Abstract
GnRH neurons are essential for reproduction, being an integral component of the hypothalamic-pituitary-gonadal axis. Progesterone (P4), a steroid hormone, modulates reproductive behavior and is associated with rapid changes in GnRH secretion. However, a direct action of P4 on GnRH neurons has not been previously described. Receptors in the progestin/adipoQ receptor family (PAQR), as well as progesterone receptor membrane component 1 (PgRMC1) and its partner serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1) mRNA binding protein 1 (SERBP1), have been shown to mediate rapid progestin actions in various tissues, including the brain. This study shows that PgRMC1 and SERBP1, but not PAQR, are expressed in prenatal GnRH neurons. Expression of PgRMC1 and SERBP1 was verified in adult mouse GnRH neurons. To investigate the effect of P4 on GnRH neuronal activity, calcium imaging was used on primary GnRH neurons maintained in explants. Application of P4 significantly decreased the activity of GnRH neurons, independent of secretion of gamma-aminobutyric acidergic and glutamatergic input, suggesting a direct action of P4 on GnRH neurons. Inhibition was not blocked by RU486, an antagonist of the classic nuclear P4 receptor. Inhibition was also maintained after uncoupling of the inhibitory regulative G protein (Gi/o), the signal transduction pathway used by PAQR. However, AG-205, a PgRMC1 ligand and inhibitor, blocked the rapid P4-mediated inhibition, and inhibition of protein kinase G, thought to be activated downstream of PgRMC1, also blocked the inhibitory activity of P4. These data show for the first time that P4 can act directly on GnRH neurons through PgRMC1 to inhibit neuronal activity.
In mammals, the pulsatile release of GnRH into the hypophyseal portal system is essential for reproductive function (1, 2). GnRH acts on gonadotrophs in the anterior pituitary to promote either LH or FSH secretion, depending on amplitude and frequency of GnRH released (3–5). A hallmark of the transition from childhood into biological adulthood is a dramatic increase in nocturnal GnRH pulse frequency in the early phases of puberty (6–8). Thereafter daytime frequencies in pulsatile GnRH secretion increase throughout puberty, whereas nighttime frequencies remain similar to early pubertal patterns (9, 10). Low levels of GnRH release during the wake period in early puberty are thought to be dependent on negative feedback from progesterone (P4), consistent with a sharp rise in circulating P4 levels in the morning (11). However, the site at which P4 acts to suppress GnRH release during early puberty is unclear.
The diurnal progesterone-sensitive mechanism during puberty is influenced by gonadal steroids, with the increase in daytime GnRH pulse frequencies correlating with a rise in testosterone levels (10, 12–14). Proper development of this diurnal system, including its sensitivity to progesterone, is important for reproductive function. In some hyperandrogenic adolescents, the GnRH pulse generator is insensitive to P4-mediated inhibition, leading to abnormal GnRH pulses and impaired fertility (11, 15). Additionally, in adult polycystic ovary syndrome patients administered a constant dosage of estradiol, the GnRH pulse generator can be insensitive to the negative feedback of P4 (16). Although these syndromes highlight the significance of P4 modulation of the GnRH pulse generator, they do not address the site of P4 action.
Indirect evidence for rapid and direct P4 control of GnRH secretion exists (17), but the mechanism by which P4 could mediate such a response was unknown. A role for the classic nuclear progesterone receptor (PGR) in progesterone modulation of the GnRH pulse generator was suggested (18), but colocalization of PGR in GnRH neurons was not detected (19). Moreover, P4 still caused acute suppression of LH levels in ovariectomized, nuclear progesterone receptor knockout mice (20), suggesting that a nonclassical, nonnuclear progesterone receptor may mediate the direct and rapid inhibition of P4 on GnRH neuronal activity.
Possible candidates for mediating rapid action of P4 on GnRH neurons include progestin/adipoQ receptors (PAQR) and progesterone receptor membrane component 1 (PgRMC1). PAQR are a family of 11 G protein-coupled, seven-transmembrane receptors (21), several of which have P4-binding capabilities (22–24). They have been suggested as mediators of rapid P4 action in sperm (25), and their expression and effects have been documented in an immortalized GnRH cell line (20). PgRMC1 and its partner serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1) mRNA binding protein 1 (SERBP1) (26), have been suggested as potential mediators of rapid progestin actions in various cells and tissues (22, 27), including those that do not express PGR, such as rat neural progenitor cells (28). The presence of PgRMC1 and SERBP1 mRNA has also been verified in several hypothalamic nuclei, including the medial preoptic area (29, 30) in which a high number of GnRH neurons are located. PAQR, in contrast, have low expression in those areas (29, 30).
This study examined the expression of PAQR and PgRMC1/SERBP1 in GnRH neurons and the role that these proteins may have in mediating an action of P4 on the GnRH pulse generator. Here we show that PgRMC1 is expressed on GnRH neurons in vivo and present evidence that P4 binding to PgRMC1 can rapidly and directly inhibit GnRH neuronal activity via a protein kinase G (PKG)-dependent mechanism.
Materials and Methods
Animals and explant cultures
All procedures were approved by the National Institute of Neurological Disorders and Stroke Animal Care and Use Committee and performed in accordance with National Institutes of Health (NIH) guidelines.
In vitro
Explants were generated as previously described (31). Briefly, embryonic day 11.5 embryos were obtained from time-mated NIH Swiss mice. Nasal pits were isolated in Gey's balanced salt solution (Life Technologies, Inc., Grand Island, NY) enriched with 50% glucose (Sigma Chemical Co., St. Louis, MO). Explants were adhered onto Permanox coverslips (Nalge Nunc International, Rochester, NY) with a plasma (Cocalico Biologicals, Reamstown, PA)/thrombin (Sigma) clot. Explants were maintained at 37 C in defined serum-free medium (SFM) in a humidified atmosphere with 5% CO2. To inhibit proliferation of olfactory neurons and nonneuronal cells, fresh media containing fluorodeoxyuridine (8 × 10−5 m; Sigma) were applied from culture day 3 to d 6. On d 6, the media were exchanged with fresh SFM and again on d 8. Explants were used between 6 and 10 d in vitro.
In vivo
Transgenic mice expressing green fluorescent protein under the control of the GnRH promoter (GnRH-GFP) were a gift from Dr. D. Spergel [University of Chicago, Biological Sciences Division, Chicago, IL (32)]. Female adult mice were euthanized in a CO2 chamber, brains removed and fixed in 4% formaldehyde (1 h), washed (PBS), and then placed in 1× PBS containing 0.1% sodium azide (NaAz) at 4 C until sectioning. Coronal brain sections (25 μm serial sections) were cut on a vibratome (Vibratome 3000 series; Vibratome Co., St. Louis, MO). Sections were stored in 12-well plates containing 1× PBS/0.1% NaAz at 4 C until staining.
Calcium imaging
Calcium imaging recordings were performed as previously described (33). Briefly, cell-permeant Calcium Green-1 AM probe (Invitrogen, Carlsbad, CA) was diluted to 2.7 mm in 20% pluronic F-127/dimethylsulfoxide (DMSO) solution (Invitrogen). This solution was then diluted in SFM for a final concentration of 13.5 μm. Explants were treated with a total of 140 μl of Calcium Green-1 AM loading solution at 37 C in a 5% CO2 humidified incubator for 20 min and washed twice with 2 ml fresh SFM (10 min each). When necessary, preincubation was included in the experimental procedure [5% CO2 humidified incubator (37 C) for a period of time, dependent on specific drug used]. After preincubation, the drug was then maintained in all solutions throughout the imaging procedure, including loading, control, treatment, and washout solutions. Explants were mounted in a perfusion chamber (Warner Instruments, Hamden, CT) and continuously superfused with medium (∼280 μl/min) using a peristaltic pump (Spectra Hardware, Inc., Westmoreland City, PA). Calcium Green-1 AM was visualized through a ×20 fluorescence objective using an inverted Nikon microscope and a charge-coupled device camera (Retiga, QImaging; Burnaby, Canada). Experiments were piloted by imaging software (iVision Spectrum; Scanalytics Inc., Rockville, MD), and pictures were acquired every 2 sec for a time period between 12 and 40 min, depending on the experiment. All recordings were terminated by a 40-mm KCl stimulation to ensure the viability of the recorded cells. Excitation wavelengths were provided via a medium-width excitation bandpass filter at 465–495 nm, and emission was monitored through a 40-nm bandpass centered on 535 nm. Fluctuations in intracellular calcium activity ([Ca2+]i) were analyzed a posteriori with iVision software. Individual GnRH neurons were identified and the region of interest marked. Intensity of Calcium Green-1 AM fluorescence (Invitrogen) was plotted, the background was subtracted, and the data were analyzed with MATLAB (Mathworks, Natick, MA).
