoform B
- POMC
- proopiomelanocortin
- pSTAT3
- phosphorylated-signal transducer and activator of transcription 3
- PTP1B
- protein tyrosine phosphatase 1B
- qRT-PCR
- quantitative real-time PCR
- UCP1
- uncoupling protein 1
- WT
- wild type.
References
- 1. Ahima RS. 2006. Obesity epidemic in need of answers. Gastroenterology 131:991. [DOI] [PubMed] [Google Scholar]
- 2. Flegal KM, Carroll MD, Kit BK, Ogden CL. 2012. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307:491–497 [DOI] [PubMed] [Google Scholar]
- 3. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- 4. Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. 1995. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543 [DOI] [PubMed] [Google Scholar]
- 5. Leshan RL, Björnholm M, Münzberg H, Myers MG. 2006. Leptin receptor signaling and action in the central nervous system. Obesity 14(Suppl 5):208S–212S [DOI] [PubMed] [Google Scholar]
- 6. Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. 1998. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547 [PubMed] [Google Scholar]
- 7. Patterson CM, Leshan RL, Jones JC, Myers MG., Jr 2011. Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res 1378:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. 2009. Leptin targets in the mouse brain. J Comp Neurol 514:518–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellacott KL, Halatchev IG, Cone RD. 2006. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology 147:3190–3195 [DOI] [PubMed] [Google Scholar]
- 10. Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. 2010. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. 2002. PTP1B regulates leptin signal transduction in vivo. Dev Cell 2:489–495 [DOI] [PubMed] [Google Scholar]
- 12. Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. 2002. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell 2:497–503 [DOI] [PubMed] [Google Scholar]
- 13. Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. 2000. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol 20:5479–5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. 2006. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12:917–924 [DOI] [PubMed] [Google Scholar]
- 15. Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W, Bence KK. 2010. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest 120:720–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delibegovic M, Bence KK, Mody N, Hong EG, Ko HJ, Kim JK, Kahn BB, Neel BG. 2007. Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol Cell Biol 27:7727–7734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Delibegovic M, Zimmer D, Kauffman C, Rak K, Hong EG, Cho YR, Kim JK, Kahn BB, Neel BG, Bence KK. 2009. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes 58:590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Owen C, Czopek A, Agouni A, Grant L, Judson R, Lees EK, Mcilroy GD, Göransson O, Welch A, Bence KK, Kahn BB, Neel BG, Mody N, Delibegovi M. 2012. Adipocyte-specific protein tyrosine phosphatase 1B deletion increases lipogenesis, adipocyte cell size and is a minor regulator of glucose homeostasis. PLoS One 7:e32700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmad F, Li PM, Meyerovitch J, Goldstein BJ. 1995. Osmotic loading of neutralizing antibodies demonstrates a role for protein-tyrosine phosphatase 1B in negative regulation of the insulin action pathway. J Biol Chem 270:20503–20508 [DOI] [PubMed] [Google Scholar]
- 20. Kenner KA, Anyanwu E, Olefsky JM, Kusari J. 1996. Protein-tyrosine phosphatase 1B is a negative regulator of insulin- and insulin-like growth factor-I-stimulated signaling. J Biol Chem 271:19810–19816 [DOI] [PubMed] [Google Scholar]
- 21. Zinker BA, Rondinone CM, Trevillyan JM, Gum RJ, Clampit JE, Waring JF, Xie N, Wilcox D, Jacobson P, Frost L, Kroeger PE, Reilly RM, Koterski S, Opgenorth TJ, Ulrich RG, Crosby S, Butler M, Murray SF, McKay RA, Bhanot S, Monia BP, Jirousek MR. 2002. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc Natl Acad Sci USA 99:11357–11362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ali MI, Ketsawatsomkron P, Belin de Chantemele EJ, Mintz JD, Muta K, Salet C, Black SM, Tremblay ML, Fulton DJ, Marrero MB, Stepp DW. 2009. Deletion of protein tyrosine phosphatase 1b improves peripheral insulin resistance and vascular function in obese, leptin-resistant mice via reduced oxidant tone. Circ Res 105:1013–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. 2007. Transcriptional control of brown fat determination by PRDM16. Cell Metab 6:38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leshan RL, Louis GW, Jo YH, Rhodes CJ, Münzberg H, Myers MG., Jr 2009. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 29:3138–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piper ML, Unger EK, Myers MG, Jr, Xu AW. 2008. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol 22:751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu Y, O'Brien WG, Lee C-C, Myers MG, Tong Q. 2012. Role of GABA release from leptin receptor-expressing neurons in body weight regulation. Endocrinology 153:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mercer JG, Moar KM, Hoggard N. 1998. Localization of leptin receptor (Ob-R) messenger ribonucleic acid in the rodent hindbrain. Endocrinology 139:29–34 [DOI] [PubMed] [Google Scholar]
