Abstract
Proopiomelanocortin (POMC) is posttranslationally processed to several peptides including α-MSH, a primary regulator of energy balance that inhibits food intake and stimulates energy expenditure. However, another POMC-derived peptide, β-endorphin (β-EP), has been shown to stimulate food intake. In this study we examined the effects of intracerebroventricular (icv) β-EP on food intake and its ability to antagonize the negative effects of α-MSH on energy balance in male rats. A single icv injection of β-EP stimulated food intake over a 2- to 6-h period during both the light and dark cycles. This effect was, however, not sustained with chronic icv β-EP infusion. In the next study, a subthreshold dose of β-EP was injected together with Nle4, d-Phe7 (NDP)-MSH after a 16-h fast, and the negative effects of NDP-MSH on refeeding and body weight gain were partially reversed. Finally, peptide interactions were studied in a chronic icv infusion model. Weight gain and food intake were significantly suppressed in the NDP-MSH group during the entire study. A subthreshold dose of β-EP antagonized these suppressive effects on food intake and weight gain for the first 3 d. However on d 4–7, β-EP no longer blocked these effects. Of note, the stimulatory effect of β-EP on feeding and its ability to antagonize MSH were specific for β-EP1–31 and were not observed with β-EP1–27. This study highlights the importance of understanding how the balance between α-MSH and β-EP is maintained and the potential role of differential POMC processing in regulating energy balance.
The hypothalamic melanocortin system, which includes both anorexigenic proopiomelanocortin (POMC) neurons and orexigenic agouti-related protein (AgRP) neurons, plays an important role in the regulation of energy balance and metabolism (1, 2). These neurons oppositely regulate food intake and energy expenditure via G protein-coupled melanocortin receptors in the brain. The POMC precursor protein is posttranslationally processed to a number of smaller peptides including α-MSH that promotes negative energy balance by both decreasing food intake and increasing energy expenditure (3–6). POMC is also processed to the endogenous opioid peptide β-endorphin (β-EP) that binds to the inhibitory G protein-coupled μ-opioid receptor (7, 8). The endogenous opioid system, consisting of the enkephalins and dynorphins, in addition to β-EP, and the μ-, δ-, and κ-opioid receptors, exerts complex effects on feeding, metabolism, nutrient selection, and reward via distinct pathways (9). However, the specific role of β-EP in energy balance and how it interacts with α-MSH is not well established. β-EP has been shown to acutely stimulate food intake after intracerebroventricular (icv) or hypothalamic injection during the light cycle (7, 10, 11) and deletion of the μ-opioid receptor protects from diet-induced obesity (12). In contrast, specific genetic deletion of β-EP yields a mildly obese phenotype (13), although under certain conditions the hedonic aspects of feeding were decreased (14). Furthermore, both POMC and AgRP neurons express μ-opioid receptors, and there is evidence that these neurons can be regulated by opioids (15–17). Hence, the role of β-EP in the regulation of energy balance is still puzzling. Although previous studies have examined the acute effects of icv β-EP during the light cycle in satiated rats, little is known about the longer term effects of icv β-EP injection during the light and dark cycles and the effects of chronic icv β-EP infusion. β-EP is a 31-amino acid peptide that can be further processed by C-terminal cleavage to yield β-EP1–27 and β-EP1–26, which have markedly reduced opioid activity (18). β-EP1–27 binds to brain opioid receptors but diminishes β-EP-induced analgesia (18). However, the effect of these peptides on feeding is relatively unknown.
Despite the fact that α-MSH and β-EP are cleaved from the same precursor peptide, their biological effects are quite different and in some cases even antagonistic. Although β-EP is an analgesic (19), α-MSH produces hyperalgesia and can blunt the analgesic effects of morphine (20, 21). In addition, α-MSH has been shown to block some of the neuroendocrine and behavioral effects of β-EP (22–25). There is also some evidence that α-MSH and β-EP can interact with respect to feeding and metabolism. For example, pretreatment with the melanocortin-receptor agonist, melanotan II, has been shown to block the acute orexigenic effects of a relatively large dose of icv β-EP (10). Although these data suggest that opioid and melanocortin systems can interact, it is yet unknown whether β-EP can antagonize the effects of α-MSH on feeding and energy balance under more physiological conditions in both an acute and chronic model. This study sought to further investigate the metabolic effects of β-EP as compared with β-EP1–27, either alone or in combination with α-MSH. First, we examined the acute effects of icv β-EP injection during both the light and dark cycles as well as the chronic effects of icv β-EP infusion. We next investigated the ability of a subthreshold dose of β-EP to attenuate the effects of α-MSH on feeding and metabolism in both an acute and chronic setting. The differences between β-EP and β-EP1–27 were also studied. Finally, we examined how infusion of these peptides modulates hypothalamic gene expression including Pomc and Agrp, as well as enzymes involved in processing the full-length POMC precursor peptide to its active metabolites, and prolylcarboxypeptidase (Prcp), which inactivates α-MSH (26, 27).
Materials and Methods
Animals
All experiments were approved by the Columbia University Institutional Animal Care and Use Committee. Male Sprague Dawley rats weighing 200–250 g were purchased from Charles River (Wilmington, MA). Animals had ad libitum access to water and LabDiet Rodent Chow 5001 (PMI Nutrition International, St. Louis, MO). Rats were acclimatized to a natural light/dark cycle.
Surgery
Rats were anesthetized with pentobarbital for icv cannula placement. For the acute icv injections (experiments 1 and 3), a 22-gauge stainless steel cannula was inserted stereotaxically into the right lateral ventricle (28). In the chronic infusion experiments (experiments 2 and 4), a 28-gauge stainless steel cannula connected to a 7-d osmotic pump (ALZET model 2001; Cupertino, CA) delivering 1 μl/h of saline was inserted stereotaxically into the right lateral ventricle (28). Animals were individually housed and allowed to recover for 5–7 d after cannula placement. After recovery, in experiments 2 and 4, minipumps were switched under isoflurane anesthesia to either 3-d pumps (ALZET model 1003D) or 7-d pumps delivering 1 μl/h. Peptides were dissolved in sterile normal saline immediately before use. Before each experiment, rats were divided into treatment groups of equivalent weight and food intake. Animals exhibiting signs of illness and consuming less than 10 g of food per day were excluded from analyses.
Peptides
Rat β-EP1–31 (022–33), β-EP1–27 (022–08), and [Nle4, d-Phe7] (NDP)-α-MSH (NDP-MSH, 043–06) were obtained from Phoenix Pharmaceuticals, Inc. (Burlingame, CA).
Experimental protocols
Experiment 1: acute effects of a single icv injection of β-EP1–31 during the light and dark cycles
Experiment 1a: effects of 0.5 and 5 μg β-EP during the light cycle.
Three groups of rats were studied during the light cycle (n = 9/group). β-EP1–31 (5 μg or 0.5 μg) or saline was injected icv between 0830 and 1100 h. Food was removed 1 h before the icv injection and returned immediately after injection. Measurements were obtained 4, 6, and 24 h after injection.
Experiment 1b: effects of 0.5, 1, and 5 μg of β-EP during the dark cycle.
In the first experiment, three groups of rats were studied (n = 7–9/group). β-EP1–31 (5 or 0.5 μg) or saline was injected icv between 1800 and 2000 h. Food was removed 2 h before icv injections and returned immediately after injection. Measurements were obtained 2, 4, and 14 h after injection. In the second experiment, two groups of rats were studied (n = 8/group). β-EP1–31 (1 μg) or saline was injected icv between 1800 and 2000 h. Measurements were obtained 2, 4, and 12 h after injection.