Drugs for calcium imaging
P4, the nuclear progesterone receptor antagonist RU486 [11β-(p-[dimethylamino]phenyl)-17β-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one], the PgRMC1 ligand AG-205 [cis-2-([1-(4-chlorophenyl)-1H-tetrazol-5-yl]thio)-1-(1,2,3,4,4a,9b-hexahydro-2,8-dimethyl-5H-pyrido[4,3-b]indol-5-yl)-ethanonecis-5-([(1-[4-chlorophenyl]-1H-tetraazol-5-yl)sulfanyl]acetyl)-2,8-dimethyl-2,3,4,4a,5,9b-hexahydro-1H-pyrido[4,3-b]indole], the PKG inhibitor Rp-8-bromo-β-phenyl-1,N2-ethenoguanosine 3′,5′-cyclic monophosphorothioate sodium salt hydrate (RP8) and the γ-aminobutyric acid (GABA)A receptor antagonist (−)-bicuculline methochloride (Bic) were obtained from Sigma. The competitive 2-amino-3-hydroxy-5-methyl-4-isoxazol propionic acid (AMPA)/kinate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), the competitive N-methyl-d-aspartate (NMDA) receptor antagonist d-(−)-2-amino-5-phosphonopentanoic acid (AP5) and the inhibitory regulative G protein (Gi/o) inhibitor pertussis toxin (PTX) were purchased from Tocris Bioscience (Bristol, UK). All stock solutions were diluted in H2O, ethanol, or DMSO per the manufacturers' instructions and stored at −20 C. Working solutions were prepared before each experiment by diluting stock solutions through serial dilutions 1:500 to 1:1 million into SFM. Final solvent dilution had no effect on GnRH neuronal activity (33).
Amplification of cDNA from single GnRH cells, explants, and brain extracts
cDNA from single GnRH cells isolated previously (34–36) were screened for the expression of PGR, membrane progesterone receptor (mPR), and PgRMC1 transcripts (Table 1). To ensure that single cell cDNA were from GnRH neurons, cDNA were screened by PCR for GnRH as well as III-tubulin (neuron specific microtubule protein), and L19 [ribosomal protein, for primer sequences see (34)]. Only cells positive for all three transcripts were examined for P4 receptors. 3′ untranslated region biased primers were first tested on cDNA prepared from adult mouse brain. Amplified PCR products were run on a 1.5% agarose gel and examined for production of an appropriate size band (Table 1). The expression of each receptor in explants and single GnRH cell cDNA (7 d in vitro) was then evaluated.
Table 1.
Primer sequences for progesterone receptors examined
| Primer Name | Primer sequence | Band size (bp) |
|---|---|---|
| PgRMC1 forward | AGGGCAGGAACAGGTATGTG | 205 |
| PgRMC1 reverse | CCAAAGGAGTATTACCCAAGACC | |
| nPR forward | AGTATGTTGGCCCCAGGACTGA | 244 |
| nPR reverse | TACACAGGCATGGAGTCTCG | |
| PAQR5 (mPRγ) forward | CTATCCAGAGCTGCCTGTCC | 330 |
| PAQR5 (mPRγ) reverse | CTACTCCATTGCTTCCCCAC | |
| PAQR6 (mPRδ) forward | GGCAGGTGGTGAAGGTCTAA | 259 |
| PAQR6 (mPRδ) reverse | TCCAAACACACAAATTGGCA | |
| PAQR7 (mPRα) forward | GAAGGGTGAGGAGTTTGCAG | 160 |
| PAQR7 (mPRα) reverse | CTAGGGCATATCAGGGACCA | |
| PAQR8 (mPRβ) forward | ACCCGTGACGCCCGTGAGAA | 459 |
| PAQR8 (mPRβ) reverse | CTGATTTTGCAGGGTGACAAACTCCAT |
Antibodies
All primary antibodies used were polyclonal, unless otherwise indicated, and diluted in 10% BSA (Gemini Bio-Products, West Sacramento, CA)/0.1% NaAz. Rabbit (Rb) anti-PgRMC1 [S1, gift from Dr. A. Wendler, Department of Clinical Pharmacology, University of Heidelberg, Mannheim, Germany (37)] and Rb anti-SERBP1 (ab55993; Abcam, Cambridge, MA) were diluted 1:600 and 1:400, respectively. Rb anti-PAQR5 (ab79517; Abcam) and Rb anti-PAQR7 (ab75508; Abcam) were diluted 1:200 and 1:500, respectively. Rb anti-PGR (ab63605; Abcam) and Rb anti-GnRH [SW-1 (38)] were diluted 1:1000. Mouse monoclonal anti-GnRH [F1D3C5, gift from Dr. A. Karande, Department of Biochemistry, Indian Institute of Science, Bangalore, India (39)] was diluted 1:4000.
Immunocytochemistry (ICC)
To ensure that recorded cells were positive for GnRH, staining was done on explants after calcium imaging with GnRH antiserum as previously described (31). Briefly, explants were fixed (4% formaldehyde, 1 h), rinsed in PBS, and placed in cryoprotectant (40) until staining. For staining, explants were washed in PBS, blocked [10% normal goat serum/0.3% Triton X-100 (NGS), 1 h], washed several times in PBS and incubated with SW-1 overnight at 4 C. Explants were then washed in PBS, incubated in biotinylated secondary antibodies (1:500 in PBS/0.3% Triton X-100, 1 h) (Vector Laboratories, Inc., Burlingame, CA), washed in PBS, and processed for avidin-biotin-horseradish peroxidase/3′3-diaminobenzidine cytochemistry.
For PgRMC1, SERBP1, PAQR5, PAQR7, and PGR staining, the above procedure was used with varying antibody dilutions to optimize staining conditions. Mouse ovary and uteri sections were used as positive controls due to established expression of various progesterone receptor proteins in these tissues (26, 41, 42).
Immunofluorescence (IF)
In vitro staining
Explants were blocked with NGS and incubated in anti-PgRMC1, anti-SERBP1, anti-PAQR5, anti-PAQR7, or anti-PGR as described above. The next day, explants were washed in PBS and incubated in Cy3-conjugated secondary antibodies (1:2000 in PBS, 1 h). Explants were then washed in PBS and incubated in anti-GnRH (F1D3C5) or NGS for 48 h (4 C). Explants were then washed in PBS, incubated in Alexa Fluor 488-conjugated secondaries (1:1000, 1 h; Invitrogen), washed, and counterstained with 4′,6′-diamino-2-phenylindole (DAPI, 1:1000).
In vivo staining
Serial vibratome brain sections from the GnRH-GFP mice were washed in PBS and placed into a 12-well plate containing NGS for 2 h. The sections were washed and incubated in anti-PgRMC1, anti-SERBP1, or NGS (negative control) for 48 h (4 C), after which sections were washed and incubated in Cy3-conjugated secondary antibodies (1:2000 in PBS, 2 h; Jackson ImmunoResearch Laboratories, West Grove, PA). Sections were counterstained with 4′,6′-diamino-2-phenylindole (1:1000; Sigma) and mounted onto glass slides for imaging.