- 28. Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. 1999. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283:1544–1548 [DOI] [PubMed] [Google Scholar]
- 29. Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. 2011. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. 2010. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285:7153–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ishibashi J, Seale P. 2010. Beige can be slimming. Science 328:1113–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sipols AJ, Baskin DG, Schwartz MW. 1995. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes 44:147–151 [DOI] [PubMed] [Google Scholar]
- 33. Schwartz MW, Sipols AJ, Marks JL, Sanacora G, White JD, Scheurink A, Kahn SE, Baskin DG, Woods SC, Figlewicz DP. 1992. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology 130:3608–3616 [DOI] [PubMed] [Google Scholar]
- 34. McGowan MK, Andrews KM, Fenner D, Grossman SP. 1993. Chronic intrahypothalamic insulin infusion in the rat: behavioral specificity. Physiol Behav 54:1031–1034 [DOI] [PubMed] [Google Scholar]
- 35. Carvalheira JB, Ribeiro EB, Araújo EP, Guimarães RB, Telles MM, Torsoni M, Gontijo JA, Velloso LA, Saad MJ. 2003. Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia 46:1629–1640 [DOI] [PubMed] [Google Scholar]
- 36. Air EL, Strowski MZ, Benoit SC, Conarello SL, Salituro GM, Guan XM, Liu K, Woods SC, Zhang BB. 2002. Small molecule insulin mimetics reduce food intake and body weight and prevent development of obesity. Nat Med 8:179–183 [DOI] [PubMed] [Google Scholar]
- 37. Woods SC, Lotter EC, McKay LD, Porte D., Jr 1979. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282:503–505 [DOI] [PubMed] [Google Scholar]
- 38. Byon JC, Kusari AB, Kusari J. 1998. Protein-tyrosine phosphatase-1B acts as a negative regulator of insulin signal transduction. Mol Cell Biochem 182:101–108 [PubMed] [Google Scholar]
- 39. Seely BL, Staubs PA, Reichart DR, Berhanu P, Milarski KL, Saltiel AR, Kusari J, Olefsky JM. 1996. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes 45:1379–1385 [DOI] [PubMed] [Google Scholar]
- 40. Bandyopadhyay D, Kusari A, Kenner KA, Liu F, Chernoff J, Gustafson TA, Kusari J. 1997. Protein-tyrosine phosphatase 1B complexes with the insulin receptor in vivo and is tyrosine-phosphorylated in the presence of insulin. J Biol Chem 272:1639–1645 [DOI] [PubMed] [Google Scholar]
- 41. Dadke S, Chernoff J. 2002. Interaction of protein tyrosine phosphatase (PTP) 1B with its substrates is influenced by two distinct binding domains. Biochem J 364:377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goldstein BJ, Bittner-Kowalczyk A, White MF, Harbeck M. 2000. Tyrosine dephosphorylation and deactivation of insulin receptor substrate-1 by protein-tyrosine phosphatase 1B. Possible facilitation by the formation of a ternary complex with the Grb2 adaptor protein. J Biol Chem 275:4283–4289 [DOI] [PubMed] [Google Scholar]
- 43. Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. 2010. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 30:2472–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morton GJ. 2007. Hypothalamic leptin regulation of energy homeostasis and glucose metabolism. J Physiol 583:437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li X, Wu X, Camacho R, Schwartz GJ, LeRoith D. 2011. Intracerebroventricular leptin infusion improves glucose homeostasis in lean type 2 diabetic MKR mice via hepatic vagal and non-vagal mechanisms. PLoS One 6:e17058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. 2004. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10:739–743 [DOI] [PubMed] [Google Scholar]
- 47. Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. 2006. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab 4:123–132 [DOI] [PubMed] [Google Scholar]
- 48. Matsuo K, Bettaieb A, Nagata N, Matsuo I, Keilhack H, Haj FG. 2011. Regulation of brown fat adipogenesis by protein tyrosine phosphatase 1B. PLoS One 6:e16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miranda S, González-Rodríguez A, Revuelta-Cervantes J, Rondinone CM, Valverde AM. 2010. Beneficial effects of PTP1B deficiency on brown adipocyte differentiation and protection against apoptosis induced by pro- and anti-inflammatory stimuli. Cell Signal 22:645–659 [DOI] [PubMed] [Google Scholar]
- 50. Xue B, Pulinilkunnil T, Murano I, Bence KK, He H, Minokoshi Y, Asakura K, Lee A, Haj F, Furukawa N, Catalano KJ, Delibegovic M, Balschi JA, Cinti S, Neel BG, Kahn BB. 2009. Neuronal protein tyrosine phosphatase 1B deficiency results in inhibition of hypothalamic AMPK and isoform-specific activation of AMPK in peripheral tissues. Mol Cell Biol 29:4563–4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Björnholm M, Münzberg H, Leshan RL, Villanueva EC, Bates SH, Louis GW, Jones JC, Ishida-Takahashi R, Bjørbaek C, Myers MG., Jr 2007. Mice lacking inhibitory leptin receptor signals are lean with normal endocrine function. J Clin Invest 117:1354–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Myers MG, Jr, Leibel RL, Seeley RJ, Schwartz MW. 2010. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 21:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matsuda J, Yokota I, Tsuruo Y, Murakami T, Ishimura K, Shima K, Kuroda Y. 1999. Developmental changes in long-form leptin receptor expression and localization in rat brain. Endocrinology 140:5233–5238 [DOI] [PubMed] [Google Scholar]