Experiment 2: chronic effects of icv infusion of β-EP1–31 for 4 d
Rats received 3-d ALZET pumps delivering either 2 or 10 μg/d β-EP1–31 or saline icv (n = 7–9/group). Food intake and body weight were recorded daily between 0900 and 1000 h. On d 4, rats were killed and trunk blood was collected. A 3-mm mediobasal hypothalamic section was dissected using a rat brain block and frozen in liquid nitrogen for quantitation of neuropeptide (Pomc, Agrp) and processing enzyme [prohormone convertase 1 (Pcsk1); prohormone convertase 2 (Pcsk2)] and degrading enzyme (Prcp) mRNA.
Experiment 3: acute effects of a single icv injection of β-EP1–31 or β-EP1–27 alone or in combination with NDP-MSH
For experiment 3, a and b, rats were fasted at 1700 h and received an icv injection approximately 16 h later. Rats were refed 1 h after injection and food intake was monitored at 2, 4, 8, and 24 h and body weight was measured at 4, 8, and 24 h.
Experiment 3a.
In the first experiment, rats received an icv injection of either 1 μg of β-EP1–31 or saline (n = 7–8). In the next experiment, rats received an icv injection of 1 μg NDP-MSH, 1 μg NDP-MSH+1 μg β-EP1–31, or saline (n = 6–7/group).
Experiment 3b.
Rats received an icv injection of 1 μg NDP-MSH, 1 μg NDP-MSH+1 μg β-EP1–31, 1 μg NDP-MSH+1 μg β-EP1–27, or saline (n = 6–8/group).
Experiment 3c.
Rats from experiment 3b were re-randomized and used for this experiment 3 d later. Rats received an icv injection of either 5 μg β-EP1–27 or saline (n = 8/group) between 0900 and 1130 h. Measurements were obtained at 2, 4, 8, and 24 h after injection.
Experiment 4: chronic effects of continuous icv infusion of NDP-MSH alone and in combination with β-EP1–31
Rats received 7-d pumps delivering 2.4 μg/d MSH-NDP, 2.4 μg/d MSH-NDP+2.4 μg/d β-EP1–31, or saline (n = 6–8/group). Food intake and body weight were recorded daily between 0900 and 1000 h. After 7 d rats were killed; trunk blood was collected and inguinal and retroperitoneal fat pads were weighed. A 3-mm mediobasal hypothalamic section was dissected for quantitation of neuropeptide (Pomc, Agrp) and processing enzyme [Pcsk1, Pcsk2, and carboxypeptidase E (Cpe)] and degrading enzyme (Prcp) mRNA.
Hormone assays
Trunk blood was collected into tubes containing EDTA immediately after decapitation and plasma was stored at −20 C. Plasma insulin and leptin concentrations were measured using RIA kits (Millipore Corp., Billerica, MA).
Measurement of hypothalamic mRNA levels
RNA isolation was performed using the RNeasy lipid tissue mini kit (QIAGEN USA, Valencia, CA) in conjunction with the ribonuclease-free deoxyribonuclease set (QIAGEN USA), and total RNA was quantified using spectrophotometry. cDNA was synthesized using the Superscript III first-strand cDNA synthesis kit (Life Technologies Corp./Invitrogen, Grand Island, NY) and was analyzed using quantitative RT-PCR performed in the Lightcycler 480 real-time PCR system (Roche Applied Science, Indianapolis, IN). Samples were normalized to β-actin. Primer sequences are as follows: β-actin forward, 5′-ctctgaaccctaaggccaaccgtgaaaa-3′, reverse, 5′-tctccggagtccatcacaatgccagtg-3′; Pomc forward, 5′-cagtgccaggacctcaccacgg-3′, reverse, 5′-cggtcccagcggaagtgaccc-3′; Agrp forward, 5′-catgccctagctacaggaag-3′, reverse, 5′-gcagtgccagcaggaca-3′; Prcp forward, 5′-gcttccgcccctatctggcagc-3′, reverse, 5′-gggccaagcaggcaaaggct-3′; Pcsk1forward, 5′-cgttcagttccaagagactc-3′, reverse, 5′-ggcagagatgcagtcattct-3′; Pcsk2 forward, 5′-ccaagcgaaaccagcttcacg-3′, reverse, 5′-catgctcgaggtagcggacg-3′; Cpe forward, 5′-gcccagggaatagatctgaac-3′, reverse, 5′-gaatgacagccttggtctc-3′.
Statistical analysis
Statistical analysis was performed with Student's t test when two groups were compared. ANOVA followed by Fisher's protected least squares difference test was used when comparing more than two groups. P < 0.05 was considered statistically significant. Results are reported as mean values ± sem.
Results
Experiment 1: acute effects of a single icv injection of ß-EP1–31 during the light and dark cycles
Experiment 1a
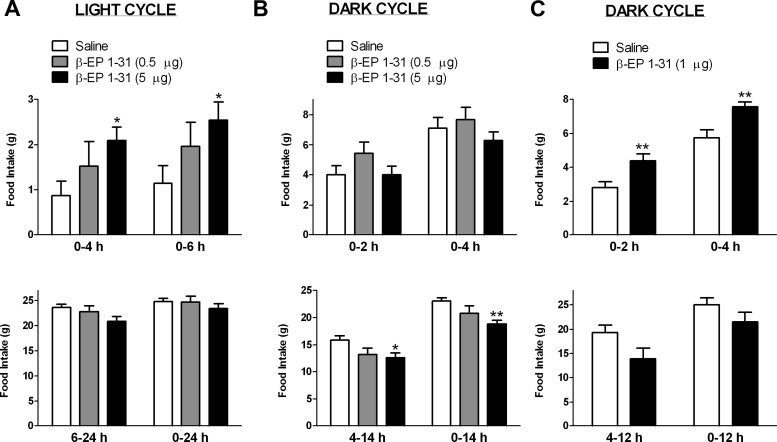
To investigate the effects of β-EP on food intake during the light cycle in satiated rats, we administered a single icv injection of β-EP1–31 (0.5 or 5 μg dose) or saline in the morning. The 5-μg dose of β-EP stimulated food intake to 240 and 221% compared with saline at the 4- and 6-h time points, respectively (P < 0.05; Fig. 1A). The 0.5-μg dose increased cumulative food intake to 175 and 171% at 4 and 6 h, respectively, but this did not reach significance. Overnight (from 6 to 24 h), food intake tended to decrease to 88% of saline levels in the 5-μg group (P = 0.06; Fig. 1A), but this was not evident in the 0.5 μg group, which consumed 96% of saline levels. Cumulative food intake was not different between groups 24 h after injection.
Fig. 1.
Effects of a single icv injection of β-EP1–31 on food intake during the light (A) and dark (B and C) cycles. A, Five micrograms of β-EP stimulated cumulative food intake at 4 and 6 h during the light cycle; the stimulation with 0.5 μg β-EP was not significant (n = 9/group) (top panel). However, between 6 and 24 h, the 5-μg dose tended to decrease food intake compared with saline (P = 0.06). Cumulative food intake was similar between all groups at 24 h (bottom panel). B, Neither the 0.5- nor the 5-μg β-EP dose stimulated food intake during the dark cycle, although a tendency was evident in the 0.5-μg dose group (P = 0.14) at 2 h. However, between 4 and 14 h, 5 μg β-EP significantly decreased food intake, and the 0.5-μg dose tended to decrease food intake (P = 0.06). Cumulative food intake was significantly lower at 14 h in the 5-μg group. C, One microgram of β-EP stimulated cumulative food intake at 2 and 4 h during the dark cycle (n = 8/group) (top panel). However, between 4 and 12 h, β-EP tended to decrease food intake (P = 0.06). Food intake was similar between groups at 12 h (bottom panel). *, P < 0.05, **, P < 0.01 vs. saline.