All images from in vitro stainings were taken on a Nikon Eclipse E800 with a Retiga SRV camera with QCapture software (QImaging). Confocal pictures from in vivo stainings were taken on a spinning disk confocal system (CSU10; Yocogawa, Sugarland, TX) mounted on an Eclipse TSE200 microscope (Nikon, Tokyo, Japan) using an electron-multiplying charge-coupled device ImageM digital camera (Hamamatsu, Hamamatsu, Shizuoka, Japan) with I-Vision software (Biovision, Mountain View, CA), and images were further analyzed using NIH ImageJ software (W. Rasband, NIH, Bethesda, MD).
Statistical analysis
Calcium oscillations, reported as peaks, were used as an indication of neuronal activity (33, 43). First, a calcium elevation is defined as a peak when its value is greater than the five previous and five subsequent points plus a minimal value representing small fluctuations in baseline (for specific values and calculation methods, see Refs. 43 and 33). The frequency of calcium oscillations was calculated as the number of detected calcium peaks per minute (PPM). Statistical analysis was performed in Prism 5 (GraphPad Software, Inc., La Jolla, CA) using a paired, two-tailed Student's t test to identify a drug effect on the peak frequency among a pool of cells. For all statistics, before-and-after values are compared in individual cells (n) from multiple explants (N) in similar groups. For groups containing fewer than 50 cells, a stringent P ≤ 0.0001 was considered significant. For groups containing more than 50 cells, a P ≤ 0.001 was considered significant.
When a statistically significant effect was observed in the treatment period compared with the control period for each set of calcium-imaging experiments, population analysis was performed to segregate the responder and nonresponder populations. The PPM for each cell during the treatment period was subtracted from the average PPM for the same cell during the control SFM period to produce a ΔPPM value. The ΔPPM for each cell was then compared with a previously determined value indicating average fluctuations in calcium response between two SFM control periods plus sd (δPPM = ±0.5). GnRH neurons whose ΔPPM fell outside the δPPM range were grouped into a responder subpopulation and analyzed separately. Where indicated, post hoc analyses were performed to evaluate subpopulation dynamics. For the post hoc analyses, an unpaired, two-tailed t test was performed on individual cells in a group using Prism 5 (GraphPad Software), and statistical significance was defined as a P ≤ 0.0001. All calcium imaging data are presented as mean ± sem. N and n represent the number of explants and cells recorded, respectively.
Results
PgRMC1 is expressed in GnRH cells
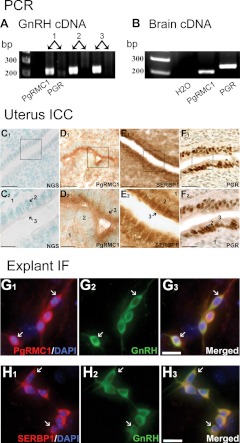
PCR was used to determine whether PAQR, PgRMC1, and/or PGR were expressed in GnRH neurons by using cDNA obtained from single GnRH cells maintained in explants (35, 44) as well as cDNA from whole explants. Brain cDNA was used as a positive control. PCR on GnRH single-cell cDNA showed bands of appropriate size in 50% of the cells for PAQR5 and in 70% of the cells for PAQR7 but not for PAQR6 or PAQR8 (n = 10, Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Brain cDNA, used as a positive control, showed bands of appropriate size for all four PAQR tested. In all single cells tested, a 205-bp band representing PgRMC1 was detected (n = 10, Fig. 1A) as well as in whole explant (data not shown) and brain cDNA (Fig. 1B). In contrast, PCR on single-cell cDNA revealed that GnRH neurons maintained in explants, like GnRH neurons in vivo (19), do not express PGR (n = 10, Fig. 1A), although a band of the appropriate size was detected by PCR in brain cDNA (Fig. 1B) and whole-explant cDNA (data not shown).
Fig. 1.
PgRMC1 and SERBP1 are expressed in GnRH neurons. PCR on cDNA from three single-cell GnRH neurons maintained in nasal explants shows bands of appropriate size for PgRMC1 but not PGR (A). Controls, Adult mouse brain cDNA shows bands for both PgRMC1 and PGR (B). A ×40 bright-field images of mouse uteri sections (C–F) used as a positive control for PgRMC1, SERBP1, and PGR ICC/IF staining. In panels C1–F1, the box represents the ×100 field shown in C2–F2; 1, Endometrial stroma, 2, endometrial glands, and 3, epithelium of the endometrium. GnRH neurons in explants express PgRMC1 (G1–G3) and SERBP1 (H1–H3). Scale bars, 25 μm in panels C1–F1; 10 μm in panels C2–F2; 5 μm in panels G and H. DAPI, 4′,6′-Diamino-2-phenylindole.
Based on PCR data, immunocytochemistry was then performed for PGR, PAQR5, PAQR7, and PgMRC1 on nasal explants. Immunocytochemistry was also performed for SERBP1. All antibodies were first optimized using uterine tissue (Fig. 1, C–F and Supplemental Fig. 1). Double-label immunofluorescence revealed that neither PAQR5 nor PAQR7 was expressed in GnRH neurons, although PAQR5 was detected in other cells in the explant (Supplemental Fig. 1). In contrast, PgRMC1 and SERBP1 were detected in most GnRH neurons (Fig. 2, G and H) and not found in other cells in the explant.
Fig. 2.
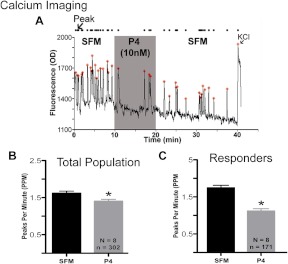
P4 rapidly inhibits GnRH neurons. Application of 10 nm progesterone to GnRH neurons in explants caused rapid inhibition of calcium oscillations (A). Analysis of the data revealed a significant decrease in PPM after P4 application (B). Population analysis indicated that only 56% of the GnRH cells responded to P4 and in this subpopulation PPM decreased by approximately 50% (C). In panels B and C, N = number of explants and n = number of GnRH neurons. *, Significantly different from previous period.
A subpopulation of GnRH neurons is rapidly inhibited by P4
Calcium-imaging experiments were used to determine whether P4 had an effect on the activity of GnRH neurons. For all functional studies, a concentration of 10 nm P4 was chosen because it is known that P4 binds to PgRMC1 with an EC50 of 10 nm (22, 45) and a dissociation constant of 11 nm-35 nm, depending on cell type (46, 47). Acute application of 10 nm P4 caused a significant decrease in GnRH [Ca2+]i oscillations (SFM: 1.62 ± 0.05; P4: 1.41 ± 0.07, n = 8, n = 302, P ≤ 0.0001, Figure 2, A and B). Population analysis revealed responder and nonresponder cells and that the responder population, comprising 56% of GnRH neurons tested previously, showed a 30–43% decrease in [Ca2+]i oscillations (SFM: 1.75 ± 0.07; P4: 1.12 ± 0.07, n = 8, n = 171, P ≤ 0.0001, Fig. 2C). Post hoc analysis of the responder and nonresponder population showed a higher frequency of spontaneous calcium transients in the responder population during the control period (responders: 1.75 ± 0.07, n = 171; nonresponders: 1.45 ± 0.07, n = 131, n = 8, P ≤ 0.05), suggesting that the two subpopulations may receive distinct input.
Neither GABAergic nor glutamatergic neurons are involved in P4's inhibitory effect
The involvement of the major inputs to GnRH cells in explants, GABA and glutamate, in the effects observed after P4 treatment were evaluated. GnRH neurons were imaged with the GABAA antagonist Bic (20 μm) (48) and/or in the presence of the AMPA receptor antagonist CNQX (49) and NMDA receptor antagonist AP5 (50) (10 μm each) diluted in SFM.
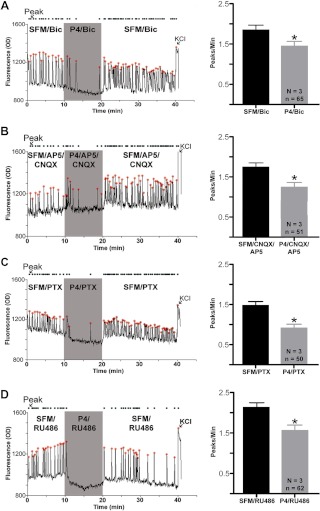
P4 in combination with Bic still caused a 23–25% decrease of [Ca2+]i oscillations in 57% of GnRH neurons (SFM/Bic 1.87 ± 0.13; P4/Bic 1.42 ± 0.12, n = 65, n = 3, P ≤ 0.0001, Fig. 3A). Post hoc analysis of control period PPM values for progesterone-responder and -nonresponder populations showed that responders exhibited a higher frequency of calcium transients than nonresponders (responders: 1.87 ± 0.13, n = 65, n = 3; nonresponders 1.16 ± 0.14, n = 49, n = 3, P ≤ 0.0001). Comparing preprogesterone PPM values of nonresponders revealed that Bic caused a significant decrease compared with SFM alone (SFM: 1.45 ± 0.07, n = 131, n = 8; Bic: 1.16 ± 0.14, n = 49, n = 3, P ≤ 0.0001). In contrast, Bic and SFM values were similar in the responder population (SFM: 1.75 ± 0.07, n = 171, n = 8; Bic: 1.87 ± 0.13, n = 65, n = 3, P = 0.19).
Fig. 3.
P4-mediated inhibition is direct and does not involve PAQR or PGR. Rapid P4-mediated inhibition of GnRH neuronal activity occurred in the presence of the GABAA antagonist Bic (A) and AMPA/NMDA antagonists CNQX/AP5 (B). Neither PTX, which uncouples Gi/o from membrane-bound receptors (C), nor RU486, a competitive inhibitor of the nuclear progesterone receptor (D), prevented the rapid P4-mediated inhibition in GnRH neurons. Left panels, Representative trace from a single GnRH neuron in the treatment group. Right panels, Grouped PPM data from the responder population. N and n, Number of explants and number of GnRH neurons, respectively. *, Significantly different from previous period.
P4 in combination with AP5/CNQX also caused a 22–25% decrease of [Ca2+]i oscillations in 44% of GnRH neurons (SFM/AP5/CNQX: 1.74 ± 0.12; P4/AP5/CNQX: 1.34 ± 0.12, n = 51, n = 3, P ≤ 0.0001, Fig. 3B). Post hoc analysis of progesterone-responder and -nonresponder subpopulations in this group showed that the responders also exhibited a higher frequency of calcium transients than nonresponders (responders: 1.74 ± 0.12, n = 51; nonresponders: 1.15 ± 0.14, n = 65, n = 3, P ≤ 0.0001). In the nonresponder population, AP5/CNQX caused a significant decrease in PPM (SFM: 1.45 ± 0.07, n = 131, n = 8; AP5/CNQX: 1.15 ± 0.14, n = 65, n = 3, P ≤ 0.0001). However, no difference was found in the responder population (SFM: 1.75 ± 0.07, n = 171, n = 8; AP5/CNQX: 1.74 ± 0.12, n = 51, n = 3, P = 0.49).
Verification of functional progesterone receptors on GnRH neurons
PAQR and PGR are not involved in P4-mediated GnRH inhibition
Our results showed that PAQR are not expressed in GnRH neurons but that PAQR5 is present on other cells. To verify the absence of functional PAQR on or indirectly influencing GnRH neurons, PTX was used to uncouple Gi/o (51–53) from membrane-bound, G protein-coupled PAQR (21). Explants were pretreated with 250 ng/ml PTX for 6 h and then imaged before and after the application of P4. After uncoupling of Gi/o, P4 still caused a 36–41% decrease in GnRH [Ca2+]i oscillations in 76% of neurons tested (SFM/PTX: 1.49 ± 0.10; P4/PTX: 0.92 ± 0.10, n = 3, n = 50, P ≤ 0.0001, Fig. 3C).
To verify the absence of functional PGR in explants that might participate in the rapid inhibition observed in GnRH neurons after P4, the synthetic steroid RU486, a potent competitive PGR antagonist (54, 55), was used. Explants were pretreated with 1 μm RU486 for 1 h. Despite inhibition of PGR, P4 caused a 24–29% inhibition of GnRH [Ca2+]i oscillations (SFM/RU486: 2.13 ± 0.12; P4/RU486: 1.56 ± 0.14, n = 3, n = 62, P ≤ 0.0001, Fig. 3D).
P4-mediated inhibition of GnRH neurons is blocked by AG-205, an antagonist of PgRMC1
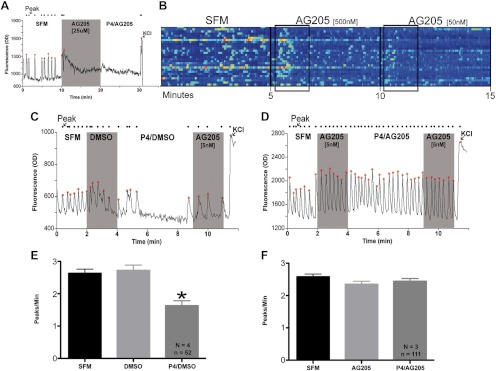
Recent literature described a compound, AG-205, that acts as a PgRMC1 ligand (56) and inhibitor (57–59) in cancer cells. AG-205 was used in the micromolar range in these previous studies. However, in the micromolar range, AG-205, but not the DMSO vehicle, caused a sharp and rapid rise in [Ca2+]i and rendered GnRH cells completely quiescent (Fig. 4A), making it impossible to distinguish any subsequent inhibitory effect of P4. A dose-response curve was performed. In the 5- to 500-nm range, AG-205 still caused a sharp and rapid spike in [Ca2+]i levels in some GnRH cells, being minimal at 5 nm. Normal [Ca2+]i oscillations were observed in the majority of cells, however, approximately 2 min after application (Fig. 4B). Therefore, a final concentration of 5 nm and an application period of 2 min were chosen to minimize unknown side effects of AG-205 on [Ca2+]i oscillations during the P4 application period.
Fig. 4.
P4-mediated inhibition is through PgRMC1. The PgRMC1 ligand and inhibitor AG-205 caused a sharp rise in intracellular calcium in the micromolar (A, shaded area). In the nanomolar range (B, boxed area), the effect was attenuated and cells were able to return to normal PPM values. P4 rapidly inhibits GnRH neuronal activity after the application of DMSO vehicle control (C and E), but inhibition is blocked by AG-205 treatment (D and F). C and D, Representative traces from single GnRH neurons. E and F, Grouped PPM data from the responder population (E) and total population (F). N and n, Number of explants and number of GnRH neurons, respectively.
GnRH neurons were imaged with either DMSO or AG-205 treatment after a 2-min SFM control period. The vehicle, or AG-205, was then maintained in the solution while P4 was applied for 5 min. Using this paradigm, neither AG-205 nor DMSO had an effect on [Ca2+]i oscillations during initial treatment (Fig. 4, C and D). Application of P4 caused rapid inhibition in GnRH cells in the DMSO group [38–42% inhibition of [Ca2+]i oscillations in 54% of GnRH neurons (SFM/DMSO: 2.72 ± 0.16; P4/DMSO: 1.63 ± 0.15, n = 4, n = 52, P ≤ 0.0001, Fig. 4E)]. In contrast, no change was detected in the activity of GnRH cells in the AG-205 group (SFM/AG-205: 2.35 ± 0.10; P4/AG-205: 2.44 ± 0.09, n = 3, n = 111, P = 0.187, Fig. 4F). Thus, AG-205 blocked P4's ability to rapidly inhibit [Ca2+]i oscillations in GnRH neurons.