Experiment 1b
To investigate the effects of β-EP on food intake during the dark cycle, we initially administered a single icv injection of β-EP1–31 (0.5 or 5 μg) or saline in the evening. The 0.5-μg dose tended to increase food intake at 2 h vs. saline (P = 0.14), whereas food intake in the 5-μg group was unchanged from saline (Fig. 1B). No differences were noted between groups at 4 h. At 14 h cumulative food intake in the 0.5-μg group was not different from saline; however, 14-h cumulative food intake was significantly less than saline in the 5-μg group (P < 0.01; Fig. 1B). We next studied the effect of 1 μg of β-EP1–31 or saline in the evening. This dose of β-EP increased food intake to 158 and 132% of saline levels at both 2 and 4 h, respectively (P < 0.01; Fig. 1C). However, between 4 and 12 h, the β-EP group consumed only 72% of saline's intake (P = 0.06) such that cumulative food intake at 12 h was similar between the saline and β-EP group.
Experiment 2: chronic effects of icv infusion of β-EP1–31 for 4 d
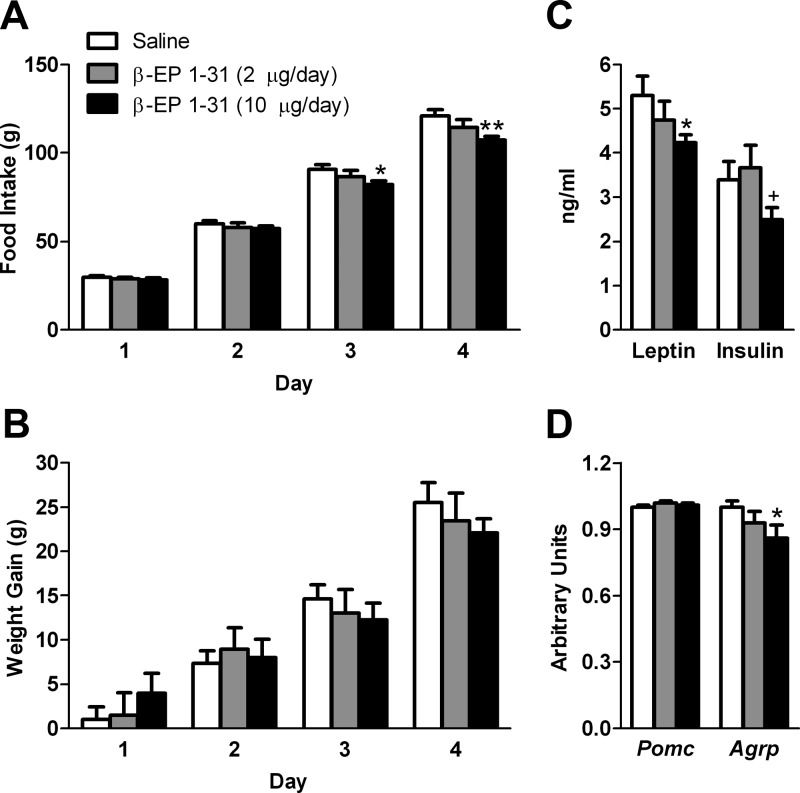
To examine the chronic effects of icv β-EP, we continuously infused either a low (2 μg/d) or high (10 μg/d) dose of β-EP1–31 via the osmotic minipump. Although we did not detect any significant increases in food intake or body weight gain during the entire treatment period, on d 1, body weight gain tended to increase with 10 μg/d β-EP (Fig. 2B). In contrast, on d 3 and 4, cumulative food intake was significantly suppressed in the 10-μg β-EP group to 90 and 89% of saline, respectively (P < 0.05, Fig. 2A). At the time the animals were killed on d 4, body weight was similar between groups (saline, 355.4 ± 4.3 g; β-EP low, 356.2 ± 8.4 g; β-EP high, 352.0 ± 4.4 g).
Fig. 2.
Effects of a 4 d icv β-EP1–31 infusion on metabolic parameters. No significant increases in cumulative food intake (A) or weight gain (B) were detected with chronic β-EP treatment at either 2 or 10 μg/d (n = 7–9/group). However, on d 3 and 4, the cumulative food intake was lower in the 10 μg/d β-EP group but not in the 2-μg/d group, compared with saline. C, Leptin levels were significantly lower and insulin levels (P = 0.10) tended to be lower in the 10-μg/d group compared with saline. D, Agrp mRNA was lower in the 10-μg/d β-EP group. *, P < 0.05, **, P < 0.01 vs. saline; +, P < 0.05 vs. 2 μg/d.
Hormone levels and hypothalamic mRNA analyses
At the time the animals were killed, leptin was suppressed to 80% of saline in the 10-μg/d β-EP group (P < 0.05; Fig. 2C); insulin was 73% of saline (P = 0.10). Leptin and insulin were not different in the 2-μg/d β-EP group vs. saline. The 10-μg dose of β-EP suppressed Agrp mRNA levels (P < 0.05; Fig. 2D), whereas Pomc mRNA levels were unchanged between groups. Furthermore, Prcp mRNA levels tended to be lower in both the 2-μg (0.93 ± 0.02, P = 0.07 vs. saline) and 10-μg β-EP (0.94 ± 0.03, P = 0.08 vs. saline) groups. Pcsk1 and Pcsk2 mRNA were also measured, but no differences were detected between any of the groups (data not shown).
Experiment 3: acute effects of a single icv injection of β-EP1–31 or β-EP1–27 alone or in combination with NDP-MSH
Experiment 3a
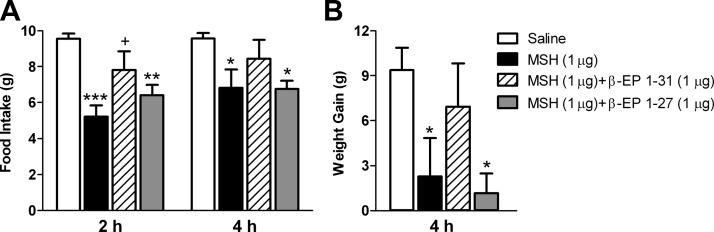
In the first experiment, performed in a fasting and refeeding model, a 1-μg icv injection of β-EP1–31 alone did not stimulate food intake or body weight gain; in fact, food intake tended to be lower at the 2-h (P = 0.10) and 4-h (P = 0.09) time points in the β-EP group (Fig. 3C). In the next experiment, three groups of rats were fasted overnight and received an icv injection of NDP-MSH (1 μg), NDP-MSH (1 μg)+β-EP1–31 (1 μg), or saline the next morning and were refed 1 h later. After 2 h, refeeding was suppressed in the MSH group to 52% of saline levels (P < 0.01; Fig. 3A). However, this suppression was reversed by concomitant β-EP treatment to 83% of saline levels (P < 0.05 vs. MSH, P = 0.23 vs. saline; Fig. 3A). By 4 h cumulative refeeding was suppressed in the MSH group to 64% of saline levels (P < 0.05; Fig. 3A); however, this suppression was still reversed by β-EP treatment (82% of saline) such that this group was not significantly different from saline. At this time point, the saline group gained 9.2 ± 1.7 g, whereas the MSH group lost 0.8 ± 1.4 g (P < 0.001 vs. saline, Fig. 3B). This effect was partly reversed in the MSH+β-EP group that gained 5.5 ± 1.2 g, which was significantly different from the MSH group (P < 0.01) but not from the saline group (P = 0.08). By 8 h, cumulative refeeding and body weight gain were still suppressed by MSH; however, this was no longer reversed by β-EP (Fig. 3, A and B). After 24 h, there were no significant differences in food intake or body weight gain between groups (data not shown).