P4-mediated inhibition of GnRH neurons is dependent on PKG, a possible signal transduction pathway for PgRMC1
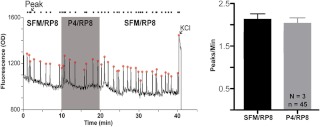
Published reports suggest that P4 acts rapidly on cells via a PKG-dependent pathway (47, 60, 61). To test whether PKG is involved in rapid P4-mediated inhibition, explants were pretreated with 3 μm of the PKG inhibitor RP8 (62) for 30 min and imaged. Inhibition of PKG prevented P4-mediated inhibition of [Ca2+]i oscillations (SFM/RP8: 2.11 ± 0.14; P4/RP8: 2.02 ± 0.14, n = 3, n = 45, P = 0.268, Fig. 5).
Fig. 5.
P4-mediated inhibition is dependent on PKG activation. The PKG inhibitor RP8 blocked P4-mediated inhibition. Left panel, Calcium oscillation trace in a single representative cell. Right panel, Grouped PPM data from total population. N and n, Number of explants and number of GnRH neurons, respectively.
PgMRC1 and SERBP1 are expressed by GnRH neurons in vivo
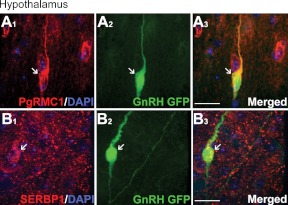
GnRH neurons maintained in explants exhibit many characteristics of GnRH neurons in vivo (63). However, the expression of PgMRC1 and SERBP1 in GnRH neurons in vivo had not been reported. Thus, the hypothalami of adult GnRH-GFP mice were examined after IF staining against the two proteins. PgRMC1- and SERBP1-positive cells (Fig. 6, A1 and B1) included GFP-positive neurons (Fig. 6, A2 and B2). Although not all GnRH neurons were positive for PgRMC1/SERBP1, double-labeled GnRH cells were not confined to a specific anatomical region and represented a large proportion of the GnRH neuronal population.
Fig. 6.
PgRMC1 and SERBP1 are expressed by GnRH cells in adult mouse hypothalamus. GnRH neurons in adult GnRH-GFP mice express PgRMC1 (A) and SERBP1 (B). Scale bars, 5 μm in panels A and B.
Discussion
This report shows that GnRH neurons in mice express the progesterone-binding receptor PgRMC1 and its binding partner SERBP1 and that P4 rapidly decreased the frequency of spontaneous baseline oscillations in [Ca2+]i in a subpopulation of GnRH neurons maintained in explants. This inhibitory effect was independent of GABAergic and glutamatergic input, suggesting for the first time that P4 can act directly on GnRH neurons. The P4-mediated inhibition was also observed after inhibition of PGR and mPR, indicating that this rapid effect occurs independently of both of these families of progesterone receptors. Inhibition, however, was blocked by AG-205, a PgRMC1 ligand, and was largely dependent on the action of PKG. These data, along with previous reports suggesting that P4 acts through PgRMC1 to cause a reduction of intercellular calcium concentrations (47, 64), are consistent with the hypothesis that P4 acts on a subpopulation of GnRH neurons through PgRMC1 to reduce calcium oscillations and thereby inhibit neuronal activity.
Progesterone has been shown to rapidly inhibit calcium influx in murine embryonic sensory neurons (65), but no direct effect of P4 on GnRH neurons had been demonstrated until now. Previous studies have shown that GnRH neurons do not express PGR (19). Evidence from studies in ewes suggested an acute, P4-mediated inhibition on GnRH/LH pulse frequency. Although the mechanism or site of action were not elucidated, P4-mediated inhibition was shown to be independent of endogenous opioid peptides and GABAA (17) but blocked by RU486 (18). The fact that P4 still caused acute suppression of LH levels in ovariectomized, nuclear progesterone receptor knockout mice (20) suggests that a nonclassical, nonnuclear progesterone receptor may mediate a direct and rapid inhibition of P4 on GnRH neuronal activity. The present study uncovers a specific mechanism that could account for such rapid inhibition: PgRMC1, possibly through a PKG-dependent pathway.
GnRH neurons maintained in explants exhibit many characteristics of GnRH cells in vivo (63) including pulsatile secretion and calcium synchronization (66–70) and, as such, have been used as a model to study GnRH neuronal activity. In this model, the major excitatory inputs to GnRH neurons are GABAergic, via GABAA receptors (8, 33, 43, 71, 72), and glutamatergic, via AMPA/NMDA receptors (63, 73, 74). P4-mediated inhibition of GnRH neurons was found to be acute and independent of GABAA and glutamate. Notably, P4 inhibition of GnRH neuronal activity occurred when both excitatory inputs were blocked, supporting the hypothesis that P4 can act directly on GnRH neurons. Post hoc analyses comparing the responder and nonresponder populations in the Bic and AP5/CNQX experiments showed that the population of GnRH neurons rapidly inhibited by P4 was not affected by blockage of either system alone but did show a decrease in PPM when both GABAergic and glutamatergic inputs were blocked simultaneously (our unpublished observation). This indicates that in the subpopulation of GnRH neurons that is responsive to progesterone, GABA and glutamate signals are compensatory. Such redundancy was not apparent in the P4 nonresponding GnRH population. The mechanism(s) underlying the differential responses to excitatory signals is presently unclear, but under all conditions in which excitatory signals were blocked, P4 inhibition of a subpopulation of GnRH neurons was detected, consistent with a direct action.
PCR and immunocytochemical data indicated that, of the many P4 receptors possible, GnRH cells expressed only PgMRC1/ SERBP1. To verify that the rapid inhibitory effect of P4 involved these receptors and not the PAQR (22–24) or PGR [which has been shown to mediate nongenomic P4 action (75) in addition to its DNA-binding activity (76)], experiments were performed disrupting Gi/o used by the PAQR and blocking PGR with its antagonist RU486 [which has no effect on mPR (22) or PgRMC1 (45, 77)]. Under these conditions, P4 still caused rapid inhibition of GnRH neuronal activity, indicating that mPR and PGR are not involved in mediating the rapid action of P4 in GnRH neurons.
Compared with the mPR and PGR, less is known about PgMRC1/SERBP1. Small interfering RNA (siRNA) studies on immortalized cells reported a 55% reduction of PgRMC1 levels (47). Because GnRH responders comprised 50% of the entire GnRH population in explants, and markers for the responders are currently unknown before P4 application, siRNA experiments were not considered; at best, 25% of the responding GnRH cells may have become unresponsive after siRNA. However, a relatively new molecule, AG-205, was recently described as a ligand to, and inhibitor of, PgRMC1 (54–56) in cancer cells. Using a similar dose of AG-205 on GnRH neurons caused a very sharp and rapid rise of [Ca2+]i that was maintained throughout the recording period. However, when used at 5 nm, AG-205 was able to block the rapid P4-mediated inhibition in GnRH neurons. Although AG-205 is a new compound and its effect on other receptors and cells has not been studied, these data support action of P4 through PgRMC1 to inhibit GnRH neuronal activity.
Previous studies indicated that PKG is activated downstream of P4 application (61). Therefore, a PKG inhibitor was used to study whether P4-mediated inhibition is dependent on PKG activation. Inhibition of PKG blocked the rapid P4-mediated inhibition of GnRH neuronal activity. Peluso and colleagues (60, 61) showed that PKG interacted with ATP synthase-β/precursor and 14-3-3σ, which the investigators suggested could result in lower intracellular ATP and thereby inhibit apoptosis in granulosa cells. Whether such an ATP-dependent mechanism, inhibited by PKG activation, could alter intracellular calcium levels in the GnRH neurons is unclear. However, this pathway is not the only PKG-dependent pathway that affects intracellular calcium. Activation of PKG can lead to phosphorylation of G protein-activated phospholipase-Cβ3, which then leads to a decrease in calcium mobilization (78) and inhibition of calcium release from intracellular stores (79). Other pathways for calcium modulation by PKG, such as inhibition of calcium influx, have also been described (80). Our finding that inhibition of PKG prevents the P4-mediated inhibition of [Ca2+]i oscillations is consistent with PKG activation downstream of P4 binding to the PgRMC1/SERBP1 complex on GnRH neurons. Although our data, taken together, complement the present literature on rapid P4-action, further studies are required to determine conclusively a direct link between PgRMC1 and PKG.