Fig. 3.
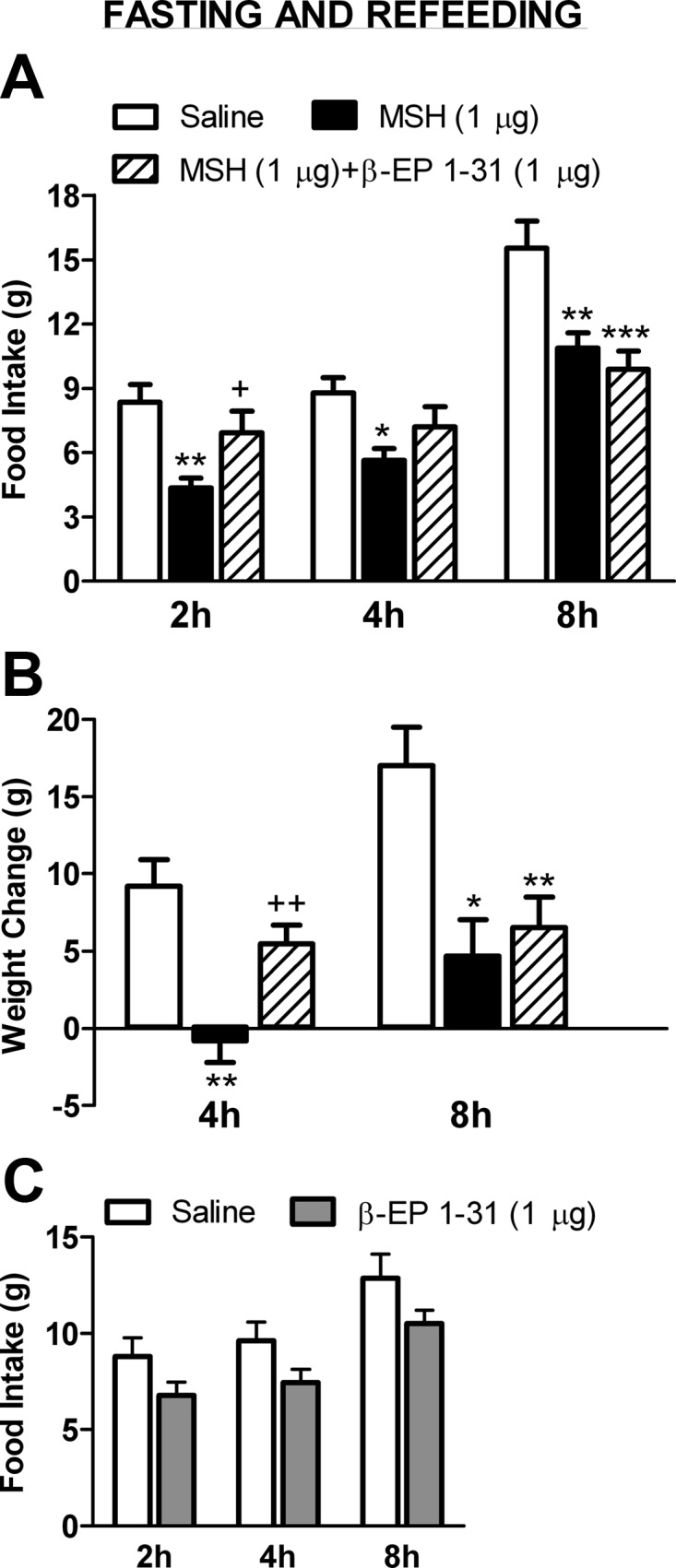
Effects of a single icv injection of β-EP1–31 on NDP-MSH-induced suppression of feeding and body weight gain. A, After an overnight fast, cumulative refeeding was significantly suppressed by a single icv injection of NDP-MSH at 2, 4, and 8 h (n = 6–7/group). Concomitant β-EP1–31 injection reversed this suppression at 2 and 4 h. At 2 h the MSH and MSH+β-EP groups were significantly different from each another. At 8 h β-EP no longer antagonized MSH's effects. B, The MSH group gained significantly less weight at 4 and 8 h compared with the saline group. This effect was reversed by β-EP at 4 h but was no longer evident at 8 h. C, A 1-μg dose of β-EP1–31 alone in a fasting and refeeding model did not stimulate food intake, in fact food intake tended to be lower at the 2 h (P = 0.10) and 4 h (P = 0.09) time points. *, P < 0.05, **, P < 0.01, ***, P < 0.001 vs. saline; +, P < 0.05, ++, P < 0.01 vs. MSH.
Experiment 3b
We next sought to investigate whether β-EP1–27 can antagonize MSH's anorexic and metabolic effects. Four groups of rats were fasted overnight and were injected icv with 1 μg of each peptide (NDP-MSH, NDP-MSH+β-EP1–31, NDP-MSH+β-EP1–27, or saline) the next morning, and rats were refed 1 h later. After 2 h, MSH suppressed food intake to 55% of saline levels (P < 0.001, Fig. 4A). Concomitant β-EP1–31 injection partly reversed this effect so that food intake was suppressed to only 82%, which was significantly different from MSH (P < 0.05) but not from saline (P = 0.10). Concomitant β-EP1–27 injection did not reverse MSH's suppressive effects; MSH+β-EP1–27 suppressed food intake to 67% of saline (P < 0.01, Fig. 4A) and was not different from MSH alone (P = 0.22). At 4 h, food intake was suppressed in the MSH group to 71% of saline (P < 0.05, Fig. 4A), and this was partly reversed by β-EP1–31 such that food intake was not different from saline (Fig. 4A); however β-EP1–27 did not reverse the effects of MSH. Weight gain at this time point was suppressed by MSH to 24% of saline levels (P < 0.05, Fig. 4B). This suppression was partly reversed by β-EP1–31 treatment to 74% of saline levels and was not different from saline (P = 0.44). MSH-induced suppression of weight gain was not reversed by β-EP1–27; in fact, weight gain in the MSH+β-EP1–27 group was suppressed to 13% of saline levels (Fig. 4B). At both the 8- and 24-h time points, cumulative food intake and weight gain were similar between groups (data not shown).
Fig. 4.
Effects of a single icv injection of β-EP1–27 or β-EP1–31 on NDP-MSH-induced suppression of feeding and body weight gain. A, After an overnight fast, cumulative refeeding was significantly suppressed by a single icv injection of NDP-MSH at 2 and 4 h (n = 6–8/group); this suppression was significantly reversed by concomitant β-EP1–31 injection but not by β-EP1–27 injection. At 2 h, the MSH and MSH+β-EP1–31 groups were significantly different from each another, but the MSH and MSH+β-EP1–27 groups were not. B, MSH significantly suppressed weight gain at 4 h, and this was reversed by concomitant β-EP1–31 injection but not by β-EP1–27. *, P < 0.05, **, P < 0.01, ***, P < 0.001 vs. saline; +, P < 0.05 vs. MSH.
Experiment 3c
To further investigate the effects of β-EP1–27 on food intake and body weight gain, we administered a single icv injection of either 5 μg β-EP1–27 or saline in the morning to fed rats. Food intake and body weight change were similar between these groups at all time points. At 2 h, the β-EP1–27 group consumed 1.4 ± 0.5 g vs. 2.0 ± 0.6 g in the saline group; at 4 h, the β-EP1–27 group consumed 2.6 ± 0.7 g vs. 3.5 ± 0.6 g in the saline group.
Experiment 4: chronic effects of continuous icv infusion of NDP-MSH alone and in combination with β-EP1–31
In this experiment, we sought to investigate whether β-EP1–31 could chronically antagonize the anorexigenic and metabolic effects of NDP-MSH. Rats received 2.4 μg/d MSH, 2.4 μg MSH+2.4 μg β-EP/d, or saline for 7 d via an osmotic minipump.
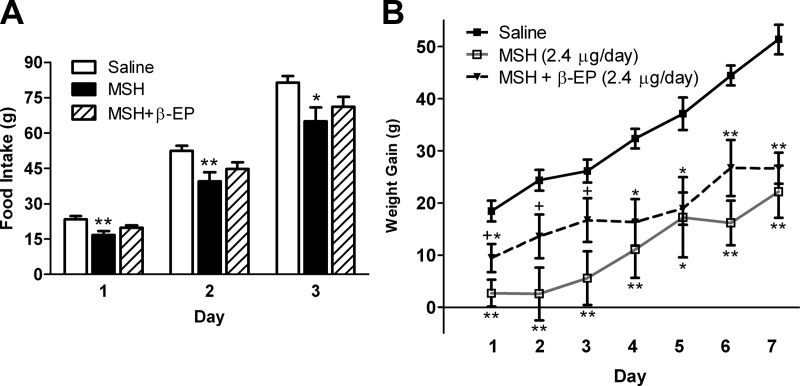
MSH significantly suppressed cumulative food intake during each day of the study. During d 1–3, this suppression was partly reversed by β-EP treatment so that the MSH+β-EP group was not significantly different from saline (Fig. 5A). During d 4–7, β-EP no longer antagonized the anorexic effects of MSH. At 7 d cumulative food intake was lower in both the MSH and MSH+β-EP groups compared with saline (Table 1).
Fig. 5.
Effects of a 7-d icv β-EP1–31 infusion on NDP-MSH-induced suppression of feeding and body weight gain. A, Chronic icv MSH infusion (2.4 μg/d) significantly suppressed cumulative food intake during the entire study (n = 6–8/group). Concomitant β-EP infusion (2.4 μg/d) partly reversed this suppression during the first 3 d of the experiment. B, Chronic icv MSH infusion significantly suppressed cumulative body weight gain during the entire study. Concomitant β-EP infusion partly reversed this suppression during the first 3 d of the experiment. However, during d 4–7, β-EP no longer antagonized MSH's effects on body weight. **, P < 0.01, *, P < 0.05 vs. saline; +, P = 0.06 vs. MSH.
Table 1.
Effects of chronic NDP-MSH, NDP-MSH+β-EP1–31, or saline infusion
| Saline | MSH | MSH+β-EP | |
|---|---|---|---|
| Food intake (g) | 196.9 ± 4.9 | 166.0 ± 10.0a | 165.1 ± 7.1a |
| Weight (g) | 387.3 ± 4.6 | 360.8 ± 10.0b | 360.1 ± 6.7b |
| Fat pads (g) | |||
| Inguinal | 3.2 ± 0.2 | 3.0 ± 0.2 | 2.6 ± 0.2b |
| RP | 2.5 ± 0.2 | 2.2 ± 0.4 | 1.6 ± 0.2b |
| Hormones (ng/ml) | |||
| Insulin | 1.7 ± 0.2 | 1.6 ± 0.2 | 1.1 ± 0.1b |
| Leptin | 2.9 ± 0.2 | 2.4 ± 0.1b | 1.8 ± 0.1a,c |
| mRNA (AU) | |||
| Pomc | 1.00 ± 0.08 | 0.82 ± 0.25 | 1.01 ± 0.17 |
| Agrp | 1.00 ± 0.06 | 1.06 ± 0.12 | 1.47 ± 0.08a,c |
| Prcp | 1.00 ± 0.02 | 0.97 ± 0.04 | 0.88 ± 0.05b |
Values are mean ± se. RP, Retroperitoneal; AU, arbitrary units.
P < 0.01 vs. saline.
P < 0.05 vs. saline.
P < 0.05 vs. MSH.
MSH significantly suppressed weight gain during each day of the study. During d 1–3, this suppression was partly reversed by β-EP treatment such that the MSH and MSH+β-EP groups tended to be different from one another (P = 0.06), and the MSH+β-EP and saline groups were not significantly different from one another on d 2 and 3 (Fig. 5B). During d 4–7, β-EP no longer antagonized the suppressive effects of MSH on body weight gain (Fig. 5B) so that by d 7 weight was equivalent between the MSH and MSH+β-EP groups (Table 1).
Fat mass, hormones, and hypothalamic mRNA analyses (Table 1)
Total fat pad mass was reduced by 10 and 27% in the MSH (P = 0.36) and MSH+β-EP (P < 0.05) groups, respectively. Leptin was suppressed in the MSH (P < 0.05) and MSH+β-EP groups (P < 0.01) vs. saline. Insulin was suppressed in the MSH+β-EP group (P < 0.05) vs. saline. Agrp mRNA levels were higher in the MSH+β-EP group vs. saline (P < 0.01) and vs. MSH alone (P < 0.05). Pomc mRNA levels were similar between groups; however the Pomc to Agrp ratio was suppressed to 71 ± 14% of saline in the MSH group (P < 0.05) and to 68 ± 7.8% in the MSH+β-EP group (P < 0.05). Furthermore, Prcp mRNA levels were decreased in the MSH+β-EP group compared with saline (P < 0.05). Pcsk1, Pcsk2, and Cpe mRNA were also measured, but no differences were detected between groups (data not shown).
Discussion
In this study, we confirm the short-term stimulatory effect of β-EP on food intake and show that this can be elicited during both the light and dark cycles. However, this effect was not sustained with chronic icv β-EP infusion. In fact, when infused at high doses, food intake was decreased. Of note, the stimulatory effect on feeding was observed only with full length β-EP and not with C-terminally cleaved β-EP1–27. Most importantly, we showed that a subthreshold dose of β-EP was able to attenuate the effects of α-MSH on food intake and body weight gain in both an acute fasting and refeeding model and during chronic icv infusion for several days. These studies show that different POMC-derived peptide products can interact to regulate energy balance and underscore the importance of understanding how the balance between these peptide products is maintained.