Despite single-cell PCR results showing 100% of GnRH neurons expressing PgRMC1 and IF data confirming that the majority of GnRH neurons maintained in explants express both PgRMC1 and SERBP1, only 57% of neurons in explants were inhibited by P4 application. The differences found between expression patterns and functional activity may be caused by modulators of events downstream of PgRMC1/SERBP1, such as PKG, that are not be expressed or active in all GnRH neurons. It is also possible that other neurons, as well as nonneuronal cells in the explant, express receptors that compete with PgRMC1 for P4 binding. Application of RU486 followed by P4 resulted in inhibition of 63% of the GnRH population. Single-cell PCR data and ICC/IF staining showed that the GnRH neurons do not express PGR. However, RU486, in addition to being an PGR antagonist, acts as an antagonist of glucocorticoid receptors (GR) (81, 82), which can also bind progesterone (83). GR are present on olfactory sensory neurons (84), which are present in nasal explants (31). If RU486 blocks P4 from binding to the GR, then more P4 might be available to bind to PgRMC1, thereby increasing the number of GnRH neurons that respond to P4.
The presence of P4-binding PAQR in explants could have a similar effect. PCR data showed that 50 and 70% of GnRH neurons were positive for PAQR5 and PAQR7, respectively, but no GnRH cells were immunopositive for either protein. The presence of mRNA alone does not necessarily indicate the presence of a functional protein because the mRNA could be posttranscriptionally modified or degraded (85, 86). However, PAQR5 was detected in non-GnRH cells in the periphery of the explant (see Supplemental Fig. 1), and although the phenotype of the PAQR5 immunopositive cells is presently unknown, the presence of P4 binding receptors on these cells could also alter the effective concentration for PgRMC1 on GnRH neurons.
Taken together, the data presented in this study indicate that direct inhibition of GnRH neuronal activity can occur by P4 via PgRMC1/SERBP1. Traditionally, the nuclear progesterone receptor has been thought to be the key player in mediating P4 action on the hypothalamic-pituitary-gonadal axis [albeit indirectly on the GnRH pulse generator (18, 19)]. Sleiter et al. (20) showed that P4 can still act on the hypothalamic-pituitary-gonadal axis in PGR-knockout mice, and although the group suggested a role for PAQR based on work in immortalized GT1–7 cells, PgRMC1 were not examined. Krebs et al. (87) found that in ovariectomized, estrogen-primed rats, P4 repressed expression of 25-Dx (PgRMC1 protein homolog) in the ventromedial hypothalamus. Because estrogen priming is necessary for induction of PGR expression (88), rapid inhibition mediated by PgRMC1 could be an initial pathway mediating P4 action on the GnRH pulse generator before activation of PGR system. Further research is needed to address the function of PgRMC1 in GnRH neurons during normal and aberrant reproductive states, including models for progesterone-insensitive hyperandrogenic adolescents and polycystic ovary syndrome patients.
Acknowledgments
We thank R. Brock (National Institute of Diabetes and Digestive and Kidney Diseases) and Dr. U. Klenke [Cellular and Developmental Neurobiology Section (CDNS), National Institute of Neurological Disorders and Stroke (NINDS)] for technical assistance with data analysis, Dr. P. Forni (CDNS, NINDS) for assistance with confocal microscopy, Marie Hickman (Newcomb Scholar, CDNS, NINDS) for assistance with in vivo brain sections, and Dr. C. Taylor-Burds (CDNS, NINDS) and the Fellows Editorial Board (National Cancer Institute) for invaluable critiques of manuscript drafts.
This work was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health (ZIA NS002824-21).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AMPA
- 2-Amino-3-hydroxy-5-methyl-4-isoxazol propionic acid
- AP5
- d-(−)-2-amino-5-phosphonopentanoic acid
- Bic
- bicuculline methochloride
- [Ca2+]i
- intracellular calcium activity
- CNQX
- 6-cyano-7-nitroquinoxaline-2,3-dione
- DMSO
- dimethylsulfoxide
- GABA
- γ-aminobutyric acid
- GnRH-GFP
- green fluorescent protein under the control of the GnRH promoter
- GR
- glucocorticoid receptor
- ICC
- immunocytochemistry
- IF
- immunofluorescence
- mPR
- membrane progesterone receptor
- NaAz
- sodium azide
- NGS
- normal goat serum
- NMDA
- N-methyl-d-aspartate
- P4
- progesterone
- PAQR
- progestin/adipoQ receptor
- PGR
- progesterone receptor
- PgRMC1
- progesterone receptor membrane component 1
- PKG
- protein kinase G
- PPM
- peaks per minute
- PTX
- pertussis toxin
- Rb
- rabbit
- RP8
- Rp-8-bromo-β-phenyl-1,N2-ethenoguanosine 3′,5′-cyclic monophosphorothioate sodium salt hydrate
- SERBP1
- serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1) mRNA binding protein 1
- SFM
- serum-free medium
- siRNA
- small interfering RNA.
References
- 1. Goodman RL, Karsch FJ. 1980. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology 107:1286–1290 [DOI] [PubMed] [Google Scholar]
- 2. Levine JE, Pau KY, Ramirez VD, Jackson GL. 1982. Simultaneous measurement of luteinizing hormone-releasing hormone and luteinizing hormone release in unanesthetized, ovariectomized sheep. Endocrinology 111:1449–1455 [DOI] [PubMed] [Google Scholar]
- 3. Gross KM, Matsumoto AM, Bremner WJ. 1987. Differential control of luteinizing hormone and follicle-stimulating hormone secretion by luteinizing hormone-releasing hormone pulse frequency in man. J Clin Endocrinol Metab 64:675–680 [DOI] [PubMed] [Google Scholar]
- 4. Marshall JC, Kelch RP. 1986. Gonadotropin-releasing hormone: role of pulsatile secretion in the regulation of reproduction. N Engl J Med 315:1459–1468 [DOI] [PubMed] [Google Scholar]
- 5. Wildt L, Häusler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. 1981. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109:376–385 [DOI] [PubMed] [Google Scholar]
- 6. Dunkel L, Alfthan H, Stenman UH, Selstam G, Rosberg S, Albertsson-Wikland K. 1992. Developmental changes in 24-hour profiles of luteinizing hormone and follicle-stimulating hormone from prepuberty to midstages of puberty in boys. J Clin Endocrinol Metab 74:890–897 [DOI] [PubMed] [Google Scholar]
- 7. Grumbach MM. 2002. The neuroendocrinology of human puberty revisited. Horm Res 57(Suppl 2):2–14 [DOI] [PubMed] [Google Scholar]
- 8. Wu FC, Butler GE, Kelnar CJ, Huhtaniemi I, Veldhuis JD. 1996. Ontogeny of pulsatile gonadotropin releasing hormone secretion from midchildhood, through puberty, to adulthood in the human male: a study using deconvolution analysis and an ultrasensitive immunofluorometric assay. J Clin Endocrinol Metab 81:1798–1805 [DOI] [PubMed] [Google Scholar]
- 9. Hall JE, Sullivan JP, Richardson GS. 2005. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab 90:2050–2055 [DOI] [PubMed] [Google Scholar]
- 10. McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. 2009. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab 94:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blank SK, McCartney CR, Chhabra S, Helm KD, Eagleson CA, Chang RJ, Marshall JC. 2009. Modulation of gonadotropin-releasing hormone pulse generator sensitivity to progesterone inhibition in hyperandrogenic adolescent girls—implications for regulation of pubertal maturation. J Clin Endocrinol Metab 94:2360–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ankarberg C, Norjavaara E. 1999. Diurnal rhythm of testosterone secretion before and throughout puberty in healthy girls: correlation with 17β-estradiol and dehydroepiandrosterone sulfate. J Clin Endocrinol Metab 84:975–984 [DOI] [PubMed] [Google Scholar]
- 13. McCartney CR. 2010. Maturation of sleep-wake gonadotrophin-releasing hormone secretion across puberty in girls: potential mechanisms and relevance to the pathogenesis of polycystic ovary syndrome. J Neuroendocrinol 22:701–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A. 2000. Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol secretion before the onset of female puberty in short children. J Clin Endocrinol Metab 85:1074–1080 [DOI] [PubMed] [Google Scholar]
- 15. Burt Solorzano CM, McCartney CR, Blank SK, Knudsen KL, Marshall JC. 2010. Hyperandrogenaemia in adolescent girls: origins of abnormal gonadotropin-releasing hormone secretion. BJOG 117:143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. 1998. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab 83:582–590 [DOI] [PubMed] [Google Scholar]
- 17. Goodman RL, Gibson M, Skinner DC, Lehman MN. 2002. Neuroendocrine control of pulsatile GnRH secretion during the ovarian cycle: evidence from the ewe. Reprod Suppl 59:41–56 [PubMed] [Google Scholar]
- 18. Skinner DC, Evans NP, Delaleu B, Goodman RL, Bouchard P, Caraty A. 1998. The negative feedback actions of progesterone on gonadotropin-releasing hormone secretion are transduced by the classical progesterone receptor. Proc Natl Acad Sci USA 95:10978–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Skinner DC, Caraty A, Allingham R. 2001. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology 142:573–579 [DOI] [PubMed] [Google Scholar]
- 20. Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. 2009. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology 150:3833–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. 2005. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol 61:372–380 [DOI] [PubMed] [Google Scholar]
- 22. Thomas P. 2008. Characteristics of membrane progestin receptor α (mPRα) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol 29:292–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Y, Bond J, Thomas P. 2003. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA 100:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. 2003. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomas P, Tubbs C, Garry VF. 2009. Progestin functions in vertebrate gametes mediated by membrane progestin receptors (mPRs): Identification of mPRα on human sperm and its association with sperm motility. Steroids 74:614–621 [DOI] [PubMed] [Google Scholar]
- 26. Zhang L, Kanda Y, Roberts DJ, Ecker JL, Losel R, Wehling M, Peluso JJ, Pru JK. 2008. Expression of progesterone receptor membrane component 1 and its partner serpine 1 mRNA binding protein in uterine and placental tissues of the mouse and human. Mol Cell Endocrinol 287:81–89 [DOI] [PubMed] [Google Scholar]
- 27. Peluso JJ. 2011. Progesterone signaling mediated through progesterone receptor membrane component-1 in ovarian cells with special emphasis on ovarian cancer. Steroids 76:903–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu L, Wang J, Zhao L, Nilsen J, McClure K, Wong K, Brinton RD. 2009. Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology 150:3186–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Intlekofer KA, Petersen SL. 2011. 17β-estradiol and progesterone regulate multiple progestin signaling molecules in the anteroventral periventricular nucleus, ventromedial nucleus and sexually dimorphic nucleus of the preoptic area in female rats. Neuroscience 176:86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Intlekofer KA, Petersen SL. 2011. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience 172:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fueshko S, Wray S. 1994. LHRH cells migrate on peripherin fibers in embryonic olfactory explant cultures: an in vitro model for neurophilic neuronal migration. Dev Biol 166:331–348 [DOI] [PubMed] [Google Scholar]
- 32. Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. 1999. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci 19:2037–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Constantin S, Wray S. 2008. Gonadotropin-releasing hormone-1 neuronal activity is independent of cyclic nucleotide-gated channels. Endocrinology 149:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giacobini P, Kopin AS, Beart PM, Mercer LD, Fasolo A, Wray S. 2004. Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J Neurosci 24:4737–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kramer PR, Guerrero G, Krishnamurthy R, Mitchell PJ, Wray S. 2000. Ectopic expression of luteinizing hormone-releasing hormone and peripherin in the respiratory epithelium of mice lacking transcription factor AP-2α. Mech Dev 94:79–94 [DOI] [PubMed] [Google Scholar]
- 36. Toba Y, Pakiam JG, Wray S. 2005. Voltage-gated calcium channels in developing GnRH-1 neuronal system in the mouse. Eur J Neurosci 22:79–92 [DOI] [PubMed] [Google Scholar]
- 37. Gerdes D, Wehling M, Leube B, Falkenstein E. 1998. Cloning and tissue expression of two putative steroid membrane receptors. Biol Chem 379:907–911 [DOI] [PubMed] [Google Scholar]
- 38. Wray S, Gähwiler BH, Gainer H. 1988. Slice cultures of LHRH neurons in the presence and absence of brainstem and pituitary. Peptides 9:1151–1175 [DOI] [PubMed] [Google Scholar]
- 39. Gangatirkar P, Gangadharan S, Narendranath A, Nagpal S, Salunke DM, Karande AA. 2002. Monoclonal antibodies to gonadotropin-releasing hormone (GnRH) inhibit binding of the hormone to its receptor. Hybrid Hybridomics 21:281–286 [DOI] [PubMed] [Google Scholar]
- 40. Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. 1986. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159 [DOI] [PubMed] [Google Scholar]
- 41. Aparicio IM, Garcia-Herreros M, O'Shea LC, Hensey C, Lonergan P, Fair T. 2011. Expression, regulation, and function of progesterone receptors in bovine cumulus oocyte complexes during in vitro maturation. Biol Reprod 84:910–921 [DOI] [PubMed] [Google Scholar]
- 42. Nutu M, Weijdegård B, Thomas P, Bergh C, Thurin-Kjellberg A, Pang Y, Billig H, Larsson DG. 2007. Membrane progesterone receptor γ: tissue distribution and expression in ciliated cells in the fallopian tube. Mol Reprod Dev 74:843–850 [DOI] [PubMed] [Google Scholar]
- 43. Moore JP, Jr, Shang E, Wray S. 2002. In situ GABAergic modulation of synchronous gonadotropin releasing hormone-1 neuronal activity. J Neurosci 22:8932–8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kramer PR, Krishnamurthy R, Mitchell PJ, Wray S. 2000. Transcription factor activator protein-2 is required for continued luteinizing hormone-releasing hormone expression in the forebrain of developing mice. Endocrinology 141:1823–1838 [DOI] [PubMed] [Google Scholar]
- 45. Peluso JJ, Liu X, Gawkowska A, Johnston-MacAnanny E. 2009. Progesterone activates a progesterone receptor membrane component 1-dependent mechanism that promotes human granulosa/luteal cell survival but not progesterone secretion. J Clin Endocrinol Metab 94:2644–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meyer C, Schmid R, Scriba PC, Wehling M. 1996. Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Eur J Biochem 239:726–731 [DOI] [PubMed] [Google Scholar]
- 47. Peluso JJ, Romak J, Liu X. 2008. Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone's antiapoptotic action in spontaneously immortalized granulosa cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology 149:534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kemp JA, Marshall GR, Woodruff GN. 1986. Quantitative evaluation of the potencies of GABA-receptor agonists and antagonists using the rat hippocampal slice preparation. Br J Pharmacol 87:677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Honoré T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, Nielsen FE. 1988. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science 241:701–703 [DOI] [PubMed] [Google Scholar]
- 50. Davies J, Watkins JC. 1982. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res 235:378–386 [DOI] [PubMed] [Google Scholar]