Our studies show that a single icv injection of β-EP stimulates food intake over a 2- to 6-h period and that this occurs in the morning in satiated rats as well as in the evening. After the initial stimulation, food intake tended to decline such that cumulative food intake the next morning was not different from controls. It is unclear whether this decline is an independent primary or secondary effect of β-EP or solely a compensatory reduction in food intake. However, when higher doses of β-EP were administered during the dark cycle, cumulative overnight food intake decreased in the absence of an acute stimulatory effect. This acute stimulatory effect of β-EP on feeding is consistent with previous reports with central injection of β-EP and other μ-opioid receptor agonists, DAMGO and morphine (9, 17, 29–31). Most studies were performed in freely fed rodents during the light cycle, but stimulatory effects have been noted in some studies during the dark cycle (31). Effects were most apparent between 2 and 4 h after opioid administration. However, when morphine was administered to food-deprived rats, food intake during refeeding was reduced, especially at higher doses of morphine that had been shown to stimulate food intake in fed rats (29). Similarly, icv DAMGO stimulated food intake in fasted mice at low doses but inhibited intake at higher doses (17). When we administered 1 μg β-EP to fasted rats, food intake was not stimulated and in fact tended to be lower, in contrast to what was observed at this dose in satiated rats. Thus, the effects of opioids on food intake appear to be dependent on feeding state. This is further illustrated in another model showing that β-EP null mice work significantly less than wild-type mice for food reinforcers under nondeprived conditions, but there was no difference when the mice were tested in a food-deprived state (32).
In our study no effects on feeding or body weight gain were detected with chronic low dose infusion of β-EP; however, with higher doses, food intake decreased by 3–4 d. This may be due to possible sedating or other behavioral effects associated with infusing a pharmacological dose of β-EP into the lateral ventricle (33, 34). However, although behavior was not formally assessed, we did not observe any obvious signs of sedation or behavioral change. Another potential explanation for the decrease in food intake during chronic β-EP infusion is the suppression in Agrp mRNA levels observed in rats infused with the higher dose of β-EP for 4 d. This decrease was noted despite a fall in leptin and insulin levels, which is usually associated with an increase in Agrp mRNA (35, 36). These data suggest that β-EP can suppress AgRP, leading to deceased food intake. AgRP neurons express μ-opioid receptors, and recent electrophysiological data show that POMC neurons release an opioid that inhibits AgRP neuronal activity (17, 37). Furthermore, there is evidence that opioids can mediate some of the downstream orexigenic effects of AgRP (38), which could be enhanced with acute opioid injection but reduced if opioid tolerance develops with chronic icv infusion. In addition, expression of Prcp, an enzyme that inactivates α-MSH, also tended to be suppressed after chronic icv β-EP infusion; this could lead to increased α-MSH and contribute to the decreased food intake observed (27).
POMC neurons also express μ-opioid receptors that exert well-established inhibitory effects on POMC neuronal activity and gene expression (16, 39–41). However, more recently presynaptic opioid receptors have been described that colocalize with γ-aminobutyric acid terminals onto POMC neurons that could lead to disinhibition of POMC neurons at low opioid concentrations (41). In addition, it appears that postsynaptic receptors are desensitized by opioids, whereas presynaptic receptors are not. Thus, the feedback regulation of POMC by β-EP is potentially quite complicated, and these mechanisms could explain the differential effects observed with various opioid administration protocols. In our study we did not detect a change in Pomc gene expression after 4 d of icv β-EP infusion but did detect a change in Agrp expression. Thus, the effects of icv β-EP on food intake appear to be biphasic, with acute stimulatory effects and longer-term inhibitory effects. The acute stimulatory effects are consistent with prior reports demonstrating acute stimulation of food intake with opioid agonists and inhibition with opioid antagonists (9, 42). The inhibitory effects of chronic β-EP infusion are consistent with effects reported with chronic morphine infusion in the rat and also with genetic studies showing that selective ablation of only the β-EP portion of Pomc yields mice that tend to be hyperphagic and obese (13, 43).
The effects of icv β-EP injection were observed only with full length β-EP1–31 and not with β-EP1–27, which possesses markedly reduced opioid activity and can even antagonize the analgesic effects of β-EP1–31 (18). Although there are no other studies of β-EP1–27 on feeding in mammalian models, high doses of β-EP1–27 did stimulate food intake in the chick but was much less potent than β-EP1–31 and was also able to antagonize β-EP1–31-induced stimulation of food intake (44). Although β-EP1–31 is the predominant form of β-EP in the hypothalamus, a significant portion is normally cleaved to β-EP1–27 and β-EP1–26 (18, 45). There is evidence that the processing enzymes, prohormone convertase 2 and carboxypeptidase E (CPE), may be responsible for the C-terminal processing of β-EP and that this process may be regulated with respect to food intake. For example, CPE-deficient mice are obese and have little C-terminal processing of β-EP (46). In addition, mice with the ablation of the transcription factor Forkhead box protein FoxO1 in POMC neurons have a lean phenotype and were found to have increased Cpe expression in the arcuate with relatively more processing of β-EP to the C-terminal forms (47). Our studies further underscore the importance of C-terminal processing of β-EP as injection of β-EP1–27 did not impact energy balance.
Despite the fact that α-MSH and β-EP1–31 are cleaved from the same precursor peptide, their biological effects are quite different and in some cases even antagonistic. This has been shown with respect to analgesia, sexual behavior, and neuroendocrine function (20–25). We therefore sought to determine how these peptides interact with respect to energy balance in both acute and chronic infusion models. We show that a subthreshold dose of β-EP, which did not increase food intake or body weight when administered alone, was able to attenuate the suppressive effects of NDP-MSH on food intake and body weight gain in both an acute fasting and refeeding model and during chronic icv infusion for several days. This was accomplished with a molar dose of β-EP that was approximately half of the molar dose of NDP-MSH that was administered. Although a previous study showed that pretreatment with the melanocortin receptor agonist melanotan II blocked the acute orexigenic effects of a relatively large dose of icv β-EP (10), this study shows that a relatively low dose of β-EP can attenuate the negative effects of MSH on energy balance, which are the predominant effects of the Pomc gene on energy balance. β-EP1–31 (but not β-EP1–27) attenuated the suppressive effects of MSH on feeding and body weight gain after an overnight fast. When infused chronically over a 7-d period, MSH suppressed food intake and body weight gain during the entire study. These suppressive effects were partially reversed by concomitant β-EP infusion for the first 3 d. However, after 3 d, β-EP no longer antagonized the effects of MSH. In fact at the time the animals were killed, the β-EP+MSH group had the lowest fat pad mass and leptin levels as well an increase in Agrp expression, consistent with the weight loss and lower leptin levels induced primarily by the MSH infusion. This is in contrast to the suppressive effects of β-EP infusion alone on Agrp expression in experiment 2. It is unclear why the antagonism of MSH by β-EP cannot be sustained after 3 d, but this may result from opioid receptor down-regulation and opioid tolerance, which has been demonstrated to occur with chronic icv β-EP infusion (33, 34). In addition, it is possible that the development of opioid tolerance leads to enhanced sensitivity to the effects of MSH infusion.
POMC is synthesized in the arcuate nucleus and processed to both α-MSH and β-EP, which are presumably released together onto downstream neurons that regulate energy balance. Although each of these peptides binds to its own distinct melanocortin or opioid receptor, respectively, there is evidence that functional antagonism may occur via a postreceptor mechanism. Melanocortin and opioid receptors are both G protein coupled, but the former is coupled to stimulatory G proteins, whereas the latter is coupled to inhibitory G proteins, resulting in increased or decreased production of cAMP, respectively (8, 48). Depending on the relative amounts of α-MSH and β-EP that are released, differential effects on neuronal activity and gene expression would be expected. At present there is no evidence for differential sorting of α-MSH and β-EP into different secretory granules. However, the first cleavage step of POMC by proconvertase 1 yields pro-ACTH and β-Lipotropin, which could potentially be sorted differently (49). In the pituitary there is evidence for a novel subcellular localization of β-Lipotropin and β-EP (but not ACTH and α-MSH) in peroxisomes; however, the mechanism by which this occurs is unclear (50). Furthermore, in the hypothalamus there is evidence for differential sorting of prothyrotropin-releasing hormone peptide products (51). Regardless of whether or not there is differential sorting of β-EP and α-MSH, there are clearly distinct mechanisms by which these peptides can be inactivated, thus altering the balance between these two peptides. Wallingford et al. (27) have shown that the enzyme prolylcarboxypeptidase (PRCP) inactivates α-MSH by removing the C-terminal valine residue. They show that Prcp null mice have a lean phenotype with elevated levels of α-MSH in the hypothalamus and that inhibition of PRCP activity decreases food intake. PRCP is highly expressed in the lateral hypothalamus and dorsomedial nucleus and to a lesser extent in the arcuate (27). Thus, PRCP could serve to modulate the strength of the α-MSH signal at melanocortin receptors on second-order neurons without affecting β-EP signaling. In contrast, C-terminal cleavage of β-EP would decrease β-EP signaling at opioid receptors on second-order neurons without affecting MSH signaling.
In summary, these studies show that the POMC-derived peptides α-MSH and β-EP can interact to regulate food intake and body weight. Although MSH has sustained negative effects on food intake and body weight gain, the short-term stimulatory effects of β-EP injection were not sustained. However, a subthreshold dose of β-EP was able to attenuate the effects of MSH on food intake and body weight gain in both an acute fasting and refeeding model and during chronic icv infusion for several days. This study highlights the importance of understanding how the balance between α-MSH and β-EP is maintained and the potential role of differential POMC processing in regulating energy balance.
Acknowledgments
This work was supported by Grant DK 080003 from the National Institutes of Health (to S.L.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AgRP
- Agouti-related protein
- CPE
- carboxypeptidase E
- DAMGO
- ([D-Ala2, N-MePhe4, Gly-ol]-enkephalin
- β-EP
- β-endorphin
- icv
- intracerebroventricular
- NDP
- Nle4, d-Phe7
- Pcsk1
- prohormone convertase 1 gene
- Pcsk2
- prohormone convertase 2 gene
- POMC
- proopiomelanocortin
- PRCP
- prolylcarboxypeptidase.
References
- 1. Lee M, Wardlaw SL. 2007. The central melanocortin system and the regulation of energy balance. Front Biosci 12:3994–4010 [DOI] [PubMed] [Google Scholar]
- 2. Cone RD. 2006. Studies on the physiological functions of the melanocortin system. Endocr Rev 27:736–749 [DOI] [PubMed] [Google Scholar]
- 3. McMinn JE, Wilkinson CW, Havel PJ, Woods SC, Schwartz MW. 2000. Effect of intracerebroventricular alpha-MSH on food intake, adiposity, c-Fos induction, and neuropeptide expression. Am J Physiol Regul Integr Comp Physiol 279:R695–R703 [DOI] [PubMed] [Google Scholar]
- 4. Seeley RJ, Burklow ML, Wilmer KA, Matthews CC, Reizes O, McOsker CC, Trokhan DP, Gross MC, Sheldon RJ. 2005. The effect of the melanocortin agonist, MT-II, on the defended level of body adiposity. Endocrinology 146:3732–3738 [DOI] [PubMed] [Google Scholar]
- 5. Pierroz DD, Ziotopoulou M, Ungsunan L, Moschos S, Flier JS, Mantzoros CS. 2002. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes 51:1337–1345 [DOI] [PubMed] [Google Scholar]
- 6. Lee M, Kim A, Chua SC, Jr, Obici S, Wardlaw SL. 2007. Transgenic MSH overexpression attenuates the metabolic effects of a high-fat diet. Am J Physiol Endocrinol Metab 293:E121–E131 [DOI] [PubMed] [Google Scholar]
- 7. Silva RM, Hadjimarkou MM, Rossi GC, Pasternak GW, Bodnar RJ. 2001. β-Endorphin-induced feeding: pharmacological characterization using selective opioid antagonists and antisense probes in rats. J Pharmacol Exp Ther 297:590–596 [PubMed] [Google Scholar]
- 8. Kieffer BL, Evans CJ. 2009. Opioid receptors: from binding sites to visible molecules in vivo. Neuropharmacology 56(Suppl 1):205–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bodnar RJ. 2004. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides 25:697–725 [DOI] [PubMed] [Google Scholar]
- 10. Grossman HC, Hadjimarkou MM, Silva RM, Giraudo SQ, Bodnar RJ. 2003. Interrelationships between mu opioid and melanocortin receptors in mediating food intake in rats. Brain Res 991:240–244 [DOI] [PubMed] [Google Scholar]
- 11. Leibowitz SF, Hor L. 1982. Endorphinergic and α-noradrenergic systems in the paraventricular nucleus: effects on eating behavior. Peptides 3:421–428 [DOI] [PubMed] [Google Scholar]
- 12. Tabarin A, Diz-Chaves Y, Carmona Mdel C, Catargi B, Zorrilla EP, Roberts AJ, Coscina DV, Rousset S, Redonnet A, Parker GC, Inoue K, Ricquier D, Pénicaud L, Kieffer BL, Koob GF. 2005. Resistance to diet-induced obesity in μ-opioid receptor-deficient mice: evidence for a “thrifty gene.” Diabetes 54:3510–3516 [DOI] [PubMed] [Google Scholar]
- 13. Appleyard SM, Hayward M, Young JI, Butler AA, Cone RD, Rubinstein M, Low MJ. 2003. A role for the endogenous opioid β-endorphin in energy homeostasis. Endocrinology 144:1753–1760 [DOI] [PubMed] [Google Scholar]
- 14. Hayward MD, Pintar JE, Low MJ. 2002. Selective reward deficit in mice lacking β-endorphin and enkephalin. J Neurosci 22:8251–8258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bouret S, Prevot V, Croix D, Jégou S, Vaudry H, Stefano GB, Beauvillain JC, Mitchell V. 1999. μ-Opioid receptor mRNA expression in proopiomelanocortin neurons of the rat arcuate nucleus. Brain Res Mol Brain Res 70:155–158 [DOI] [PubMed] [Google Scholar]
- 16. Zheng SX, Bosch MA, Rønnekleiv OK. 2005. μ-Opioid receptor mRNA expression in identified hypothalamic neurons. J Comp Neurol 487:332–344 [DOI] [PubMed] [Google Scholar]