- 51. Barbieri JT, Cortina G. 1988. ADP-ribosyltransferase mutations in the catalytic S-1 subunit of pertussis toxin. Infect Immun 56:1934–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bokoch GM, Katada T, Northup JK, Hewlett EL, Gilman AG. 1983. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem 258:2072–2075 [PubMed] [Google Scholar]
- 53. Casey PJ, Graziano MP, Gilman AG. 1989. G protein βγ subunits from bovine brain and retina: equivalent catalytic support of ADP-ribosylation of α subunits by pertussis toxin but differential interactions with Gs α. Biochemistry 28:611–616 [DOI] [PubMed] [Google Scholar]
- 54. Baulieu EE. 1991. The steroid hormone antagonist RU486. Mechanism at the cellular level and clinical applications. Endocrinol Metab Clin North Am 20:873–891 [PubMed] [Google Scholar]
- 55. Leonhardt SA, Boonyaratanakornkit V, Edwards DP. 2003. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids 68:761–770 [DOI] [PubMed] [Google Scholar]
- 56. Yoshitani N, Satou K, Saito K, Suzuki S, Hatanaka H, Seki M, Shinozaki K, Hirota H, Yokoyama S. 2005. A structure-based strategy for discovery of small ligands binding to functionally unknown proteins: combination of in silico screening and surface plasmon resonance measurements. Proteomics 5:1472–1480 [DOI] [PubMed] [Google Scholar]
- 57. Ahmed IS, Rohe HJ, Twist KE, Mattingly MN, Craven RJ. 2010. Progesterone receptor membrane component 1 (Pgrmc1): a heme-1 domain protein that promotes tumorigenesis and is inhibited by a small molecule. J Pharmacol Exp Ther 333:564–573 [DOI] [PubMed] [Google Scholar]
- 58. Ahmed IS, Rohe HJ, Twist KE, Craven RJ. 2010. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. J Biol Chem 285:24775–24782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mir SU, Ahmed IS, Arnold S, Craven RJ. 2012. Elevated progesterone receptor membrane component 1/σ-2 receptor levels in lung tumors and plasma from lung cancer patients. Int J Cancer 131:E1–E9 [DOI] [PubMed] [Google Scholar]
- 60. Peluso JJ, Liu X, Romak J. 2007. Progesterone maintains basal intracellular adenosine triphosphate levels and viability of spontaneously immortalized granulosa cells by promoting an interaction between 14-3-n]3σ and ATP synthase β/precursor through a protein kinase G-dependent mechanism. Endocrinology 148:2037–2044 [DOI] [PubMed] [Google Scholar]
- 61. Peluso JJ, Pappalardo A. 2004. Progesterone regulates granulosa cell viability through a protein kinase G-dependent mechanism that may involve 14-3-]3σ. Biol Reprod 71:1870–1878 [DOI] [PubMed] [Google Scholar]
- 62. Wei JY, Cohen ED, Yan YY, Genieser HG, Barnstable CJ. 1996. Identification of competitive antagonists of the rod photoreceptor cGMP-gated cation channel: β-phenyl-1,N2-etheno-substituted cGMP analogues as probes of the cGMP-binding site. Biochemistry 35:16815–16823 [DOI] [PubMed] [Google Scholar]
- 63. Constantin S, Klenke U, Wray S. 2010. The calcium oscillator of GnRH-1 neurons is developmentally regulated. Endocrinology 151:3863–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peluso JJ, Fernandez G, Pappalardo A, White BA. 2002. Membrane-initiated events account for progesterone's ability to regulate intracellular free calcium levels and inhibit rat granulosa cell mitosis. Biol Reprod 67:379–385 [DOI] [PubMed] [Google Scholar]
- 65. Viéro C, Méchaly I, Aptel H, Puech S, Valmier J, Bancel F, Dayanithi G. 2006. Rapid inhibition of Ca2+ influx by neurosteroids in murine embryonic sensory neurones. Cell Calcium 40:383–391 [DOI] [PubMed] [Google Scholar]
- 66. Duittoz AH, Batailler M. 2000. Pulsatile GnRH secretion from primary cultures of sheep olfactory placode explants. J Reprod Fertil 120:391–396 [PubMed] [Google Scholar]
- 67. Funabashi T, Daikoku S, Shinohara K, Kimura F. 2000. Pulsatile gonadotropin-releasing hormone (GnRH) secretion is an inherent function of GnRH neurons, as revealed by the culture of medial olfactory placode obtained from embryonic rats. Neuroendocrinology 71:138–144 [DOI] [PubMed] [Google Scholar]
- 68. Moore JP, Jr, Wray S. 2000. Luteinizing hormone-releasing hormone (LHRH) biosynthesis and secretion in embryonic LHRH. Endocrinology 141:4486–4495 [DOI] [PubMed] [Google Scholar]
- 69. Terasawa E, Keen KL, Mogi K, Claude P. 1999. Pulsatile release of luteinizing hormone-releasing hormone (LHRH) in cultured LHRH neurons derived from the embryonic olfactory placode of the rhesus monkey. Endocrinology 140:1432–1441 [DOI] [PubMed] [Google Scholar]
- 70. Terasawa E, Schanhofer WK, Keen KL, Luchansky L. 1999. Intracellular Ca(2+) oscillations in luteinizing hormone-releasing hormone neurons derived from the embryonic olfactory placode of the rhesus monkey. J Neurosci 19:5898–5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Constantin S, Caligioni CS, Stojilkovic S, Wray S. 2009. Kisspeptin-10 facilitates a plasma membrane-driven calcium oscillator in gonadotropin-releasing hormone-1 neurons. Endocrinology 150:1400–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wray S, Fueshko SM, Kusano K, Gainer H. 1996. GABAergic neurons in the embryonic olfactory pit/vomeronasal organ: maintenance of functional GABAergic synapses in olfactory explants. Dev Biol 180:631–645 [DOI] [PubMed] [Google Scholar]
- 73. Clarkson J, Herbison AE. 2006. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol Cell Endocrinol 254–255:32–38 [DOI] [PubMed] [Google Scholar]
- 74. Iremonger KJ, Constantin S, Liu X, Herbison AE. 2010. Glutamate regulation of GnRH neuron excitability. Brain Res 1364:35–43 [DOI] [PubMed] [Google Scholar]
- 75. Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. 1998. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J 17:2008–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tsai MJ, O'Malley BW. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- 77. Engmann L, Losel R, Wehling M, Peluso JJ. 2006. Progesterone regulation of human granulosa/luteal cell viability by an RU486-independent mechanism. J Clin Endocrinol Metab 91:4962–4968 [DOI] [PubMed] [Google Scholar]
- 78. Xia C, Bao Z, Yue C, Sanborn BM, Liu M. 2001. Phosphorylation and regulation of G-protein-activated phospholipase C-β 3 by cGMP-dependent protein kinases. J Biol Chem 276:19770–19777 [DOI] [PubMed] [Google Scholar]
- 79. Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, Hofmann F, Ruth P. 2000. Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Iβ. Nature 404:197–201 [DOI] [PubMed] [Google Scholar]
- 80. Francis SH, Busch JL, Corbin JD, Sibley D. 2010. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62:525–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Spitz IM, Bardin CW. 1993. Mifepristone (RU 486)—a modulator of progestin and glucocorticoid action. N Engl J Med 329:404–412 [DOI] [PubMed] [Google Scholar]
- 82. Zhang J, Tsai FT, Geller DS. 2006. Differential interaction of RU486 with the progesterone and glucocorticoid receptors. J Mol Endocrinol 37:163–173 [DOI] [PubMed] [Google Scholar]
- 83. Karalis K, Goodwin G, Majzoub JA. 1996. Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. Nat Med 2:556–560 [DOI] [PubMed] [Google Scholar]
- 84. Wei Y, Zhang C, Miao X, Xing F, Liu X, Zhao H, Zhan X, Han D. 2009. Effects of glucocorticoid on cyclic nucleotide-gated channels of olfactory receptor neurons. J Otolaryngol Head Neck Surg 38:90–95 [PubMed] [Google Scholar]
- 85. Wu X, Brewer G. 2012. The regulation of mRNA stability in mammalian cells: 2.0. Gene 500:10–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Malter JS. 2001. Regulation of mRNA stability in the nervous system and beyond. J Neurosci Res 66:311–316 [DOI] [PubMed] [Google Scholar]
- 87. Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. 2000. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci USA 97:12816–12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. 1990. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]