- 17. Barnes MJ, Argyropoulos G, Bray GA. 2010. Preference for a high fat diet, but not hyperphagia following activation of μ opioid receptors is blocked in AgRP knockout mice. Brain Res 1317:100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nicolas P, Li CH. 1985. β-Endorphin-(1–27) is a naturally occurring antagonist to etorphine-induced analgesia. Proc Natl Acad Sci USA 82:3178–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loh HH, Tseng LF, Wei E, Li CH. 1976. β-Endorphin is a potent analgesic agent. Proc Nat Acad Sci USA 73:2895–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sandman CA, Kastin AJ. 1981. Intraventricular administration of MSH induces hyperalgesia in rats. Peptides 2:231–233 [DOI] [PubMed] [Google Scholar]
- 21. Contreras PC, Takemori AE. 1984. Antagonism of morphine-induced analgesia, tolerance and dependence by α-melanocyte-stimulating hormone. J Pharmacol Exp Ther 229:21–26 [PubMed] [Google Scholar]
- 22. Wardlaw SL, Smeal MM, Markowitz CE. 1986. Antagonism of β-endorphin-induced prolactin release by α-melanocyte-stimulating hormone and corticotropin-like intermediate lobe peptide. Endocrinology 119:112–118 [DOI] [PubMed] [Google Scholar]
- 23. Wardlaw SL, Ferin M. 1990. Interaction between β-endorphin and α-melanocyte-stimulating hormone in the control of prolactin and luteinizing hormone secretion in the primate. Endocrinology 126:2035–2040 [DOI] [PubMed] [Google Scholar]
- 24. Khorram O, McCann SM. 1986. Interaction of α-melanocyte-stimulating hormone with β-endorphin to influence anterior pituitary hormone secretion in the female rat. Endocrinology 119:1071–1075 [DOI] [PubMed] [Google Scholar]
- 25. Hughes AM, Everitt BJ, Herbert J. 1988. The effects of simultaneous or separate infusions of some pro-opiomelanocortin-derived peptides (β-endorphin, melanocyte stimulating hormone, and corticotrophin-like intermediate polypeptide) and their acetylated derivatives upon sexual and ingestive behaviour of male rats. Neuroscience 27:689–698 [DOI] [PubMed] [Google Scholar]
- 26. Wardlaw SL. 2011. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur J Pharmacol 660:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. 2009. Prolylcarboxypeptidase regulates food intake by inactivating α-MSH in rodents. J Clin Invest 119:2291–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee M, Kim A, Conwell IM, Hruby V, Mayorov A, Cai M, Wardlaw SL. 2008. Effects of selective modulation of the central melanocortin-3-receptor on food intake and hypothalamic POMC expression. Peptides 29:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanger DJ, McCarthy PS. 1980. Differential effects of morphine on food and water intake in food deprived and freely-feeding rats. Psychopharmacology 72:103–106 [DOI] [PubMed] [Google Scholar]
- 30. McLean S, Hoebel BG. 1983. Feeding induced by opiates injected into the paraventricular hypothalamus. Peptides 4:287–292 [DOI] [PubMed] [Google Scholar]
- 31. Bhakthavatsalam P, Leibowitz SF. 1986. Morphine-elicited feeding: diurnal rhythm, circulating corticosterone and macronutrient selection. Pharmacol Biochem Behav 24:911–917 [DOI] [PubMed] [Google Scholar]
- 32. Low MJ, Hayward MD, Appleyard SM, Rubinstein M. 2003. State-dependent modulation of feeding behavior by proopiomelanocortin-derived β-endorphin. Ann NY Acad Sci 994:192–201 [DOI] [PubMed] [Google Scholar]
- 33. Tseng LF, Loh HH, Li CH. 1977. Human β-endorphin: development of tolerance and behavioral activity in rats. Biochem Biophys Res Commun 74:390–396 [DOI] [PubMed] [Google Scholar]
- 34. Bhargava HN. 1981. Inhibition of tolerance to the pharmacological effects of human β-endorphin by prolyl-leucyl-glycinamide and cyclo(leucylglycine) in the rat. J Pharmacol Exp Ther 218:404–408 [PubMed] [Google Scholar]
- 35. Wilson BD, Bagnol D, Kaelin CB, Ollmann MM, Gantz I, Watson SJ, Barsh GS. 1999. Physiological and anatomical circuitry between Agouti-related protein and leptin signaling. Endocrinology 140:2387–2397 [DOI] [PubMed] [Google Scholar]
- 36. Korner J, Savontaus E, Chua SC, Jr, Leibel RL, Wardlaw SL. 2001. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol 13:959–966 [DOI] [PubMed] [Google Scholar]
- 37. Yang Y, Atasoy D, Su HH, Sternson SM. 2011. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 146:992–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brugman S, Clegg DJ, Woods SC, Seeley RJ. 2002. Combined blockade of both μ- and κ-opioid receptors prevents the acute orexigenic action of Agouti-related protein. Endocrinology 143:4265–4270 [DOI] [PubMed] [Google Scholar]
- 39. Kelly MJ, Loose MD, Ronnekleiv OK. 1990. Opioids hyperpolarize β-endorphin neurons via μ-receptor activation of a potassium conductance. Neuroendocrinology 52:268–275 [DOI] [PubMed] [Google Scholar]
- 40. Markowitz CE, Berkowitz KM, Jaffe SB, Wardlaw SL. 1992. Effect of opioid receptor antagonism on proopiomelanocortin peptide levels and gene expression in the hypothalamus. Mol Cell Neurosci 3:184–190 [DOI] [PubMed] [Google Scholar]
- 41. Pennock RL, Hentges ST. 2011. Differential expression and sensitivity of presynaptic and postsynaptic opioid receptors regulating hypothalamic proopiomelanocortin neurons. J Neurosci 31:281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levine AS, Billington CJ. 1989. Opioids. Are they regulators of feeding? Ann NY Acad Sci 575:209–219; discussion 219–220 [DOI] [PubMed] [Google Scholar]
- 43. Gosnell BA, Krahn DD. 1993. The effects of continuous morphine infusion on diet selection and body weight. Physiol Behav 54:853–859 [DOI] [PubMed] [Google Scholar]
- 44. Yanagita K, Shiraishi J, Fujita M, Bungo T. 2008. Effects of N-terminal fragments of β-endorphin on feeding in chicks. Neurosci Lett 442:140–142 [DOI] [PubMed] [Google Scholar]
- 45. Jaffe SB, Sobieszczyk S, Wardlaw SL. 1994. Effect of opioid antagonism on β-endorphin processing and proopiomelanocortin-peptide release in the hypothalamus. Brain Res 648:24–31 [DOI] [PubMed] [Google Scholar]
- 46. Berman Y, Mzhavia N, Polonskaia A, Devi LA. 2001. Impaired prohormone convertases in Cpe(fat)/Cpe(fat) mice. J Biol Chem 276:1466–1473 [DOI] [PubMed] [Google Scholar]
- 47. Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, DePinho RA, Wardlaw SL, Accili D. 2009. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med 15:1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cone RD, Mountjoy KG, Robbins LS, Nadeau JH, Johnson KR, Roselli-Rehfuss L, Mortrud MT. 1993. Cloning and functional characterization of a family of receptors for the melanotropic peptides. Ann NY Acad Sci 680:342–363 [DOI] [PubMed] [Google Scholar]
- 49. Pritchard LE, White A. 2007. Neuropeptide processing and its impact on melanocortin pathways. Endocrinology 148:4201–4207 [DOI] [PubMed] [Google Scholar]
- 50. Höftberger R, Kunze M, Voigtländer T, Unterberger U, Regelsberger G, Bauer J, Aboul-Enein F, Garzuly F, Forss-Petter S, Bernheimer H, Berger J, Budka H. 2010. Peroxisomal localization of the proopiomelanocortin-derived peptides β-lipotropin and β-endorphin. Endocrinology 151:4801–4810 [DOI] [PubMed] [Google Scholar]
- 51. Perello M, Stuart R, Nillni EA. 2008. Prothyrotropin-releasing hormone targets its processing products to different vesicles of the secretory pathway. J Biol Chem 283:19936–19947 [DOI] [PMC free article] [PubMed] [Google Scholar]